Abstract
The ammonia transporter family member, Rh B Glycoprotein (RhBG/Rhbg), is essential for ammonia transport by the rodent kidney, but in the human kidney mRNA but not protein expression has been reported. Because ammonia transport is fundamental for acid-base homeostasis, the current study addressed RhBG expression in the human kidney. Two distinct RhBG mRNA sequences have been reported, with different numbers of consecutive cytosines at nt1265 and thus encoding different carboxy-tails. Sequencing the region of difference in both human kidney and liver mRNA showed eight sequential cytosines, not seven as in some reports. Knowing the correct mRNA sequence for RhBG, we then assessed RhBG protein expression using antibodies against the correct amino acid sequence. Immunoblot analysis demonstrated RhBG protein expression in human kidney and immunohistochemistry identified basolateral RhBG in connecting segment (CNT) and the cortical and outer medullary collecting ducts. Colocalization of RhBG with multiple cell-specific markers demonstrated that that CNT cells and collecting duct type A intercalated cells express high levels of RhBG, and type B intercalated cells and principal cells do not express detectable RhBG. Thus, these studies identify the correct mRNA and thus protein sequence for human RhBG and show that the human kidney expresses basolateral RhBG protein in CNT, type A intercalated cells, and non-A, non-B cells. We conclude that RhBG can mediate an important role in human renal ammonia transport.
Keywords: renal ammonia excretion, ammonia transport, acid-base homeostasis, RhBG
renal ammonia excretion is the primary component of basal net acid excretion and changes in ammonia excretion are the primary component of the renal response to the majority of acid-base perturbations (38). Importantly, renal ammonia excretion involves integrated intrarenal ammoniagenesis and epithelial cell transport involving essentially all renal epithelial cells (34, 37, 38). The final site of ammonia transport occurs in the collecting duct, which secretes 60–80% of total urinary ammonia (9, 37). Thus, understanding the molecular mechanisms of collecting duct ammonia secretion is fundamental to understanding acid-base homeostasis.
Our understanding of collecting duct ammonia transport has undergone fundamental changes in the last several years. Almost 20 years ago collecting duct ammonia secretion was shown to involve parallel NH3 and H+ secretion, without significant levels of NH4+ permeability (6, 17). For many years, the NH3 permeability was assumed to be due to lipid-phase NH3 diffusion. However, recent studies have generated a new understanding of the molecular mechanisms of collecting duct ammonia secretion. In particular, the nonerythroid glycoproteins, Rh B Glycoprotein and Rh C Glycoprotein, are ammonia-specific transporters (6, 17, 37, 38) expressed in the distal convoluted tubule (DCT), connecting segment (CNT), and the collecting duct (7, 26, 31). In animal models, multiple conditions with altered renal ammonia transport have parallel changes in Rhbg and Rhcg expression (11, 12, 16, 22, 27). Definitive evidence for the functional significance of Rhbg and Rhcg expression was shown in studies in which genetic deletion of either Rhbg or Rhcg altered basal ammonia metabolism and impaired ammonia excretion in response to an acid load (3, 4, 19, 20).
However, it has not been clear that RhBG has a role in human renal ammonia excretion because RhBG protein to date has not been detected in the human kidney. In the initial report identifying RhBG, the human kidney was shown to express relatively high levels of RhBG mRNA (23). A subsequent study confirmed RhBG mRNA expression, but it was unable to detect RhBG protein in the human kidney (5). Because metabolic acidosis increases mouse renal Rhbg protein expression and genetic deletion of Rhbg from intercalated cells alters both basal and acidosis-stimulated ammonia excretion (3), determining whether RhBG protein, in addition to RhBG mRNA, is present in the human kidney is important.
Thus, the purpose of the current studies was to examine RhBG expression in the human kidney. First, two different reported mRNA sequences, which encode differing carboxy-terminal sequences, have been reported for human RhBG; we determined which is actually present. We then identified that a vector commonly used for expressing RhBG encodes a frame-shift mutation of RhBG. Next, we used multiple antibodies to show RhBG protein expression in the human kidney. Finally, we determined RhBG's specific cellular localization in the human kidney. Our results indicate that RhBG is present in the human kidney, and it is expressed in the CNT and in type A and non-A, non-B intercalated cells, a pattern essentially identical to its expression in the mouse and rat kidney.
METHODS
Antibodies.
Peptides comprising amino acids 37–51, 207–221, and 427–441 of RhBG were generated by the Interdisciplinary Center for Biotechnology Research of the University of Florida using standard techniques, coupled to keyhole limpet hemocyanin, and used to generate polyclonal antibodies (Cocalico Biological, Reamstown, PA). These antibodies were then affinity-purified using standard techniques and are referred to as p37, p207, and p427 in this report. Affinity-purified antibodies to human RhBG, aa 35–84, referred to as p35, were obtained from Aviva Systems Biology (San Diego, CA; ARP49502_P050). Fiona Karet, Ph.D., supplied antibodies to the a4 subunit of H+-ATPase; Susan Wall, M.D., (Emory University, Atlanta, GA) supplied antibodies to pendrin; and David Ellison, M.D., (Oregon Health Sciences University, Portland, OR) supplied antibodies to thiazide-sensitive cotransporter (NCC). The peptide used to generate p35 was obtained from Aviva Systems Biology.
Human tissue samples.
We obtained normal human kidney mRNA from Strategene (Santa Clara, CA) and from Clontech Laboratories (Mountain View, CA), and normal human liver mRNA from Clontech Laboratories. We obtained human renal total protein lysates from Clontech Laboratories and renal membrane lysates from Novus Biologicals (Littleton, CO). Normal human kidney samples for immunohistochemistry were from unused portions of nephrectomy specimens from treatment of renal cell carcinoma from the pathology laboratory of the Gainesville Veterans Affairs Medical Center. IRB approval for use of human renal samples was obtained from the University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System Institutional Review Boards.
Animal samples.
Mice with floxed Rhbg gene generated in our laboratory and described previously (2, 3) and expressing Cre-recombinase under control of the Ksp-cadherin promoter (19, 21, 29) were used to generate mice with collecting duct-specific Rhbg knockout (CD-Rhbg-KO). Littermates that did not express Cre-recombinase were used as control mice. All breeding was performed in the University of Florida College of Medicine Transgenic Animal Facility by trained personnel. Genotyping was performed using DNA obtained from tail-clip samples using procedures we detailed previously (3, 19). All animal studies were approved by the University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System Institutional Animal Care and Use Committees.
Mouse tissue preparation for immunolabeling.
Mice were anesthetized with inhalant isoflurane. Kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP), then cut transversely into several 2- to 4-mm-thick slices, and immersed 24 h at 4°C in the same fixative. Kidney samples from each animal were embedded in polyester wax made using polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol, and 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunoblotting procedure.
Immunoblot analysis was performed using standard techniques previously described in detail (3, 10, 19, 20, 35, 36). Briefly, 10 μg of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.6), and incubated at 4°C overnight with primary antibody diluted in nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining. After being washed, membranes were exposed to secondary antibody, goat anti-rabbit IgG (Millipore, Billerica, MA), and conjugated to horseradish peroxidase at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 5.0, software (Kodak Scientific Imaging, New Haven, CT). Absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Immunohistochemistry.
Immunolocalization was accomplished by using immunoperoxidase procedures similar to those detailed previously (3, 10, 19, 20, 35, 36), with exceptions as noted below. Tissue deglycosylation involved treatment with glycoprotein denaturing buffer (New England Biolabs, Ipswich, MA) for 20 min at 100°C, rinsing in PBS, treatment with peptide:N-glycosidase F (PNGase F; P0704L, New England Biolabs) 1–25 U/μl, 1% NP-40 in 0.05 M Na phosphate buffer for 2 h at 37°C, and followed by being rinsed in PBS. Tissues then underwent several washes in PBS containing 1% BSA, 0.05% saponin, and 0.2% gelatin, followed by incubation with Serum-Free Protein Block (DakoCytomation) for 15 min, and then incubation at 4°C overnight with primary antibody. After several washes in PBS containing 0.1% BSA, 0.05% saponin, and 0.2% gelatin, the sections were washed in PBS and incubated for 1 h with polymer-linked peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA), again washed with PBS, and then exposed to diaminobenzidine for 5 min. The sections were washed in distilled water and then dehydrated in a graded series of ethanols and xylene and mounted with Permount. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon Instruments, Melville, NY). Color correction, automatic contrast adjustment, and figure generation were performed using Adobe Photoshop, versions CS and CS5.
Double immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (10, 11, 13). Briefly, tissue sections were labeled with the first primary antibody following the procedure described above, using Vector SG (Vector Laboratories) as the chromogen to produce a blue label. After the Vector SG reaction, sections were washed in distilled water and then blocked by incubating the sections in 3% H2O2 at room temperature for 45 min and Serum-Free Protein Block as described in the single label procedure. The above procedure was repeated with the substitution of a second primary antibody and the substitution of DAB for Vector SG. The sections were then washed with glass-distilled water, dehydrated with xylene, mounted, and observed by light microscopy.
CLIP-tag RhBG in pcDNA5/FRT.
The plasmid expressing human RhBG in pEGFP-C1 was a generous gift of C.-H Huang, New York Blood Center (23). The region expressing RhBG was fused to the C-terminus of the CLIP-tag using XhoI and NotI sites of pCLIPm vector (New England BioLabs) by using Pfu Ultra High-Fidelity DNA polymerase (Stratagene, La Jolla, CA) for PCR amplification using primers (XhoI) 5′-GCC-CTC-GAG-ATG-GCC-GGG-TCT-CCT-AGC-3′ and (NotI) 5′-CGT-CGC-GGC-CGC-TTA-GGC-CTG-AGT-GTC-TGC-CT-3′. With the use of the available EcoRV site on the N-terminus of CLIP-tag and the NotI site on the C-terminus of RhBG, the CLIP-tag RhBG was subcloned into pcDNA5/FRT for use with the Flp-In CV-1 cell line (Invitrogen, Grand Island, NY). We generated c-RhBG and c-GFP-RhBG using Quik-Change II Site-directed Mutagenesis Kit (Stratagene) according to the manufacturer's instructions.
Cell culture.
HEK-293 and CV-1 cells were grown in DMEM/F12, 10% fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES, and pen/strep. With the use of Lipofectamine 2000, cells in suspension were cotransfected with CLIP-tag c-RhBG or c-GFP-RhBG in pcDNA5/FRT.
RNA and cDNA for sequencing.
Total RNA from human kidney and liver was obtained from Clontech and an additional sample from human kidney was obtained from Stratagene to confirm the results. CV-1 cells were grown to confluence, rinsed with PBS, and immediately used to isolate RNA using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was generated from RNA using SuperScript III First-Strand Synthesis for RT-PCR according to the procedure recommended by the manufacturer (Invitrogen). Primers: forward primer, 5′-ACCTCCATGGGATGCCGGGG-3′ and reverse primer, 5′-ACTTGGTCCTCGTAGTGCTGGGA-3′ were used to amplify the region from nucleotide 1028–1299. DNA sequencing was performed by the University of Florida Interdisciplinary Center for Biological Research. Sequence alignment was performed using CluxtalX, v2.1, software (18).
Protein antigenicity prediction.
Protein antigenicity was predicted using AbDesigner software from Mark Knepper, M.D., Ph.D. and used as recently reported (25).
RESULTS
Sequence of RhBG mRNA.
Two sequences for human RhBG mRNA that differ in whether the codons starting at nucleotide 1265 contain a stretch of seven or eight consecutive cytosine bases have been reported (Fig. 1A). This difference results in a frame-shift mutation distal to the nucleotide sequence difference, causing a completely different amino acid sequence in the carboxy-tails of the translated proteins, beginning at amino acid 425 (Fig. 1B). To determine the correct sequence for human RhBG mRNA and protein, we sequenced the relevant nucleotides in mRNA from human kidney and liver. These results are shown in Fig. 1, C-E. Sequencing mRNA from human kidney from two different sources and from human liver showed eight cytosines beginning at nucleotide 1265, identical to that observed in published sequence CAI12178.1 and differing from that for NM_020407.4. We then analyzed the human DNA sequence and identified eight cytosines at the relevant site in chromosome 1 (1). These results indicate the correct mRNA sequence for human RhBG has eight consecutive cytosines at nucleotide 1265. Thus, the correct carboxy-terminal sequence for RhBG, beginning at amino acid 410, is GGGLLLKLPF-LDSPPRLPAL-RGPSSLAGAW-RA, not GGGLLLKLPF-DSPPDSQHY-EDQVHWQVPG-EHEDKAQRP-LRVEEADTQA as predicted from mRNA sequence NM_020407.1 (Fig. 1B).
Fig. 1.
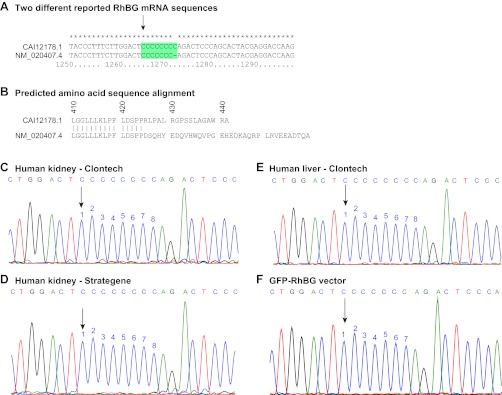
Multiple sequence alignment of reported and experimentally identified mRNA and DNA sequences for human RhBG. A: sequence alignment of the 2 different reported mRNA sequences for human RhBG, NM_020407.4 and CAI12178.1. Alignment between nucleotides 1250 and 1299 is shown. Beginning at nucleotide 1265 (arrow) there are 7 consecutive cytosine bases in NM 020407.4 and a series of 8 consecutive cytosine bases in CAI12178.1 sequence. These differences result in a frame-shift alteration in the translated protein sequence and thereby encode differing carboxy-tails beginning at amino acid 425 (B). C–E: results of sequencing human kidney mRNA from 2 different sources and human hepatic mRNA. Sequencing tracings of nucleotides 1258–80 are shown. All samples showed a sequence of 8 consecutive cytosine bases beginning at nucleotide 1265 (arrow). These results are consistent with reported mRNA sequence CAI12178.1, and not with sequence NM_020407.4. F: sequencing of GFP-RhBG vector, a vector commonly used for heterologous human RhBG expression. GFP-RhBG vector had the erroneous sequence with 7, not 8, cytosines at the corresponding site.
GFP-RhBG vector sequence.
A vector commonly used for expressing human RhBG is GFP-RhBG (23). To use this vector it is essential to determine whether it contains the correct or incorrect mRNA sequence. We sequenced the GFP-RhBG vector, using the same primers, and identified a stretch of only seven cytosines beginning at corresponding nucleotide 1265, not eight as present in human RhBG mRNA (Fig. 1F). The lack of the eighth cytosine causes a frame-shift mutation in the expressed protein, giving rise to a protein in which the carboxy-terminus differs beginning at amino acid 425.
To enable expression of the correct human RhBG mRNA, we used site-directed mutagenesis to add an additional cytosine. Sequencing the resulting vector indicated presence of eight consecutive cytosines at nucleotide 1265 (data not shown). We use the terms c-RhBG and c-GFP-RhBG to refer to the corrected human RhBG and GFP-RhBG vector, respectively.
Antibody characterization.
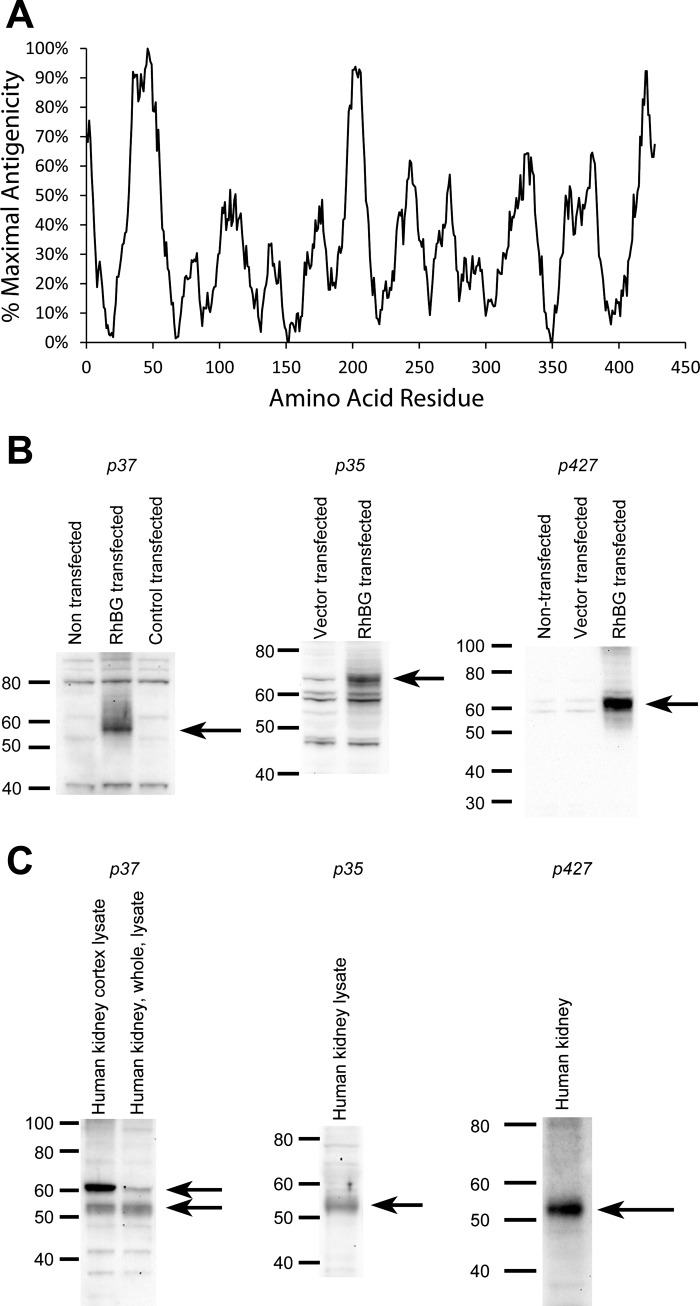
Our next set of studies sought to develop and characterize antibodies against human RhBG. First, we used NHLBI-AbDesigner software to predict antigenicity of the RhBG protein (25). Three sites of peak antigenicity were identified, at the first extracellular loop at approximately residue 46, in the fourth intracellular loop, at approximately residue 200, and in the carboxy-terminus (Fig. 2A). We generated peptides of 15 amino acid length corresponding to each of these peaks and generated rabbit polyclonal anti-peptide antibodies using standard techniques. We then verified that these polyclonal antibodies recognize human RhBG. To do so, we used the c-GFP-RhBG and c-RhBG vectors to express human RhBG in CV-1 and in HEK-293 cells; control cells expressed empty vector. In preliminary studies, antibodies to p207 did not function well by ELISA and were not used for further experiments. Figure 2B shows the results of studies examining the p37, p427, and the p35 antibodies. Immunoblot analysis showed expression of protein of molecular weight of ∼55–61 kDa, the expected molecular weight of glycosylated RhBG, in cells transfected with RhBG and ∼70 kDa in cells transfected with c-GFP-RhBG, with the difference in molecular weight likely related to mobility differences related to coexpression of GFP. Proteins detected at lower abundance in nontransfected or control vector-transfected cells may represent endogenous Rhbg protein. More importantly, these studies confirm that these antibodies to different regions of human RhBG recognize RhBG protein.
Fig. 2.
Prediction of antigenicity of 15 amino acid residue peptides along the human RhBG protein. A: use of NHLBI AbDesigner software (25) to predict antigenicity of 15 amino acid residue peptides, beginning at residue 1 and continuing through residue 427. Relative antigenicity is shown, with maximal antigenicity plotted as 100%. B: characterization of anti-peptide RhBG antibodies. B, left: immunoblot of CV-1 cells either nontransfected, transfected with c-RhBG, or transfected with control vector and immunoblotted with anti-p37 antibody. Arrow shows detection of ∼57-kDa RhBG protein. Bands at ∼41 and 80 kDa are either nonspecific or may represent detection of endogenous Rhbg protein in monomeric and dimeric nonglycosylated forms. B, middle: immunoblot of CV-1 cells transfected either with control vector or with c-GFP-RhBG vector and immunoblotted with p35 antibody. Arrow shows detection of ∼70-kDa GFP-RhBG protein. The increase in apparent molecular weight likely reflects differences in mobility related to inclusion of GFP in protein. B, right: protein from nontransfected HEK cells or from HEK cells transfected either with vector or with c-RhBG. Immunoblot analysis shows expression of ∼61-kDa RhBG protein. The slight difference in molecular weight compared with A may reflect differences in glycosylation in different cell types. C, left: immunoblot analysis of human kidney proteins with antibody p37, and it demonstrates identification of ∼54- and 61-kDa bands. C, middle: immunoblot analysis with p35 antibody, and it demonstrates identification of ∼54-kDa protein. C, right: immunoblot analysis using p427 antibody, and it demonstrates expression of 55-kDa protein. Slight variations likely reflect interexperimental variations in mobility.
To further characterize antibodies p35 and p37, we generated mice with collecting duct-specific Rhbg deletion. We generated these mice using Cre-loxP techniques, using mice with floxed Rhbg genes we characterized previously (2, 3) and using mice expressing Cre-recombinase under control of the Ksp-cadherin promoter (14, 29). In previous studies examining Rhcg, we showed that the Ksp-cadherin promoter yields collecting duct-specific gene deletion (19, 21). Examination of mice homozygous for floxed Rhbg alleles and expressing Ksp-cadherin-Cre using mouse Rhbg-specific antibodies confirmed collecting duct-specific Rhbg deletion. Mice homozygous for floxed Rhbg alleles but not expressing Ksp-cadherin-Cre (control mice) had normal Rhbg expression (data not shown). Immunohistochemistry of kidneys from control mice with intact Rhbg expression using antibodies p35 or p37 demonstrated a normal pattern of basolateral Rhbg expression (Fig. 3). In mice with collecting duct-specific Rhbg deletion, no collecting duct Rhbg expression was detectable using antibodies p35 or p37. These observations further demonstrate specificity of these antibodies for Rhbg. Antibody p427 did not label mouse tissues, consistent with no significant homology between the peptide used to generate this antibody and mouse Rhbg (data not shown).
Fig. 3.
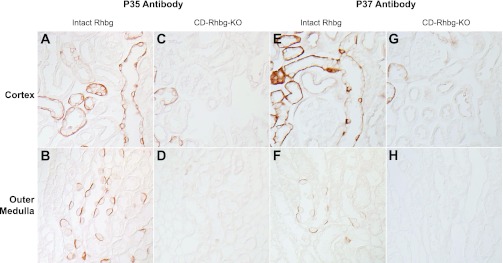
Immunohistochemistry in kidney tissues in mice with intact and collecting duct (CD)-specific Rhbg deletion (CD-Rhbg-KO). A and B: immunohistochemistry of the cortex and outer medulla of mouse kidney with intact Rhbg expression using antibody p35. Basolateral Rhbg immunolabel is easily evident. C and D: similar studies using antibody p35, except performed in mouse kidney with CD-Rhbg-KO. No significant immunolabel is present in the CD; minimal residual immunolabel is present in the connecting segment (CNT) and distal convoluted tubule (DCT), segments that retain a low level of Rhbg expression in this genotype (unpublished observations). E and F: immunohistochemistry of mouse kidney with intact Rhbg expression using antibody p37 in the cortex and outer medulla. Again, basolateral Rhbg immunolabel is easily evident. G and H: similar studies using antibody p37, except performed in mouse kidney with CD-Rhbg-KO. No significant immunolabel is present in the CD; minimal residual immunolabel is present in the CNT and DCT, segments that retain a low level of Rhbg expression in this genotype. Mice with intact Rhbg expression expressed floxed Rhbg alleles and did not express Cre-recombinase. Mice with CD-Rhbg-KO expressed floxed Rhbg alleles and expressed Cre-recombinase under control of Ksp-cadherin promoter. All tissues were treated with peptide:N-glycosidase F (PNGase F) as described in methods.
Human kidney RhBG protein expression.
Next, we determined whether RhBG protein is present in the human kidney. Immunoblot analysis of human renal proteins using antibodies against epitopes in both the first extracellular loop and the carboxy terminus identified RhBG protein expression (Fig. 2C). Identification of a slight difference in the molecular weight with the p37 antibody may reflect slight differences in affinity for differentially glycosylated forms of RhBG.
Cellular expression of RhBG in the human kidney.
To understand RhBG's role in human renal ammonia transport, it is necessary to determine its cellular expression pattern. Thus, we determined RhBG's cellular expression in the human kidney using immunohistochemistry. In general, antibody p35 provided the highest quality immunohistochemistry and was used for the majority of studies, except when detailed otherwise.
Initial studies with p35 antibody identified either no or very low levels of detectable RhBG immunolabel in the human kidney (Fig. 4, A and B). Analysis of RhBG antigenicity predicted the most antigenic sites were in the first extracellular loop, which include the putative N-linked glycosylation site. This raised the possibility that the extracellular glycosylation might impair access of the antibody to antigenic sites on the protein.
Fig. 4.
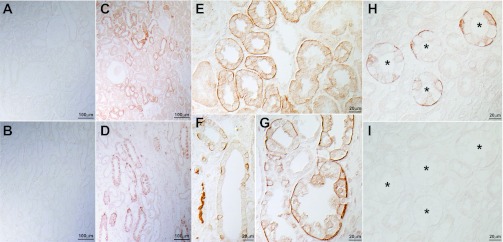
RhBG immunolabel expression in the human kidney. A and B: low-power micrograph of human kidney cortex and outer medulla, respectively, examined for RhBG immunolabel in the absence of deglycosylation. RhBG immunolabel was not detectable. C and D: low-power micrograph of human kidney cortex and outer medulla, respectively, examined for RhBG immunolabel after deglycosylation with PNGase F. Abundant RhBG immunolabel is present in a subpopulation of epithelial segments in both the cortex and outer medulla. E: high-power magnification of cortex and demonstrates strong basolateral RhBG immunolabel in the CNT. F: high-power magnification of cortical collecting duct (CCD). Abundant RhBG immunolabel is present in a subpopulation of cells. G: high-power magnification of outer medulla. Strong RhBG immunolabel is present in a subpopulation of cells in the outer medullary collecting duct (OMCD). H and I: effect of preincubation of p35 with immunizing peptide. H: no peptide was used and strong basolateral immunolabel is present. I: human kidney in which p35 was preincubated with immunizing peptide, and no significant immunolabel is observed. Antibody p35 was used for these studies. *Collecting duct lumen.
Protein deglycosylation is a routine procedure performed in studies utilizing immunoblot analysis of proteins. We treated human kidney samples with PNGase F, an enzyme that specifically cleaves N-linked glycosylation sites. Immunohistochemistry of PNGase F-treated tissues revealed abundant RhBG immunolabel in a subpopulation of cells (Fig. 4, C and D). No RhBG immunolabel was identifiable in glomerular, vascular, or interstitial cells or in tubular epithelial cells with an apical brush border, i.e., proximal tubular cells. The immunolabel pattern in the cortex suggested expression in CNT and in a subpopulation of collecting duct cells. In the outer medulla, only a subpopulation of cells in the outer medullary collecting duct (OMCD) expressed detectable immunolabel. Preincubating the antibody with the immunizing peptide blocked immunolabel, providing evidence of specificity of the immunolabel reaction (Fig. 4, H-I). Thus, the human kidney expresses basolateral RhBG in the distal nephron segments, including CNT, cortical collecting duct (CCD), and OMCD. Moreover, removal of N-linked carbohydrates is necessary to enable antibody recognition, confirming human RhBG protein is glycosylated and that the antibody used for these studies recognizes the first extracellular loop region where N-linked glycosylation is present.
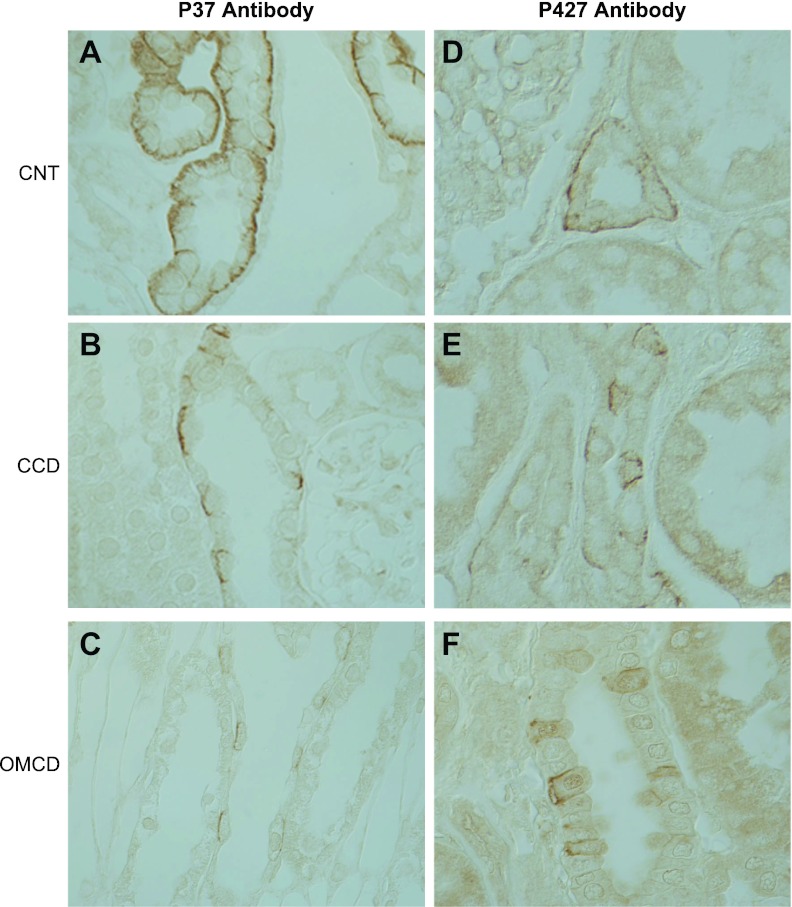
To further confirm these studies, we also performed immunohistochemistry using p37 and p427 antibody to the first extracellular loop and the carboxy-terminus of RhBG, respectively. Antibody p37, targeted to the glycosylated first extracellular loop, required deglycosylation, whereas p427, targeted to the carboxy-terminus, did not (data not shown). Both antibodies gave immunolabel similar to that observed with p35 antibody (Fig. 5).
Fig. 5.
Immunohistochemistry of human kidney using p37 and p427 antibody. A–C: immunohistochemistry using antibody p37 directed against epitopes present on the 1st extracellular loop of RhBG. A: high-power magnification of CNT and demonstrates strong basolateral RhBG immunolabel. B and C: high-power magnification of CCD and medullary collecting duct (MCD), respectively, and demonstrate strong basolateral RhBG immunolabel in a subpopulation of cells. D–F: immunohistochemistry using antibody p427 to the carboxy-tail of RhBG. D: high-power micrograph of CNT and demonstrates strong and homogeneous basolateral RhBG immunolabel. E: high-power micrograph of CCD demonstrating strong basolateral RhBG immunolabel in a subpopulation of cells. F: high-power micrograph of OMCD demonstrating strong basolateral RhBG immunolabel in a subpopulation of cells. Kidney sections tested with p37 underwent deglycosylation before treatment with primary antibody; those treated with p427 did not.
Double immunolabel with CCD-specific markers.
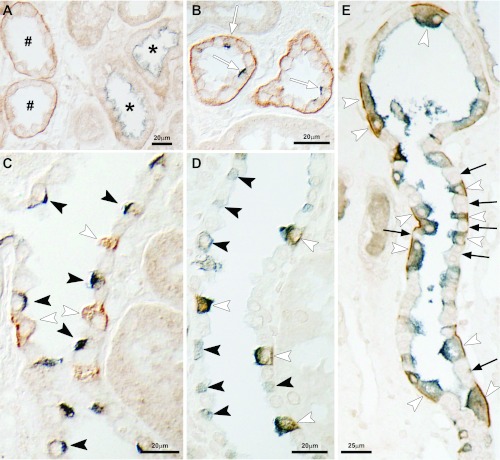
The collecting duct consists of two fundamentally different cell types, intercalated cells and principal cells. To determine which collecting duct cell populations expressed RhBG, we performed double immunolabel using cell-specific markers. Figure 6 summarizes these findings. NCC-positive cells, identifying DCT cells, were generally negative for basolateral RhBG, indicating lack of substantial RhBG expression in the DCT (Fig. 6A). In the CNT, both pendrin-positive cells, which predominantly are non-A, non-B type intercalated cells (10), and pendrin-negative cells, which include both the majority cell type (CNT cells) and type A intercalated cells, expressed basolateral RhBG immunolabel (Fig. 6B). In the CCD, in contrast, pendrin-positive cells, which predominantly are type B intercalated cells (10), did not express detectable RhBG immunolabel (Fig. 6C). All cells with intense apical H+-ATPase immunolabel expressed strong basolateral RhBG immunolabel in the CNT, CCD, and OMCD (Fig. 6, D and E). Intercalated cells with diffuse or basolateral H+-ATPase immunolabel did not express detectable RhBG (Fig. 6D), nor did cells without H+-ATPase immunoreactivity. In additional studies, using higher concentrations of primary antibody, we did not find detectable RhBG immunolabel in human collecting duct principal cells. Thus, CNT cells, type A intercalated cells, and non-A, non-B intercalated cells express RhBG, but type B intercalated cells and principal cells do not.
Fig. 6.
Double immunolabel of RhBG with cell-specific markers. A: double immunolabel of RhBG (brown) with thiazide-sensitive cotransporter (NCC; blue) in cortical convoluted tubules. NCC-positive tubules (*), identifying DCT segments, which do not express detectable RhBG immunolabel. In contrast, NCC-negative tubules in the cortical labyrinth (#), identifying CNT cells, do express basolateral RhBG immunolabel. B: CNT with double immunolabel for RhBG (brown) and pendrin (blue). CNT cells with apical pendrin, i.e., non-A, non-B intercalated cells, express basolateral RhBG immunolabel (white arrows). C: CCD with double immunolabel for RhBG (brown) and pendrin (blue). Cells with apical pendrin immunolabel, identifying B intercalated cells, do not express detectable RhBG (black arrowheads). D: CCD with double immunolabel for RhBG (brown) and H+-ATPase (blue). Type A intercalated cells with apical H+-ATPase immunolabel express basolateral RhBG (white arrowheads), whereas type B intercalated cells, with diffuse cytoplasmic or basolateral H+-ATPase immunolabel, do not express detectable RhBG (black arrowheads). E: outer medulla labeled for H+-ATPase (blue) and RhBG (brown). Type A intercalated cells, identified by apical H+-ATPase immunolabel, express basolateral Rhbg immunolabel (white arrowheads). Principal cells, lacking apical H+-ATPase immunolabel, do not express detectable RhBG immunolabel (arrows). Antibody p35 was used to immunolocalize RhBG in these studies.
DISCUSSION
This study provides several important lines of new evidence regarding expression of the ammonia transporter family member, RhBG, in the human kidney. First, these studies address differences in the reported mRNA, and thus amino acid, sequence for human RhBG and identify the correct sequences. Second, they show that RhBG protein is present in the human kidney and that basolateral RhBG is present specifically in the CNT, in non-A, non-B intercalated cells, and in type A intercalated cells in the CCD and the OMCD. Thus, RhBG protein is present in the human kidney and is likely to mediate an important role in human ammonia metabolism.
RhBG/Rhbg is one of two nonerythroid Rh glycoproteins that recent studies identified as playing important roles in mammalian ammonia transport. Numerous studies in rodent models have shown that Rhbg is expressed in distal nephron segments, from the distal convoluted tubule through the collecting duct (7, 26, 31, 37, 38). Notably, 60–80% of total urinary ammonia is secreted in this site, making it a critical site for urinary ammonia excretion (9, 37). Several lines of evidence indicate Rhbg mediates an important role in renal ammonia excretion. Studies utilizing intercalated cell-specific Rhbg gene deletion showed Rhbg is involved in basal ammonia excretion (3). Multiple models of altered ammonia excretion showed that Rhbg expression changed in parallel with ammonia excretion in a wide variety of conditions in which ammonia excretion was altered, including metabolic acidosis (3, 20, 27), reduced renal mass (16), and in hypokalemia (2). Finally, studies examining intercalated cell-specific Rhbg deletion showed impaired ammonia excretion in response to metabolic acidosis (3) and to hypokalemia (2). Thus, RhBG/Rhbg expression appears critical for renal ammonia transport and maintenance of acid-base homeostasis.
The current study provides the first demonstration that RhBG is present in the human kidney. The evidence for RhBG expression includes identification of mRNA expression, confirmed by sequencing specific portions of the RhBG gene, immunoblot analysis using multiple antibodies directed against distinct epitopes, and immunohistochemistry studies demonstrating its cellular localization of RhBG. These findings, in concert with findings previously discussed showing that Rhbg in the mouse is necessary for normal basal ammonia metabolism (3), for acidosis-stimulated ammonia excretion (3), and also for hypokalemia-stimulated ammonia excretion (2), indicate that RhBG is likely to mediate critical roles in human renal ammonia metabolism and excretion.
In contrast to the A-type intercalated cell and the non-A, non-B intercalated cell, RhBG expression was not observed in the type B intercalated cell. This lack of detectable RhBG expression in type B intercalated cells is consistent with findings in the mouse and rat kidney (27, 31). The lack of RhBG in type B intercalated cells in the human kidney is also consistent with observations that these cells do not express the other nonerythroid Rh glycoprotein, RhCG/Rhcg, either in the human kidney (10) or in the rat and mouse kidneys (15, 31). Thus, RhBG is expressed in the human kidney in cells that mediate critical roles in urine acidification and transcellular ammonia secretion. The lack of RhBG expression in the type B intercalated cell further supports a model in which this cell type is involved in bicarbonate secretion and chloride reabsorption, and not in urine acidification and ammonia secretion (8, 32, 33).
One difference in the localization of Rhbg/RhBG in the human kidney from that in the mouse and rat kidney may relate to expression in principal cells. In both the mouse and rat kidney, principal cells express Rhbg (3, 27, 31), and in the mouse principal cell Rhbg expression increases and contributes to both basal and acidosis-stimulated ammonia excretion (3). In the human kidney, we did not detect RhBG protein in principal cells. Similarly, the related Rh glycoprotein Rhcg/RhCG is present in and contributes to rat and mouse principal cell transcellular ammonia secretion (11, 15, 16, 19, 20, 27, 28, 31), but is not detectable in human principal cells (10). Thus, significant Rhbg/RhBG and Rhcg/RhCG proteins are expressed in the rodent kidney principal cell, but not in the human principal cell, suggesting the principal cell's role in collecting duct ammonia secretion may differ in rodent and human kidney. However, it is important to emphasize we cannot exclude that human principal cells express RhBG, but at levels below that detectable using current techniques. In particular, differences in the tissue preservation techniques involved with using human nephrectomy specimens, including delays in fixation, use of immersion vs. perfusion fixation, differences in fixatives, neutral-buffered formalin vs. PLP, and differences in embedding media, paraffin vs. wax, may all contribute to decreased antigen preservation and result in failure to detect low levels of RhBG expression in the human kidney.
Although a previous report (5) was unable to detect RhBG protein expression in the human kidney, the current study identified at least one reason why. One of the two antibodies used in this previous study (5) was directed against the presumed carboxy-terminus sequence, but this sequence was based on a mRNA sequence with seven, not eight, cytosines beginning at nucleotide 1265. The current study shows that the correct mRNA sequence has eight cytosines starting at nucleotide 1265. Why previous reports of mRNA sequence for RhBG reported seven, not eight, cytosines at this site is unclear, but may have resulted from a “proof-reading” error during the initial cloning of RhBG, which is a rare, but known, event. Importantly, use of a mRNA sequence with seven cytosines results in a “frame-shift” error in the corresponding protein's carboxy-terminus. As a result, the antibody generated against a peptide sequence in the carboxy-tail in this previous study (5) recognized an epitope not present in human RhBG protein, thereby explaining the inability to detect RhBG protein in the human kidney. Tests of the antibody against a heterologously expressed protein were positive, but this is likely because the protein expressed was transcribed from a mRNA sequence with only seven cytosines at nucleotide 1265 encoding a protein with a carboxy-terminus similar to that used for generating the anti-peptide antibody, but different from that in human RhBG protein.
Identification of the correct mRNA sequence, and thus amino acid sequence, for RhBG has important implications for studies examining regulation of human RhBG. In particular, one study reported that Tyr-429 mediates phosphorylation-regulated transport activity and membrane insertion of RhBG (30). However, Tyr-429 is present only in the protein that results from transcription of the erroneous RhBG cDNA with seven cytosines and does not exist in native human RhBG. Similarly, the postulated PDZ-binding motif at 455-DTQA-458 (30) does not exist in native human RhBG. Another study reported an ankyrin G interaction domain at the 419-FLD-421 sequence (24). Again, this study examined human RhBG with the incorrect carboxy-tail. The differences in the carboxy-tail that the current manuscript identified are immediately adjacent to the proposed ankyrin G interaction domain; whether the tertiary structure of the correct carboxy-tail alters the tertiary structure of the 419-FLD-421 sequence and whether this might alter ankyrin binding at this sequence is unknown.
In summary, the current study provides important new information regarding the role of RhBG in human ammonia metabolism. RhBG is present in sites critical for ammonia excretion, and its expression is greatest in acid- and ammonia-secreting intercalated cells. Thus, RhBG is likely to mediate an important role in human renal ammonia metabolism, and thereby in acid-base homeostasis. Furthermore, we identified the correct mRNA and, by extension, carboxy terminus amino acid sequence, which has important implications for identifying and studying the molecular mechanisms regulating RhBG-mediated ammonia transport.
GRANTS
These studies were supported by funds from the National Institutes of Health (DK-045788), Merit Review Grant program of the Department of Veterans Affairs (1I01BX000818), and the National Research Foundation of Korea (2011-0016068).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.-H.H., H.-W.L., M.E.H., J.W.V., and I.D.W. conception and design of research; K.-H.H., H.-W.L., M.E.H., F.M.W., G.O., B.P.C., and W.L.C. performed experiments; K.-H.H., H.-W.L., M.E.H., J.W.V., and I.D.W. analyzed data; K.-H.H., H.-W.L., M.E.H., J.W.V., and I.D.W. interpreted results of experiments; K.-H.H., H.-W.L., J.W.V., and I.D.W. edited and revised manuscript; K.-H.H., H.-W.L., M.E.H., B.P.C., W.L.C., J.W.V., and I.D.W. approved final version of manuscript; H.-W.L. and I.D.W. prepared figures; I.D.W. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Mark Knepper, M.D., Ph.D. for making the AbDesigner software results available before public release of the software.
REFERENCES
- 1.Authors unknown Homo sapiens chromosome 1 genomic contig, GRCh37.p9 Primary Assembly. http://www.ncbi.nlm.nih.gov/nucleotide/224514980?report=gbwithparts&from=7827683&to=7843279&RID=4WEJCKNR01N Last accessed: Sept. 10, 2012
- 2.Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID. Intercalated cell-specific Rh B glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. Am J Physiol Renal Physiol 304: F422–F431, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B Glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Brown ACN, Hallouane D, Mawby WJ, Karet FE, Saleem MA, Howie AJ, Toye AM. RhCG is the major putative ammonia transporter expressed in human kidney and RhBG is not expressed at detectable levels. Am J Physiol Renal Physiol 296: F1279–F1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBose TD, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol 1: 1193–1203, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Eladari D, Chambrey R, Frische S, Vallet M, Edwards A. Pendrin as a regulator of ECF and blood pressure. Curr Opin Nephrol Hypertens 18: 356–362, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia-reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol 293: F1342–F1354, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Han KH, Lee HW, Handlogten ME, Bishop JM, Levi M, Kim J, Verlander JW, Weiner ID. Effect of hypokalemia on renal expression of the ammonia transporter family members, Rh B Glycoprotein and Rh C Glycoprotein, in the rat kidney. Am J Physiol Renal Physiol 301: F823–F832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han KH, Lee SY, Kim WY, Shin JA, Kim J, Weiner ID. Expression of the ammonia transporter family members, Rh B Glycoprotein and Rh C Glycoprotein, in the developing rat kidney. Am J Physiol Renal Physiol 299: F187–F198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, Aronson PS. Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol Renal Physiol 277: F599–F610, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Knepper MA. NH4+ transport in the kidney. Kidney Int 40: S95–S102, 1991 [PubMed] [Google Scholar]
- 18.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C Glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID. Renal ammonia excretion in response to hypokalemia: effects of collecting duct-specific Rh C Glycoprotein deletion. Am J Physiol Renal Physiol 304: F410–F421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Lopez C, Metral S, Eladari D, Drevensek S, Gane P, Chambrey R, Bennett V, Cartron JP, Van Kim C, Colin Y. The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J Biol Chem 280: 8221–8228, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Pisitkun T, Hoffert JD, Saeed F, Knepper MA. NHLBI-AbDesigner: an online tool for design of peptide-directed antibodies. Am J Physiol Cell Physiol 302: C154–C164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Sohet F, Colin Y, Genetet S, Ripoche P, Metral S, Le Van Kim C, Lopez C. Phosphorylation and ankyrin-G binding of the C-terminal domain regulate targeting and function of the ammonium transporter RhBG. J Biol Chem 283: 26557–26567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Wagner C, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Arch 458: 137–156, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens 14: 480–484, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Weiner ID, Verlander JW. Renal and hepatic expression of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein. Acta Physiol Scand 179: 331–338, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner ID, Verlander JW. Molecular physiology of the Rh ammonia transport proteins. Curr Opin Nephrol Hypertens 19: 471–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]