Abstract
Renal artery stenosis (RAS) is an important cause of chronic renal dysfunction. Recent studies have underscored a critical role for CCL2 (MCP-1)-mediated inflammation in the progression of chronic renal damage in RAS and other chronic renal diseases. In vitro studies have implicated p38 MAPK as a critical intermediate for the production of CCL2. However, a potential role of p38 signaling in the development and progression of chronic renal disease in RAS has not been previously defined. We sought to test the hypothesis that inhibition of p38 MAPK ameliorates chronic renal injury in mice with RAS. We established a murine RAS model by placing a cuff on the right renal artery and treated mice with the p38 inhibitor SB203580 or vehicle for 2 wk. In mice treated with vehicle, the cuffed kidney developed interstitial fibrosis, tubular atrophy, and interstitial inflammation. In mice treated with SB203580, the RAS-induced renal atrophy was reduced (70% vs. 39%, P < 0.05). SB203580 also reduced interstitial inflammation and extracellular matrix deposition but had no effect on the development of hypertension. SB203580 partially blocked the induction of CCL2, CCL7 (MCP-3), CC chemokine receptor 2 (CCR2), and collagen 4 mRNA expression in the cuffed kidneys. In vitro, blockade of p38 hindered both TNF-α and TGF-β-induced CCL2 upregulation. Based on these observations, we conclude that p38 MAPK plays a critical role in the induction of CCL2/CCL7/CCR2 system and the development of interstitial inflammation in RAS.
Keywords: renal artery stenosis, fibrosis, atrophy, CCL2, CCR2
renal artery stenosis (RAS) is an important cause of chronic renal dysfunction and hypertension. Atherosclerosis is the most common etiology for the development of RAS. RAS has been identified in 6.8% of individuals over 65 years of age and in almost 40% of patients with a history of coronary or peripheral vascular disease. RAS is a major risk factor for death from cardiovascular disease as well as for the development of chronic renal failure (37). Optimal management of patients with RAS is limited by a lack of understanding of fundamental mechanisms underlying the development of chronic renal disease.
Recent studies have underscored a critical role for inflammation in the development of chronic renal disease. Of the many signaling pathways that may lead to inflammation, both in vitro and in vivo studies have implicated a critical role of the p38 MAPK pathway in the development of renal inflammation and fibrosis (18, 43, 45, 50). p38 Is activated by many stimuli that have been implicated in the development of chronic renal damage in RAS, including ANG II, reactive oxygen species, TNF-α, IL-1, and TGF-β1 (22). It has been reported that administration of small molecule inhibitors of p38 might protect kidneys from injury and slow disease progression in several experimental renal disease models (1, 18, 21–23). For example, Stambe et al. (42) reported that a specific p38α inhibitor, NPC 31169, inhibits renal fibrosis in a rat unilateral ureteric obstruction model.
There is considerable experimental evidence that CCL2 plays a critical role in regulation of inflammatory responses to acute and chronic injury. CCL2 recruits monocytes and subpopulations of T cells during inflammation, and upregulation of CCL2 is highly correlated with renal inflammation and interstitial fibrosis in many renal diseases including diabetic nephropathy (46), glomerulonephritis (41), RAS (6), and ureteral obstruction (25). CCL2 exerts its functions through binding to its receptor CC chemokine receptor 2 (CCR2). CCR2 is predominantly expressed in monocytes but has also been seen in other cell types such as vascular smooth muscle cells (44), endothelial cells (48), fibroblasts (16), and mesangial cells (MC) (40). CCL2 can directly stimulate extracellular matrix production in cultured MC (31), thereby providing a link between inflammation and fibrogenesis in chronic renal disease. We, and others, have demonstrated that p38 MAPK is critical for TNF-α or TGF-β-induced CCL2 expression in vitro and in vivo (5, 10, 14, 24).
Although we have previously shown that the development of interstitial fibrosis and tubular atrophy in mice with RAS is associated with induction of CCL2 and influx of inflammatory cells, a potential role of p38 in this process has not previously been addressed. We sought to test the hypothesis that inhibition of p38 signaling would block CCL2 expression, reduce renal inflammation, and protect the cuffed kidney from the renal damage in mice with RAS.
MATERIALS AND METHODS
Animals.
Studies were conducted in 43 male C57BL/6 mice (NCI-Frederick, Frederick, MD), 6–8 wk of age, weighing 20–25 g. All animal procedures were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and the study protocol was approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee.
Surgical procedures.
Mice were anesthetized with a ketamine-xylazine mixed cocktail (100 mg/kg ip ketamine, 10 mg/kg ip xylazine). The skin of the back was shaved and wiped clean with topical antiseptic and alcohol. The right kidney was exposed through a small flank incision 2 cm in length and externalized. The renal artery was isolated, and a small segment was dissected free of the renal vein. RAS surgeries were performed on 36 mice as previously described (47). Briefly, a 0.5-mm length of 0.36-mm (external diameter) × 0.20-mm (internal diameter) polytetrafluoroethylene tube (Braintree Scientific, Braintree, MA) was cut open lengthwise and put around the right main renal artery approximately equidistant to the aorta and renal bifurcation. The cuff was then closed and held in place by two 10-0 nylon circumferential sutures (Surgical Specialties, Reading, PA). Sham surgeries without placement of a cuff were performed on 13 mice. The reduction of renal artery blood flow was monitored by pulse-wave Doppler ultrasound using Vevo 770 (Visualsonics, Toronto, Canada). In general, the blood flow was reduced by 60–80% (47).
p38 MAPK inhibitors.
The p38 inhibitor SB203580 was purchased from Selleck Chemicals (Houston, TX) and was made into a stock of 4 mg/ml solution in DMSO. Mice were weighed, and SB203580 (10 mg/kg ip) along with an equivalent volume of 0.9% saline was administered daily after the surgery. For vehicle-only treatment, equivalent volume of DMSO and 0.9% saline were administered. For in vitro experiments, the p38 inhibitor SB202190 was obtained from Calbiochem (La Jolla, CA), and 10 μM SB202190 was added into the medium 1 h before the treatment of TNF-α or TGF-β.
Blood pressure measurement.
Blood pressure was noninvasively measured by determining the tail blood volume with a volume pressure-recording sensor and an occlusion tail cuff (CODA System, Kent Scientific, Torrington, CT) (12). Measurements were taken in conscious mice before surgery 1 and 2 wks following RAS or sham surgery.
Plasma renin activity assay.
Blood from inferior vena cava was collected in Vacutainer EDTA tubes on ice. The plasma fraction was separated by centrifugation and stored at −80°C until assay. Renin activity in plasma was measured via production of ANG I from angiotensinogen using a GammaCoat Plasma Renin Activity Kit (DiaSorin, Stillwater, MN) according to the manufacturer's instructions. Porcine angiotensinogen (cat. no. A2283; Sigma-Aldrich, St. Louis, MO) substrate was used for the assay.
Histology and immunohistochemistry.
Animals were killed at 2 wk after the RAS or sham surgery. Mice were weighed and anesthetized with ketamine-xylazine, and the cuffed and contralateral kidneys were excised. The kidneys were weighed, and portions were either fixed for histopathological analysis and immunohistochemical staining or snap frozen in liquid nitrogen for Western blot analysis.
Renal tissue was fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin per standard techniques. Sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin and Masson's trichrome. Immunostains were performed for CD3 (DakoCytomation, Carpinteria, CA) and collagen 4 (Acris Antibodies, San Diego, CA). A Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) was used for blocking, secondary antibody, and amplification steps. NovaRed (Vector) was used for color development followed by hematoxylin counterstain. To facilitate consistency between staining batches, we stained the slides on the Dako Autostainer, an automated staining machine.
Assessment of histopathological features.
Sections were analyzed in a blinded and random fashion using a Leica DMLB microscope (Leica Microsystems), a Micropublisher 3.3 RTV camera (Q-Imaging, Surrey, BC), and the MetaVue Imaging System (Universal Imaging, Downington, PA). Glomerular, interstitial, tubular, and vascular features of renal tissue were assessed on hematoxylin and eosin and trichrome-stained slides. Quantitative analyses of histopathological manifestations of renal injury were performed in a blinded fashion using the MetaVue Image Analysis System (version 6.3r2; Universal Imaging, Downington, PA).
The atrophy area of kidneys was assessed on hematoxylin and eosin-stained slides and was compared with the entire cortical surface area. Glomerular planar surface area and interstitial fibrosis measurements were determined on trichrome-stained slides. Glomeruli were visualized using a magnification, ×20/0.50, and the area was measured using MetaVue's Trace Region function to map out the capillary loop. Only glomeruli with a vascular pole were taken into account.
Quantitative analysis of trichrome-positive extracellular matrix and collagen 4 deposition was performed with the MetaVue Imaging System, as previously described (9). In brief, the entire cortex was systematically analyzed as a series of consecutive fields. For each field, a region of interest containing tubules and interstitium, but excluding glomeruli and large vessels, was defined. The total area of each region of interest was calculated as the percentage of positively staining material relative to the area of the entire region of interest. Results for each region of interest, obtained over the entire renal cortex, were averaged to provide a percentage of total cortical tubulointerstitial area.
Western blot analysis.
Tissues were homogenized in 1 × lysis buffer (Cell Signaling, Danvers, MA) with protease inhibitors (protease inhibitor cocktail; Roche Applied Science, Indianapolis, IN) and phosphatase inhibitors (phosphatase inhibitor cocktail 2; Sigma, St. Louis, MO). Homogenates were centrifuged at 10,000 g for 10 min at 4°C, and the resulting supernatants were used for analysis. Protein concentrations were determined using the Lowry method. Equal amounts of lysate, denatured in loading buffer for 5 min at 100°C, were subjected to SDS-PAGE in the Criterion system (Bio-Rad Laboratories) followed by transfer to polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked with 5% milk in Tris-buffered saline (TBS) containing 0.5% Tween 20 and incubated with primary antibodies for MK2, phospho-MK2, tubulin (Cell Signaling) and CCL2 (Abbiotec, San Diego, CA), followed by horseradish peroxidase-conjugated secondary antibodies (Southern Biotech, Birmingham, AL). The blots were then visualized by exposure to X-ray film using the enhanced chemiluminescense Western blot detection reagents and analysis system (Amersham Biosciences, Piscataway, NJ).
Quantitative real-time PCR.
Total RNA was extracted from kidney tissues or cells using the RNeasy Mini Plus Kit (Qiagen, Valenica, CA) according to the manufacturer's instructions. Total RNA was quantitated using spectrophotometry (NanoDrop; NanoDrop Technologies, Wilmington, DE). We first amplified the according genes by PCR using the primer pairs listed as following: m-CCL2 forward: 5′-AGCACCAGCACCAGCCAACTC-3′, reverse: 5′-TGGATGCTCCAGCCGGCAACT-3′; m-CCL7: forward: 5′-AGAAGCAAGGCCAGCACAGAGT-3′, reverse: 5′-GAGCAGCAGGCACAGAAGCGT-3′; m-TGF-β1: forward: 5′-TTGCCGAGGGTTCCCGCTCT-3′, reverse: 5′-CCTCCCGGGCGTCAGCACTA-3′; m-CCR2: forward: 5′-TCAGCTGCCTGCAAAGACCAGA-3′, reverse: 5′-CATACGGTGTGGTGGCCCCT-3′; m-TNF-α: forward: 5′-GGGACAAGGCTGCCCCGACT-3′, reverse: 5′-TCCTTGGGGCAGGGGCTCTT-3′; m-Col4a1: forward: 5′-TGAAGGCAGGGGAGCTGCGA-3′, reverse: 5′-GCCAACGAAGCGGGGTGTGT-3′; m-GAPDH: forward: 5′-GCACAGTCAAGGCCGAGAAT-3′, reverse: 5′-GCCTTCTCCATGGTGGTGAA-3′; m/r-18S: forward: 5′-CTCAACACGGGAAACCTCAC-3′, reverse: 5′-CGCTCCACCAACTAAGAACG-3′; r-CCL2: forward: 5′-TAGCATCCACGTGCTGTCTC-3′, reverse: 5′-CATTCAAAGGTGCTGAAGTCC-3′; r-CCR2: forward: 5′-AGGGGGCCACCACACCGTAT-3′, reverse: 5′-AGCCCAGAATGGGAGTGTGAGCA-3′; r-Col4a: forward: 5′-ATTCCTTTGTGATGCACACCAG-3′, reverse: 5′-AAGCTGTAAGCATTCGCGTAGTA-3′. The PCR resulting products were then purified by QIAquick PCR purification kit (Qiagen). These PCR products were confirmed by DNA sequencing. Purified PCR products were quantitated by spectrophotometry and the copy number calculated as follows: 6.02 × 1023 (copies/mol) × DNA amount (g)/ [DNA length (bp) × 660 (g/mol per bp)]. Based on the calculated copy number of each gene, we make a series of standards ranging from 1×102 to 1×108 copies/μl. With these standards, we quantified the gene expression in these tissues and cells by absolute real-time quantitative PCR. In brief, first-strand cDNA was prepared from 1 μg total RNA using an iScript cDNA synthesis Kit (Bio-Rad, Hercules, CA). All real-time RT-PCR reactions were conducted in a total volume of 20 μl using SYBR Green ER qPCR SuperMix (Invitrogen, Carlsbad, CA) and the gene expression levels in each sample were quantified by absolute real-time quantitative PCR with the Bio-Rad iQ5 Gradient Real Time PCR system. Each reaction was in triplicate. The standard curve and data analysis were produced using Bio-Rad iQ5 software. The copy number of each gene was double-normalized to GAPDH and 18S rRNA.
ELISA.
The concentration of the secreted CCL2 in the culture medium was determined using the BioSource MCP-1 rat ELISA kit (Life Technologies, Grand Island, NY). MC were treated with or without TNF-α and/or p38 inhibitor as indicated. Media were collected from wells at 6 h, and cell debris was removed by centrifuge. Samples were assayed in triplicate for CCL2 concentration following manufacturer's protocol. Final protein concentrations were averages of triplicate samples corrected for background media or negative control values.
Statistical analysis.
Data are presented as means ± SE. Comparisons between different groups were performed using Student's t-test for parametric data and the Mann-Whitney U-test for nonparametric data. P values < 0.05 were considered statistically significant.
RESULTS
Blockade of p38 signaling has no effect on blood pressure, cardiac hypertrophy, or plasma renin activity in 2K1C mice.
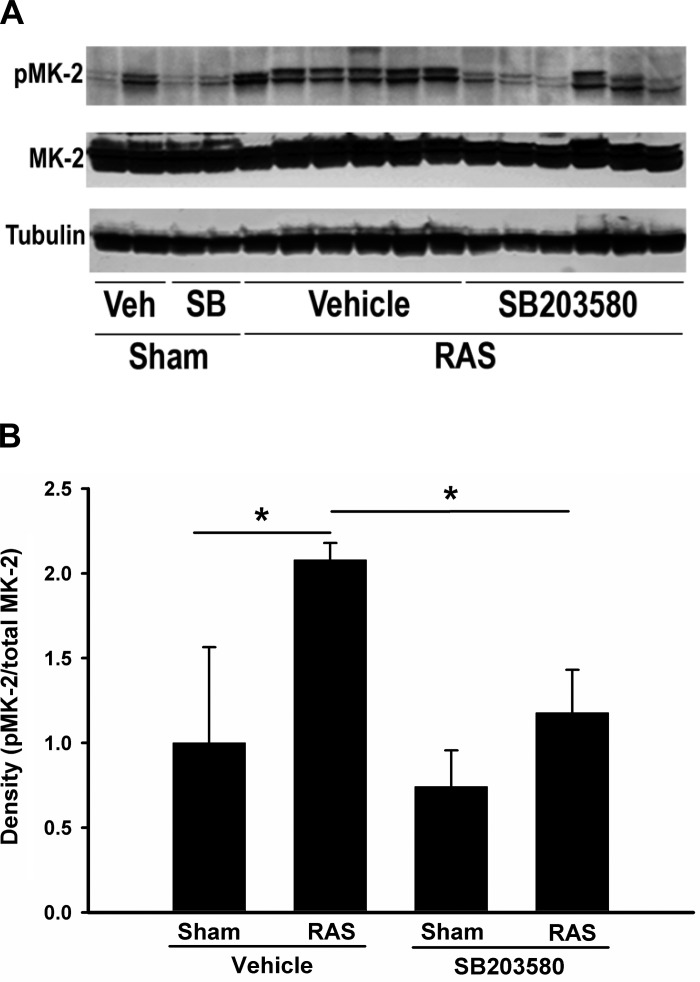
To investigate the role of p38 signaling pathway in the development of RAS, we used a specific p38 inhibitor, SB203580, to block the activity of p38. SB203580 does not affect the phosphorylation of p38, but prevents ATP binding to p38α and p38β and blocks the activation of downstream kinases (7). Unilateral RAS was established through placement of a polytetrafluoroethylene cuff on the right renal artery, as previously described (47). Sham surgeries consisted of exposure and manipulation of the right renal artery, but without placement of a cuff. Sham or RAS mice were treated with SB203580 (10 mg·kg−1·day−1) or vehicle for 2 wk. These mice were evaluated at 2 wk after sham or RAS surgeries. We assessed p38 activity by measuring phosphorylated MAPKAPK-2 (MK-2), a downstream mediator of p38 signaling, via Western blot analysis. Phosphorylated MK-2 was increased in all the RAS kidneys with vehicle treatment, but this elevation was blocked or reduced with SB203580 treatment, verifying the in vivo efficacy of this inhibitor in preventing p38 activation (Fig. 1). After 2 wk, the mean body weight of RAS mice was slightly lower than that of sham mice (21.2 ± 0.4 vs. 22.8 ± 0.6, P < 0.05). However, inhibition of p38 by SB203580 did not alter the mean body weight in either sham or RAS group after 2 wk of treatment (Table 1).
Fig. 1.
Treatment of SB203580 inhibits renal artery stenosis (RAS)-induced p38 activity. A: Western blot analysis from renal homogenates from sham or stenotic kidneys with or without SB203580 treatment for 2 wk were probed with and antibodies as indicated. Images are blots of representative experiments. Phospho-MK-2 level is an indicator for p38 MAPK activity. Tubulin was used as loading control. B: phospho-MK-2 (p-MK-2) and total MK-2 density were quantitated from Western blot analysis above and relative ratios of p-MK-2 /total MK-2 are illustrated. Values are means ± SE, *P < 0.05.
Table 1.
Characteristics of sham and renal artery stenosis (RAS) mice with or without 2-wk treatment of SB203580
|
Vehicle |
SB203580 |
|||
|---|---|---|---|---|
| Sham, n = 6 | RAS, n = 17 | Sham, n = 7 | RAS, n = 21 | |
| Body weight, g | 22.8 ± 0.6 | 21.2 ± 0.4 | 22.6 ± 0.7 | 21.9 ± 0.4 |
| Heart weight, mg | 108 ± 2 | 117 ± 3 | 104 ± 3 | 118 ± 2 |
| Kidney weight, mg | ||||
| Sham/cuffed kidney | 147 ± 8 | 63 ± 5a | 139 ± 6 | 87 ± 7b |
| Contralateral kidney | 137 ± 6 | 157 ± 5c | 134 ± 4 | 154 ± 4 |
| Glomerular area, μm2 | ||||
| Sham/cuffed kidney | 4124 ± 158 | 2594 ± 197d | 4213 ± 203 | 3011 ± 120e |
| Contralateral kidney | 3976 ± 171 | 4356 ± 240 | 4116 ± 348 | 4380 ± 256 |
Values are means ± SE. sham, sham-operated; RAS, renal artery stenosis.
P < 0.05 vs. sham with vehicle treated,
P < 0.05 vs. RAS with vehicle treated,
P < 0.05 vs. sham with vehicle treated,
P < 0.05 vs. sham with vehicle treated,
P < 0.05 vs. RAS with DMSO treated.
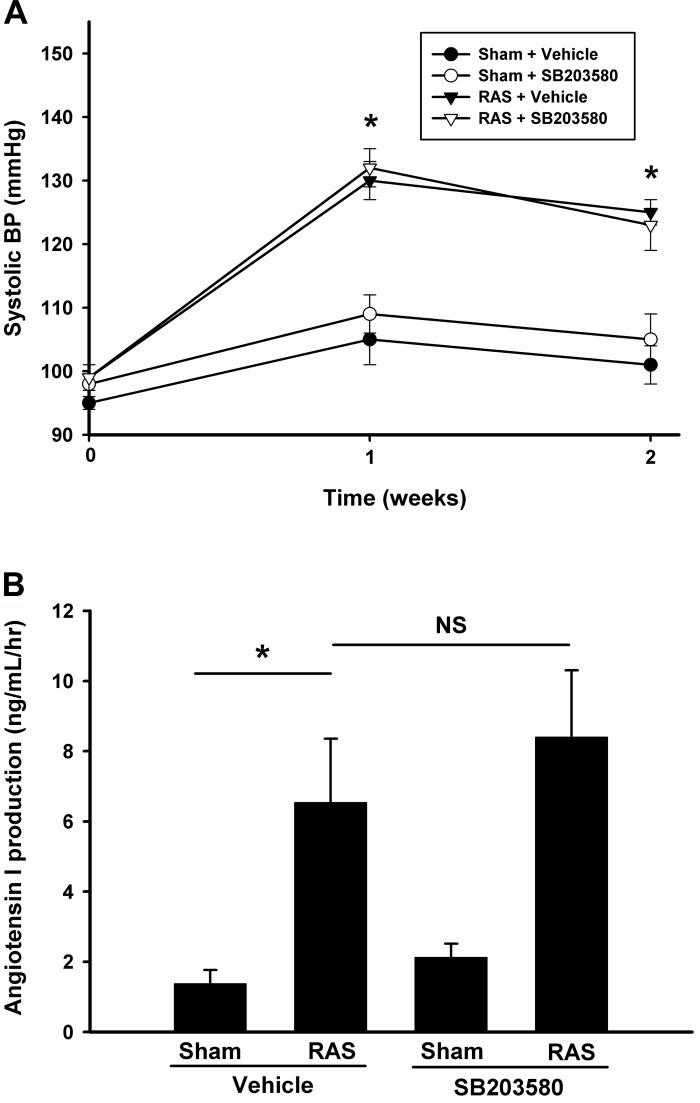
As expected, mice subjected to RAS surgery developed hypertension within 1 wk (systolic pressure 130 ± 3 vs. 105 ± 4, P < 0.05). However, treatment of SB203580 did not alter the mean systolic blood pressure in either sham or RAS mice, suggesting SB203580 is not critical for the development of high blood pressure in RAS. Similarly, 2 wk after the operation, the blood pressure in RAS mice remained elevated; treatment with SB203580 still had no significant effect on mean systolic blood pressure (Fig. 2A). Plasma renin activity, as assessed by ANG I content, was increased in RAS mice compared with those in sham mice. However, inhibition of p38 by SB203580 did not alter renin activity, suggesting that p38 does not modulate the renin-angiotensin-aldosterone pathway (Fig. 2B). Similarly, we observed that mean heart weight was increased in RAS mice (117 ± 3 vs. 108 ± 2 mg, P < 0.05), but SB203580 had no effect on the mean heart weight in either sham or RAS group (Table 1), suggesting p38 inhibition did not alter RAS-induced cardiac hypertrophy.
Fig. 2.
Inhibition of p38 does not affect blood pressure and plasma activity. A: mice systolic blood pressure was measured 1 day before and 1 and 2 wk after the surgery in different groups as indicated. Values are means ± SE. Blood pressure (BP) was significantly increased in mice at 1 and 2 wk after RAS surgery with or without SB203580 treatment. *P < 0.01. B: 2 wk after sham or RAS surgery, renin activity in EDTA plasma was assessed via production of ANG I from exogenously added angiotensinogen substrate using GammaCoat Renin Activity 125I RIA Kit (Diasorin, Stillwater, MN). Values are means ± SE, *P < 0.05, NS, not statistically significant.
Inhibition of p38 attenuates renal fibrosis and tubular atrophy in RAS mice.
In accordance with our previous observations, the cuffed kidneys of vehicle-treated mice developed renal atrophy, as evidenced by a 57% reduction in weight compared with sham controls (P < 0.05) (Table 1). Treatment with SB203580 reduced the extent of renal atrophy (mean kidney weights 87 ± 7 mg in SB203580 treated mice vs. 63 ± 5 mg in vehicle treated mice; P < 0.05). The glomeruli evaluated in sections obtained from the cuffed kidneys of vehicle-treated mice were decreased in size. Mean glomerular area had a 37% reduction in stenotic kidneys with vehicle treatment. However, this reduction of mean glomerular area was partially inhibited by p38 inhibition (Table. 1). As we have previously observed, the contralateral kidney underwent compensatory hyperplasia (6) but this process was not significantly affected by SB203580 treatment.
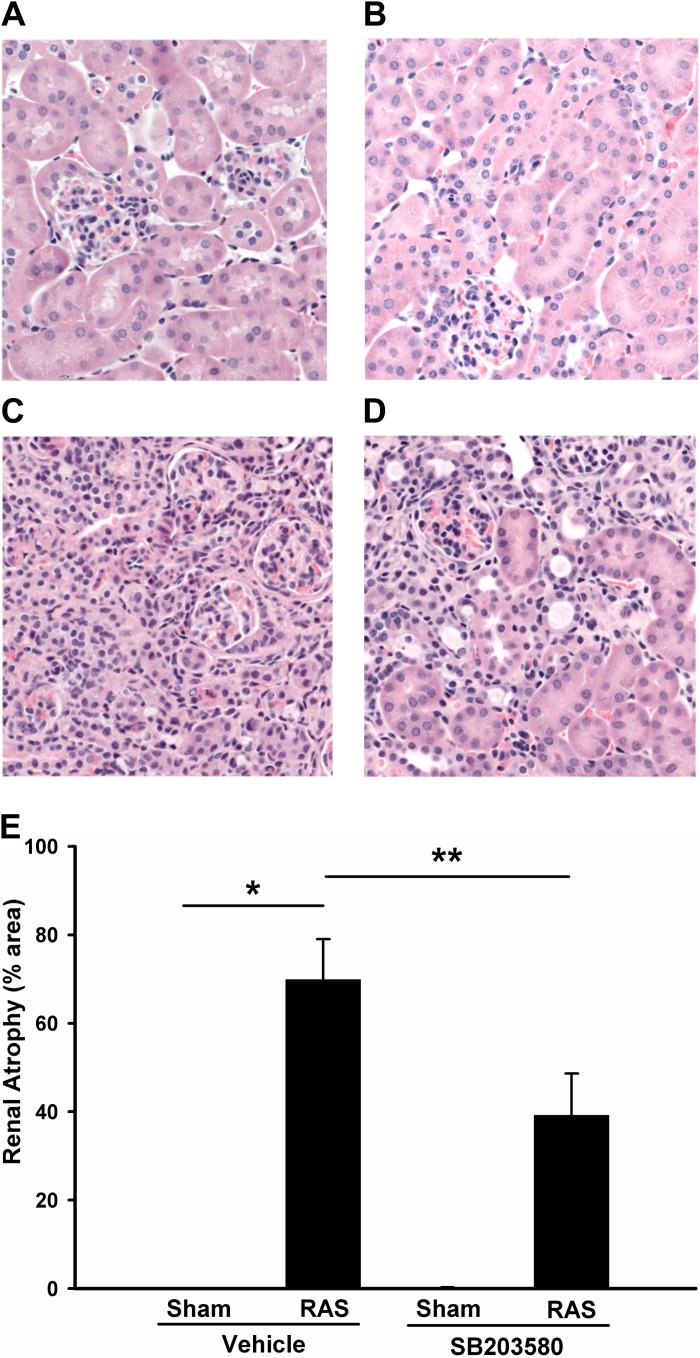
Histologic analysis of the cuffed kidney of vehicle-treated mice showed generalized tubular atrophy, characterized by decreased glomerular spacing, flattening and simplification of tubular epithelium, thickening of tubular basement membranes, and focal interstitial inflammatory infiltrates (Fig. 3C). The mean percentage of tubular atrophy in cuffed kidneys of vehicle-treated mice was 70%. Treatment with SB203580 reduced the atrophy rate in RAS kidneys to 39% (P < 0.05), suggesting that p38 is critical for the development of renal tubular atrophy in RAS (Fig. 3, D and E).
Fig. 3.
Inhibition of p38 attenuates renal atrophy in RAS mice. Histological sections obtained from sham and RAS mice with or without 2 wk SB203580 treatment were stained with hematoxylin and eosin. Representative images from each group are shown (magnification, ×400). A: sham operated, vehicle treated; B: sham operated, SB203580 treated; C: RAS, vehicle treated; D: RAS, SB203580 treated. E: atrophy rate (atrophy/total) from each group was quantitated via MetaVue image analysis system and visualized. Values are means ± SE, *P < 0.01, **P < 0.05.
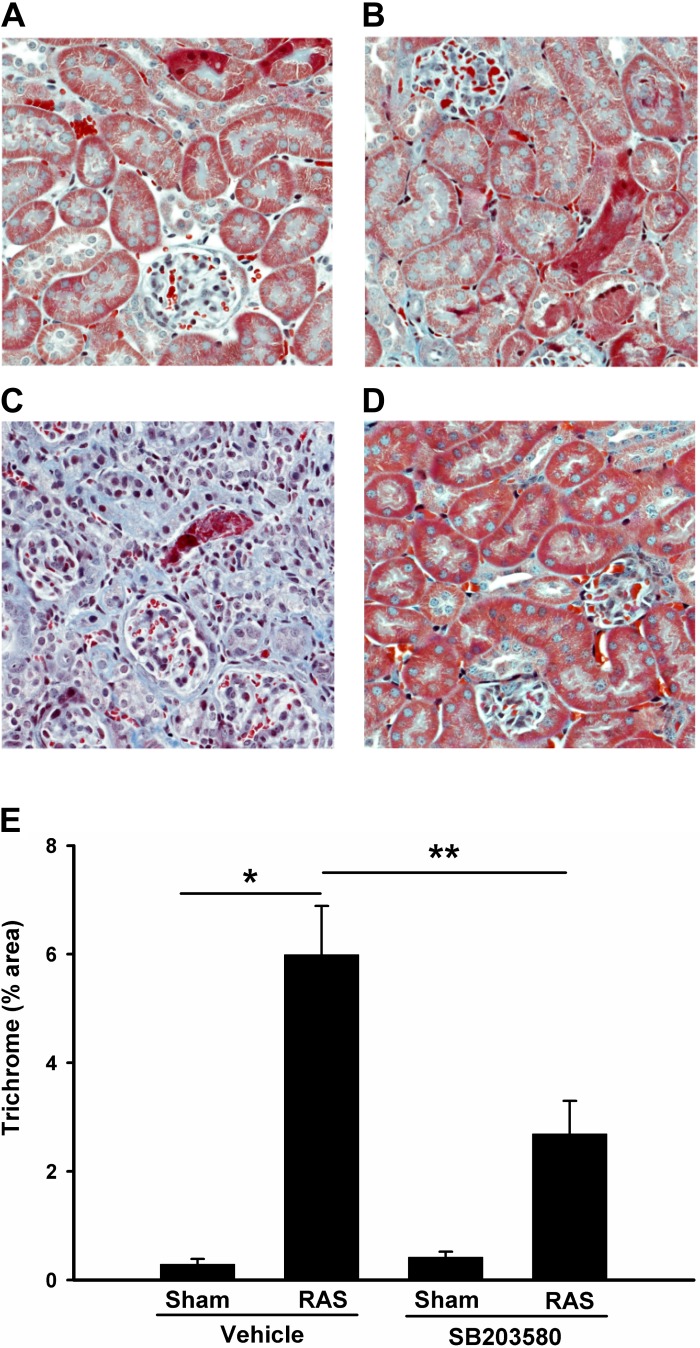
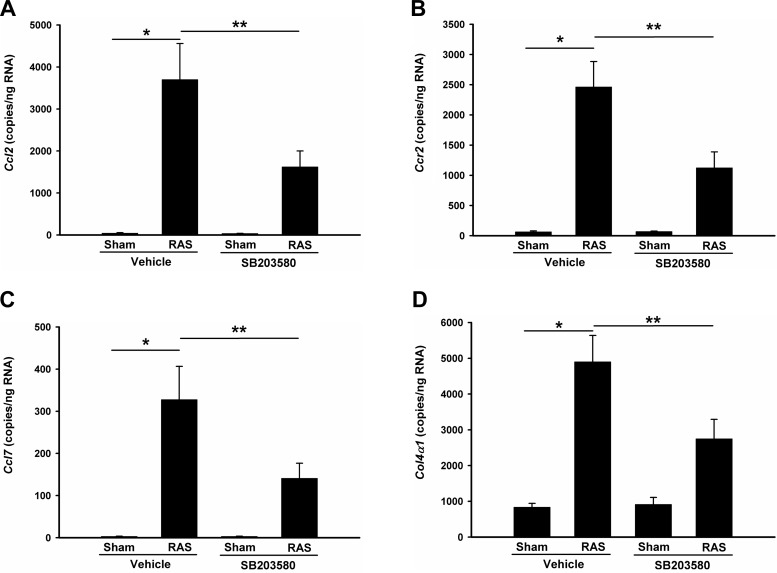
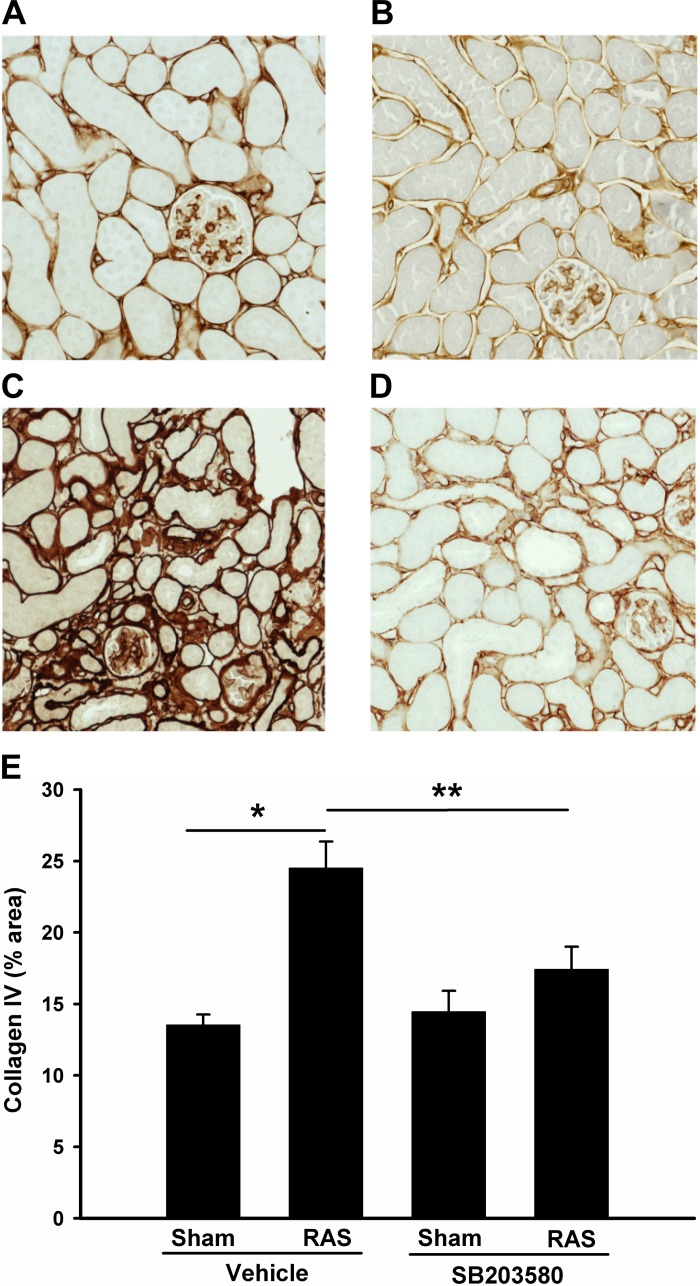
The cuffed kidneys showed a significant increase in interstitial extracellular matrix deposition, as assessed by quantitative analysis of trichrome-stained histologic sections (6.0 ± 0.9% vs. 0.3 ± 0.1%; P < 0.01; Fig. 4, A and C). Treatment with SB203580 reduced extracellular matrix deposition by over 50% (6.0 ± 0.9% RAS vs. 2.7 ± 0.6%; P < 0.05, Fig. 4, D and E). Accordingly, this reduction in extracellular matrix deposition was associated with a 50% decrease in collagen 4 mRNA expression (Fig. 8F). Immunohistochemical staining for collagen 4 also confirmed that collagen deposition was increased in the stenotic kidneys but was reduced by p38 inhibition (Fig. 5, A–E).
Fig. 4.
Inhibition of p38 partially blocks renal fibrosis in RAS mice. Histological sections were prepared as Fig. 3 but stained with trichrome. Representative images from each group are shown (magnification, ×400). A: sham operated, vehicle treated; B: sham operated, SB203580 treated; C: RAS, vehicle treated; D: RAS, SB203580 treated. E: %collagen staining (blue) from each group was quantitated via MetaVue image analysis system and illustrated. Values are means ± SE. *P < 0.01; **P < 0.05.
Fig. 8.
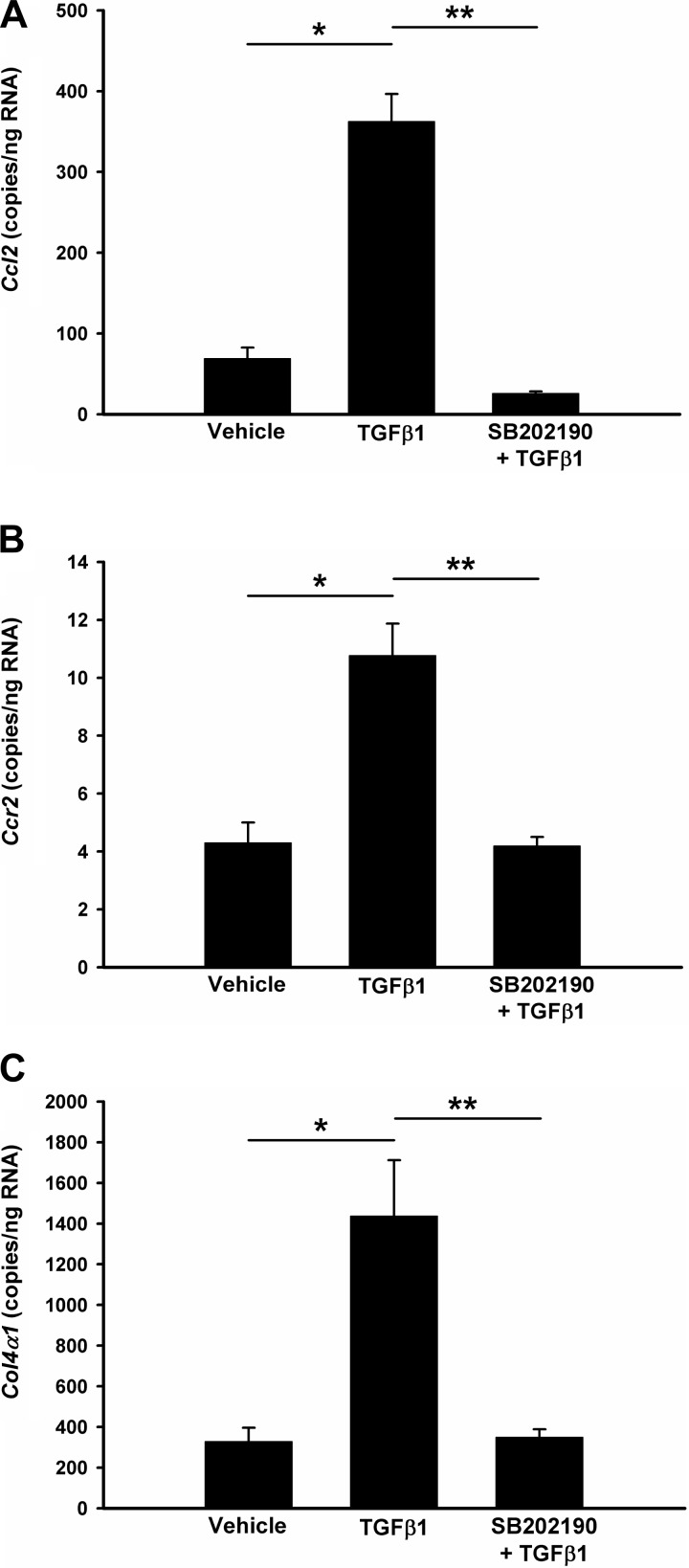
p38 blockade affects CCL2/CCL7/CCR2 expressions in RAS mice. Gene expression was measured by absolute quantitation real-time PCR and the gene copy numbers in 1 μg total RNA were double-normalized to GAPDH and 18S rRNA. A: CCL2; B: CCR2; C: CCL7; D: Col4a1. CCR2, CC chemokine receptor 2. Values are means ± SE, *P < 0.01, **P < 0.05, NS: not statistically significant.
Fig. 5.
Inhibition of p38 reduces interstitial collagen 4 deposition in stenotic kidneys. Histological sections were prepared as Fig. 3 but immunostained with collagen 4. Representative images from each group are shown (magnification, ×200). A: sham operated, vehicle treated; B: sham operated, SB203580 treated; C: RAS, vehicle treated; D: RAS, SB203580 treated. E: %collagen 4 staining (brown) from each group was quantitated via MetaVue image analysis system and illustrated. Values are means ± SE. *P < 0.01; **P < 0.05.
Inhibition of p38 reduces expression of CCL2 in RAS mice.
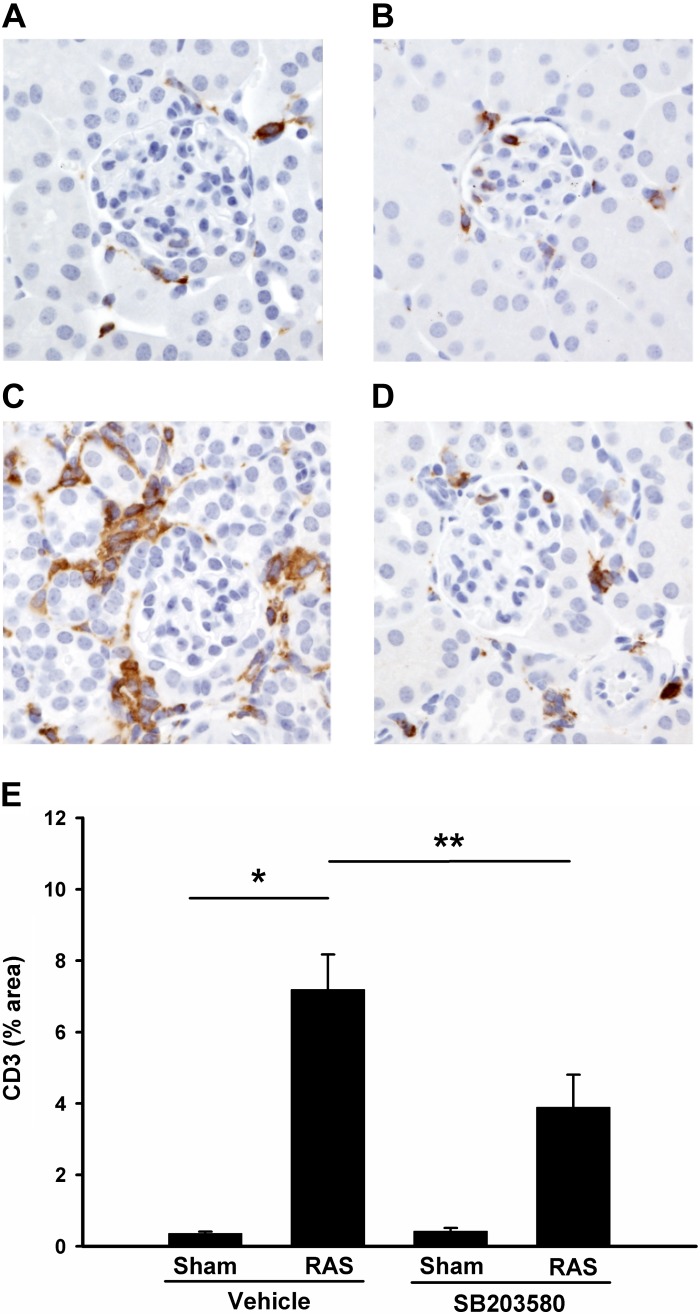
In other experimental models, inhibition of p38 reduces the inflammatory response to tissue injury (23, 30). In our previous studies, we found that the development of renal atrophy in the stenotic kidneys was associated with induction of CCL2 and the influx of inflammatory cells, including macrophages and T cells (6). We therefore sought to determine whether blockade of p38 signaling reduces renal inflammation in the 2K1C model. Based on our previous studies, the predominant inflammatory cell type present at 2 wk following RAS surgery is the CD3+ T lymphocyte, which is followed by macrophage infiltration at later time points. We found that SB203580-mediated reduction in renal atrophy was associated with decreased interstitial infiltration with T cells, as evidenced by CD3 staining (Fig. 6, A–E).
Fig. 6.
Inhibition of p38 reduces T cells infiltration in stenotic kidneys. Histological sections were prepared as Fig. 3 but immunostained with CD3. Representative images from each group are shown (magnification, ×400). A: sham operated, vehicle treated; B: sham operated, SB203580 treated; C: RAS, vehicle treated; D: RAS, SB203580 treated. E: %CD3 staining (brown) from each group was quantitated via MetaVue Image Analysis System and illustrated. Values are means ± SE, *P < 0.01, **P < 0.05.
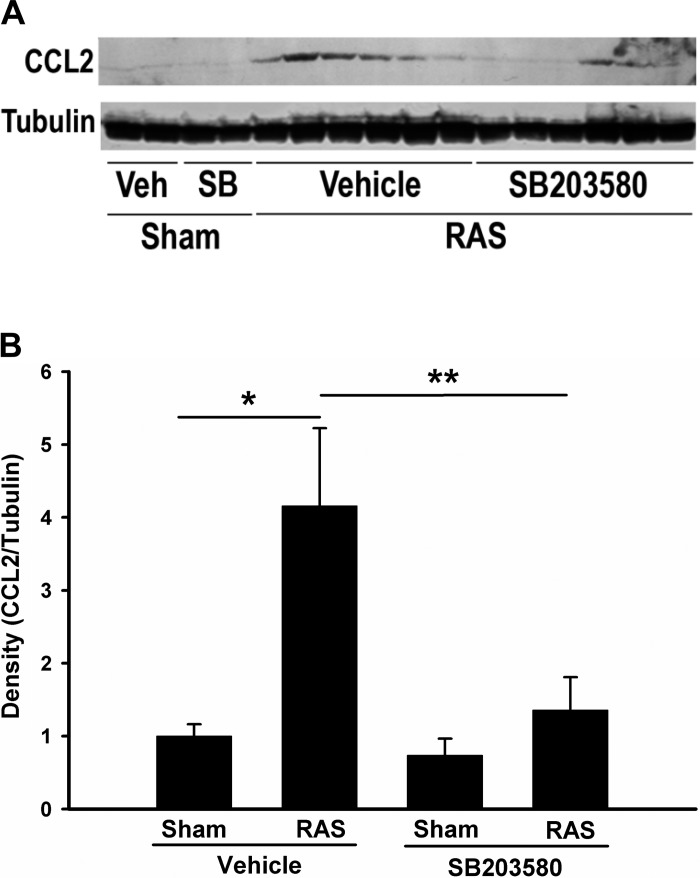
In accordance with our previous findings, CCL2 production was significantly increased in stenotic kidneys, but this upregulation was attenuated through inhibition of p38 with SB203580 treatment (Fig. 7, A and B). Quantitative real-time PCR data confirmed a similar trend of CCL2 expression at the mRNA level (Fig. 8A). We also investigated the expression of other cytokines and chemokines known to direct inflammation and fibrosis in chronic renal injury (TNF-α, TGF-β1, TGF-β2, TGF-β3, IL-6, CCL5, CCL7, and CCL8), their receptors (TGF-βR1, TGF-βR2, and CCR2), adhesion molecule (Fn1 and ICAM-1) and extracellular matrix components (Col1a1, Col3a1, and Col4a1). The expression of all of these genes was increased in the cuffed kidneys of mice subjected to RAS (data not shown). Blockade of p38 partially blocked the upregulation of CCL2, CCL7, CCR2, and Col4a1 (Fig. 8, A–D). Like CCL2, CCL7 is a secreted chemokine which attracts monocytes during inflammation. CCR2 is the major receptor for both CCL2 and CCL7. Given the fact that Col4a1 expression is regulated by CCL2 (34), these data suggest that p38 is critical for activation of CCL2/CCL7/CCR2 signaling pathway in stenotic kidneys and blockade of p38 signaling attenuates the renal inflammation in RAS through interruption of this pathway.
Fig. 7.
Inhibition of p38 reduces the upregulation of CCL2 in RAS mice. A: immunoblot analysis of CCL2 expression. Western blot analysis from renal homogenates from sham or stenotic kidneys with or without SB203580 treatment for 2 wk were probed with antibodies as indicated. Images are blots of representative experiments. B: densitometric quantitation of above blots. CCL2 levels were normalized to tubulin and relative density was illustrated Values are means ± SE, *P < 0.01, **P < 0.05.
Inhibition of p38 reduces CCL2 expression in vitro.
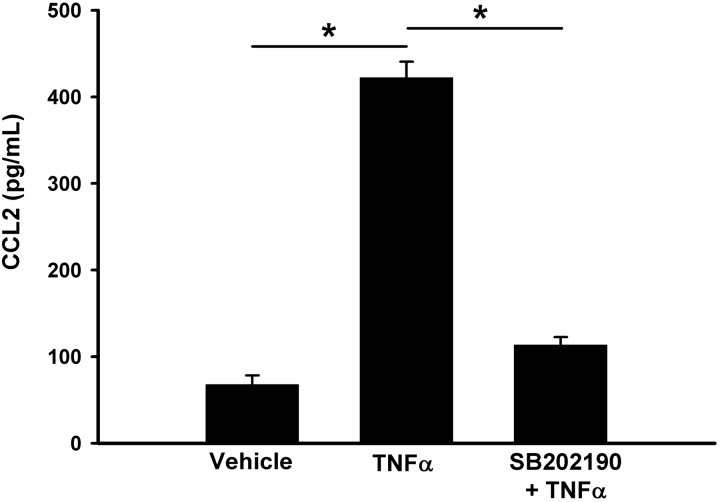
To confirm the critical role of p38 on CCL2 expression, we then performed a series of in vitro studies in cultured rat renal MC. Consistent with our previous findings (5), we found that TGF-β is a potent stimulus for CCL2 production by MC. However, TGF-β-mediated induction of CCL2 was abolished by SB202190 (Fig. 9A). As expected, TGF-β also induced CCR2 and collagen 4a expression but inhibition of p38 by SB202190 reduced them to baseline levels (Fig. 9C). Measurement of CCL2 secretion by ELISA showed a similar pattern for TNF-α-stimulated CCL2 in rat MC. TNF-α increased CCL2 production by over fivefold, but this upregulation was reduced to baseline levels by SB202190 (Fig. 10).
Fig. 9.
Inhibition of p38 blocks TGF-β-induced upregulation of CCL2 and col4 expression in rat glomerular mesangial cells (MC). MC were pretreated with vehicle (0.1% DMSO) or p38 inhibitor SB202190 for 1 h, followed by 10 ng/ml TGF-β1 for 6 h. Total RNA was isolated and the copy number of each gene in 1 μg total RNA was measured by absolute quantitation real-time PCR and normalized by 18S rRNA. CCL2 (A); Col4a (B); CCR2 (C). Values are means ± SE, *P < 0.01; **P < 0.05.
Fig. 10.
Inhibition of p38 reduces TNF-α-stimulated CCL2 expression in rat MC. MC were pretreated with vehicle or p38 inhibitor SB202190 for 1 h, followed by 10 ng/ml TNF-α for 6 h. The concentration of CCL2 secreted into the culture medium was quantified by using a rat-specific CCL2 ELISA kit. Values are means ± SE, *P < 0.01.
DISCUSSION
RAS, most commonly caused by atherosclerosis, remains an important cause of renal dysfunction and is associated with significant cardiovascular morbidity and mortality. Despite numerous studies, the mechanisms underlying irreversible renal injury and progressive deterioration of kidney function have not been fully elucidated in patients with RAS. It has been well recognized that the activation of the renin-angiotensin-aldosterone pathway results in elevation of blood pressure and contributes to both cardiac and kidney injury. Renal ischemia and oxidative stress are also potent proinflammatory stimuli and have been shown to play an important role in RAS-mediated chronic renal damage (15, 26).
Previous reports have indicated that inhibition of p38 MAPK may reduce tissue inflammation. Furthermore, inhibition of p38 has also been shown to reduce fibrosis in several models of chronic renal diseases (29, 33, 36, 38, 49). Although these studies have supported a beneficial effect of p38 inhibition to prevent renal disease progression, inhibition of p38 exacerbates renal injury in a remnant kidney model (31). A potential role of p38 inhibition in preventing chronic renal damage in the setting of RAS has not previously been defined. In our studies, we demonstrated that p38 MAPK is activated following induction of RAS and that blockade of p38 activity partially reduces interstitial fibrosis, tubular atrophy, and interstitial inflammation in the stenotic kidney.
In our studies, we did not observe a significant effect of p38 inhibition on blood pressure on either sham or RAS mice (Fig. 2). Previous reports have suggested that inhibition of p38 MAPK could reduce blood pressure in a renal ANG II-infusion hypertension model (1, 11, 35). Similar to the RAS model, the ANG II-infusion model presents with elevated blood pressure and heart and kidney damage. However, there are some significant differences between these models. Although the ANG activities in the 2K1C RAS model are elevated, there may be ANG II-independent pathways that promote renal damage in this model. In particular, inflammation, which plays a critical role in the progression of renal damage, may occur through both ANG II-dependent and independent pathways. In this study, we found p38 inhibition did not significantly change renin-1 activity and systolic blood pressure in mice 2 wk after the RAS surgery, suggesting that the effects of p38 on the damage of stenotic kidneys do not depend on the renin-angiotensin-aldosterone system. Consistent with our findings, de Borst et al. (8) have reported that inhibition of p38 did not affect the blood pressure in high renin homozygous transgenic rats, another widely-used hypertension animal model. Given the diverse cellular pathways which p38 MAPK affects, it is conceivable that p38 inhibition has different influences on blood pressure in different disease models.
Our studies highlight a critical role for CCL2/CCL7/CCR2 system in the progression of RAS. The expression of CCL2/CCL7/CCR2 is induced following induction of RAS and is associated with the influx of inflammatory cells. CCR2 is the receptor for both CCL2 and CCL7 and its role in fibrosis has been defined in several disease models by applications of specific receptor antagonists and gene knockout animal models (20, 27, 51). CCL2/CCR2 may induce intercellular adhesion molecule 1 (ICAM-1) expression, leading to enhanced monocyte adhesion (13). Furthermore, CCL2/CCR2 has also been demonstrated to regulate collagen expression and deposition in extracellular matrix (21, 34). CCL7 is an important mediator of fibrosis in systemic sclerosis (32), but its role in renal fibrosis is still not clear. Given the fact that the induction of CCL2/CCL7/CCR2 in stenotic kidney was partially blocked by p38 inhibition, we propose that CCL2/CCL7/CCR2 system is a critical mediator for p38-activated fibrogenesis in RAS. In accordance with observations made by other investigators (2, 19), our in vitro studies confirmed that p38 is an essential signaling intermediate for TGF-β and TNF-α-induced CCL2 expression. Therefore, both our in vivo and in vitro data suggest p38 is important for induction of CCL2/CCL7/CCR2 system, which plays a critical role in renal fibrosis in RAS.
The underlying mechanism by which p38 regulates the expression of proinflammatory cytokines is not fully understood. It has been suggested that p38 might control the mRNA turnover of these cytokines. Many proinflammatory cytokines are encoded by mRNAs that contain destabilizing AUUUA motifs in the 3′ untranslated regions. The RNA binding protein tristetraprolin recognizes these motifs and directs mRNA for rapid degradation (4). p38 Phosphorylates and activates MK-2, which further leads to phosphorylation and inactivation of tristetraproli. Thus, p38 activation can stabilize the mRNAs of these proinflammatory cytokines and increase their expression. On the other hand, inhibition of p38 leads to activation of tristetraproli and decreases the expression of these cytokines by accelerating the mRNA turnover (3). We found that p38 inhibition reduced the RAS-induced elevation of several cytokines such as CCL2 and CCL7. On the other hand, other cytokines (IL-6, CCL5, and CCL8) appear to be induced in a p38-independent fashion. These data suggest there are other signals to mediate the elevation of these cytokines in RAS kidneys. Given that p38 inhibition only partially blocked the tubular atrophy and interstitial fibrosis, it is likely that other signaling pathways also contribute to the development of atrophy and fibrosis in this model.
In summary, the data reported here demonstrate that p38 MAPK activity is essential for inflammation and fibrosis development in stenotic kidneys in RAS. Inhibition of the p38 signaling pathway reduces T lymphocyte infiltration and expression of proinflammatory cytokines, such as CCL2 and CCL7. In vitro experiments on rat MC have also shown that CCL2 might be a critical mediator of p38 signaling. There has been recent interest in the potential clinical use of low molecular weight inhibitors of MAPK signaling pathways as anti-inflammatory agents (17, 36, 39). A low molecular weight p38 inhibitor has recently been used to treat humans with idiopathic pulmonary fibrosis (28). Based on these considerations, we propose that low molecular weight p38 inhibitors may have therapeutic benefit in preventing irreversible renal dysfunction in patients with RAS.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant P01-HL-85307 and National Center for Research Resources Grant CTSA-UL1-RR024150. Dr. Diping Wang was supported by a Ruth L. Kirschstein National Research Service Award National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-007013-35.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.W., G.M.W., P.Y., B.E.K., J.C., K.B., K.R.L., and C.E.G. performed experiments; D.W., G.M.W., J.C., and J.P.G. analyzed data; D.W., J.C., and J.P.G. interpreted results of experiments; D.W. and G.M.W. prepared figures; D.W., G.M.W., V.D.G., L.O.L., S.C.T., K.A.N., and J.P.G. edited and revised manuscript; D.W., G.M.W., P.Y., B.E.K., J.C., K.B., K.R.L., C.E.G., V.D.G., L.O.L., S.C.T., K.A.N., R.D.S., and J.P.G. approved final version of manuscript; V.D.G., L.O.L., S.C.T., K.A.N., R.D.S., and J.P.G. conception and design of research; J.P.G. drafted manuscript.
ACKNOWLEDGMENTS
We thank Cherish Grabau for excellent secretarial assistance.
REFERENCES
- 1. Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CP, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol 49: 362– 368, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bian ZM, Elner SG, Yoshida A, Kunkel SL, Su J, Elner VM. Activation of p38, ERK1/2 and NIK pathways is required for IL-1β and TNF-α-induced chemokine expression in human retinal pigment epithelial cells. Exp Eye Res 73: 111– 121, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR. Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol Cell Biol 26: 2408– 2418, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrick DM, Lai WS, Blackshear PJ. The tandem CCCH zinc finger protein tristetraprolin and its relevance to cytokine mRNA turnover and arthritis. Arthritis Res Ther 6: 248– 264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng J, Diaz Encarnacion MM, Warner GM, Gray CE, Nath KA, Grande JP. TGF-β1 stimulates monocyte chemoattractant protein-1 expression in mesangial cells through a phosphodiesterase isoenzyme 4-dependent process. Am J Physiol Cell Physiol 289: C959– C970, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of 2-kidney, 1-clip hypertension. Am J Physiol Renal Physiol 297: F1055– F1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773: 1358– 1375, 2007 [DOI] [PubMed] [Google Scholar]
- 8. de Borst MH, Navis G, de Boer RA, Huitema S, Vis LM, van Gilst WH, van Goor H. Specific MAP-kinase blockade protects against renal damage in homozygous TGR(mRen2)27 rats. Lab Invest 83: 1761– 1770, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Diaz Encarnacion MM, Griffin MD, Slezak JM, Bergstralh EJ, Stegall MD, Velosa JA, Grande JP. Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am J Transplant 4: 248– 256, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Diaz Encarnacion MM, Warner GM, Cheng J, Gray CE, Nath KA, Grande JP. n-3 Fatty acids block TNF-α-stimulated MCP-1 expression in rat mesangial cells. Am J Physiol Renal Physiol 300: F1142– F1151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebrahimian T, Li MW, Lemarie CA, Simeone SM, Pagano PJ, Gaestel M, Paradis P, Wassmann S, Schiffrin EL. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension: regulation of oxidative stress. Hypertension 57: 245– 254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288– 1291, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Giunti S, Pinach S, Arnaldi L, Viberti G, Perin PC, Camussi G, Gruden G. The MCP-1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int 69: 856– 863, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gordon JR. TGF-β1 and TNF-α secreted by mast cells stimulated via the FcepsilonRI activate fibroblasts for high-level production of monocyte chemoattractant protein-1 (MCP-1). Cell Immunol 201: 42– 49, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 346: 1954– 1962, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol 163: 2193– 2201, 1999 [PubMed] [Google Scholar]
- 17. Ivanenkov YA, Balakin KV, Tkachenko SE. New approaches to the treatment of inflammatory disease: focus on small-molecule inhibitors of signal transduction pathways. Drugs R D 9: 397– 434, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Jin N, Wang Q, Zhang X, Jiang D, Cheng H, Zhu K. The selective p38 mitogen-activated protein kinase inhibitor, SB203580, improves renal disease in MRL/lpr mouse model of systemic lupus. Int Immunopharmacol 11: 1319– 1326, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Kawano H, Kim S, Ohta K, Nakao T, Miyazaki H, Nakatani T, Iwao H. Differential contribution of three mitogen-activated protein kinases to PDGF-BB-induced mesangial cell proliferation and gene expression. J Am Soc Nephrol 14: 584– 592, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 165: 237– 246, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis J Exp Med 185: 1371– 1380, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma FY, Liu J, Nikolic-Paterson DJ. The role of stress-activated protein kinase signaling in renal pathophysiology. Braz J Med Biol Res 42: 29– 37, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Ma JY, Medicherla S, Kerr I, Mangadu R, Protter AA, Higgins LS. Selective p38α mitogen-activated protein kinase inhibitor attenuates lung inflammation and fibrosis in IL-13 transgenic mouse model of asthma. J Asthma 1: 31– 44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matoba K, Kawanami D, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K. Rho-kinase mediates TNF-α-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun 402: 725– 730, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Meng XM, Huang XR, Xiao J, Chung AC, Qin W, Chen HY, Lan HY. Disruption of Smad4 impairs TGF-β/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int 81: 266– 279, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, Seta F, Capone ML, Tacconelli S, Palatresi S, Bencini C, Del Vecchio C, Mansueto G, Arosio E, Santonastaso CL, Lechi A, Morganti A, Patrono C. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation 106: 2800– 2805, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 167: 4368– 4377, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Moran N. p38 kinase inhibitor approved for idiopathic pulmonary fibrosis. Nat Biotechnol 29: 301, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Munoz L, Ramsay EE, Manetsch M, Ge Q, Peifer C, Laufer S, Ammit AJ. Novel p38 MAPK inhibitor ML3403 has potent anti-inflammatory activity in airway smooth muscle. Eur J Pharmacol 635: 212– 218, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Nishida M, Okumura Y, Sato H, Hamaoka K. Delayed inhibition of p38 mitogen-activated protein kinase ameliorates renal fibrosis in obstructive nephropathy. Nephrol Dial Transplant 23: 2520– 2524, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Ohashi R, Nakagawa T, Watanabe S, Kanellis J, Almirez RG, Schreiner GF, Johnson RJ. Inhibition of p38 mitogen-activated protein kinase augments progression of remnant kidney model by activating the ERK pathway. Am J Pathol 164: 477– 485, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ong VH, Evans LA, Shiwen X, Fisher IB, Rajkumar V, Abraham DJ, Black CM, Denton CP. Monocyte chemoattractant protein 3 as a mediator of fibrosis: Overexpression in systemic sclerosis and the type 1 tight-skin mouse. Arthritis Rheum 48: 1979– 1991, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Oto T, Calderone A, Li Z, Rosenfeldt FL, Pepe S. p38 Mitogen-activated protein kinase inhibition reduces inflammatory cytokines in a brain-dead transplant donor animal model. Heart Lung Circ 18: 393– 400, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Park J, Ryu DR, Li JJ, Jung DS, Kwak SJ, Lee SH, Yoo TH, Han SH, Lee JE, Kim DK, Moon SJ, Kim K, Han DS, Kang SW. MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am J Physiol Renal Physiol 295: F749– F757, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Park JK, Fischer R, Dechend R, Shagdarsuren E, Gapeljuk A, Wellner M, Meiners S, Gratze P, Al-Saadi N, Feldt S, Fiebeler A, Madwed JB, Schirdewan A, Haller H, Luft FC, Muller DN. p38 mitogen-activated protein kinase inhibition ameliorates angiotensin II-induced target organ damage. Hypertension 49: 481– 489, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Pettus LH, Wurz RP. Small molecule p38 MAP kinase inhibitors for the treatment of inflammatory diseases: novel structures and developments during 2006–2008. Curr Top Med Chem 8: 1452– 1467, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 344: 431– 442, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Schieven GL. The p38α kinase plays a central role in inflammation. Curr Top Med Chem 9: 1038– 1048, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dental Res 86: 800– 811, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Schwarz M, Wahl M, Resch K, Radeke HH. IFN-γ induces functional chemokine receptor expression in human mesangial cells. Clin Exp Immunol 128: 285– 294, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stahl RA, Thaiss F, Disser M, Helmchen U, Hora K, Schlondorff D. Increased expression of monocyte chemoattractant protein-1 in anti- thymocyte antibody-induced glomerulonephritis. Kidney Int 44: 1036– 1047, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ. The role of p38α mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol 15: 370– 379, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Stambe C, Nikolic-Paterson DJ, Hill PA, Dowling J, Atkins RC. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J Am Soc Nephrol 15: 326– 336, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kubler W, Kreuzer J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-κB and activator protein-1. Arterioscler Thromb Vasc Biol 22: 914– 920, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Wada T, Furuichi K, Sakai N, Hisada Y, Kobayashi K, Mukaida N, Tomosugi N, Matsushima K, Yokoyama H. Involvement of p38 mitogen-activated protein kinase followed by chemokine expression in crescentic glomerulonephritis. Am J Kidney Dis 38: 1169– 1177, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58: 1492– 1499, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302: F1455– F1464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells : implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol 19: 2085– 2093, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs 18: 1893– 1905, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Zhu XY, Daghini E, Chade AR, Napoli C, Ritman EL, Lerman A, Lerman LO. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol 18: 1209– 1217, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 168: 2953– 2962, 2002 [DOI] [PubMed] [Google Scholar]