Abstract
High NaCl activates the transcription factor nuclear factor of activated T cells 5 (NFAT5), leading to increased transcription of osmoprotective target genes. Kinases PKA, PI3K, AKT1, and p38α were known to contribute to the high NaCl-induced increase of NFAT5 activity. We now identify another kinase, GSK-3β. siRNA-mediated knock-down of GSK-3β increases NFAT5 transcriptional and transactivating activities without affecting high NaCl-induced nuclear localization of NFAT5 or NFAT5 protein expression. High NaCl increases phosphorylation of GSK-3β-S9, which inhibits GSK-3β. In GSK-3β-null mouse embryonic fibroblasts transfection of GSK-3β, in which serine 9 is mutated to alanine, so that it cannot be inhibited by phosphorylation at that site, inhibits high NaCl-induced NFAT5 transcriptional activity more than transfection of wild-type GSK-3β. High NaCl-induced phosphorylation of GSK-3β-S9 depends on PKA, PI3K, and AKT, but not p38α. Overexpression of PKA catalytic subunit α or of catalytically active AKT1 reduces inhibition of NFAT5 by GSK-3β, but overexpression of p38α together with its catalytically active upstream kinase, MKK6, does not. Thus, GSK-3β normally inhibits NFAT5 by suppressing its transactivating activity. When activated by high NaCl, PKA, PI3K, and AKT1, but not p38α, increase phosphorylation of GSK-3β-S9, which reduces the inhibitory effect of GSK-3β on NFAT5, and thus contributes to activation of NFAT5.
Keywords: hypertonicity, p38α, phosphorylation, transactivation, nuclear localization
high nacl and other forms of hypertonicity stress cells and, when excessive, are lethal. Interstitial NaCl normally is very high in kidney medullas and can vary considerably (4). Interstitial fluid is also normally hypertonic in some other organs and systemic hypertonicity occurs in some pathophysiological states, but such hypertonicity is not nearly as great as in the kidney medulla (46). Adaptation to hypertonicity depends critically on the transcription factor nuclear factor of activated T cells 5 (NFAT5; also called TonEBP or OREBP) (36, 41, 44), which activates expression of osmoprotective genes (4). The osmoprotective genes include ones involved in accumulation of compatible organic osmolytes, which reduce elevated intracellular ionic strength and normalize cell volume (4), and a chaperone (HSP70) that protects proteins from misfolding (65). Hypertonicity increases NFAT5 activity by elevating its transactivating activity (17), its nuclear localization (36, 44), its abundance (44), and its phosphorylation (9, 17, 20, 21, 31). Kinases, including ATM (32), PI3K-1A (30), c-Abl (21), CDK5 (20), AKT1 (58), PKA (16), and p38α (35, 73), and phosphatases, including SHP-1 (74), contribute to signaling high NaCl-induced activation of NFAT5. In addition to its role in activating expression of osmoprotective genes, NFAT5 is also involved in biological processes that apparently do not involve hypertonic stress, including cardiac development and function (42), vascular smooth muscle migration (26), skeletal muscle myogenesis (47), cancer progression and metastasis (7, 22), replication of HIV-1, HIV-2, and multiple simian immunodeficiency (53, 54), SFFVp virus replication (67), and expression of multiple Toll-like receptor-induced genes (5).
Glycogen synthase kinase-3β, GSK-3β (GSK3B), is a ubiquitously expressed serine/threonine kinase that was originally characterized as phosphorylating and inactivating glycogen synthase, the rate-limiting enzyme of glycogen synthesis (28). Since then, GSK-3β has been found to regulate a wide variety of biological processes such as function of neurons (29), immunological responses (63), cardiac hypertrophy (8), and cancer (43). The pleiotropic effects of GSK-3β involve regulation of many transcription factors, such as CREB, neurogenin 2, SMAD1, c-Jun, β-catenin (29), and NFAT1–4 (63). GSK-3β differs from most other protein kinases in that it is most active in its resting state, resulting in inhibition of its target transcription factors. When cells are stimulated, GSK-3β is inhibited, resulting in activation of its substrates. The activity of GSK-3β is inhibited by phosphorylation of serine residues, of which serine 9 is most studied (29). Many kinases, including PKA, PI3K, AKT1, and p38α, inhibit GSK-3β associated with phosphorylation of GSK-3β-S9 (34). High NaCl increases phosphorylation of GSK-3β-S9 in human embryonic kidney 293 (HEK293) cells (30), but it decreases the phosphorylation in renal medullary interstitial cells (55).
The present study was undertaken to determine whether high NaCl-induced phosphorylation and inhibition of GSK-3β in HEK293 cells contribute to high NaCl-induced activation of NFAT5 and whether PKA, PI3K, AKT1, and p38α are involved.
MATERIALS AND METHODS
Cells and chemicals.
HEK293 cells, purchased from American Type Culture Collection (ATCC), and HEK293FT cells (Invitrogen) were incubated in Eagle's minimal essential medium plus 10% fetal bovine serum in 5% CO2-95% air at 37°C and used between passages 38 and 48. We chose HEK293 and HEK293FT cells because of their good transfection efficiency. HEK293 cells stably expressing a luciferase reporter containing three osmotic response elements (OREs) from the aldose reductase gene or the same elements mutated to prevent specific DNA binding (72) were maintained in the same medium plus 2 μg/ml blasticidin and used between passages 39 and 44. Mouse inner medullary collecting duct cells (mIMCD3; gift of Dr. Steven R. Gullans) (56) were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 low glucose (1:1) containing 10% fetal bovine serum and 2 mM l-glutamine in 5% CO2-95% air at 37°C and used between passages 14 and 20. GSK-3β−/− mouse embryonic fibroblasts (gift of Dr. James R. Woodgett) were incubated in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum in 5% CO2-95% air at 37°C and used between passages 22 and 32. HeLa cells (ATCC) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum in 5% CO2-95% air at 37°C and used between passages 6 and 10. We used HeLa cells for Duolink in situ assays because HeLa cells do not detach from the plates during the multiple washings that the assay requires, whereas HEK293 and HEK293FT cells do detach. The initial osmolality of media was 290 mosmol/kgH2O for HEK293 cells and 300 mosmol/kgH2O for mIMCD3 cells, GSK-3β-null embryonic fibroblasts, and HeLa cells. All experiments were performed on subconfluent cells. SB203580 was purchased from Calbiochem. H89, wortmannin, and triciribin were purchased from Sigma.
Plasmids, siRNAs, transfections, and luciferase activity.
Plasmids coding for hemagglutinin (HA)-tagged GSK-3β [pcDNA3-GSK-3β-HA (27), “GSK-3β-HA,” or “GSK-3β”], its mutant [pcDNA3-GSK-3β-S9/A-HA (27), “S9/A-HA”], Flag-tagged constitutively active mitogen-activated protein kinase kinase 6 [pcDNA3-MKK6 (glu)-Flag (51), “caMKK6”], and HA-tagged constitutively active serine/theronine kinase AKT1 [pLNCX-Myr-AKT1-HA (52), “caAKT1”] were purchased from Addgene. The plasmid coding for the catalytic subunit α of protein kinase A (pcDNA3-PKAcα, “PKAcα”) was a gift of Dr. Sankar Ghosh (71). The plasmid coding for mitogen-activated protein kinase p38α (pEBG-p38α, “p38α-GST”) was a gift of Dr. Yusen Liu (73). The plasmid NFAT5-V5 was described previously (32). The human ORE-X and NFAT5 transactivation luciferase reporters were previously described (17, 32). siRNA against human GSK-3β was purchased from Santa Cruz Biotechnology. The control siRNA was described previously (30). Transfections of siRNAs and DNA plasmids were done with Lipofectamine 2000, using the recommended ratio of siRNA or DNA to Lipofectamine 2000 (Invitrogen). All transfections were done by adding cell suspensions to plated complex of DNA or siRNA with Lipofectamine 2000 (reverse transfection). Luciferase activity was measured as previously described (72).
Western analysis and antibodies.
Cells were preincubated for 60 min with the chemical inhibitors before changing NaCl. After treatment, cells were washed once with ice-cold PBS adjusted to the osmolality of the medium by adding NaCl. For measuring phosphorylation, the samples were collected in Phosphosafe buffer (EMD Chemicals). For measuring NFAT5 protein abundance, the samples were collected with 1% Triton X-100, 150 mM NaCl, and 50 mM Tris·HCl, pH 7.4. All buffers were supplemented with a protease inhibitor tablet (Roche) immediately before use. Samples were analyzed by Western blotting and quantified by infrared imaging (Odyssey, Li-Cor). Antibodies against NFAT5 (catalog no. SC-13035) and Brg-1 (catalog no. SC-17796) were from Santa Cruz Biotechnology. Antibodies against GSK-3β (catalog no. 9315), GSK-3β-S9-P (catalog no. 9336), MKK6 (catalog no. 9264), p38 (catalog no. 9212), p38-P (catalog no. 9216), p38α (catalog no. 9218), PKA catalytic subunit α (catalog no. 5842), GAPDH (catalog no. 2118), and AKT1 (catalog no. 2920) were from Cell Signaling. β-Tubulin (catalog no. T8660) was from Sigma. Mouse anti-GSK-3α/β (catalog no. 44–610) was from Invitrogen.
NFAT5 transactivating activity and nuclear localization.
NFAT5 transactivating activity was analyzed in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system, as previously described (17). For measuring NFAT5 nuclear localization, cells were transfected with GSK-3β siRNA or cotransfected with plasmids coding for NFAT5-V5 and GSK-3β-HA for 24 h and then divided into two dishes and incubated for an additional 22 h. In one dish, the medium was replaced with fresh culture medium (290 mosmol/kgH2O) and the other with medium at 500 mosmol/kgH2O (NaCl added) for 2 h. Cells were washed once with ice-cold PBS adjusted to the osmolality of the medium by adding NaCl. The cytoplasmic and nuclear proteins were extracted separately using NE-PER (Pierce). NFAT5 in each fraction was measured by Western blot analysis and the nuclear-to-cytoplasmic ratio was calculated from the concentrations in cytoplasmic and nuclear extracts and the relative volumes of the extracts (15). The cytoplasmic protein β-tubulin and nuclear protein Brg-1 were monitored in each extract to exclude the possibility that the ratio was affected by inadequate separation of nuclear and cytoplasmic proteins.
GSK-3β activity.
After treatments, HEK293 cells were washed once with ice-cold PBS adjusted to the osmolality of the medium by adding NaCl and lysed with ice-cold 1% Triton X-100, 150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 2.0 μM NaF, 2.0 μM Na3VO4, and protease inhibitor cocktail tablet (Roche). GSK-3β was immunoprecipitated with rabbit anti-GSK-3β antibody (Cell Signaling, catalog no. 9315) at 4°C for 2 h. The immunoprecipitates were washed twice with the lysis buffer and once with the assay buffer. GSK-3β bound to agarose beads was incubated with its substrate (Promega, catalog no. V1991), according to the manufacturer's protocol. ADP released by the reaction into the supernatant was measured with the ADP-Glo Kinase Assay system (Promega, catalog no. V9101), according to the manufacturer's instruction. The beads were stripped with SDS-loading buffer at 95°C for 5 min and the immunoprecipitated GSK-3β was detected by Western blotting, quantified by infrared imaging (Odyssey, Li-Cor). The measured GSK-3β activity was normalized by the relative amounts of immunoprecipitated GSK-3β.
Coimmunoprecipitation.
HEK293 cells stably expressing NFAT5-V5 (32) were treated with the normotonic culture medium or hypertonic medium (NaCl added) for 2 h. After being washed once with ice-cold normotonic PBS or hypertonic PBS buffer (NaCl added), cells were collected in a lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, and 1% Triton X-100 plus protease inhibitor tablet. The supernatant was incubated with agarose-conjugated mouse anti-V5 antibody (ABD Serotec, catalog no. MCA1360) at 4°C overnight, and then the agarose beads were gently washed twice with ice-cold lysis buffer. Proteins were stripped from the beads by SDS-loading buffer at 95°C for 5 min and analyzed by immunoblotting, using rabbit anti-GSK-3β (Cell Signaling, catalog no. 9315) and rabbit anti-SHP-1 (Santa Cruz Biotechnology, catalog no. SC-287).
Duolink in situ.
Cells were fixed with 4% formaldehyde for 15 min at room temperature and for an additional 45 min on ice, permeablized with 0.5% Triton X-100 in PBS at room temperature for 15 min, blocked with Odyssey blocking buffer (Li-Cor) at room temperature for 30 min, and incubated with the mouse anti-V5 (1:250, ABD Serotec) and rabbit anti-GSK-3β (1:100, Cell Signaling). The rest of the analysis was performed according to the manufacturer's protocol (Olink Bioscience).
Statistics.
Data are expressed as means ± SE. The results were normalized to the 290 mosmol/kgH2O control and were log transformed for paired t-test or repeated-measures ANOVA with Student-Newman-Keuls post hoc comparison for multiple comparisons. P < 0.05 is considered significant.
RESULTS
Effect of GSK-3β on the transcriptional activity of NFAT5.
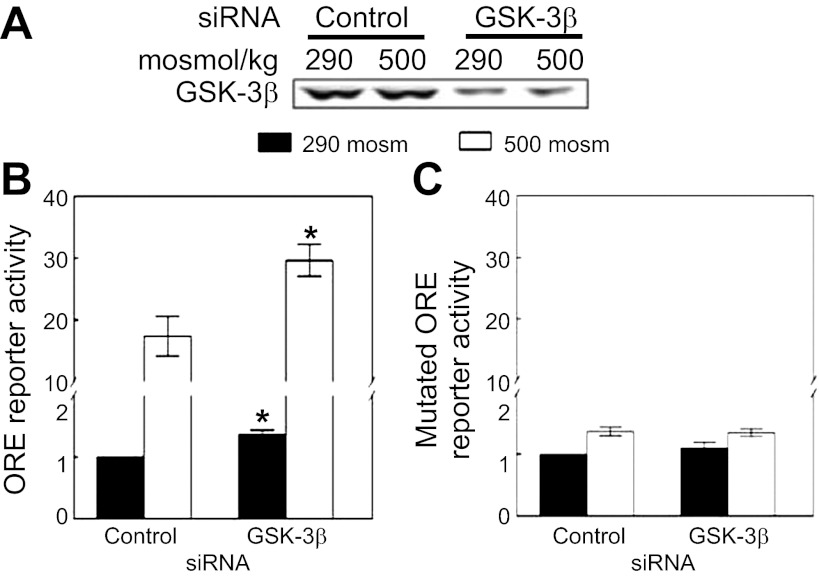
We tested the effect of siRNA-mediated knockdown of GSK-3β on transcriptional activity of NFAT5 by the use of HEK293 cells that stably express a luciferase reporter containing OREs. OREs are specific NFAT5 DNA binding sites. Knockdown of GSK-3β (Fig. 1A) increases NFAT5 transcriptional activity both at 290 and 500 mosmol/kgH2O, NaCl added (Fig. 1B). Knockdown of GSK-3β has no effect on the mutant ORE reporter at either tonicity (Fig. 1C). We conclude that GSK-3β contributes to regulation of NFAT5 transcriptional activity.
Fig. 1.
Glycogen synthase kinase (GSK)-3β negatively regulates high NaCl-induced nuclear factor of activated T cells 5 (NFAT5) transcriptional activity. Human embryonic kidney (HEK)293 cells stably expressing a luciferase reporter of NFAT5 transcriptional activity were transfected with siRNA against GSK-3β or control siRNAs for 32 h, and then osmolality was increased to 500 mosmol/kgH2O (NaCl added) or left at 290 mosmol/kgH2O for 16 h. We tested siRNAs from Santa Cruz Biotechnology, Cell Signaling, and Dharmacon, finding similar results. The result using the siRNA from Santa Cruz Biotechnology is displayed here. A: siRNA decreases GSK-3β protein. B: knockdown of GSK-3β significantly increases NFAT5 transcriptional activity at both 290 and 500 mosmol/kgH2O (NaCl added) in HEK293 cells stably expressing a luciferase reporter containing a wild-type osmotic response element (ORE). C: siRNA has no significant effect at either osmolality when the reporter contains an ORE element mutated to prevent binding by NFAT5 (*P < 0.05, compared with control siRNAs, repeated-measures ANOVA, n = 3).
Effect of GSK-3β on NFAT5 transactivating activity.
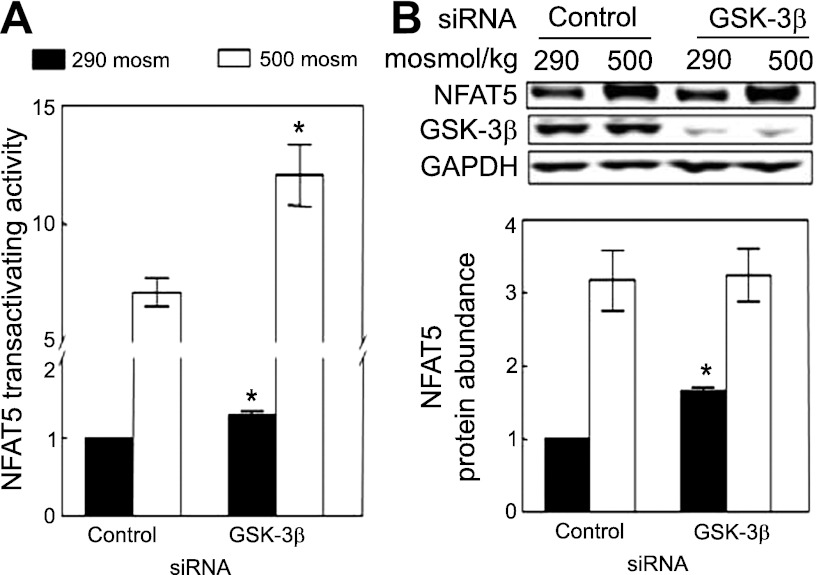
High NaCl increases transactivating activity of NFAT5, as measured with a binary reporter system expressing the NFAT5 transactivating domain (17). The activity in this assay depends on the expressed recombinant NFAT5 transactivation domain and is independent of native NFAT5. We used HEK293 cells that stably express the reporter to test for a role of GSK-3β. siRNA-mediated knockdown of GSK-3β increases transactivating activity of NFAT5 both at 290 and 500 mosmol/kgH2O (Fig. 2A). We conclude that GSK-3β contributes to regulation of NFAT5 transactivating activity.
Fig. 2.
A: siRNA-mediated knockdown of GSK-3β increases high NaCl-induced elevation of NFAT5 transactivating activity. As in Fig. 1B except that we measured NFAT5 transactivating activity in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system. B: siRNA-mediated knockdown of GSK-3β increases NFAT5 protein abundance at 290 mosmol/kgH2O. HEK293 cells were transfected with siRNA against GSK-3β for 32 h at 290 mosmol/kgH2O, and the medium was changed for 16 h, either maintaining it at 290 mosmol/kgH2O or increasing it to 500 mosmol/kgH2O (NaCl added). *P < 0.05 compared with control siRNA, repeated-measures ANOVA, n = 3.
Effect of GSK-3β on NFAT5 protein abundance.
High NaCl increases the amount of NFAT5 protein (44). We used siRNA-mediated knockdown to test for a role of GSK-3β in the high NaCl-induced increase of expression of NFAT5 protein abundance in HEK293 cells. Knockdown of GSK-3β increases NFAT5 protein at 290 mosmol/kgH2O, but it does not affect the high NaCl-induced increase of NFAT5 protein (Fig. 2B). Thus, we find that GSK-3β reduces NFAT5 protein expression under normotonic conditions, but do not find that GSK-3β contributes to the high NaCl-induced increase of NFAT5 protein.
Lack of effect of GSK-3β on nuclear localization of NFAT5.
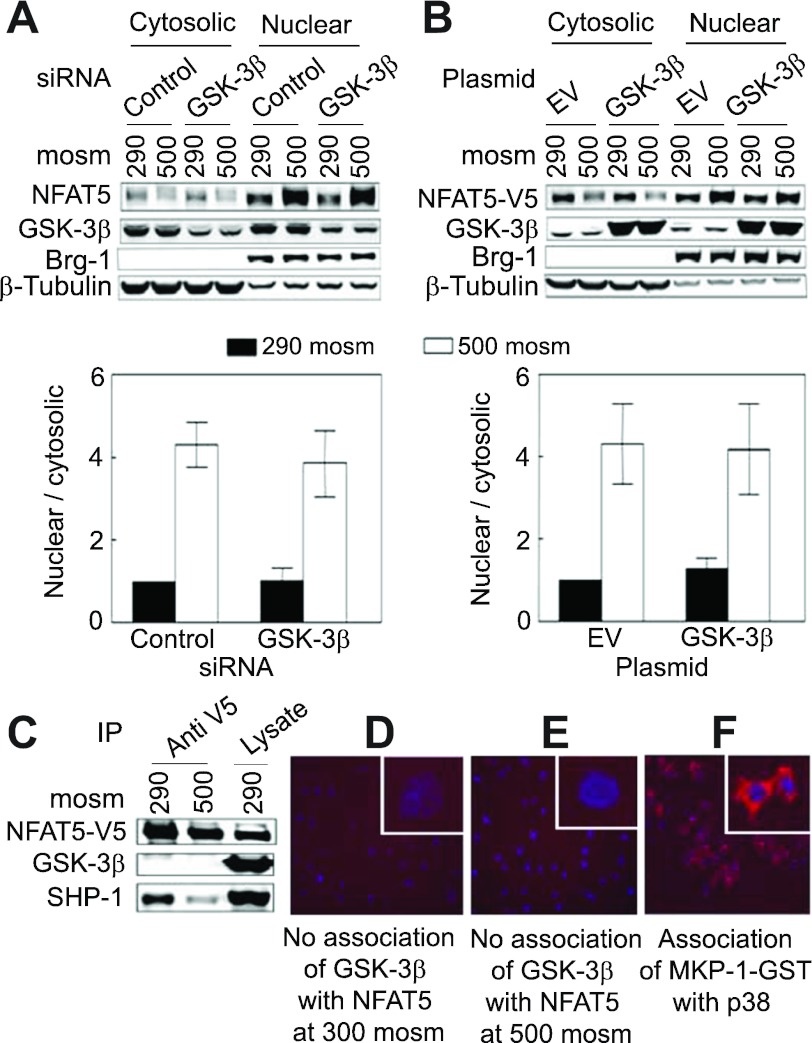
High NaCl increases the nuclear localization of NFAT5 (36, 44). GSK-3β inhibits the transcriptional activity of the other NFATs (NFAT1–4) by reducing their nuclear localization (3). However, siRNA-mediated knockdown of GSK-3β has no significant effect on high NaCl-induced nuclear localization of endogenous NFAT5 (Fig. 3A), nor does overexpression of GSK-3β affect nuclear localization of recombinant NFAT5-V5 (Fig. 3B).
Fig. 3.
A and B: GSK-3β does not affect NFAT5 nuclear localization. A: HEK293 cells were transfected with siRNA against GSK-3β or control siRNAs and treated as in Fig. 1 except that osmolality was increased for only 2 h. Proteins were extracted separately from cytoplasm and nuclei and then analyzed by Western blotting, and the nuclear-to-cytoplasmic ratio of NFAT5 was calculated. Note that the subcellular distributions of β-tubulin (cytoplasmic marker) and Brg-1 (nuclear marker) are unaffected by NaCl concentration. B: as in A except that HEK293FT cells were transiently cotransfected with plasmids coding for GSK-3β-HA and NFAT5-V5. C–E: lack of evidence for direct association of GSK-3β with NFAT5. C: NFAT5-V5 was immunoprecipitated from HEK293 cells that stably express it (32) after the medium was replaced for 2 h either with the same medium at 290 or an otherwise identical medium at 500 mosmol/kgH2O (NaCl added). SHP-1 coimmunoprecipitates with NFAT5-V5, but GSK-3β does not. D and E: after HeLa cells were transfected with NFAT5-V5, they were transferred for 24 h to Lab-Tek chamber slides for an additional 22 h before the medium was replaced for 2 h either with the same medium at 290 or an otherwise identical medium at 500 mosmol/kgH2O (NaCl added). Then, Duolink in situ assay was performed. No association (red staining) is detected between NFAT5-V5 and GSK-3β. F: positive control for the Duolink assay confirming in HeLa cells the previously observed (73) direct association of p38 with MKP-1-GST in mouse inner medullary collecting duct cells (mIMCD3) cells.
Tests of physical association of GSK-3β with NFAT5.
We used anti-V5 to immunoprecipitate NFAT5-V5 from HEK293 cells that stably express NFAT5-V5 (32). GSK-3β does not coimmunopreciptate with NFAT5-V5, although as observed previously (74), SHP-1 does (Fig. 3C). Duolink in situ is an alternative way to detect association between proteins (61). In agreement with the results of immunoprecipitation, Duolink in situ does not detect physical association of NFAT5-V5 with GSK-3β (Fig. 3, D and E). As a positive control, Duolink in situ does detect the previously reported (73) association of MKP-1 with p38 (Fig. 3F). Thus, we fail to find evidence for direct physical association of GSK-3β with NFAT5.
Effect of high NaCl on phosphorylation of GSK-3β-S9 and on activity of GSK-3β.
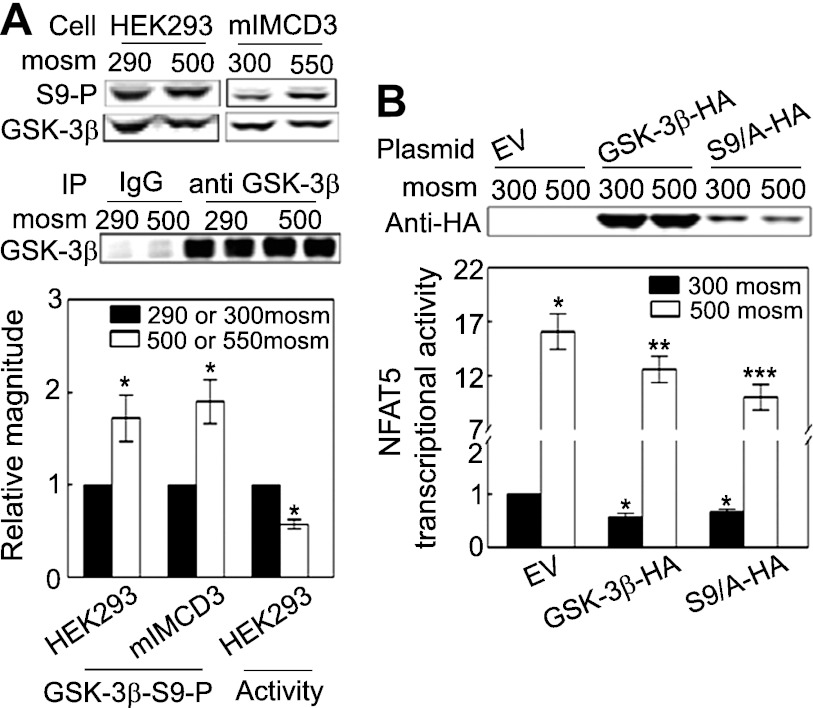
We confirm our previous finding (30) that high NaCl increases phosphorylation of GSK-3β-S9 in HEK293 cells (Fig. 4A). To test whether the effect is limited to HEK293 cells, we also tested mIMCD3 cells, finding the same result (Fig. 4A). The increased phosphorylation is accompanied by decreased GSK-3β kinase activity (Fig. 4A). We conclude that high NaCl increases phosphorylation of GSK-3β-S9, which inhibits GSK-3β activity.
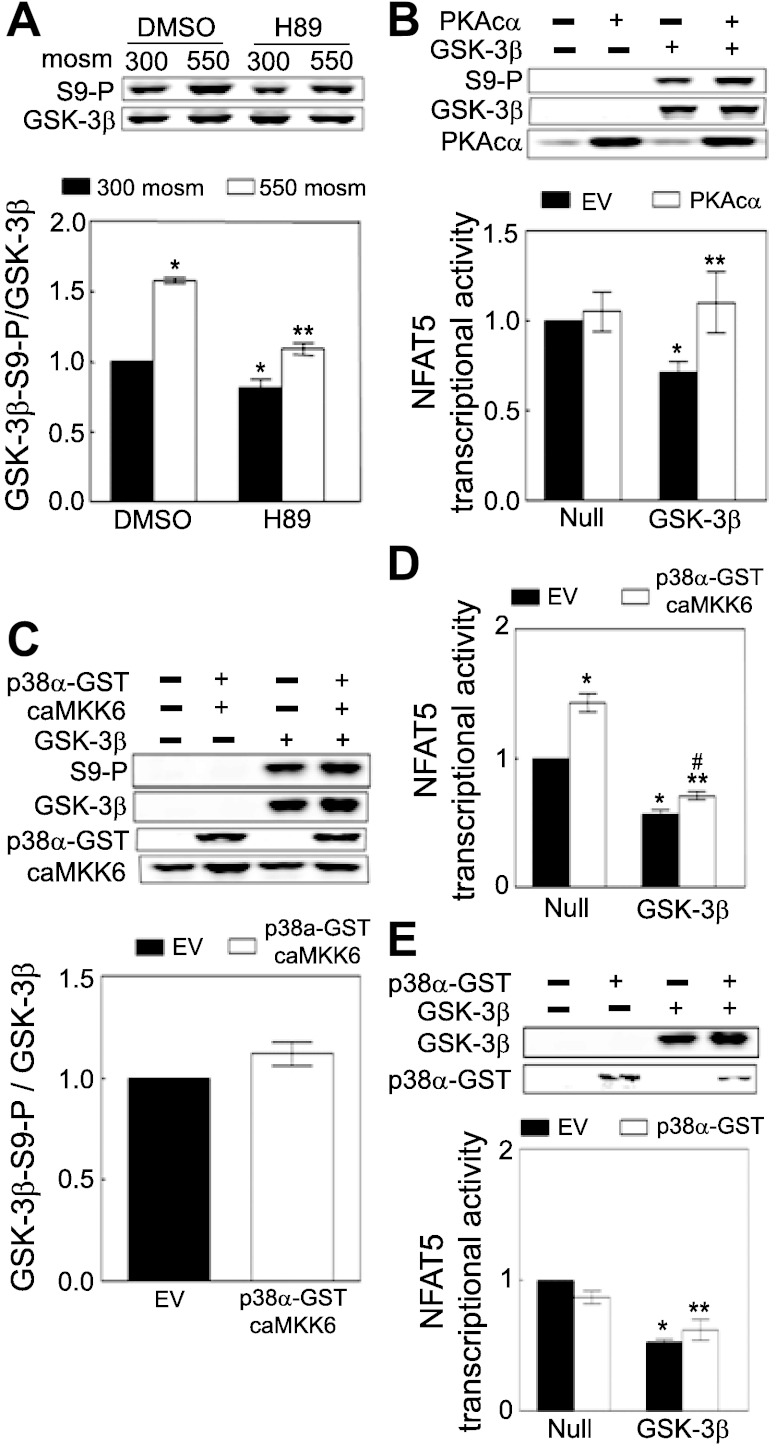
Fig. 4.
High NaCl increases phosphorylation of GSK-3β-S9 and decreases GSK-3β activity. A: HEK293 cells: medium was replaced for 30 min with the same medium at 290 or an otherwise identical medium at 500 mosmol/kgH2O (NaCl added); mIMCD3 cells: medium was replaced for 30 min either with the same medium at 300 or an otherwise identical medium at 550 mosmol/kgH2O (NaCl added). GSK-3β-S9 phosphorylation was measured by Western blot analysis of the ratio of phospho-GSK-3β/total GSK-3β. The activity of GSK-3β immunoprecipitated from HEK293 cells was measured with the ADP Glo Kinase Assay system (*P < 0.05 compared with respective control, paired t-test, n = 3 for phosphorylation assay and n = 4 for activity assay). B: overexpression of wild-type GSK-3β-HA reduces NFAT5 transcriptional activity in GSK-3β-null MEF cells, and overexpression of GSK-3β-S9A reduces NFAT5 transcriptional activity even more. GSK-3β−/− mouse embryonic fibroblasts (MEFs) were cotransfected with a luciferase reporter of NFAT5 transcriptional activity (ORE-X) together either with the empty vector (EV) pcDNA3, pcDNA3-GSK-3β-HA wild-type (GSK-3β-HA), or pcDNA3-GSK-3β-S9/A-HA (S9A-HA) for 24 h, and then osmolality was increased to 500 mosmol/kgH2O (NaCl added) or left at 300 mosmol/kgH2O for an additional 24 h before ORE-X reporter activity was measured. *P < 0.05 vs. EV at 300 mosmol/kgH2O. **P < 0.05 vs. EV at 500 mosmol/kgH2O. ***P < 0.05 vs. GSK-3β-HA at 500 mosmol/kgH2O, repeated-measures ANOVA, n = 4.
Effect on NFAT5 transcriptional activity of expressing wild-type and S9A-mutant GSK-3β in GSK-3β-null cells.
Since GSK-3β that is not phosphorylated on serine 9 inhibits NFAT5 transcriptional activity, we supposed that mutant GSK-3β-S9A, which cannot be phosphorylated at position 9, might also reduce NFAT5 transcriptional activity. To examine that possibility, we transfected GSK-3β-null cells with empty vector, wild-type GSK-3β-HA, or GSK-3β-S9A-HA (S9A-HA). Overexpression of GSK-3β-HA reduces NFAT5 transcriptional activity relative to empty vector (Fig. 4B). Overexpression of GSK-3β-S9A-HA reduces NFAT5 transcriptional activity even more. We suppose that the great abundance of overexpressed GSK-3β-HA prevents it from being completely phosphorylated. In agreement with this, GSK-3β-S9A-HA, which cannot be phosphorylated at position 9, inhibits even more efficiently than GSK-3β-S9-HA. NFAT5 activity is regulated by multiple signaling pathways (see Fig. 7). Since GSK-3β is only one component of these pathways, it is not surprising that overexpression of the GSK-3β-S9A mutant only partially inhibits NFAT5 activity.
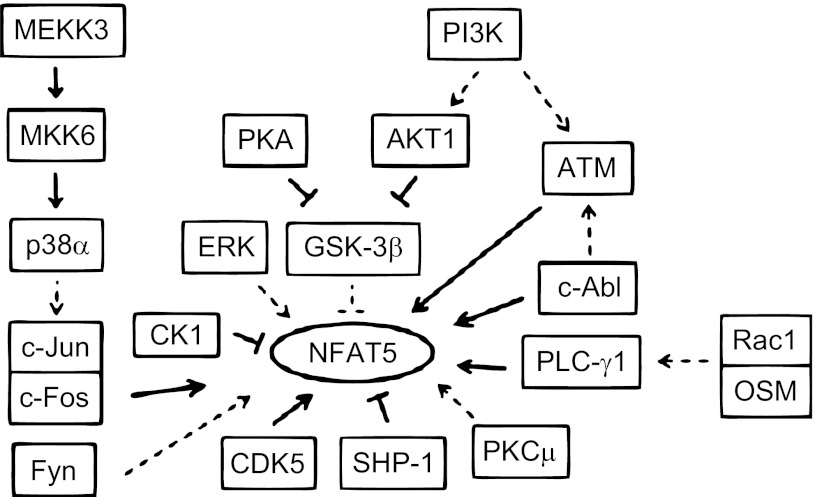
Fig. 7.
Summary of kinases and phosphatases known to regulate NFAT5 and of proteins that interact with them. All the depicted interactions are in the context of hypertonicity. Bold lines indicate direct interaction with another component of a pathway or with NFAT5 itself. Dashed lines indicate that the effect is not known to be direct.
Effect of PKA on phosphorylation of GSK-3β-S9 and on the contribution of GSK-3β to regulation of NFAT5 transcriptional activity.
High NaCl increases PKA activity, which contributes to high NaCl-induced increase of NFAT5 transcriptional and transactivating activities (16). PKA can catalyze phosphorylation of GSK-3β (13), suggesting that inhibition of GSK-3β activity by PKA might contribute to the high NaCl-induced increase of NFAT5 transcriptional activity. We applied H89 (10 μM), a direct inhibitor of PKA, but not of GSK-3β (1) to test that possibility. H89 reduces phosphorylation of GSK-3β-S9 at both NaCl concentrations (Fig. 5A), supporting the idea that PKA contributes to high NaCl-induced phosphorylation of GSK-3β-S9. We next tested the possibility that PKA contributes to high NaCl-induced increase of NFAT5 transcriptional activity by phosphorylating GSK-3β. We overexpressed the catalytic subunit α of PKA (PKAcα) in GSK-3β-null cells without or with reconstitution of GSK-3β, keeping the osmolality at 300 mosmol/kgH2O so that any wild-type GSK-3β-HA would be active. Overexpression of PKAcα does not affect NFAT5 transcriptional activity in GSK-3β-null cells that are not reconstituted (“Null” in Fig. 5B). However, reconstitution of the cells with GSK-3β reduces NFAT5 transcriptional activity (“GSK-3β, EV” in Fig. 5B), which is reversed by overexpression of PKAc (“GSK-3β, PKAcα” in Fig. 5B), accompanied by increased phosphorylation of GSK-3β-S9 (top panel in Fig. 5B). We conclude that PKA contributes to activity of NFAT5 by increasing phosphorylation of GSK-3β-S9, which reduces inhibitory GSK-3β activity.
Fig. 5.
A: PKA contributes to high NaCl-induced phosphorylation of GSK-3β-S9. mIMCD3 cells were preincubated with 0.1% DMSO (control) or 10 μM PKA inhibitor H89 in DMSO for 60 min at 300 mosmol/kgH2O, and then the medium was changed for 30 min to an identical one or an otherwise identical one in which osmolality was increased to 500 mosmol/kgH2O (NaCl added). Phospho-GSK-3β-S9 and total GSK-3β were measured by Western blot analysis. *P < 0.05 compared with DMSO at 300 mosmol/kgH2O. **P < 0.05, compared with DMSO at 550 mosmol/kgH2O, repeated-measures ANOVA, n = 3. B: PKA increases phosphorylation of GSK-3β-S9 which contributes to high NaCl-induced increase of NFAT5 transcriptional activity. GSK-3β-null MEFs were cotransfected at 300 mosmol/kgH2O with a luciferase reporter of NFAT5 transcriptional activity (ORE-X), GSK-3β-HA (GSK-3β), or EV (Null), and catalytically active PKA (PKAcα) or EV for 24 h, and then the medium was refreshed for an additional 24 h before luciferase activity was measured. *P < 0.05 vs. EV in the null cells. **P < 0.05 vs. EV in the GSK-3β reconstituted group, GSK-3β, repeated-measures ANOVA, n = 4. Expression of PKAcα and GSK-3β-HA was identified by anti-PKA and anti-GSK-3β antibodies, respectively (top). Reconstitution of the null cells with GSK-3β inhibits NFAT5 transcriptional activity, but PKA activity increases phosphorylation of GSK-3β and reverses the inhibition. (C to E). The stimulatory effect of p38α on NFAT5 activity is not mediated by inhibitory phosphorylation of GSK-3β-S9. C and D: GSK-3β-null MEFs were cotransfected at 300 mosmol/kgH2O with a luciferase reporter of NFAT5 transcriptional activity (ORE-X), GSK-3β-HA (GSK-3β), or EV (Null), and p38α-GST plus catalytically active MKK6 (caMKK6) or their EVs as in B, before measurement of luciferase activity and Western blot analysis. *P < 0.05 vs. EV in the null cells. **P < 0.05 vs. EV in the GSK-3β reconstituted group, GSK-3β. #P < 0.05 vs. p38α-GST and caMKK6 in the null cells, repeated-measures ANOVA, n = 3. E: GSK-3β-null MEFs were cotransfected at 300 mosmol/kgH2O with a luciferase reporter of NFAT5 transcriptional activity (ORE-X), GSK-3β-HA (GSK-3β) or EV (Null), and p38α-GST or its EV as in B, and then luciferase activity was measured. *P < 0.05 vs. EV in the null cells. **P < 0.05 vs. p38α-GST in the null cells, repeated-measures ANOVA, n = 3.
Lack of involvement of p38α in regulation of NFAT5 transcriptional activity by GSK-3β.
High NaCl increases p38α activity, which contributes to the high NaCl-induced increase of NFAT5 transcriptional activity (35, 73). p38 Has been reported to catalyze phosphorylation of GSK-3β (40, 45), suggesting that inhibition of GSK-3β activity by p38α might contribute to high NaCl-induced increase of NFAT5 transcriptional activity. To test that possibility, we overexpressed p38α together with catalytically active MKK6 (caMKK6), which activates p38α, in GSK-3β-null cells at 300 mosmol/kgH2O. p38α Does not significantly affect phosphorylation of GSK-3β-S9 (Fig. 5C). When GSK-3β-null cells are not reconstituted (“Null”), transfection of p38α plus caMKK6 increases NFAT5 transcriptional activity, demonstrating that p38 can activate NFAT5 independent of GSK-3β (Fig. 5D). Simply reconstituting the cells (“GSK-3β”) reduces NFAT5 transcriptional activity, confirming that GSK-3β negatively regulates NFAT5 activity. When the null cells are reconstituted so that they express GSK-3β, not only does increasing p38 activity (by transfection of p38α plus caMKK6) not significantly increase phosphorylation of GSK-3β-S9 (Fig. 5C), but NFAT5 transcriptional activity is increased much less than in the absence of GSK-3β (Fig. 5D). Incidentally, overexpression of p38α alone without caMKK6 to activate it has no significant effect on NFAT5 transcriptional activity with or without reconstitution of GSK-3β (Fig. 5E). We conclude that p38α-induced increase of NFAT5 activity is independent of GSK-3β.
Effect of PI3K and AKT1 on phosphorylation of GSK-3β-S9 and NFAT5 transcriptional activity.
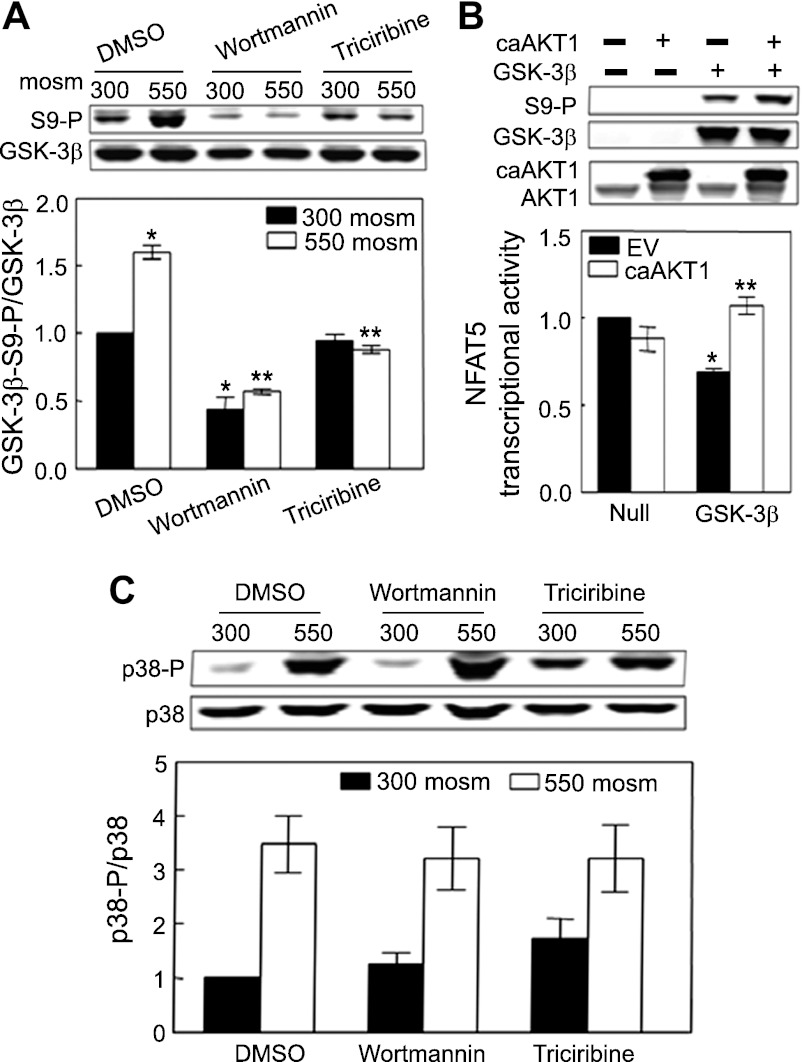
PI3K and its downstream kinase AKT1, which are known to inactivate GSK-3β by increasing its phosphorylation (34), are also known to contribute to high NaCl-induced activation of NFAT5 (30, 58), suggesting that GSK-3β might mediate the activation of NFAT5 by PI3K and AKT1. We inhibited PI3K and AKT1 to test this possibility. Wortmannin (200 nM), an inhibitor of PI3K, reduces phosphorylation of GSK-3β-S9 in mIMCD3 cells both at 300 mosmol/kgH2O and when osmolality is increased to 550 mosmol/kgH2O by adding NaCl (Fig. 6A). Triciribine (10 μM), an inhibitor of AKT, reduces phosphorylation of GSK-3β-S9 at 550 but not at 300 mosmol/kgH2O (Fig. 6A). We suppose that triciribine does not reduce phosphorylation of GSK-3β-S9 at 300 mosmol/kgH2O because AKT1 activity is already low at that osmolality. Furthermore, overexpression of catalytically active AKT1 (caAKT1) in unreconstituted GSK-3β-null MEF cells (Null) at 300 mosmol/kgH2O does not affect NFAT5 transcriptional activity, but, when the cells are reconstituted, overexpression of caAKT1 increases NFAT5 transcriptional activity significantly (GSK-3β; Fig. 6B). Also, caAKT1 increases phosphorylation of GSK-3β in reconstituted cells (Fig. 6B, top panel), as previously reported (12). Incidentally, neither wortmannin nor triciribine significantly affects high NaCl-induced phosphorylation of p38 (Fig. 6C). We conclude that PI3K and AKT1 contribute to the high NaCl-induced increase of NFAT5 transcriptional activity by phosphorylating GSK-3β-S9, which reduces inhibition of NFAT5 by GSK-3β.
Fig. 6.
A: PI3 kinase and AKT1 contribute to high NaCl-induced phosphorylation of GSK-3β-S9. mIMCD3 cells were preincubated with 0.1% DMSO (control), 200 nM wortmannin (PI3K inhibitor), or 10 μM triciribine (AKT1 inhibitor) for 60 min at 300 mosmol/kgH2O, and then the medium was changed for 30 min to the identical one or to an otherwise identical one at 550 mosmol/kgH2O (NaCl added). Phospho-GSK-3β-S9 and total GSK-3β were measured by Western blot analysis. *P < 0.05 vs. DMSO at 300 mosmol/kgH2O. **P < 0.05 vs. DMSO at 550 mosmol/kgH2O, repeated-measures ANOVA, n = 3. Inhibition of PI3K or AKT1 prevents the high NaCl-induced increase of phosphorylation of GSK-3β-S9. B: overexpression of caAKT1 eliminates the inhibitory effect of GSK-3β on NFAT5 transcriptional activity accompanied by increased phosphorylation of GSK-3β-S9. GSK-3β-null MEFs were transfected at 300 mosmol/kgH2O with luciferase reporter of NFAT5 transcriptional activity (ORE-X), GSK-3β-HA (GSK-3β), or EV and caAKT1 or EVs for 24 h, and then the medium was replaced with one still at 300 mosmol/kgH2O for an additional 24 h, before measuring luciferase activity. Lysate was immunoblotted to verify expression of caAKT1, GSK-3β, and phosphorylation of GSK-3β-S9 (top). *P < 0.05 vs. EV in null cells. **P < 0.05 vs. EV in GSK-3β reconstituted cells, repeated-measures ANOVA, n = 3. C: wortmannin or triciribine has no significant effect on phosphorylation of p38. mIMCD3 cells were treated as in A (n = 3).
DISCUSSION
GSK-3β is a widely expressed and highly conserved serine/threonine protein kinase (34). Over 100 cytoplasmic and nuclear proteins were proposed as substrates of GSK-3 (29, 34), including NFATc, but not NFAT5, which we identify here. GSK-3β is inhibited by phosphorylation at S9, and also at other sites, including S389 (60) and T43 (10). Phosphorylation of GSK-3β within its N-terminal region creates a “pseudosubstrate” that intramolecularly binds to a “phosphoprotein binding pocket” within the active site of the kinase, suppressing activity by occluding substrate access to the binding pocket (18). High NaCl increases phosphorylation of GSK-3β-S9 in HEK293 cells and mIMCD3 cells and decreases GSK-3β activity as measured in HEK293 cells (Fig. 4A). It is noteworthy that the effect of high NaCl may be cell specific, since the opposite effect was observed with the renal medullary interstitial cells (55). The phosphorylation that inactivates GSK-3β is increased by protein kinases and is decreased by phosphatase-1 (29, 69). Accordingly, siRNA-mediated knockdown of phosphatase-1 increases NFAT5 activity (74). GSK-3β is one of the handful of the over 500 known protein kinases that has a strong preference for substrates that are already primed by phosphorylation at a serine/threonine near the GSK-3 target residue (29, 34). GSK3β preferentially phosphorylates proteins and peptides at serine or threonine residues that flank another phosphoserine or phosphothreonine, located four residues C-terminal to the GSK-3β site (50, 64).
Regulation of NFAT5 activity by GSK-3β.
We considered whether GSK-3β could directly regulate NFAT5 activity by inhibitory phosphorylation of serines and/or threonines in NFAT5. We attempted to identify such serines and threonines by searching for sites that match the consensus GSK-3β phosphorylation pattern. Unfortunately, the GSK-3β consensus pattern of [ST]-X-X-X-p[ST] (11) does not discriminate sufficiently to distinguish between the 216 serines in NFAT5 (41 of which match the consensus) and the 111 threonines (11 of which match the consensus). Various search engines predict GSK-3 phosphorylation sites based on other criteria in addition to amino acid pattern. Predicted sites in NFAT5 include S145 (NetPhosK, PhosphoMotif Finder, GPS 2.1), S744 (PhosphoMotif Finder, GPS 2.1), S842 (PhosphoMotif Finder), S1168 (PhosphoMotif Finder, GPS 2.1), S1364 (NetPhosK, PhosphoMotif Finder, GPS 2.1), and S1436 (NetPhosK, PhosphoMotif Finder, GPS 2.1). We do not know whether any of these sites is phosphorylated at 300 because of GSK-3β activity and becomes dephosphorylated because high NaCl inhibits GSK-3β. Direct GSK-3β-induced inhibition of NFAT5 when NaCl is not elevated presumably requires priming by phosphorylation of a serine or threonine distal to the GSK-3β site. Dephosphorylation of the priming site could itself contribute to activation of NFAT5. We are unaware of protein kinases, besides GSK-3β, whose inhibition by high NaCl contributes to activation of NFAT5. However, our siRNA screen (74) identified 16 protein phosphatases whose knockdown decreases activation of NFAT5 by high NaCl, making them candidates for such a role. Finally, we cannot rule out the possibility that regulation of NFAT5 by GSK-3β is indirect, involving intermediate downstream signaling proteins, and we failed to find evidence that NFAT5 and GSK-3β directly interact with each other (Fig. 3, C to F).
GSK-3β affects nuclear localization of NFAT1–4, but not NFAT5.
All the members of the NFAT family are important substrates of GSK-3β, but the effect of GSK-3β on nuclear localization of NFAT1–4 differs from that on nuclear localization of NFAT5. All of the NFATs reside in the cytoplasm and translocate to the nucleus upon activation. However, phosphorylation of serines in serine-proline repeats, conserved in the amino terminus of NFAT1–4, controls their nuclear localization. GSK-3β phosphorylates these serine residues, which promote nuclear exit of NFAT1–4 and result in inhibition of NFAT1–4 transcriptional activity (3). Calcineurin dephosphorylates the serine residues, which increase nuclear localization of NFAT1–4 (2, 57). Unlike NFAT1–4, NFAT5 does not contain serine-proline repeats in its amino terminus (24, 44). Instead, high NaCl-induced phosphorylation of other amino acids regulates nuclear localization of NFAT5. For example, phosphorylation of tyrosine 143 (21, 31, 74), threonine 135 (20), and serines 155 and 158 (66) regulates nuclear import and export of NFAT5. The difference in amino acid composition explains why GSK-3β affects nuclear localization of NFAT5 differently from nuclear localization of NFAT1–4.
Regulation of NFAT5 by PKA via GSK-3β.
Activation of PKA by high NaCl contributes to the high NaCl-induced increase of NFAT5 transcriptional and transactivating activities (17). Our present results reveal that inhibition of GSK-3β is in the signaling pathway that is involved, consistent with the previous finding that GSK-3β is a substrate of PKA (13). PKA inhibits GSK-3β by phosphorylating it on serine 9 (13). High NaCl increases phosphorylation of GSK-3β-S9 (Fig. 5A). Inhibition of PKA by H89 inhibits the increased phosphorylation (Fig. 5A). Also, catalytically active PKA does not increase NFAT5 activity unless GSK-3β is expressed (Fig. 5B). Thus, the contribution of PKA to high NaCl-induced increase of NFAT5 transcriptional activity is mediated through GSK-3β.
PI3K and AKT1 regulate NFAT5 via GSK-3β.
High NaCl activates PI3K and its downstream target AKT1, which contributes to the high NaCl-induced increase of NFAT5 activity (30, 58). The previously known pathway was via ATM (32). High NaCl activates ATM, and suppression of PI3K-IA reduces activation of ATM by high NaCl. Thus, the contribution of PI3K to high NaCl-induced activation of NFAT5 occurs, at least in part, through a PI3K–ATM–NFAT5 pathway (30, 32). Now, we find evidence for an additional pathway, namely PI3K–AKT1–GSK-3β–NFAT5. Inhibition of PI3K by wortmannin or of AKT1 by triciribine reduces phosphorylation of GSK-3β-S9 (Fig. 6A). Also, overexpression of caAKT1 increases NFAT5 transcriptional activity in GSK-3β-null cells only if they are reconstituted with GSK-3β (GSK-3β; Fig. 6B). We conclude that PI3K phosphorylates GSK-3β-S9 via AKT1, which reduces the inhibitory effect of GSK-3β on NFAT5.
Contribution of p38α to high NaCl-induced activation of NFAT5 is independent of GSK-3β.
Studies using a p38 inhibitor, SB203580, reached the conclusion that p38 increases the inhibitory phosphorylation of GSK-3β-S9 induced by hepatocyte growth factor (40) and veratridine (45). However, we do not find that overexpression of p38α increases phosphorylation of GSK-3β-S9 (Fig. 5C). SB203580 reduces adiponectin-induced phosphorylation of GSK-3β at yet another site, namely, S389 (68). That raises the possibility that high NaCl-induced activation of p38α could inhibit GSK-3β by phosphorylation of GSK-3β-S389. However, overexpression of p38α does not reverse the inhibitory effect of GSK-3β on NFAT5 transcriptional activity (Fig. 5D), a finding independent of what site in GSK-3β might be phosphorylated by p38. We conclude that the stimulatory effect of p38α on NFAT5 is not mediated by inhibition of GSK-3β. Along the same line, inhibition of GSK-3β is also independent of p38 in cyclic strain of vascular smooth muscle cells (25). Although previous studies showed that AKT1 can act upstream of p38 (38), we and others demonstrated that wortmannin and triciribine have no significant effect on high NaCl-induced stimulatory phosphorylation of p38 (Fig. 6C) (58), indicating that the effect of PI3K and AKT1 on GSK-3β and NFAT5 is independent of p38α.
Summary of protein kinases and phosphatases that regulate NFAT5 activity.
Proteomic, immunoblotting, and mutational analyses revealed that the tonicity-dependent phosphorylation of multiple serine, threonine, and tyrosine residues in NFAT5 contributes to regulation of its transcriptional activity (20, 21, 31, 32, 66, 74). Therefore, it is not surprising that NFAT5 is regulated by multiple kinases and phosphatases. In Fig. 7, we summarize the kinases and phosphatases that we presently know regulate NFAT5. All interactions depicted are in the context of hypertonicity. Bold lines represent direct interaction between components of a pathway or between a signaling molecule and NFAT5. Dashed lines indicate effects not known to be direct. Hypertonicity induces a MAPK cascade that begins with autophosphorylation of MEKK3 at S526 and culminates in MKK6-mediated activation of p38α (19). MEKK3 and MKK6 activate NFAT5 transcriptional activity via p38α (35, 48, 73). No site directly phosphorylated by p38α has been identified in NFAT5, but p38α does mediate coactivation of NFAT5 transcriptional and transactivating activities via c-Jun and c-Fos. c-Jun and c-Fos associate physically with NFAT5 and the AP1 site that flanks an NFAT5 ORE in several genes (33). Fyn and ERK increase NFAT5 transactivating activity (35, 62). The mechanism by which PKCμ (39) activates NFAT5 transcription is unknown. Fyn, ERK, and PKCμ probably activate NFAT5 indirectly through other signaling molecules. CK1 and CDK5 directly phosphorylate NFAT5 at S158 and T135, which produces complementary results (20, 66). CK1 stimulates NFAT5 export from the nucleus at low NaCl (66), whereas CDK5 stimulates its localization to the nucleus at high NaCl (20). c-Abl and PLC-γ1 act directly on NFAT5. c-Abl phosphorylates NFAT5 on Y143, which facilitates binding of PLC-γ1, and stimulates nuclear localization of NFAT5 (21, 31). SHP-1 phosphatase, which is inhibited by high NaCl, opposes the action of c-Abl by dephosphorylating NFAT5-Y143 and thus reduces nuclear localization of NFAT5 (74). c-Abl and PLC-γ1 stimulate NFAT5 transactivating activity, and SHP-1 inhibits it (21, 31, 74). PLC-γ1 mediates the increase of NFAT5 transactivating activity caused by Rac and OSM (75). c-Abl and PI3K both contribute to high NaCl-induced activation of ATM (21, 30). ATM in turn increases NFAT5 nuclear localization (70) and also increases NFAT5 transactivating activity by phosphorylating NFAT5-S1247 and -S1367 (32).
Perspectives
The finding that GSK-3β acts downstream of multiple signaling pathways that have distinct effects raises the question of how GSK-3β can selectively signal to NFAT5 in the context of hypertonicity. It has been proposed that GSK is distributed between scaffolding proteins and other structures so that each system has its own population of GSK-3 molecules “assigned” to it, and this effectively insulates the signals since the GSK-3 subpopulations do not intermingle or exchange (34). The same possibility applies to other hypertonicity-induced mediators of NFAT5 activity, such as p38α, PKA, and ATM, each of which is involved in numerous signaling pathways. In addition to compartmentalization, the multiple transducers that signal hypertonicity compose a system in which the combined effect provides a specificity that is lacking in the individual components.
Cells in various organs such as thymus and spleen (23), intervertebral discs (37), and, most notably, the kidney medulla (14) are normally exposed to a hyperosmotic environment. Cells in general also are impacted by the hyperosmolality of hypernatremia (49), diabetes mellitus (6), and inflammatory bowel disease (59) and in other conditions (46). Based on the present study, further analysis of the role of GSK-3β may help to understand how cells cope with the hyperosmotic stress associated with these various physiological and pathophysiological conditions.
GRANTS
This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, and by a grant-in-aid from National Kidney Foundation/National Capital Area.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Z., M.B.B., and J.D.F. conception and design of research; X.Z. and H.W. performed experiments; X.Z., M.B.B., and J.D.F. analyzed data; X.Z., M.B.B., and J.D.F. interpreted results of experiments; X.Z. prepared figures; X.Z. drafted manuscript; X.Z., M.B.B., and J.D.F. edited and revised manuscript; X.Z., M.B.B., and J.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Steven R. Gullans (Excel Venture Management, Boston, MA) for mIMCD3, Dr. James R. Woodgett (Ontario Cancer Institute, Toronto, Canada) for GSK-3β−/− mouse embryonic fibroblasts, Dr. Yusen Liu (Ohio State University, Columbus, OH) for pEBG and pEBG-p38α plasmids, Dr. Sankar Ghosh (Yale University, New Haven, CT), and Dr. David Scott (Uniformed Services University, Bethesda, MD) for use of cell culture equipment.
REFERENCES
- 1.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol 29: 1039–1045, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beals CR, Clipstone NA, Ho SN, Crabtree GR. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev 11: 824–834, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1934, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Buxade M, Lunazzi G, Minguillon J, Iborra S, Berga-Bolanos R, Del VM, Aramburu J, Lopez-Rodriguez C. Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J Exp Med 209: 379–393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos MV, Bastos M, Martins T, Leitao P, Lemos M, Carvalheiro M, Ruas A. Diabetic hyperosmolality. Retrospective study of 60 cases. Acta Med Port 16: 13–19, 2003 [PubMed] [Google Scholar]
- 7.Chen M, Sinha M, Luxon BA, Bresnick AR, O'Connor KL. Integrin α6β4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem 284: 1484–1494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H, Woodgett J, Maamari M, Force T. Targeting GSK-3 family members in the heart: a very sharp double-edged sword. J Mol Cell Cardiol 51: 607–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl SC, Handler JS, Kwon HM. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol 280: C248–C253, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell 19: 159–170, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116: 1175–1186, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebert AD, Wechselberger C, Frank S, Wallace-Jones B, Seno M, Martinez-Lacaci I, Bianco C, De SM, Weitzel HK, Salomon DS. Cripto-1 induces phosphatidylinositol 3′-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3beta in human cervical carcinoma cells. Cancer Res 59: 4502–4505, 1999 [PubMed] [Google Scholar]
- 13.Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA 97: 11960–11965, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol 16: 1583–1592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraris JD, Burg MB. Tonicity-regulated gene expression. Methods Enzymol 428: 279–296, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ferraris JD, Persaud P, Williams CK, Chen Y, Burg MB. cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/osmotic response element-binding protein. Proc Natl Acad Sci USA 99: 16800–16805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraris JD, Williams CK, Persaud P, Zhang Z, Chen Y, Burg MB. Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc Natl Acad Sci USA 99: 739–744, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell 7: 1321–1327, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fritz A, Brayer KJ, McCormick N, Adams DG, Wadzinski BE, Vaillancourt RR. Phosphorylation of serine 526 is required for MEKK3 activity, and association with 14–3-3 blocks dephosphorylation. J Biol Chem 281: 6236–6245, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gallazzini M, Heussler GE, Kunin M, Izumi Y, Burg MB, Ferraris JD. High NaCl-induced activation of CDK5 increases phosphorylation of the osmoprotective transcription factor TonEBP/OREBP at threonine 135, which contributes to its rapid nuclear localization. Mol Biol Cell 22: 703–714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallazzini M, Yu MJ, Gunaratne R, Burg MB, Ferraris JD. c-Abl mediates high NaCl-induced phosphorylation and activation of the transcription factor TonEBP/OREBP. FASEB J 24: 4325–4335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germann S, Gratadou L, Zonta E, Dardenne E, Gaudineau B, Fougere M, Samaan S, Dutertre M, Jauliac S, Auboeuf D. Dual role of the ddx5/ddx17 RNA helicases in the control of the pro-migratory NFAT5 transcription factor. Oncogene 31: 4536–4549, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101: 10673–10678, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graef IA, Gastier JM, Francke U, Crabtree GR. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc Natl Acad Sci USA 98: 5740–5745, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guha S, Cullen JP, Morrow D, Colombo A, Lally C, Walls D, Redmond EM, Cahill PA. Glycogen synthase kinase 3 beta positively regulates Notch signaling in vascular smooth muscle cells: role in cell proliferation and survival. Basic Res Cardiol 106: 773–785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halterman JA, Kwon HM, Zargham R, Schoppee Bortz PD, Wamhoff BR. Nuclear factor of activated T cells 5 regulates vascular smooth muscle cell phenotypic modulation. Arterioscler Thromb Vasc Biol 31: 2287–2296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374: 617–622, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Hughes K, Pulverer BJ, Theocharous P, Woodgett JR. Baculovirus-mediated expression and characterisation of rat glycogen synthase kinase-3 beta, the mammalian homologue of the Drosophila melanogaster zeste-white 3sgg homeotic gene product. Eur J Biochem 203: 305–311, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci 11: 539–551, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: role in osmoprotective transcriptional regulation. Proc Natl Acad Sci USA 103: 8882–8887, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irarrazabal CE, Gallazzini M, Schnetz MP, Kunin M, Simons BL, Williams CK, Burg MB, Ferraris JD. Phospholipase C-gamma1 is involved in signaling the activation by high NaCl of the osmoprotective transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 906–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irarrazabal CE, Liu JC, Burg MB, Ferraris JD. ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 101: 8809–8814, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irarrazabal CE, Williams CK, Ely MA, Birrer MJ, Garcia-Perez A, Burg MB, Ferraris JD. Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J Biol Chem 283: 2554–2563, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci 4: 40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS. Fyn and p38 signaling are both required for maximal hypertonic activation of the OREBP/TonEBP. J Biol Chem 277: 46085–46092, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Ko BC, Turck CW, Lee KW, Yang Y, Chung SS. Purification, identification, and characterization of an osmotic response element binding protein. Biochem Biophys Res Commun 270: 52–61, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Kraemer J, Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine (Phila Pa 1976) 10: 69–71, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Liao Y, Hung MC. Regulation of the activity of p38 mitogen-activated protein kinase by Akt in cancer and adenoviral protein E1A-mediated sensitization to apoptosis. Mol Cell Biol 23: 6836–6848, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim YS, Lee JS, Huang TQ, Seo JS. Protein kinase Cmu plays an essential role in hypertonicity-induced heat shock protein 70 expression. Exp Mol Med 40: 596–606, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J 16: 950–962, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA 96: 7214–7219, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mak MC, Lam KM, Chan PK, Lau YB, Tang WH, Yeung PK, Ko BC, Chung SM, Chung SK. Embryonic lethality in mice lacking the nuclear factor of activated T cells 5 protein due to impaired cardiac development and function. PLos One 6: e19186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills CN, Nowsheen S, Bonner JA, Yang ES. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front Mol Neurosci 4: 47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 96: 2538–2542, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemoto T, Miyazaki S, Kanai T, Maruta T, Satoh S, Yoshikawa N, Yanagita T, Wada A. Nav-Ca2+ influx-induced increased phosphorylations of extracellular signal-regulated kinase (ERK) and p38 attenuate tau phosphorylation via glycogen synthase kinase-3beta: priming of Nav1.7 gating by ERK and p38. Eur J Pharmacol 640: 20–28, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Neuhofer W. Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr Genomics 11: 584–590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Connor RS, Mills ST, Jones KA, Ho SN, Pavlath GK. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J Cell Sci 120: 149–159, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Padda R, Wamsley-Davis A, Gustin MC, Ross R, Yu C, Sheikh-Hamad D. MEKK3-mediated signaling to p38 kinase and TonE in hypertonically stressed kidney cells. Am J Physiol Renal Physiol 291: F874–F881, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med 124: 197–203, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Picton C, Woodgett J, Hemmings B, Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett 150: 191–196, 1982 [DOI] [PubMed] [Google Scholar]
- 51.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 96: 2110–2115, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ranjbar S, Jasenosky LD, Chow N, Goldfeld AE. Regulation of Mycobacterium tuberculosis-dependent HIV-1 transcription reveals a new role for NFAT5 in the toll-like receptor pathway. PLoS Pathog 8: e1002620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ranjbar S, Tsytsykova AV, Lee SK, Rajsbaum R, Falvo JV, Lieberman J, Shankar P, Goldfeld AE. NFAT5 regulates HIV-1 in primary monocytes via a highly conserved long terminal repeat site. PLoS Pathog 2: e130, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao R, Hao CM, Breyer MD. Hypertonic stress activates glycogen synthase kinase 3beta-mediated apoptosis of renal medullary interstitial cells, suppressing an NFkappaB-driven cyclooxygenase-2-dependent survival pathway. J Biol Chem 279: 3949–3955, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Fluid Electrolyte Physiol 265: F416–F424, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Rinschen MM, Klokkers J, Pavenstadt H, Neugebauer U, Schlatter E, Edemir B. Different effects of CsA and FK506 on aquaporin-2 abundance in rat primary cultured collecting duct cells. Pflügers Arch 462: 611–622, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Roth I, Leroy V, Kwon HM, Martin PY, Feraille E, Hasler U. Osmoprotective transcription factor NFAT5/TonEBP modulates nuclear factor-kappaB activity. Mol Biol Cell 21: 3459–3474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schilli R, Breuer RI, Klein F, Dunn K, Gnaedinger A, Bernstein J, Paige M, Kaufman M. Comparison of the composition of faecal fluid in Crohn's disease and ulcerative colitis. Gut 23: 326–332, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 320: 667–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thymiakou E, Episkopou V. Detection of signaling effector-complexes downstream of bmp4 using PLA, a proximity ligation assay. J Vis Exp pii: 2631, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res 22: 965–974, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53: 130–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Roach PJ. Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3. Dominant role of the phosphorylation of Ser-640 (site-3a). J Biol Chem 268: 23876–23880, 1993 [PubMed] [Google Scholar]
- 65.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol 22: 5753–5760, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu S, Wong CC, Tong EH, Chung SS, Yates JR, III, Yin Y, Ko BC. Phosphorylation by casein kinase 1 regulates tonicity-induced osmotic response element-binding protein/tonicity enhancer-binding protein nucleocytoplasmic trafficking. J Biol Chem 283: 17624–17634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamaguchi K, Itoh K, Ohnishi N, Itoh Y, Baum C, Tsuji T, Nagao T, Higashitsuji H, Okanoue T, Fujita J. Engineered long terminal repeats of retroviral vectors enhance transgene expression in hepatocytes in vitro and in vivo. Mol Ther 8: 796–803, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J Biol Chem 286: 44913–44920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem 278: 33067–33077, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z, Ferraris JD, Irarrazabal CE, Dmitrieva NI, Park JH, Burg MB. Ataxia telangiectasia-mutated, a DNA damage-inducible kinase, contributes to high NaCl-induced nuclear localization of transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 289: F506–F511, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Zhou X, Ferraris JD, Cai Q, Agarwal A, Burg MB. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 289: F377–F385, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Zhou X, Ferraris JD, Dmitrieva NI, Liu Y, Burg MB. MKP-1 inhibits high NaCl-induced activation of p38 but does not inhibit the activation of TonEBP/OREBP: opposite roles of p38alpha and p38delta. Proc Natl Acad Sci USA 105: 5620–5625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X, Gallazzini M, Burg MB, Ferraris JD. Contribution of SHP-1 protein tyrosine phosphatase to osmotic regulation of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 7072–7077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X, Izumi Y, Burg MB, Ferraris JD. Rac1/osmosensing scaffold for MEKK3 contributes via phospholipase C-gamma1 to activation of the osmoprotective transcription factor NFAT5. Proc Natl Acad Sci USA 108: 12155–12160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]