Abstract
Improper macrophage activation is pathogenically linked to various metabolic, inflammatory, and immune disorders. Therefore, regulatory proteins controlling macrophage activation have emerged as important new therapeutic targets. We recently demonstrated that netrin-1 regulates inflammation and infiltration of monocytes and ameliorates ischemia-reperfusion-induced kidney injury. However, it was not known whether netrin-1 regulates the phenotype of macrophages and the signaling mechanism through which it might do this. In this study, we report novel mechanisms underlying netrin-1's effects on macrophages using in vivo and in vitro studies. Overexpression of netrin-1 in spleen and kidney of transgenic mice increased expression of arginase-1, IL-4, and IL-13 and decreased expression of COX-2, indicating a phenotypic switch in macrophage polarization toward an M2-like phenotype. Moreover, flow cytometry analysis showed a significant increase in mannose receptor-positive macrophages in spleen compared with wild type. In vitro, netrin-1 induced the expression of M2 marker expression in bone marrow-derived macrophages, peritoneal macrophages, and RAW264.7 cells, and suppressed IFNγ-induced M1 polarization and production of inflammatory mediators. Adoptive transfer of netrin-1-treated macrophages suppressed inflammation and kidney injury against ischemia-reperfusion. Netrin-1 activated PPAR pathways and inhibition of PPAR activation abolished netrin-1-induced M2 polarization and suppression of cytokine production. Consistent with in vitro studies, administration of PPAR antagonist to mice abolished the netrin-1 protective effects against ischemia-reperfusion injury of the kidney. These findings illustrate that netrin-1 regulates macrophage polarization through PPAR pathways and confers anti-inflammatory actions in inflammed kidney tissue.
Keywords: macrophage polarization, netrin-1, PPARγ, ischemia-reperfusion injury, acute kidney injury
tissue-resident macrophages show tremendous plasticity in their response to external stimuli. The local metabolic and immune microenvironment is known to contribute to this plasticity (5). Macrophages express distinct patterns of surface receptors and metabolic enzymes in response to different stimuli, and these ultimately generate the diversity of macrophage functions and phenotypes. Broadly, there are two distinct polarization states of macrophages, M1 and M2, that have been characterized (4, 11, 23). LPS and IFNγ can promote macrophage differentiation to a “classical” or M1 phenotype (11). The M1 activation pattern is associated with tissue destruction and inflammation and is responsible for upregulating pro-inflammatory cytokines and increasing the production of reactive nitrogen species and reactive oxygen species (3). In contrast, the “alternative” or M2 activation phenotype of macrophages is induced in response to IL-4 and IL-13. M2-polarized macrophages dampen the inflammatory process by producing anti-inflammatory factors, such as IL-10 and TGF-β1. M2 macrophages also upregulate mannose receptor (MR), C type 2 (Mrc2c), and IL-1 receptor antagonist (IL-1RA), and scavenger receptors, such as cluster of differentiation 36 (CD36), as well as increased expression of arginase-1 (Arg-1). The M2 phenotype is thought to promote tissue repair after inflammation and/or injury (3, 11, 23).
Recent studies have demonstrated that the anti-inflammatory molecule netrin-1 suppresses monocyte migration and function in both acute and chronic inflammatory diseases. Moreover, netrin-1 is highly induced in tubular epithelial cells after reperfusion injury and partial deficiency of netrin-1 exacerbates ischemia-reperfusion injury (6, 16). Overexpression of netrin-1 tubular epithelial cells also protects kidney against ischemia-reperfusion injury (21). Netrin-1 was capable of inhibiting leukocyte migration in response to multiple chemotactic stimuli. This broad inhibitory effect was not due to a direct interaction of netrin-1 with the chemoattractants (10). More recent data also suggest that netrin-1 suppresses the release of cytokines and chemokines from different immune cells through regulation of NF-κB activation and COX-2 expression (Ranganathan PV, Jayakumar C, Mohamed R, Dong Z, Ramesh G. Unpublished observations). However, it was not known whether netrin-1 can directly regulate macrophage polarization, thereby suppressing inflammation. Moreover, the signaling mechanism through which it regulates macrophage phenotype is unknown. Since netrin-1 is anti-inflammatory and suppresses NF-κB activation, cytokine production, and COX-2 expression, which are associated with macrophage polarization, we hypothesized that netrin-1 induces M2 polarization, thereby controling inflammation and possibly enhancing regeneration. Using bone marrow-derived macrophages(BMM), peritoneal macrophages, and RAW264.7 cells, we demonstrate that netrin-1 induces M2 macrophage polarization and suppresses IFNγ-induced M1 polarization and the associated inflammatory cytokine production. In vitro studies were validated in vivo using netrin-1 transgenic animals that overexpress netrin-1 in spleen and kidney. Moreover, for the first time, we demonstrate that netrin-1 activates PPAR pathways to mediate its effect on macrophage polarization and anti-inflammatory effects.
MATERIALS AND METHODS
Renal ischemia-reperfusion.
Netrin-1 transgenic mice were characterized for transgene expression and phenotype as described previously (12, 21). Eight-week-old male netrin-1 transgenic mice, which overexpress chicken netrin-1 in kidney proximal tubular epithelial cells and spleen under control of the fatty acid binding protein promoter or their litter mate or C57BL/6J mice (8–9 wk of age, The Jackson Lab, Bar Harbor, ME), were anesthetized with pentobarbital sodium (50 mg/kg body wt, intraperitoneally) and were placed on a heating pad to maintain body temperature at 37°C. Both renal pedicles were identified through dorsal incisions and clamped for 30 min. Reperfusion was confirmed visually upon release of the clamps. As a control, sham-operated animals were subjected to the same surgical procedure except that the renal pedicles were not clamped. Surgical wounds were closed, and mice were given 1 ml of warm saline intraperitoneally. Mice were kept in a warm incubator until they regained consciousness. Some animals received in vitro polarized bone marrow-derived macrophages (BMM) (500,000 cells/animals) intravenously 1 h before renal pedicle clamping. Urine and kidney tissue were collected 24 h after reperfusion and processed for ELISA and RNA isolation. The Institutional Animal Care and Use Committee of the Georgia Health Sciences University approved all of the protocols and procedures using animal. The approval number is BR10-10-369.
Macrophage isolation and culture.
Peritoneal macrophages were isolated by injecting mice with 3 ml of RPMI medium, gently massaging the belly, and then aspirating the fluid. Cells were resuspended in RPMI medium 1640 containing 10% FCS and plated at a cell density of 1–5 × 105 cells per well of a six-well plate. Twenty-four hours after being plated, nonadherent cells were removed by washing, and fresh medium was replaced.
Bone marrow derived-macrophages were obtained as described previously (7). Briefly, femur and tibia were dissected out in DMEM containing 10% FCS, and antibiotics and were flushed to remove bone marrow cells. After lysing the red cells, the remaining cells were counted and plated in a T-25 flask, and 10 ng/ml of macrophage colony stimulating factor (M-CSF) was added. After culturing overnight, nonadherent cells were collected, washed, and plated in 60-mm petri plates with 10 ng/ml of M-CSF in DMEM containing 10% FBS. After 10 days, cells were washed, and the adherent cells were released and removed with 0.1% EDTA. Cells were counted and replated in six wells at a density of 2 × 105 cells/well. The resulting BMMs were judged to be >98% pure based on F4/80 staining. For in vitro polarization, BMMs were stimulated with either 100 U/ml murine recombinant IFNγ (Prospec) or 250 ng/ml murine recombinant netrin-1 (R&D Systems) for 24–48 h. To track injected M1 or M2 cells, the cells were labeled using the PKH26 Red Fluorescence Cell Linker Kit (Sigma) according to the manufacturer's instructions and analyzed by flow cytometry.
Raw 264.7 cells were cultured in RPMI medium containing 10% FBS. At 80% confluency, cells were treated with different drugs in the presence or absence of netrin-1. Forty-eight hours after treatment, cells and supernatant were harvested and subjected to cytokine and gene expression analysis. To determine netrin-1 effects on LPS-induced cytokine production, RAW264.7 cells were stimulated with LPS (10 ng/ml) with/without netrin-1 for 48 h.
Flow cytometry.
To quantify the M2 polarized macrophage in transgenic spleen and in vitro from netrin-1-treated bone marrow-derived macrophages, spleen were minced into fragments of 1 mm3 and digested with 2 mg/ml collagenase I and 100 U/ml DNase I. The digested spleens were then sequentially passed through 100- and 40-μm mesh. Red blood cells in the resulting renal cells were lysed using red blood cell lysis buffer (Sigma). Spleen macrophages were stained using the following fluorochrome-labeled antibodies: anti-F4/80 (ebiosciences)-APC and rat anti-mouse mannose receptor (CD206) antibody conjugated with Alexa-488 (Abserotec). Fc receptors on leukocytes were blocked before staining with rat anti-FcR (CD16/CD32) (BD Biosciences). After renal leukocytes were stained for surface markers, the cells were fixated and analyzed by using FACSCalibur, and the data were analyzed using Cyflogic V.1.2.1 software.
Quantification of mRNA by real-time RT-PCR.
Real-time RT-PCR was performed in an Applied Biosystems 7700 Sequence Detection System (Foster City, CA). Total RNA (1.5 μg) was reverse transcribed in a reaction volume of 20 μl using Omniscript RT kit and random primers. The product was diluted to a volume of 150 μl, and 6-μl aliquots were used as templates for amplification using the SYBR Green PCR amplification reagent (Qiagen) and gene-specific primers. The primer sets used were mouse TNF-α (forward: GCATGATCCGCGACGTGGAA; reverse: AGATCCATGCCGTTG GCCAG), MCP-1 (forward: ATGCAGGTCCCTGTCATG; reverse: GCTTGAGGTGGTTGTGGA), mannose receptor (forward: CCCAAGGGCTCTTCTAAAGCA; reverse: CGCCGGCACCTATCACA), arginase-1 (forward: CTCCAAGCCAAAGTCCTTAGAG; reverse: GGAGCTGTCATTAGGGACATC), IL-13 (forward: CAGCATGGTATGGAGTGTGG; reverse: GTGGGCTACTTCGATTTTGG), and IL-10 (forward:ATGCCTGGCTCAGCACTG; reverse: GTCCTGCATTAAGGAGTCG). The amount of DNA was normalized to the β-actin signal amplified in a separate reaction (forward primer: AGAGGGAAATCGTGCGTGAC; reverse: CAATAGTGATGACCTGGCCGT).
Renal function.
Renal function was assessed by measurements of serum creatinine (catalog no: DZ072B; Diazyme Labs).
Cytokine and chemokine measurements.
Cytokines and chemokines in plasma were measured using ELISA array kit from SABiosciences and ELISA kit from eBioscience.
Western blot analysis.
Protein extraction from RAW264.7 cells and Western blot analysis were carried out as described previously (14, 20). The membrane was probed with rabbit anti-PPARα (Assay Biotech), PPARβ (Abcam), and PPARγ (Cell Signaling) antibody (R&D System). Proteins were detected using enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech). Protein loading was normalized to GAPDH expression using anti-mouse GAPDH antibody (Sigma-Aldrich, St. Louis, MO).
Histology and immunostaining.
Kidney tissue was fixed in buffered 10% formalin for 12 h and then embedded in paraffin wax. For assessment of injury, 5 μM sections were stained with periodic acid-Schiff (PAS) followed by hematoxylin. Tubular injury was assessed in PAS-stained sections using a semiquantitative scale (15) in which the percentage of cortical tubules showing epithelial cell necrosis, brush-border loss, cast formation, and apoptotic bodies in the cortex was assigned a score: 0 = normal; 1 = <10%; 2 = 10–25%; 3 = 26–75%; 4 = >75%. Ten fields of ×40 magnification were examined and averaged. The individual scoring of the slides was blinded to the genotype of the animal. To quantify leukocyte infiltration, sections were stained with rat anti-mouse neutrophil antibody (Abcam, Cambridge, MA) (1:200 dilution) or a isotype-matched normal rat IgG2b (R&D Systems, Minneapolis, MN) followed by goat anti-rat biotin conjugate. Color was developed after incubation with ABC reagent (Vector Lab). Stained sections were photographed, and five ×40 fields of neutrophils were examined for quantification of leukocytes. Kidney tissue was fixed in buffered 10% formalin for 12 h and then embedded in paraffin wax. For assessment of injury, 5 μM sections were stained with periodic acid-Schiff (PAS) followed by hematoxylin. Color was developed after incubation with ABC reagent (Vector Lab). Stained sections were photographed using an Olympus inverted microscope with color charge-coupled device camera.
Statistical methods.
All assays were performed in duplicate. The data are reported as means ± SE. Statistical significance was assessed by an unpaired, two-tailed Student t-test for single comparison or ANOVA for multiple comparisons.
RESULTS
Netrin-1 overexpression increases M2 marker expression and M2 polarizing cytokines in spleen and kidney.
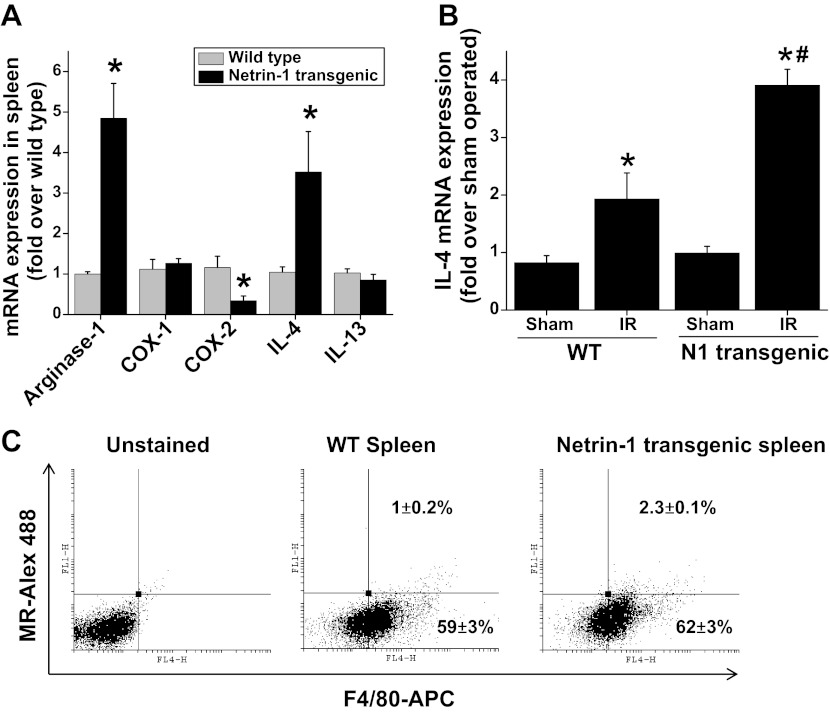
To determine the influence of netrin-1 overexpression on macrophage polarization in vivo, we determined M2 marker expression. Transgenic overexpression of netrin-1 in mouse spleen and kidney increased expression of the M2 markers arginase-1 and IL-4 but suppressed COX-2 expression (Fig. 1A). The Th2 cytokines IL-4 and IL-13 are known to suppress IFNγ-induced M1 polarization but at the same time induce M2 polarization. Our previous results had shown that administration of cisplatin causes a large increase in both IL-4 and IL-13 expression in netrin-1 transgenic animals but not in WT animals (13). In addition, ischemia-reperfusion also strongly induced the expression of IL-4 in kidney (Fig. 1B).
Fig. 1.
Transgenic overexpression of netrin-1 increases M2 macrophage polarization and IL-4 expression. A: expression of M1 and M2 markers and M2-polarizing cytokines in normal wild-type (WT) and netrin-1 transgenic spleen was determined by RT-PCR. Netrin-1 overexpression increases expression of arginase-1 and IL-4 but downregulates COX-2 expression. *Significant difference vs. WT spleen (P < 0.001). B: IL-4 expression in WT and netrin-1 transgenic mouse kidney subjected to sham and ischemia-reperfusion (IR). Netrin-1 overexpression enhanced IR-induced IL-4 expression in kidney. *Significant difference vs. sham (P < 0.05). #Significant difference vs. WT IR (P < 0.05). C: netrin-1 overexpression polarizes macrophages to M2 as determined by flow cytometry using mannose receptor (MR) and F4/80 markers under basal condition. Netrin-1 overexpression increased the number of MR-positive macrophages significantly. Values are means ± SE (n = 4–6).
Netrin-1 overexpression induces macrophages toward M2 polarization in spleen.
M2 polarized macrophages were quantified by flow cytometry as described in materials and methods. Netrin-1 overexpression in spleen induced a significant increase in M2-polarized macrophages compared with WT mice (Fig. 1C).
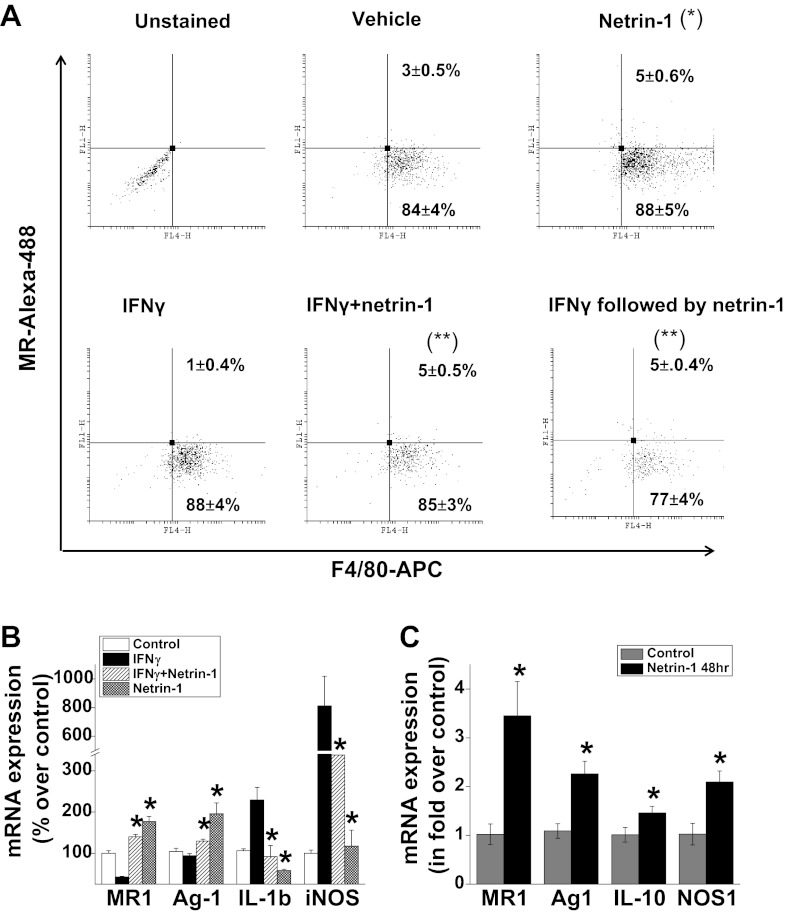
Netrin-1 induces M2 polarization in vitro and increases M2 marker expression.
To determine directly whether netrin-1 can induce macrophage M2 polarization and suppress IFNγ-induced M1 polarization, bone marrow-derived macrophages were subjected to different treatments. Netrin-1 treatment increased the population of M2 polarized macrophages, whereas addition of IFNγ reduced the number of M2 polarized macrophages as determined by surface expression of mannose receptor by flow cytometry (Fig. 2A). When netrin-1 and IFNγ were added at the same time, netrin-1 suppressed IFNγ-induced downregulation of mannose receptor expression. Moreover, when netrin-1 was added after M1 polarization by IFNγ, netrin-1 completely reversed the M1 polarization to the M2 phenotype.
Fig. 2.
Netrin-1 induced M2 polarization and suppressed IFNγ-induced M1 polarization of macrophages. A: bone marrow-derived macrophages were treated with netrin-1 or IFNγ or together for a period of 48 h and then analyzed by flow cytometry. To determine whether netrin-1 can reverse IFNγ-induced M1 polarized macrophages to M2 polarized state, IFNγ was treated for 24 h, washed, and had netrin-1 added, and was incubated for an additional 48 h. Netrin-1 induced a significant increase in number of M2-polarized macrophages compared with vehicle treatment. In addition, netrin-1 also suppressed IFNγ-induced M1 polarization as well as reversed M1-polarized macrophages to M2 polarization. *Signficant difference vs. vehicle-treated group (P < 0.05). **Significant difference vs. IFNγ-treated group (P < 0.001) (n = 3). B: peritoneal macrophages were treated with netrin-1 or IFNγ or together for 48 h. M1 and M2 expression were analyzed by real-time PCR. Netrin-1 increased M2 marker expression but suppressed IFNγ-induced M1 marker expression. *Significant difference vs. IFNγ-treated group (P < 0.05). C: RAW264.7 cells were treated with netrin-1 or vehicle, and M1 and M2 expression were analyzed by real-time PCR. Netrin-1 treatment increased M2 marker expression. Values are means ± SE (n = 4). *Significant difference vs. control group (P < 0.05).
Consistent with the in vivo data and studies with BMM, netrin-1 addition also induced M2 polarization in peritoneal macrophages (Fig. 2B) and in the RAW264.7 cell line (Fig. 2C). Netrin-1 increased expression of M2 markers (MR, arginase-1, and IL-10) but suppressed expression of M1 marker [inducible NO synthase (iNOS) and IL-1β] in both peritoneal macrophages and RAW264.7 cells.
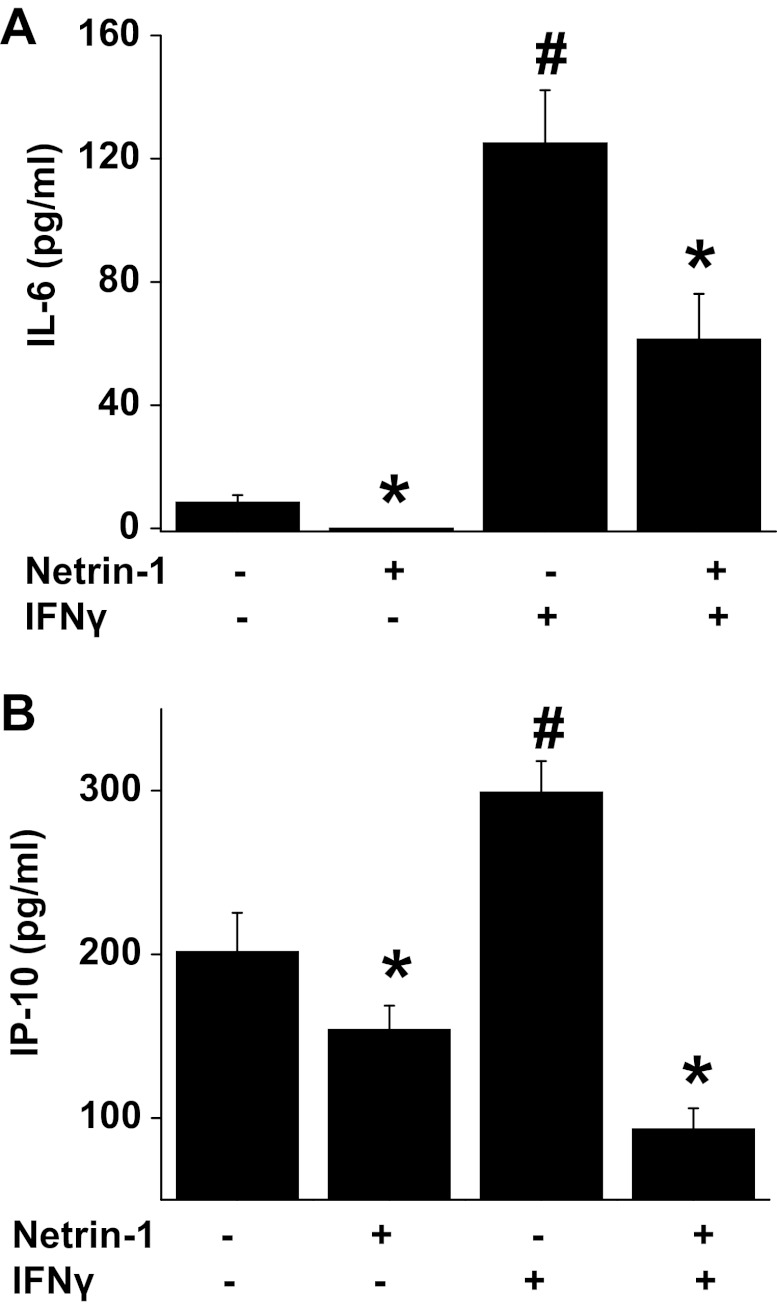
Netrin-1 suppresses IFNγ-induced cytokine and chemokine production.
To determine whether polarization of macrophages is associated with changes in cytokine and chemokine production, IL-6 and IP-10 were quantified in supernatants after treating BMM with vehicle, netrin-1, IFNγ, or IFNγ + netrin-1. Our results show that netrin-1 suppressed both basal level and the IFNγ-induced increase in IL-6 and IP-10 secretion (Fig. 3). IP-10 expression was shown to be associated with M1 phenotype of macrophages consistent with IFNγ-induced M1 polarization (17).
Fig. 3.
Netrin-1 suppressed IFNγ-induced cytokine and chemokine production. Bone marrow-derived macrophages were treated with vehicle, netrin-1, IFNγ, or a combination of netrin-1 and IFNγ for 48 h. Cytokine and chemokine levels in the supernatant were determined by ELISA. Netrin-1 suppressed both basal and IFNγ-induced IL-6 (A) and IP-10 (B) production. Values are means ± SE (n = 4). *Significant difference vs. control (P < 0.05). #Significant difference vs. all other groups (P < 0.05).
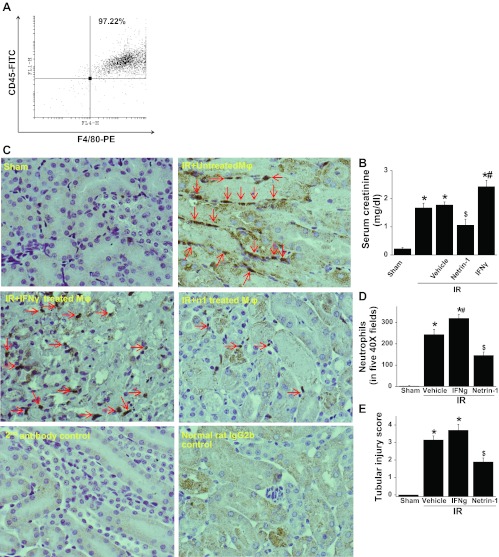
In vitro M2 polarized macrophages treated with netrin-1 suppresses ischemic acute kidney injury and inflammation in mice.
To determine whether in vitro M2-polarized macrophages have a protective role in vivo, bone marrow-derived macrophages were treated with vehicle, netrin-1, or IFNγ for a period of 48 h. Cells were harvested, washed, and counted. Then 500,000 cells were injected intravenously 1 h before renal pedicle clamping. Administration of bone marrow-derived macrophages before surgery, which were treated in vitro with netrin-1, suppressed kidney injury (Fig. 4B). However, administration of vehicle-treated macrophages did not suppress ischemia-reperfusion injury of kidney. Moreover, administration of IFNγ-treated macrophages exacerbated ischemic acute kidney injury. Neutrophil infiltration into the kidney was also suppressed by administration of netrin-1-treated macrophages but not by administration of IFNγ- or vehicle-treated macrophages. There was no staining seen with isotype-matched normal rat IgG as a primary antibody or secondary antibody alone in control or sham-operated animals' kidneys (Fig. 4C). Quantification of neutrophil staining in kidney is shown in Fig. 4D. Improved kidney function was associated with better preservation of kidney morphology in mice that received netrin-1-treated macrophage compared with animals that received vehicle or IFNγ-treated macrophages (Fig. 4E).
Fig. 4.
Adoptive transfer of netrin-1-treated bone marrow-derived macrophages suppressed ischemia-reperfusion injury of the kidney. A: flow cytometry analysis of bone marrow-derived macrophages showing >97% are positive for F4/80 and CD45 surface markers. B: adoptive transfer of netrin-1-treated but not vehicle-treated macrophages suppressed kidney injury as measured by serum creatinine levels. Transfer of IFNγ-treated macrophages exacerbated kidney injury. C: neutrophil infiltration into the kidney was suppressed by administration of netrin-1-treated macrophages but not IFNγ-treated macrophages. There was no staining seen with isotype-matched normal rat IgG as a primary antibody or secondary antibody alone in control or sham-operated animals kidney. D: quantification of neutrophil staining in kidney. Adoptive transfer of netrin-1-treated but not vehicle-treated macrophages suppressed neutrophils infiltration into the kidney injury. Transfer of IFNγ-treated macrophages increased more neutrophil infiltration. E: tubular necrosis was quantified as described in materials and methods. Values are means ± SE (n = 4–6). Significant difference vs. sham: *P < 0.001; $P < 0.05. #Significant difference vs. vehicle-treated macrophage group (P < 0.05).
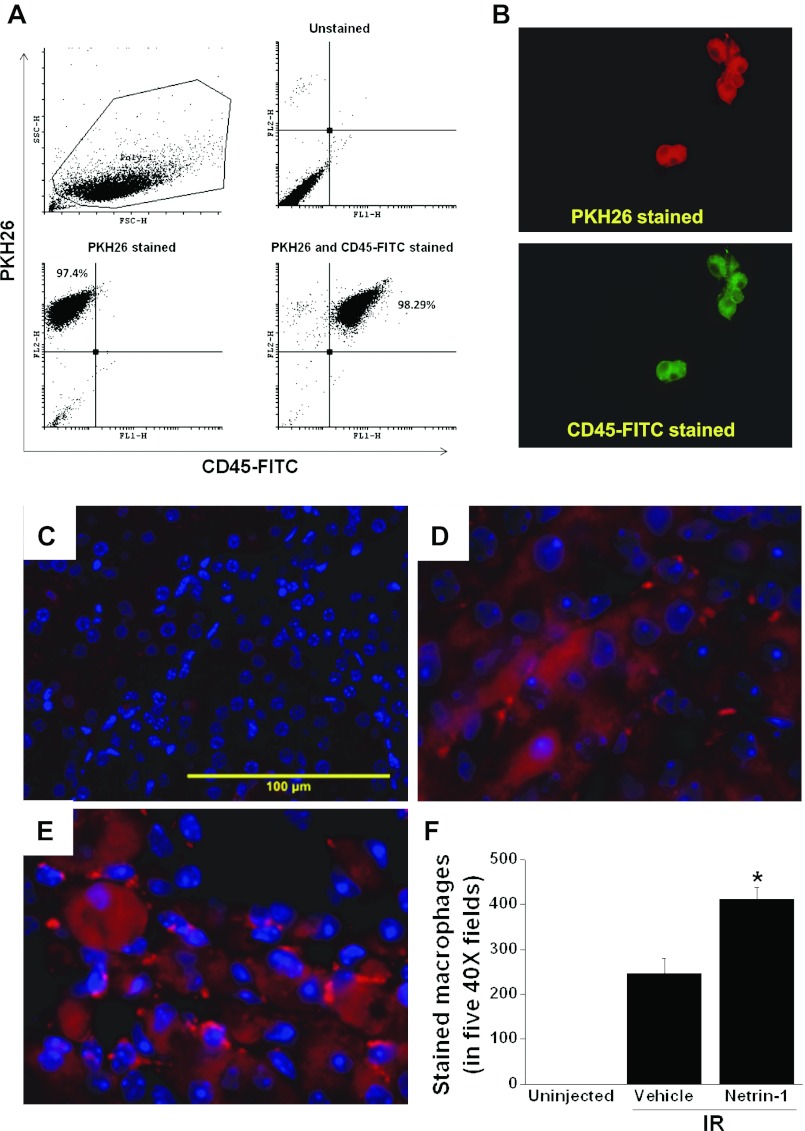
To determine whether adoptively transferred macrophages infiltrate into kidney interstitium, vehicle-treated and netrin-1-treated macrophages were labeled with PKH26 Red fluorescent linker as described in materials and methods and were then injected into mice 1 h before surgery. Uniform labeling of fluorescent dye was observed (Fig. 5, A and B). Infiltrating labeled cells were visualized in kidney at 24 h after reperfusion. The netrin-1-treated macrophage infiltration was increased in the kidney interstitium (Fig. 5E) compared with vehicle-treated macrophage (Fig. 5D), whereas no stained cells were seen in un-injected control mice kidney (Fig. 5C), suggesting a paracrine role of polarized macrophages in protecting kidney against ischemia-reperfusion injury.
Fig. 5.
Adoptively transferred bone marrow-derived macrophages are present in the interstitium of the kidney. Bone marrow-derived macrophages were labeled with red dye as described in materials and methods. A: flow cytometry analysis of macrophage labeled with red dye and CD45-FITC showing uniform and complete labeling of differentiated macrophages. B: photomicrograph of labeled macrophages. Magnification = ×400. C: un-injected control kidney section showing no stained macrophages. D: kidney section showing labeled (red) vehicle-treated macrophages in the interstitium following ischemia-reperfusion injury. E: kidney section showing labeled (red) netrin-1-treated macrophages in the interstitium following ischemia-reperfusion injury. F: quantification of labeled macrophages in the kidney sections. *Significant difference vs. vehicle-treated macrophages (P < 0.05) (n = 3).
Netrin-1 activates anti-inflammatory pathways that are known to regulate macrophage polarization and inflammation.
The signaling pathway through which netrin-1 suppresses inflammation is not known. Although netrin-1 induced an increase in cAMP levels, which is known to inhibit leukocyte migration, it is not clear whether this pathway alone can suppress cytokine and chemokine production, suppression of COX-2 expression, and modulation of monocyte phenotypes. To determine whether an additional pathway mediates the anti-inflammatory action of netrin-1 in leukocytes and other cells, 40 different signaling pathways were screened using a signaling array from SA biosciences. The signaling array contains 40 different plasmids where response elements were cloned upstream of the luciferase gene. RAW 264.7 macrophages were transfected and treated with netrin-1, and luciferase activities were measured. As shown in Fig. 6, three anti-inflammatory pathways were activated consistently. These include the PPARs (plasmid has a combination of PPARα, PPARβ/δ, and PPARγ response elements), glucocorticoid response element (GRE), and retinoic acid response elements (RXRs) (Fig. 6). We further analyzed the role of PPAR pathways due to its known role in macrophage polarization. PPARs are known to dimerize with RXR for DNA binding and transactivation. PPARs are members of a nuclear-hormone-receptor superfamily; they transduce a wide variety of signals, including environmental, nutritional, and inflammatory events into a defined and ordered set of cellular responses at the level of gene transcription. Various types of fatty acid metabolites of arachidonic acid can bind and activate PPARs. Recent evidence has indicated an important role for PPARs in the control of various types of inflammatory response (2). These functions are mediated by several mechanisms, which include the abilities of the PPARs to transrepress the activities of many activated transcription factors (NF-κB), signal transducers and activators of transcription (STATs), activator protein 1 (AP1), and nuclear factor of activated T-cells (NFAT), transcriptional upregulation of NF-κB inhibitor IκB, and the ability of PPAR-RXR heterodimers to inhibit phosphorylation of the MAPK (JNK and p38) cascade.
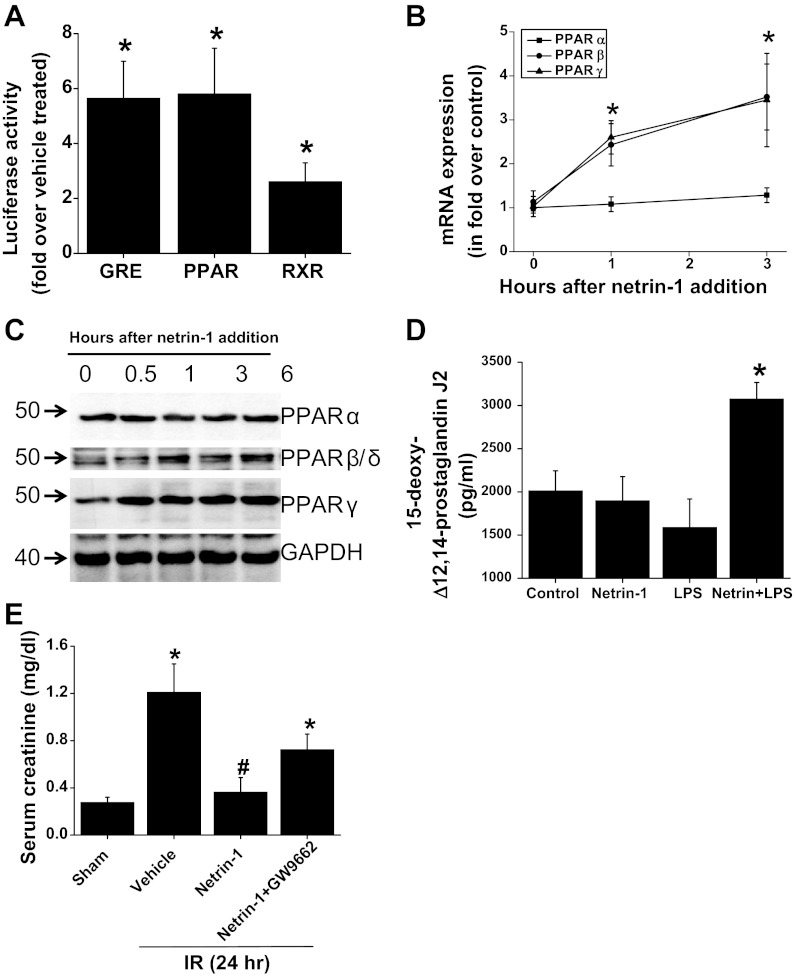
Fig. 6.
Netrin-1 activates PPAR pathways in macrophages, and PPAR pathways mediate part of the protective effects of netrin-1. A: netrin-1 increased PPAR, glucorticoid receptor, and RXR pathways as measured by luciferase activity as described in materials and methods. *Significant difference vs. vehicle-treated group (P < 0.05). B: time course of netrin-1-induced expression of PPARα, -β, and -γ in RAW264.7 cells. Netrin-1 significantly increased PPARβ and PPARγ expression but not PPARα expression. *Significant difference vs. 0 h (P < 0.001). C: Western blot analysis of PPARα, -β, and -γ protein expression with netrin-1 treatment. Netrin-1 increased PPARβ and PPARγ but not PPARα protein expression compared with 0 h. D: netrin-1 increased PPARγ ligand production in the presence of TLR4 receptor stimulation. *Signficant difference vs. all other groups (P < 0.01). E: administration of PPARγ antagonist abolished part of netrin-1 protective effects against ischemia-reperfusion injury of the kidney. Significant difference vs. sham: *P < 0.05; #P < 0.05 (n = 4–6).
Our results show that netrin-1 increases the expression of both PPARβ/δ and PPARγ in macrophages. In addition, netrin-1 induced the PPARγ ligand 15-deoxy-Δ12,14-prostaglandin J2 in the presence of TLR4 activation. Neither TLR4 activation alone nor netrin-1 increases 15-deoxy-Δ12,14-prostaglandin J2 levels in macrophages (Fig. 6). Administration of netrin-1 suppressed ischemia-reperfusion injury, which was abolished by PPARγ antagonist. In vitro, addition of netrin-1 strongly induced PPARβ and -γ expression and activation.
To determine whether PPARγ is the mediator of netrin-1 protective function against ischemia-reperfusion injury, mice were treated with netrin-1 and PPARγ antagonist (GW9662) and subjected to ischemia-reperfusion injury. Netrin-1 administration protected kidney against ischemia-reperfusion injury compared with vehicle treatment (Fig. 6E). Administration of PPARγ antagonist abolished part of the netrin-1 protective effects, suggesting a role for PPARγ in mediating the netrin-1 effects in vitro and in vivo.
PPAR antagonist abolishes netrin-1-mediated suppression of IFNγ-induced M1 polarization and cytokine production.
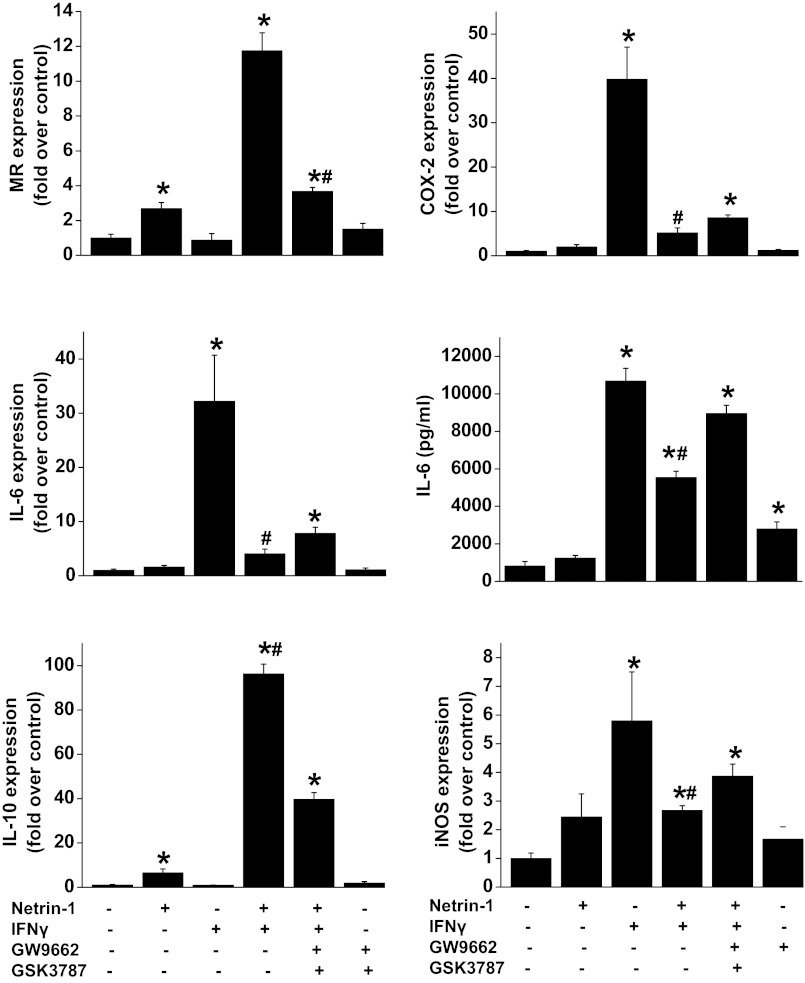
To determine whether netrin-1-mediated suppression of IFNγ-induced cytokine production and M1 polaization can be abolished with PPAR antagonist (GW9662: PPARγ antagonist and GSK3797: PPARβ antagonist), RAW264.7 cells were subjected to different treatments, and gene expression was analyzed by real-time PCR. As shown in Fig. 7, netrin-1 treatment increased expression of M2 markers (MR and IL-10), whereas IFNγ treatment induced M1 markers (COX-2, iNOS, and IL-6). Addition of netrin-1 with IFNγ suppressed M1 marker expression but enhanced M2 marker expression compared with cells treated with IFNγ alone. Importantly, when cells were treated with PPARβ and PPARγ antagonist along with netrin-1 and IFNγ, the M2 polarizing effects of netrin-1 were abolished, suggesting the important role of PPAR pathways in mediating netrin-1 effects in macrophages.
Fig. 7.
IFNγ-induced M1 marker expression and cytokine production are suppressed by netrin-1, and the effect of netrin-1 was abolished with PPAR antagonist. RAW264.7 cells were treated with netrin-1, IFNγ, netrin-1 + IFNγ, or PPARβ and -γ antagonist with netrin-1 and IFNγ for 48 h. Gene expression was analyzed by real-time PCR. IL-6 in the supernatant was quantified by ELISA. GW9662, PPARγ antagonist; GSK3797, PPARβ antagonist. Values are means ± SE (n = 4). *Significant difference vs. vehicle-treated group (P < 0.05). #Signficant difference vs. netrin-1 + IFNγ + GW9662 + GSK3787 group (P < 0.05).
DISCUSSION
Macrophages, the sentinels of innate immunity, take residence in nearly every tissue and display marked heterogeneity in their cell surface markers, location, and function (4, 5, 11). These long-lived resident tissue macrophages perform a variety of functions, including host defense, clearance of cellular debris, remodeling of tissues, and regulation of the inflammatory response. Although circulating monocytes are known to give rise to resident tissue macrophages (5), the regulatory pathways that direct the specification of macrophages into distinct functional subsets are largely unknown. We recently demonstrated that netrin-1 regulates the inflammatory response of macrophages and ameliorates ischemia-reperfusion-induced kidney inflammation and injury (19). In this study, we report for the first time that a novel mechanism underlies netrin-1 actions on macrophages. Our results show that netrin-1 treatment of macrophages induced M2 marker expression and suppressed IFNγ-induced M1 marker expression. Adoptive transfer of in vitro polarized macrophages into mice before ischemia-reperfusion suppressed kidney injury, inflammation, and neturophil infiltration. Moreover, transgenic mice that overexpress netrin-1 in spleen showed an increase in the number of M2 polarized macrophages, which was associated with enhanced IL-4, IL-13, and arginase-1 expression but downregulation of COX-2 expression. Interestingly, netrin-1 activated the PPAR and glucocorticoid pathways, known anti-inflammatory macrophage-polarizing signaling pathways. Inhibition of the PPAR pathways abolished netrin-1 effects on macrophages. Moreover, administration of PPARγ antagonist abolished the netrin-1 protective effects against ischemia-reperfusion injury of the kidney.
The presence of a large number of infiltrating monocytes in kidney interstitium is a hallmark of acute kidney injury. Activated inflammatory monocytes play a critical role in the development of tissue injury and subsequent development of chronic kidney disease (8, 9). Subsequent to infiltration of inflammatory monocytes, they differentiate into a less inflammatory phenotype depending on the mileu and are known to play a role in the repair process. Consistent with this view, our laboratories earlier results had shown that netrin-1 overexpression protects kidney from acute kidney injury (13, 21) and acute kidney injury-induced chronic kidney disease (unpublished observations). The present study also documents the presence of an M2-polarizing mileu in netrin-1 transgenic spleen and kidney. Moreover, there is a significant increase in the number of M2-polarized macrophages in the spleen. Interestingly, the baseline expression of IL-4 in kidney is not increased, whereas IL-4 expression in spleen is increased. The reason for this difference is not clear, but it may be due to polarized expression of transgene netrin-1 in tubular epithelial cells of the kidney, where netrin-1 protein is not accessible for interstitial cell under basal condition compared with diffusible nature in spleen. However, whether netrin-1 regulates macrophage polarization through upregulation of IL-4 and IL-13 or directly through PPAR pathways is not clear. Our in vivo PPARγ inhibition studies suggest a direct role of the PPAR pathway in netrin-1-mediated protective effects. IL-4 and IL-13 are known to activate STAT6, which induces expression of PPARβ and PPARγ. Activation of PPARβ and PPARγ mediates induction of STAT6 and M2 markers such as MR and arginase-1 (1, 18). Therefore, both direct and indirect mechanisms may operate in netrin-1 polarization of macrophages in vivo.
Netrin-1 addition to macrophage culture suppressed IFNγ-induced M1 polarization, M1 marker expression, and cytokine production. Moreover, netrin-1 is able to reverse M1-polarized macrophages to M2 phenotypes, suggesting that netrin-1-mediated anti-inflammatory function can be effective irrespective of the phenotype of macrophages. Interestingly, the expression of IL-10 and MR is enhanced when netrin-1 and IFNγ were added together, suggesting potentiation of netrin-1 effects. However, the pathways that are responsible for this is not clear. Consistent with our in vivo studies, administration of netrin-1 protected kidney from injury and inflammation. Consistent with our observation, Lee et al. (7) demonstrated that iNOS-positive proinflammatory (M1) macrophages are recruited into the kidney in the first 48 h after ischemia-reperfusion injury, whereas arginase 1- and MR-positive, non-inflammatory (M2) macrophages predominate at later time points (7). In addition, depletion of macrophages before ischemia-reperfusion diminishes kidney injury. Infusion of IFNγ-stimulated, bone marrow-derived macrophages into macrophage-depleted mice at the time of kidney reperfusion restored injury to the level seen without macrophage depletion, suggesting that proinflammatory macrophages worsen kidney damage. In vitro studies showed that IFNγ-stimulated proinflammatory macrophages begin to express markers of M2 macrophages when cocultured with renal tubular cells (7). Interestingly, netrin-1 is highly induced after ischemia-reperfusion injury (16). Therefore, it is possible that tubular epithelial-derived netrin-1 may participate in reprogramming macrophages in vivo.
Netrin-1-mediated activation of PPARs pathways is new and was previously unknown. The mechanism through which netrin-1 activates PPAR is not clear. However, netrin-1 induced both PPAR expression as well as production of PPAR ligand. In addition, PPARs also regulated at the level of phosphorylation. Netrin-1 is known to increase Akt activation (22). Akt pathways influence the phosphorylation and DNA-binding activity of PPARs. Therefore, netrin-1 may affect PPAR activation at different levels, depending on the cells and presence of other stimuli. PPARs are known to dimerize with RXR for DNA binding and transactivation. RXR is also activated by netrin-1, suggesting that there may be a common pathway through which netrin-1 may enhance its anti-inflammatory activity. Recent evidence has indicated an important role for PPARs in the control of various types of inflammatory response (2). These functions are mediated by several mechanisms, which include the abilities of the PPARs to transrepress the activities of many activated transcription factors (e.g., NF-κB, STATs, AP1, and NFAT), transcriptional upregulation of NF-κB inhibitor IκB, and the ability of PPAR-RXR heterodimers to inhibit phosphorylation of the MAPK (JNK and p38) cascade. Consistent with these earlier reports, our recent studies have shown that netrin-1 suppressed IκBα degradation and activation of NF-κB in both epithelial cells and macrophages (Ranganathan PV, Jayakumar C, Mohamed R, Dong Z, Ramesh G. Unpublished observations). Moreover, netrin-1 overexpression also suppressed ischemia-reperfusion-induced STAT3 activation in the epithelial cells (15a), which further supports that netrin-1-induced activation of PPAR may play an important role in suppressing inflammation.
In conclusion, netrin-1 reprograms macrophages toward an anti-inflammatory phenotype both in vitro and in vivo. Adoptive transfer of reprogrammed macrophages into mice suppressed ischemia-reperfusion injury of the kidney. The macrophage reprogramming and netrin-1 protective effect in vivo was abolished with PPAR antagonist. Netrin-1 represents a new and novel anti-inflammatory therapy for ischemic and other forms of organ injury.
GRANTS
This work was supported by an R01 grant (7R01 DK-083379-02) from the National Institute of Diabetes and Digestive and Kidney Disease to G. Ramesh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.R., C.J., and G.R. performed experiments; P.R. and G.R. analyzed data; P.R. and G.R. interpreted results of experiments; P.R. and G.R. prepared figures; P.R. and G.R. drafted manuscript; P.R., C.J., and G.R. approved final version of manuscript; C.J. and G.R. edited and revised manuscript; G.R. conception and design of research.
REFERENCES
- 1. Chawla A. Control of macrophage activation and function by PPARs. Circ Res 106: 1559– 1569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daynes RA, Jones DC. Emerging roles of PPARS in inflammation and immunity. Nat Rev Immunol 2: 748– 759, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593– 604, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23– 35, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953– 964, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Grenz A, Dalton JH, Bauerle JD, Badulak A, Ridyard D, Gandjeva A, Aherne CM, Brodsky KS, Kim JH, Tuder RM, Eltzschig HK. Partial netrin-1 deficiency aggravates acute kidney injury. PLos One 6: e14812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317– 326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L, Huang L, Sung Ss Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte//macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526– 1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Seminars Nephrol 30: 268– 277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, Kinane TB. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA 102: 14729– 14734, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity 23: 344– 346, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Mohamed R, Jayakumar C, Ranganathan PV, Ganapathy V, Ramesh G. Kidney proximal tubular epithelial-specific overexpression of netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol 181: 1991– 2002, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajasundari A, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression in kidney proximal tubular epithelium ameliorates cisplatin nephrotoxicity. Lab Invest 91: 1717– 1726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166– F174, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166– F174, 2005 [DOI] [PubMed] [Google Scholar]
- 15a. Ranganathan P, Jayakumar C, Ramesh G. Kidney epithelial cells netrin-1 suppresses AKI-induced interstitial fibrosis and glomerulosclerosis through suppression of IL-6/STAT3 signaling. Am J Physiol Renal Physiol. (First published February 13, 2013). doi:10.1152/ajprenal.00650.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reeves WB, Kwon O, Ramesh G. Netrin-1 and kidney injury. II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol 294: F731– F738, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42: 717– 727, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM, Barak Y, Schwabe J, Nagy L. STAT6 transcription factor is a facilitator of the nuclear receptor PPAR-regulated Gene expression in macrophages and dendritic cells. Immunity 33: 699– 712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol 185: 3750– 3758, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Wang W, Brian RW, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol 294: F739– F747, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang W, Reeves WB, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am J Pathol 175: 1010– 1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Reeves WB, Ramesh G. Netrin-1 increases proliferation and migration of renal proximal tubular epithelial cells via the UNC5B receptor. Am J Physiol Renal Physiol 296: F723– F729, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Harris DCH. Macrophages in Renal Disease. J Am Soc Nephrol 22: 21– 27, 2011 [DOI] [PubMed] [Google Scholar]