Abstract
NADPH oxidase (Nox) isoforms have been implicated in contributing to diabetic microvascular complications, but the functional role of individual isoforms in diabetic kidney are unclear. Nox2, in particular, is highly expressed in phagocytes and may play a key inflammatory role in diabetic kidney disease. To determine the role of Nox2, we evaluated kidney function and pathology in wild-type (WT; C57BL/6) and Nox2 knockout (KO) mice with type 1 diabetes. Diabetes was induced in male Nox2 KO and WT mice with a multiple low-dose streptozotocin protocol. Groups were studied for kidney disease after 8 and 20 wk of diabetes. Hyperglycemia and body weights were similar in WT and Nox2 KO diabetic mice. All functional and structural features of early and later stage diabetic kidney disease (albuminuria, mesangial matrix, tubulointerstitial disease, and gene expression of matrix and transforming growth factor-β) were similar in both diabetic groups compared with their respective nondiabetic groups, except for reduction of macrophage infiltration and monocyte chemoattractant protein-1 in the diabetic Nox2 KO mice. Systolic blood pressure by telemetry was surprisingly increased in Nox2 KO mice; however, the systolic blood pressure was reduced in the diabetic WT and Nox2 KO mice by tail-cuff. Interestingly, diabetic Nox2 KO mice had marked upregulation of renal Nox4 at both the glomerular and cortical levels. The present results demonstrate that lack of Nox2 does not protect against diabetic kidney disease in type 1 diabetes, despite a reduction in macrophage infiltration. The lack of renoprotection may be due to upregulation of renal Nox4.
Keywords: NADPH oxidase, albuminuria, oxidative stress, matrix, Nox4, Nox2, nephropathy
the nadph oxidase (nox) system is considered a key contributor to generation of reactive oxygen species (ROS) in many cell types and tissues (29). The first Nox isoform to be identified was Nox2 (i.e., gp91phox) in neutrophils and macrophages (18). However, recent studies have identified Nox2 at varying expression levels in numerous cell types, including endothelial, vascular smooth muscle, mesangial, and tubular epithelial cells (4, 13, 16). There are presently five identified isoforms of Nox, plus the related Duox1 and Duox2 isoforms (5, 8, 11). Nox1 is primarily found in endothelial cells and is stimulated by angiotensin II to generate ROS. Nox3 is fetal with only low-level expression in adult tissues. Nox4 was initially identified in the kidney, but is now found to be ubiquitous. Nox5 is expressed in human endothelial cells, but not by rodents. Several studies have implicated the Nox system in diabetic microvascular complications, such as nephropathy (7, 10, 12, 15, 27).
The functional role of the Nox system in mediating diabetic microvascular complications has been deduced primarily using inhibitors such as apocynin and diphenylene iodonium in both cell and animal models. Apocynin inhibited albuminuria and renovascular dysfunction of diabetes, protects against glucose-induced ROS production in podocytes, and reduced podocyte apoptosis in the db/db mouse model of type 2 diabetes (32). As apocynin primarily appears to inhibit assembly of Nox2 and potentially also Nox1 and Nox3, the effects of apocynin may be independent of Nox4. The role of Nox4 in diabetic kidney disease was also examined using injection of anti-sense oligonucleotides to Nox4, and a protective effect was demonstrated in the streptozotocin (STZ) rat model of type 1 diabetes (15). Although these prior studies demonstrate a potential role for Nox, the specific contribution of Nox2 has not been determined. We have, therefore, investigated the role of Nox2 in onset of diabetic kidney using wild-type (WT) and Nox2 knockout (KO) mice.
MATERIALS AND METHODS
Animal studies.
Male C57BL/6J WT and age-matched Nox2 KO mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were given standard rodent chow and water ad libitum. A cohort of 2-mo-old male WT and Nox2 KO mice were made diabetic with a multiple low-dose STZ protocol (60 mg·kg−1·day−1 × 5 days) using guidelines proposed by the Animal Models of Diabetic Complications Consortium (2, 35). Kidney structure and function were studied after 8 and 20 wk of diabetes. Blood glucose was measured using the Accu-Chek meter system (Roche). Portions of liver, heart, and kidney were snap-frozen in liquid nitrogen for RNA and protein isolation. An aliquot of kidney was frozen in OCT for immunostaining. All animal procedures were approved by the Institutional Animal Care and Use Committee of University of California, San Diego, and the Animal Ethics committee of the Alfred Hospital Medical Research and Education precinct and Baker IDI Heart and Diabetes Institute.
Urine albumin and hydrogen peroxide measurements.
Twenty-four-hour urine samples were collected from mice maintained in Nalgene metabolic cages after 8 wk of diabetes. Urine albumin and creatinine were measured using commercial kits (Albuwell and Creatinine companion kit, Exocell, Philadelphia, PA). Urine and serum creatinine were measured by HPLC, as previously described (3), according to the Animal Models of Diabetic Complications Consortium guideline. Creatinine clearance was estimated as the ratio of daily urinary creatinine excretion to plasma creatinine concentration, and expressed as milliliters per minute per square meter surface area. Urinary hydrogen peroxide was measured as an index of oxidative stress using the Amplex red assay, according to the manufacturer's instructions (Invitrogen, Eugene, OR) and as previously described (31).
Immunofluorescent staining.
Frozen kidney was cut into 4- to 6-μm sections, fixed using 4% paraformaldehyde and blocked with 5% fetal bovine serum for 1 h at room temperature. Sections were incubated overnight at 4°C with anti-Nox4 (Novus Biological), anti-collagen type IV (Abcam), anti-transforming growth factor (TGF)-β 1/2/3 (Santa Cruz Biotechnology), anti-fibronectin (FN) (Sigma-Aldrich), anti-F4/80 (Sigma-Aldrich) or anti-Nox2 (Santa Cruz Biotechnology,) antibodies, washed with ice-cold PBS, and then incubated with a rabbit or goat secondary antibody conjugated to Alexa Fluor dye (Molecular Probes) for 1 h in a humidified chamber in the dark. Sections were visualized by a confocal LSM 510 microscope (Zeiss).
Western blotting.
Kidney cortex was homogenized by sonication in lysis buffer (120 mM NaCl, 1 mM EGTA in PBS, pH 7.4), lysed for 20 min on ice, and centrifuged (15,000 rpm for 30 min). Proteins were separated by electrophoresis on polyacrylamide gels and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline with Tween for 1 h at 24°C. Membranes were incubated overnight at 4°C with anti-Nox4 (Novus) and anti-β-actin (Sigma) antibodies, followed by application of secondary antibodies and visualization by chemiluminescence. Results were normalized to β-actin levels in the same sample.
RNA isolation and quantitative real-time PCR.
Total RNA was extracted from kidney cortex using the TRIzol reagent (Invitrogen). One microgram of total RNA was reverse-transcribed, and RNA expression levels were quantified by real-time RT-PCR using a sequence detection system (Prism 7700; Applied Biosystems, Foster City, CA), as previously described (36). PCR was carried out using the following primers: Nox1, Nox2, Nox3, Nox4, p22phox, p47phox, monocyte chemoattractant protein-1 (MCP-1), α1-type IV collagen, FN, and TGF-β1. For quantitative analysis, the samples were normalized to 18S rRNA and β-actin expression using the ΔΔCT value method.
Morphological analysis.
The left kidney was fixed in 4% buffered formalin and then embedded in paraffin, cut into 4-μm sections, and stained with periodic-acid-Schiff (PAS) reagent. For evaluating glomerular size and mesangial matrix area, 25 randomly selected glomeruli in the outer cortex of each kidney section were evaluated by point-counting techniques, as described previously (30). Briefly, the microscopic image of the kidney was overlaid with grids. The intersections of grids were counted and grouped as follows: capillary lumen, PAS-positive area, or nucleus. The number of all of the grid intersections was calculated for glomerular size. The PAS-positive area was defined as the mesangial matrix area. Tubulointerstitial area (TIA) was estimated at the corticomedullary junction using a point-counting system (14). For each field, 100 points were assessed on a 1-cm2 eyepiece graticule with 10 equidistant gridlines. Six fields were analyzed per kidney at ×200. Results are expressed as the percentage of tubulointerstitial space within the area assessed. TIA was calculated according to the following equation: TIA = number of tubulointerstitial grid intersections/total number of intersections. Measurements were performed and analyzed by an observer blind to the experimental treatment and group.
Statistical analysis.
Data are expressed as means ± SE. Differences between groups were evaluated for significance using independent t-test or one-way ANOVA with the Student-Newman-Keuls post hoc test. P < 0.05 was considered significant.
RESULTS
Metabolic analysis and expression of Nox isoforms/regulatory subunits in Nox2 KO and WT mice.
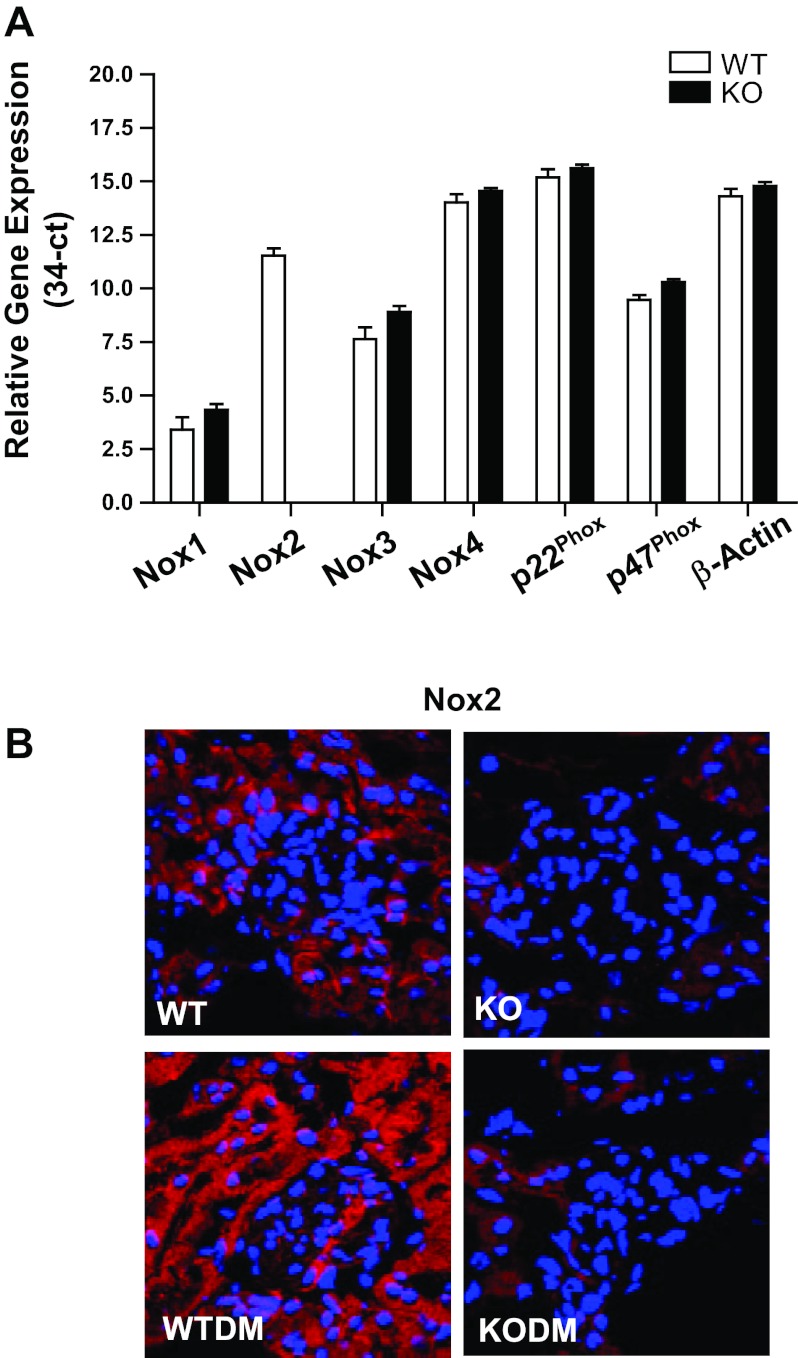
Nox isoforms and subunit expression were evaluated in the WT and the Nox2 KO mouse kidney cortex (Fig. 1A). In WT kidneys, Nox2 and Nox4 were the most abundantly expressed Nox isoforms. In the Nox2 KO mouse kidney, Nox2 was predictably absent, and there was no change in other Nox isoforms.
Fig. 1.
Expression of NADPH oxidase (Nox) isoforms and their regulatory subunits in kidney cortex of Nox2 knockout (KO) and wild-type (WT) mice. A: RNA expression of Nox subunits in kidney cortex of WT controls and Nox2 KO mice after 8 wk of diabetes, as assessed by quantitative RT-PCR Ct values for transcripts expressed as (34-Ct), using 34 as a theoretical “no-expression” value for quantitative RT-PCR. Values are means ± SE; n = 4 for each group. Open bars, WT mice; solid bars, Nox2 KO mice. B: immunofluorescence imaging with anti-Nox2 antibody was performed on frozen kidney sections from mice after 8 wk of diabetes. Nox2 (red); nuclei (blue). Original magnification, ×63. WTDM, WT diabetic mice; KODM, Nox2 KO diabetic mice.
Diabetes was induced in male 8-wk-old Nox2 KO and WT mice by intraperitoneal injection of 60 mg/kg body wt of STZ over 5 consecutive days. Mice displayed a significant increase of blood glucose levels 2 wk after STZ treatment. There was no difference in the rate of conversion to diabetes, with 97% (29/30) in the WT group and 95% (36/38) in the Nox2 KO group becoming hyperglycemic (>300 mg/dl). The degree of hyperglycemia and weight change was similar in both WT and Nox2 KO diabetic groups. Body weight was lower in both groups of diabetic mice compared with nondiabetic mice. There is a tendency for an increase in kidney weight in both diabetic groups after 8 wk of diabetes. A significant increase in the kidney-to-body weight ratio, a measure of kidney hypertrophy, was observed in both WT and Nox2 KO diabetic mice compared with genotype-matched nondiabetic mice (Table 1). By immunofluorescence, Nox2 protein was localized primarily in tubular cells in the kidney and increased in diabetic WT mice (Fig. 1B).
Table 1.
Physiological characteristics of WT and Nox2 KO mice after diabetes
| n | Duration of DM, wk | Body Weight, g | Blood Glucose, mg/dl | Kidney Weight, g | Kidney/Body Weight, % | |
|---|---|---|---|---|---|---|
| WT | 30 | 8 | 28.4 ± 0.7 | 194.0 ± 13.6 | 0.31 ± 0.06 | 0.54 ± 0.02 |
| WTDM | 20 | 8 | 24.6 ± 0.4* | 517.8 ± 18.7* | 0.34 ± 0.04 | 0.69 ± 0.02* |
| KO | 24 | 8 | 28.8 ± 0.4 | 207.5 ± 15.4 | 0.35 ± 0.05 | 0.60 ± 0.03 |
| KODM | 38 | 8 | 23.8 ± 0.4*† | 571.8 ± 12.8*† | 0.40 ± 0.07 | 0.84 ± 0.03*† |
| WT | 10 | 20 | 34.1 ± 0.91 | 219.6 ± 7.38 | 0.377 ± 0.025 | 0.55 ± 0.06 |
| WTDM | 10 | 20 | 26.6 ± 0.61* | 388.8 ± 11.88* | 0.487 ± 0.013* | 0.92 ± 0.07* |
| KO | 10 | 20 | 32.1 ± 0.72 | 176.4 ± 13.86 | 0.459 ± 0.055 | 0.71 ± 0.01 |
| KODM | 10 | 20 | 24.4 ± 1.41*† | 419.4 ± 32.4† | 0.431 ± 0.015 | 0.88 ± 0.04*† |
Values are means ± SE; n, no. of mice. WT, wild-type mice; WTDM, streptozotocin (STZ)-induced diabetic wild-type mice; KO, Nox2 knockout mice; KODM, STZ-induced diabetic Nox2 knockout mice; DM, diabetes mellitus.
P < 0.05 vs. WT.
P < 0.05 vs. KO.
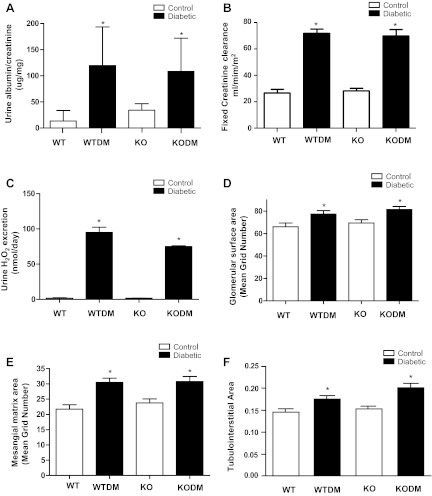
Effects of Nox2 deletion on urinary albuminuria, creatinine clearance, and urine hydrogen peroxide.
After 8 wk of diabetes, there was an increase in albuminuria in both WT and Nox2 KO mice. The albumin-to-creatinine ratio was significantly (P <0.05) increased in both diabetic WT mice and diabetic Nox2 KO mice compared with nondiabetic animals (Fig. 2A). After 20 wk of diabetes, the induction of diabetes was associated with an increased in creatinine clearance (hyperfiltration) in both diabetic WT mice and diabetic Nox2 KO mice compared with nondiabetic animals (Fig. 2B). Hydrogen peroxide in urine was measured as an index of renal oxidative stress. After 8 wk of diabetes, there was a 15-fold increase of urinary hydrogen peroxide excretion in both the WT mice and the Nox2 KO mice (Fig. 2C).
Fig. 2.
Effects of Nox2 deletion on urinary albuminuria, creatinine clearance, oxidative stress, and glomerular and tubulointerstitial pathology. Urinary albumin-to-creatinine ratio (A) and urine H2O2 excretion (C) were measured in diabetic mice 8 wk after induction of diabetes. WT mice, n = 30; WTDM mice, n = 24; Nox2 KO mice, n = 24; KODM mice, n = 38. Glomerular surface area (D) and mesangial matrix area (E) were measured by morphometric analysis of glomeruli in kidneys after 8 wk of diabetes. Creatinine clearance by HPLC (B) and tubulointerstitial area (F) using a point-counting system were measured after 20 wk of diabetes. Values are group means ± SE; n = 5 for each group in B, D, E, and F. *P < 0.05 vs. control group.
Glomerular and tubulointerstitial pathology in both diabetic Nox2 KO and WT mice.
Glomerular histology was normal in Nox2 KO mice. Diabetes-induced glomerular enlargement was of similar magnitude in both WT diabetic and Nox2 KO diabetic mice after 8 wk of diabetes (Fig. 2D). Glomerular mesangial matrix, quantified by PAS staining, was increased to a similar extent in WT and Nox2 KO diabetic mice after 8 wk of diabetes (Fig. 2E). To determine whether chronic features of diabetic kidney disease could be affected by Nox2, mice were also studied at 20 wk of diabetes. There was a modest but significant increase in TIA in both WT and Nox2 KO diabetic mice after 20 wk of diabetes, but no significant difference between WT and Nox2 KO diabetic mice (Fig. 2F).
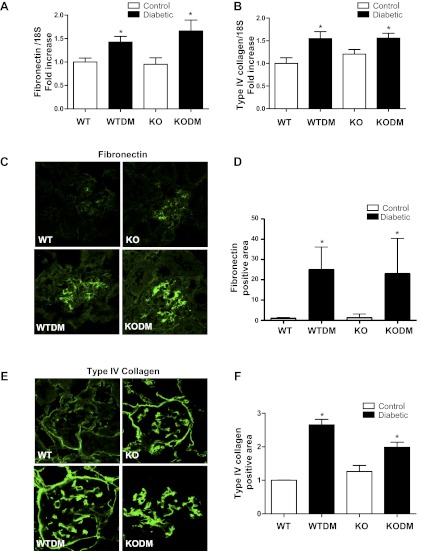
Expression of FN and type IV collagen in diabetic Nox2 KO and WT mice.
Real-time PCR showed a similar diabetes-induced increase in gene expression of the extracellular matrix proteins FN and type IV collagen in glomeruli from both WT and Nox2 KO mice (Fig. 3, A and B). Localization of FN and type IV collagen by immunofluorescence indicated that glomerular FN and type IV collagen were significantly increased in both WT and Nox2 KO diabetic mice (Fig. 3, C–F).
Fig. 3.
The deposition of matrix proteins in glomeruli of diabetic mice. The expression levels of fibronectin (A) and type IV collagen (B) mRNAs in kidney cortex were determined by quantitative RT-PCR from mice after 8 wk of diabetes. Data were normalized to 18S. n = 4. Immunofluorescence image and quantification with anti-fibronectin (C and D) and anti-type IV collagen (E and F) antibodies was performed on frozen sections from mice after 8 wk of diabetes. Quantification of glomerular protein (green) pixel density divided by glomerular area is shown. Original magnification, ×63. Data are expressed as fold induction (4 images per mouse, 3 male mice/group). Values are group means ± SE. *P < 0.05 vs WT.
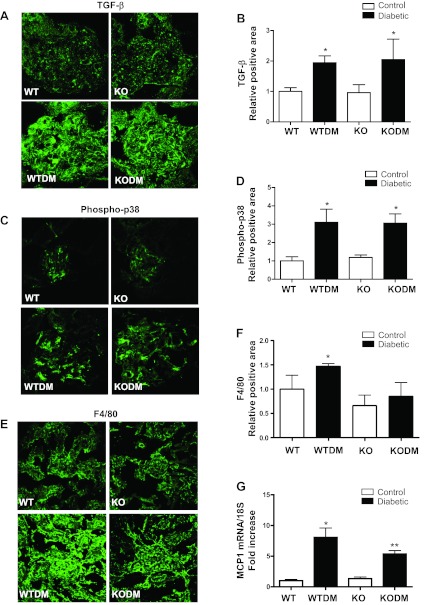
Regulation of TGF-β, phospho-p38 MAPK, macrophages, and MCP-1 in diabetic Nox2 KO and WT mice.
TGF-β, phospho-p38 MAPK, MCP-1, and macrophage infiltration were examined to determine whether specific mediators of fibrosis and inflammation were affected by Nox2 deletion. There was a significant and similar induction of glomerular TGF-β and phospho-p38 MAPK expression under diabetic conditions in both WT and Nox2 KO mice (Fig. 4, A–D). The numbers of macrophages were significantly increased only in WT diabetic mice (Fig. 4 E and F). Real-time PCR showed that MCP1 gene expression was stimulated in WT diabetic kidney but significantly less in the Nox2 KO diabetic mice (Fig. 4G).
Fig. 4.
Expression of transforming growth factor (TGF)-β, phospho-p38 MAPK, F4/80, and monocyte chemoattractant protein (MCP)-1. Immunofluorescence images with quantitative analysis of TGF-β (A and B), phospho-p38 MAPK (C and D) in the glomeruli, and F4/80 (E and F) of kidney cortex were performed on frozen sections from mice after 8 wk of diabetes. Sections were visualized by confocal microscopy. Original magnification, ×63. Quantification of glomerular protein (green) pixel density divided by glomerular area is shown. Data are expressed as fold induction (4 images per mouse, 3 mice/group). G: MCP-1 mRNA expression showed in kidney cortex by quantitative RT-PCR. Data, normalized to 18S, are presented as the fold-increase relative to control. Values are group means ± SE; n = 4 for each group. *P < 0.05 vs. WT. **P < 0.05 vs. all other groups.
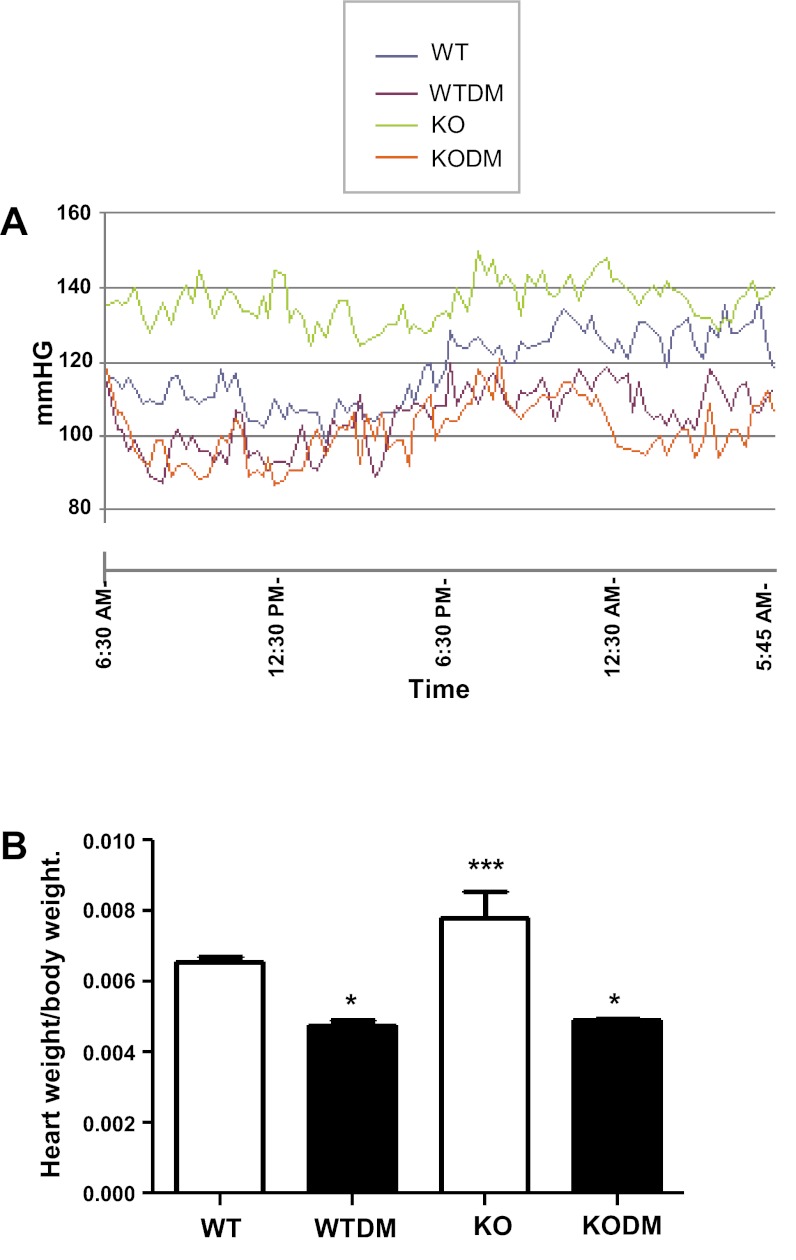
Blood pressure and the heart weight-to-body weight ratio in WT and Nox2 KO mice.
Systolic blood pressure was increased in Nox2 KO mice compared with WT mice when measured by either tail-cuff or radiotelemetry (Fig. 5A and Table 2). Diabetes (8 wk) increased systolic blood pressure in both WT and Nox2 KO mice when measured by the tail-cuff method. However, this finding was not confirmed by radiotelemetry, which recorded a significant reduction in systolic blood pressure in both diabetic groups (Table 2). The expected reduction in blood pressure during sleep was found in all groups, although there was a blunting of the somnolent drop in the Nox2 KO mice without diabetes. The heart rate was significantly lower in both groups of diabetic mice. Heart weight-to-body weight ratio was significantly greater in the KO mice and was reduced in the diabetic mice vs. WT control mice (Fig. 5B).
Fig. 5.
Daily profiles of systolic blood pressure by radiotelemetry (A) and heart weight-to-body weight ratio (B) in WT and Nox2 KO mice after 8 wk of diabetes (n = 10). Values are group means ± SE. *P < 0.0001 vs. control group. ***P < 0.0001 vs. all other groups.
Table 2.
Systolic BP, heart rate, and body weight for WT and Nox2 KO mice after 8-wk diabetes
| Systolic BP by Tail-cuff, mmHg | Systolic BP by Radiotelemetry, mmHg | Heart Rate, beats/min | Body Weight, g | |
|---|---|---|---|---|
| WT | 116.32 ± 1.65 | 118.05 ± 1.174 | 505.50 ± 8.474 | 26.82 ± 2.38 |
| WTDM | 135.32 ± 3.36* | 104.98 ± 1.588* | 412.10 ± 7.290* | 24.46 ± 2.39* |
| KO | 136.21 ± 4.29* | 136.06 ± 2.834* | 484.60 ± 9.708* | 24.01 ± 3.31 |
| KODM | 141.41 ± 3.18* | 101.35 ± 1.25*† | 408.86 ± 7.246*† | 24.31 ± 2.37 |
Values are means ± SE; n = 10 for all groups. BP, blood pressure.
P < 0.05 vs. WT.
P < 0.05 vs. KO.
Nox4 expression in the glomeruli of Nox2 KO diabetic mice.
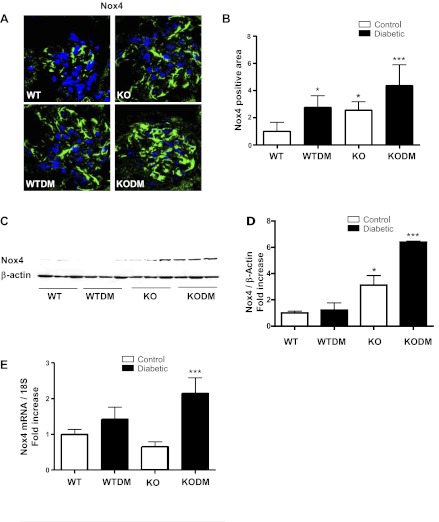
To address the lack of renal protection in Nox2 KO diabetic mice, we investigated compensation by other components of the NAPDH oxidase system. There was no significant increase in gene expression by real-time PCR of any of the Nox isoforms or Nox subunits in the Nox2 KO diabetic kidney after 8 wk of diabetes (data not shown). However, by immunostaining, there was a marked upregulation of glomerular Nox4 (Fig. 6, A and B). Overlay studies using a podocyte-specific antibody indicated that the increase in glomerular Nox4 was primarily in podocytes. In addition, immunoblot analysis of renal cortical protein was performed. There was a marked increase in cortical Nox4 in the Nox2 KO diabetic kidney cortex (Fig. 6, C and D). Real-time PCR revealed a significant induction in Nox4 expression in 20-wk diabetic Nox2 KO mice compared with diabetic WT mice (Fig. 6E). There was a significant increase in Nox4 protein in the Nox2 KO mouse kidney by immunoblotting and immunofluorescence staining. However, there was no increase by real-time PCR for Nox4 gene expression in 8 wk (Fig. 1A) and 20 wk of nondiabetic kidney (Fig. 6E).
Fig. 6.
Expression of Nox4 was increased in diabetic Nox2 KO mice. A: immunofluorescence imaging with anti-Nox4 antibody was performed on frozen sections from mice after 8 wk of diabetes. Sections were visualized by confocal microscopy. Original magnification, ×63. B: quantification of glomerular protein (green) pixel density divided by glomerular area is shown. Data are expressed as fold induction (4 images per mouse, 3 mice/group). The protein levels of Nox4 were analyzed by Western blot analysis (C) and quantitative analysis (D) of Nox4 protein levels and Nox4 gene expression (E) by quantitative RT-PCR in the kidney cortex after 20 wk of diabetes. Values are group means ± SE; n = 3 for each group. *P < 0.05 vs. WT. ***P < 0.05 vs. all other groups.
DISCUSSION
Our results show that albuminuria, H2O2 production, renal and glomerular hypertrophy, induction of TGF-β, phospho-p38 MAPK, and extracellular matrix expression in glomeruli are similarly increased in WT and Nox2 KO diabetic mice. Diabetic glomerular and tubulointerstitial disease were also not affected by deletion of Nox2.
Nox2 KO mice exhibited a relatively normal phenotype, other than showing mild hypertension, as detected by both tail-cuff plethysmography and radiotelemetry. Generation of ROS by endothelial Nox2 has been associated with hypertension (22), so deletion of Nox2 would not be predicted to promote hypertension, unless compensatory mechanisms are activated to result in hypertension. Deletion of Nox2 also did not alter the general manifestations of type 1 diabetes, such as hyperglycemia and weight loss. It was notable that diabetic mice, both WT and Nox2 KO, were hypotensive by radiotelemetry, but hypertensive by tail-cuff plethymography. The hypotensive consequences of STZ-induced diabetes in rodents are well documented in conscious animals (17, 34), highlighting concerns that tail-cuff plethysmography can be inaccurate in diabetic rodents due to a reliance on assumptions of consistent mechanical properties of the skin and collagen in the tail.
Nox2 KO mice have been reported to exhibit reduced superoxide production in organs, such as the heart, lungs, and brain, and were protected from various pathophysiological conditions, such as cardiac hypertrophy, hypoxic pulmonary hypertension, and surgically induced brain injury (9, 20, 21). However, as Nox2 KO diabetic mice had no reduction in functional and structural features of diabetic renal disease, our results suggest that Nox2 is not critical for disease pathogenesis, and that other Nox family members may be of greater importance in the pathogenesis of diabetic kidney disease. Nox4 may be of particular interest, as there was a marked increase in Nox4 mRNA and protein in the renal cortex and specifically in the glomeruli of diabetic Nox2 KO mice. Prior work suggests that diabetes leads to increased expression of Nox subunits Nox4 and p22phox, which may be responsible for overproduction of ROS and the renal damage seen in diabetes (19, 25). Renal Nox4 was upregulated in a cortex-specific manner, and Nox4 contributed to increased ROS generation, p38 phosphorylation, and FN and TGF-β expression in kidneys from type 2 diabetic db/db mice, while knockdown of Nox4 by siRNA in vitro decreased TGF-β and FN production in mouse proximal tubules (6, 27). At the time of the preparation of this paper, the role of Nox4 in diabetic kidney disease was recently examined using pharmacological therapy and with Nox4 KO mice. GKT136901, a Nox1/4 inhibitor, attenuates the development of nephropathy in db/db mice (28). A renoprotective effect of the Nox1/4 inhibitor may be beneficial through reduced oxidative damage and decreased ERK1/2 activation. (28). Surprisingly, a separate study found no protective kidney benefit in the Nox4-deficient mouse in models of unilateral ureteral obstruction or STZ-induced diabetes (1), and an additional study found that Nox4-deficient mice had enhanced fibrosis after unilateral ureteral obstruction, perhaps due to lack of nuclear factor-erythroid 2-related factor 2 and hypoxia-inducible factor-1α upregulation (23). Thus it appears that Nox4 above baseline does contribute to diabetic kidney disease; however, baseline levels of Nox4 may also provide protective effects.
The mechanism that drives upregulation of Nox4 expression in the diabetic mouse kidney after deletion of Nox2 has yet to be defined. It was recently shown that Nox2 and Nox4 each compensate for the deficiency of each other in endothelial cells of the lung (24). It will be of interest to determine whether deletion or overexpression of Nox4 alters Nox2 levels and affects the overall pattern of diabetic kidney disease. Further studies are in progress to unravel the role of Nox4 and related signaling pathways activated by hyperglycemia in regulating Nox4 expression and ROS production in kidney.
The diabetes-induced infiltration of macrophages into the kidney was reduced in diabetic Nox2 KO mice. This may reflect a reduction in macrophage recruitment, as gene expression of the chemotactic factor MCP-1 was significantly lower in kidneys of diabetic Nox2 KO mice compared with diabetic WT mice. As MCP-1 production can occur in other cells, including podocytes in glomeruli of diabetic kidney (33) and activation of MCP-1 gene expression is mediated by ROS (19, 26), Nox2-derived ROS may be involved in pathways that enhance production of MCP-1 from macrophages, podocytes, and other cells in the diabetic kidney. The lack of Nox2-derived ROS has the potential to reduce the overall numbers of infiltrating inflammatory cells into the kidney. However, while reduced macrophage infiltration may be predicted to attenuate renal pathology in diabetes, the degree of glomerular and tubulointerstitial disease was not affected in the Nox2 KO diabetic mice.
In summary, we have demonstrated that glomerular mesangial matrix expansion, tubulointerstitial disease, and albuminuria were not ameliorated in diabetic Nox2 KO mice. These results suggest that ablating Nox2 activity does not protect against the major features of diabetic kidney disease.
GRANTS
This study was supported by grants from the National Institutes of Health (R01 DK-053867, 1DP3DK-094352-01 and U01 DK-060995), a VA Merit Award, and the Juvenile Diabetes Research Foundation to K. Sharma.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.-H.Y., K.A.J.-D., D.B., and K.S. conception and design of research; Y.-H.Y., S.O., S.L., D.B., and T.N. performed experiments; Y.-H.Y., S.O., D.B., T.N., and K.S. analyzed data; Y.-H.Y., S.O., and K.S. interpreted results of experiments; Y.-H.Y. prepared figures; Y.-H.Y. and K.S. drafted manuscript; Y.-H.Y. and K.S. edited and revised manuscript; Y.-H.Y., K.A.J.-D., and K.S. approved final version of manuscript.
REFERENCES
- 1. Babelova A, Avaniadi D, Jung O, Fork C, Beckmann J, Kosowski J, Weissmann N, Anilkumar N, Shah AM, Schaefer L, Schroder K, Brandes RP. Role of Nox4 in murine models of kidney disease. Free Radic Biol Med 53: 842–853, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Breyer M, Bottinger E, Brosius Fr Coffman T, Harris R, Heilig C, Sharma KA. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-alpha and -gamma agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol Dial Transplant 21: 2399–2405, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Cohen MP, Sharma K, Guo J, Eltayeb BO, Ziyadeh FN. The renal TGF-beta system in the db/db mouse model of diabetic nephropathy. Exp Nephrol 6: 226–233, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci 73: 1813–1824, 2003 [DOI] [PubMed] [Google Scholar]
- 8. De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Deng S, Kruger A, Kleschyov AL, Kalinowski L, Daiber A, Wojnowski L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic Biol Med 42: 466–473, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Ding H, Hashem M, Triggle C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur J Pharmacol 561: 121–128, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J Biol Chem 274: 37265–37269, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 46: 1428–1437, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res 71: 289–299, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Giunti S, Calkin AC, Forbes JM, Allen TJ, Thomas MC, Cooper ME, Jandeleit-Dahm KA. The pleiotropic actions of rosuvastatin confer renal benefits in the diabetic Apo-E knockout mouse. Am J Physiol Renal Physiol 299: F528–F535, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Gorlach A, Brandes RP, Bassus S, Kronemann N, Kirchmaier CM, Busse R, Schini-Kerth VB. Oxidative stress and expression of p22phox are involved in the up-regulation of tissue factor in vascular smooth muscle cells in response to activated platelets. FASEB J 14: 1518–1528, 2000 [PubMed] [Google Scholar]
- 17. Hebden RA, Bennett T, Gardiner SM. Pressor sensitivities to vasopressin, angiotensin II, or methoxamine in diabetic rats. Am J Physiol Regul Integr Comp Physiol 253: R726–R734, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Hilenski L, Clempus R, Quinn M, Lambeth JD, Greindling K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14: S227–S232, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett 414: 228–232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol 106: 527–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nlandu Khodo S, Dizin E, Sossauer G, Szanto I, Martin PY, Feraille E, Krause KH, de Seigneux S. NADPH-Oxidase 4 Protects against Kidney Fibrosis during Chronic Renal Injury. J Am Soc Nephrol 23: 1967–1976, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 288: F1144–F1152, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Satriano JA, Shuldiner M, Hora K, Xing Y, Shan Z, Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest 92: 1564–1571, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Sedeek M, Gutsol A, Montezano AC, Burger D, Nguyen Dinh Cat A, Kennedy CR, Burns KD, Cooper ME, Jandeleit-Dahm K, Page P, Szyndralewiez C, Heitz F, Hebert RL, Touyz RM. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin Sci (Lond) 124: 191–202, 2013 [DOI] [PubMed] [Google Scholar]
- 29. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seyer-Hansen K, Hansen J, Gundersen HJ. Renal hypertrophy in experimental diabetes. A morphometric study. Diabetologia 18: 501–505, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 33. Tarabra E, Giunti S, Barutta F, Salvidio G, Burt D, Deferrari G, Gambino R, Vergola D, Pinach S, Perin PC, Camussi G, Gruden G. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes 58: 2109–2118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tomlinson KC, Gardiner SM, Bennett T. Blood pressure in streptozotocin-treated Brattleboro and Long-Evans rats. Am J Physiol Regul Integr Comp Physiol 258: R852–R859, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Williams KJ, Qiu G, Usui HK, Dunn SR, McCue P, Bottinger E, Iozzo RV, Sharma K. Decorin deficiency enhances progressive nephropathy in diabetic mice. Am J Pathol 171: 1441–1450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int 76: 32–43, 2009 [DOI] [PubMed] [Google Scholar]