Abstract
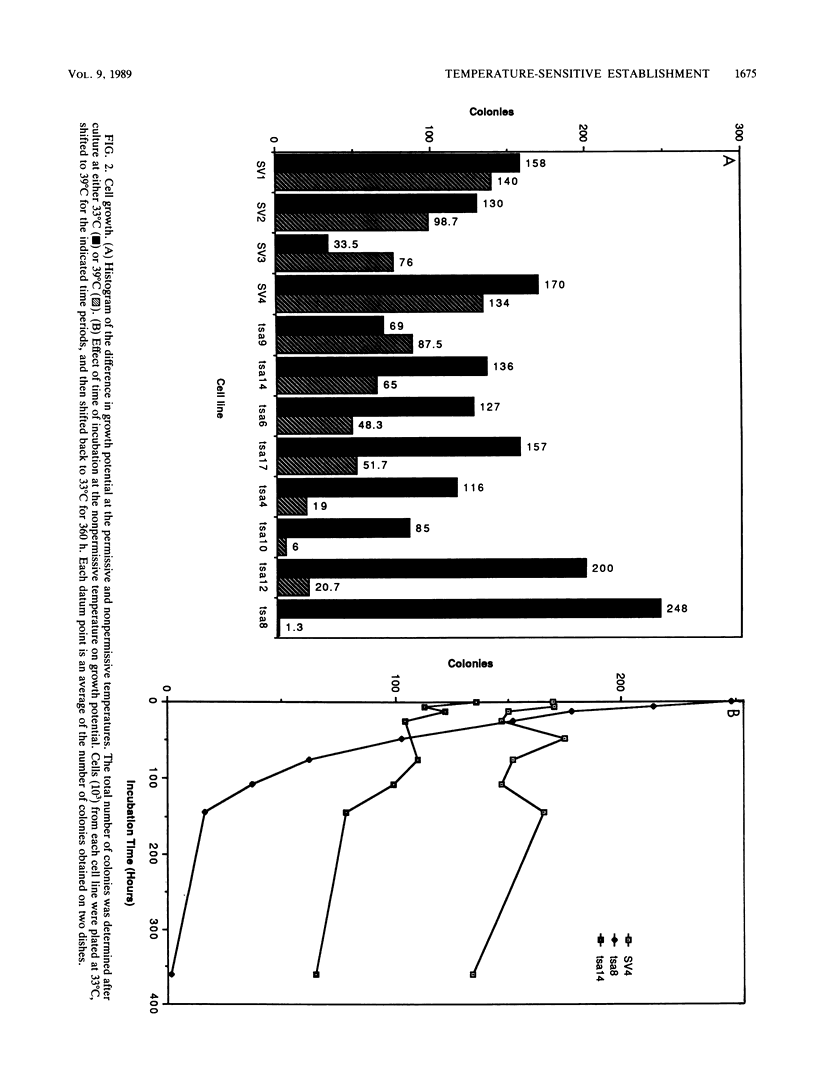
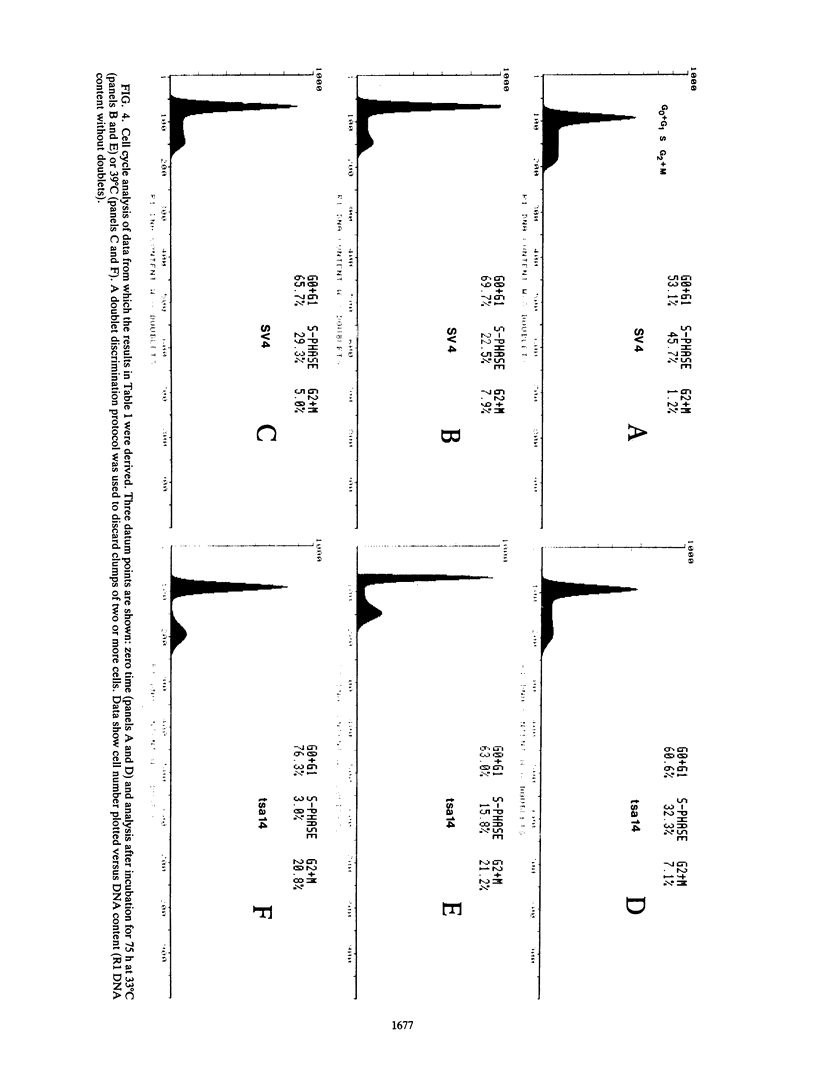
The thermolabile large T antigen, encoded by the simian virus 40 early-region mutant tsA58, was used to establish clonal cell lines derived from rat embryo fibroblasts. These cell lines grew continuously at the permissive temperature but upon shift-up to the nonpermissive temperature showed rapidly arrested growth. The growth arrest occurred in either the G1 or G2 phase of the cell cycle. After growth arrest, the cells remained metabolically active as assayed by general protein synthesis and the ability to exclude trypan blue. The inability of these cell lines to divide at the nonpermissive temperature was not readily complemented by the exogenous introduction of other nuclear oncogenes. This finding suggests that either these genes establish cells via different pathways or that immortalization by one oncogene results in a finely balanced cellular state which cannot be adequately complemented by another establishment gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Phillips P. D., Brooks K. M. Cellular senescence: factors modulating cell proliferation in vitro. Basic Life Sci. 1985;35:241–253. doi: 10.1007/978-1-4899-2218-2_15. [DOI] [PubMed] [Google Scholar]
- Davies J., Jimenez A. A new selective agent for eukaryotic cloning vectors. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1089–1092. doi: 10.4269/ajtmh.1980.29.1089. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen K., Jat P. S., Valtz N., Levy D., McKay R. Immortalization of precursor cells from the mammalian CNS. Neuron. 1988 Aug;1(6):439–448. doi: 10.1016/0896-6273(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gelfant S. A new concept of tissue and tumor cell proliferation. Cancer Res. 1977 Nov;37(11):3845–3862. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman S. D., Cristofalo V. J. Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Physiol. 1985 Oct;125(1):122–126. doi: 10.1002/jcp.1041250116. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grove G. L., Cristofalo V. J. Characterization of the cell cycle of cultured human diploid cells: effects of aging and hydrocortisone. J Cell Physiol. 1977 Mar;90(3):415–422. doi: 10.1002/jcp.1040900305. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T., Ruley H. E. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Cepko C. L., Mulligan R. C., Sharp P. A. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986 Apr;6(4):1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol. 1986 Sep;59(3):746–750. doi: 10.1128/jvi.59.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Watanabe S., Yoshiike K. Immortalization of primary rat cells by human papillomavirus type 16 subgenomic DNA fragments controlled by the SV40 promoter. Virology. 1988 Jul;165(1):321–325. doi: 10.1016/0042-6822(88)90694-0. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Sharp P. A. Transcription control by oncogenes. Cell. 1985 May;41(1):3–5. doi: 10.1016/0092-8674(85)90049-2. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Kaufman R. J., Sharp P. A. Regulation of transcription of the adenovirus EII promoter by EIa gene products: absence of sequence specificity. Mol Cell Biol. 1984 Oct;4(10):1970–1977. doi: 10.1128/mcb.4.10.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Ruley H. E. Role of c-myc in the transformation of REF52 cells by viral and cellular oncogenes. Oncogene. 1987;2(1):41–48. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Marcus M., Fainsod A., Diamond G. The genetic analysis of mammalian cell-cycle mutants. Annu Rev Genet. 1985;19:389–421. doi: 10.1146/annurev.ge.19.120185.002133. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. A., Gardes M., Feunteun J. Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology. 1983 May;127(1):74–82. doi: 10.1016/0042-6822(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Miller J. S., Kimelman D., Cepko C. L., Lemischka I. R., Mulligan R. C. Individual adenovirus type 5 early region 1A gene products elicit distinct alterations of cellular morphology and gene expression. J Virol. 1985 Nov;56(2):404–413. doi: 10.1128/jvi.56.2.404-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sedivy J. M., Capone J. P., RajBhandary U. L., Sharp P. A. An inducible mammalian amber suppressor: propagation of a poliovirus mutant. Cell. 1987 Jul 31;50(3):379–389. doi: 10.1016/0092-8674(87)90492-2. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988 Jun;7(6):1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Lathangue N. B., McLachlan D. R., Smith B. J., Anderton B. H., Dowding A. J. Chromatin proteins share antigenic determinants with neurofilaments. J Neurochem. 1985 Jan;44(1):149–154. doi: 10.1111/j.1471-4159.1985.tb07124.x. [DOI] [PubMed] [Google Scholar]