Summary
Cell motility is a cornerstone of embryogenesis, tissue remodeling and repair, and cancer cell invasion. It is generally thought that migrating cells grab and exert traction force onto the extracellular matrix in order to pull the cell body forward. While previous studies have shown that myosin II deficient cells migrate efficiently, whether these cells exert traction forces during cell migration in the absence of the major contractile machinery is currently unknown. Using an array of micron-sized pillars as a force sensor and shRNA specific to each myosin II isoform (A and B), we analyzed how myosin IIA and IIB individually regulate cell migration and traction force generation. Myosin IIA and IIB localized preferentially to the leading edge where traction force was greatest, and the trailing edge, respectively. When individual myosin II isoforms were depleted by shRNA, myosin IIA deficient cells lost actin stress fibers and focal adhesions, whereas myosin IIB deficient cells maintained similar actin organization and focal adhesions as wild-type cells. Interestingly, myosin IIA deficient cells migrated faster than wild-type or myosin IIB deficient cells on both a rigid surface and a pillar array, yet myosin IIA deficient cells exerted significantly less traction force at the leading edge than wild-type or myosin IIB deficient cells. These results suggest that, in the absence of myosin IIA mediated force-generating machinery, cells move with minimal traction forces at the cell periphery, thus demonstrating the remarkable ability of cells to adapt and migrate.
Key words: Cell migration, Focal adhesion, Myosin II, Traction force, Force sensor
Introduction
Many epithelial cells have an ability to migrate on extracellular matrix. This is an essential requirement for normal tissue development and repair, as well as for epithelial derived cancer cells to invade into the surrounding matrix during metastasis. Cell migration is a multi-step process requiring new membrane extensions at the leading edge, the formation of new adhesions to the substrate, and contractions at the trailing edge to move cell body forward (Ridley et al., 2003). A critical component of cell migration is thought to be the actin–myosin force-generating machinery. Using the actin network as a track, myosin II motors cross-link and contract the actin network, thus generating forces.
Myosin IIA and IIB are the predominant isoforms of myosin II in many cells. These two myosin II isoforms differ in motor properties and expression levels, therefore, each isoform appears to play distinct roles in cell migration (Conti and Adelstein, 2008; Vicente-Manzanares et al., 2009). Myosin IIA deficiency leads to a broader lamellipodium (Even-Ram et al., 2007; Sandquist et al., 2006; Vicente-Manzanares et al., 2007), and in many cell types, faster cell speeds relative to corresponding wild-type cells (Doyle et al., 2012; Even-Ram et al., 2007; Sandquist et al., 2006), although a slower than wild-type cell speed was observed in a breast cancer cell line (Betapudi et al., 2006). In contrast, myosin IIB deficient cells migrate similar to (Doyle et al., 2012; Even-Ram et al., 2007) or slower than wild-type cells (Sandquist et al., 2006), and myosin IIB is thought to regulate cell polarity during cell migration (Lo et al., 2004; Vicente-Manzanares et al., 2008). While myosin II is clearly important for cell migration, it is unclear how myosin II deficiency affects traction force generation in migrating cells.
We sought to analyze how myosin II deficient MDCK cells exert traction forces in order to migrate. Our analysis demonstrates that myosin IIA deficient cells adhere to the substrate without distinct focal adhesions while expressing integrin β1 levels that were similar to wild-type and myosin IIB deficient cells. Furthermore, myosin IIA deficient cells migrate faster than wild-type or myosin IIB deficient cells on a fibronectin-coated glass surface and pillar array. Using a miniature force sensor, we show that myosin IIA deficient cells do not exert detectable traction forces onto the substrates, whereas myosin IIB deficient cells exert traction forces similar to wild-type cells. This unique cell motility of myosin IIA deficient cells is rescued by the expression of exogenous myosin IIA, suggesting that myosin IIA, and not other off-target shRNA artifacts, is important for observed traction force generation. This ability of epithelial cells to migrate without the core force generating machinery demonstrates the unique adaptation of cell migration mechanisms.
Results
Myosin IIA and IIB localization along the sites of traction force transmission
Since myosin II is required for force generation, we analyzed the relative localization of myosin IIA and IIB with respect to the sites of force generation in migrating cells. Using a micro-fabricated force sensor, and MDCK cells with dual expression of mCherry-tagged myosin IIA and GFP-tagged myosin IIB, we were able to simultaneously visualize and directly compare the relative localization of myosin isoforms and traction force exerting sites. Unlike the focal adhesion proteins, which localizes directly to the force-bearing sites of the pillars (Tan et al., 2003; Uemura et al., 2011), myosin IIA and IIB localized away from the bending pillars (Fig. 1A,B). Myosin IIA proteins were more concentrated at the leading edge, whereas myosin IIB proteins were more concentrated at the trailing edge (Fig. 1B). Quantification of myosin IIA and IIB signals along the representative migrating cell (Fig. 1B) confirms that the myosin IIA signal (magenta) localized along the leading edge of membrane extensions where myosin IIB signal (green) was absent (Fig. 1C). In addition, myosin IIB signal was the highest at the trailing edge (Fig. 1C). The leading edge of the migrating cell exerted significantly more traction force than the trailing edge (Fig. 1C, bottom), suggesting that myosin IIA and IIB play distinct roles in the generation of traction forces at leading and trailing edges, respectively.
Fig. 1. Myosin IIA and B differentially localized in migrating cells.

(A) MDCK cell expressing both myosin IIA-mCherry and myosin IIB-GFP migrating on the pillar array. (B) The overlay image of myosin IIA (magenta) and IIB (green) with arrows indicating the directions and magnitudes of traction force exerted by the cell. Scale bar: 10 µm. (C) Fluorescence intensity profile of myosin IIA (magenta) and IIB (green), and local traction force profile (bottom) along the migrating cell shown in A and B.
Myosin IIA deficient cells are devoid of focal adhesions
Using shRNA expressing plasmids, we generated stable MDCK cell lines expressing shRNA for myosin IIA and IIB (Shih and Yamada, 2010). These cell lines have significantly reduced myosin IIA or IIB level without affecting the other isoform as determined by Western blot (Fig. 2A). In a previous study, we demonstrated myosin IIA deficient cells are surprisingly fast crawlers on a collagen coated coverslip, but are not motile in a 3D collagen matrix (Shih and Yamada, 2010). However, myosin IIB deficient cells migrated similarly to the wild-type cells both on a 2D surface and in a 3D matrix (Shih and Yamada, 2010). Since myosin II is required for the generation of traction forces, we further investigated cell migration of myosin IIA and IIB deficient cells.
Fig. 2. Myosin IIA deficient cells lack focal adhesions.

(A) Western blots of Myosin IIA (MIIA) or IIB (MIIB) shRNA transfected clones. Selective and complete knockdown of IIA and IIB relative to wild-type (WT) cells are shown in anti-myosin IIA (MIIA) or IIB (MIIB) blot. Similar levels of paxillin (Pax) and integrin β1 (β1) are observed. Tubulin blot of the same sample is used as a loading control. (B) Representative immunofluorescence images of wild-type, shRNA myosin IIA, or IIB transfected cells using anti-paxillin antibody (red) and phalloidin (green). Scale bar: 10 µm.
On a fibronectin-coated glass coverslip, wild-type MDCK cells developed actin stress fibers that terminated at paxillin-positive focal adhesions (Fig. 2B). While myosin IIB deficient cells had similar actin and focal adhesion organization as wild-type cells, myosin IIA deficient cells had a thinner actin network that lacked distinct stress fibers (Fig. 2B). The focal adhesions of myosin IIA deficient cells were minimal in size compared to wild-type or myosin IIB deficient cells (Fig. 2B). These results suggest that the contractility of myosin IIA, and not myosin IIB, is required for the development of stress fibers and large focal adhesions in these cells.
The predominant effect of myosin IIA knockdown and the lack of phenotype in myosin IIB deficient cells may be due to the difference in the amounts of myosin II isoforms. Using the GFP-tagged myosin IIA or IIB overexpressing MDCK cell line as a reference, we compared the relative intensity of endogenous myosin IIA and IIB bands in the parental cell line. By ensuring that the band intensities are within a linear range (supplementary material Fig. S1A), we measured relative protein levels of endogenous myosin isoforms in the parental cells and GFP-tagged myosin isoforms in the overexpressed cells using myosin IIA and IIB antibodies (supplementary material Fig. S1B). Then, we immunoblotted two GFP-tagged myosin IIA and IIB expressing cells using anti-GFP antibody to compare the relative level of GFP-tagged myosin IIA and IIB in these cell lines (supplementary material Fig. S1B), which in turn enabled us to compare the relative protein levels of endogenous IIA and IIB in the parental cell line. We found that the myosin IIA level was five times greater than the myosin IIB level in the parental cell line. The higher level of myosin IIA over myosin IIB in MDCK cells may explain why myosin IIA deficiency resulted in a more significant phenotype than myosin IIB deficiency.
Myosin IIA deficient cells migrate faster than wild-type or myosin IIB deficient cells
While many wild-type and myosin IIB deficient cells had a typical epithelial morphology, myosin IIA deficient cells had broad lamellipodia extensions with a distinct trailing edge (Fig. 3A), which is consistent with previous studies on a collagen-coated glass coverslip (Shih and Yamada, 2010). Despite the absence of focal adhesions in myosin IIA deficient cells, these cells migrated faster and more persistently (determined by the ratio of end point displacement and total path length) than wild-type or myosin IIB deficient cells on a fibronectin-coated glass coverslip (Fig. 3B). Furthermore, on a fibronectin coated pillar array, myosin IIA deficient cells also migrated faster and more persistently than wild-type or myosin IIB deficient cells (Fig. 3C), suggesting that the enhanced migration potential of myosin IIA deficient cells is evident on both stiff glass and softer PDMS pillar substrates. Altogether, these results suggest that the absence of focal adhesions in myosin IIA deficient cells may be more advantageous for rapid cell migration on 2D surfaces. Note, however, that this rapid cell migration of myosin IIA deficient cells is unique to 2D cell migration, and the phenotypes are different in cell migration in a 3D environment (see Discussion).
Fig. 3. Myosin IIA deficient cells migrate at a faster rate than wild-type and myosin IIB knockdown cells.

(A) Representative bright-field images of wild-type (WT), shRNA myosin IIA (shRNA MIIA) or IIB (shRNA MIIB) cells on a fibronectin-coated glass coverslip. Scale bar: 10 µm. (B) Cell migration speed and persistency of wild-type (WT), shRNA myosin IIA (shRNA MIIA) or IIB (shRNA MIIB) cells on a fibronectin-coated glass coverslip. Cells were imaged for 12 hours and single cells were tracked to calculate average speeds of individual cells, and the persistency of migration path was calculated based on the ratios of the distance between starting and ending points and the total path length. *P<0.05. (C) Cell migration speed and persistency of wild-type (WT), shRNA myosin IIA (shRNA MIIA) or IIB (shRNA MIIB) cells on a fibronectin-coated micro-pillar array. *P<0.05.
Myosin IIA deficient cells migrate despite reduced traction forces at cell periphery
On a fibronectin-coated glass coverslip and pillar array, myosin IIA deficient cells migrated faster than myosin IIB deficient cells (Fig. 3). To analyze the traction force of migrating myosin II deficient cells, these cells were plated on fibronectin-coated pillar arrays and analyzed for traction force generation. Both myosin IIA and IIB cells migrated on the force sensing pillar array, but with one key distinction. Interestingly, myosin IIA deficient cells moved on the surface of pillar array, but with minimal deflection of pillars at the leading edge (Fig. 4A). Similar to the wild-type cells, myosin IIB deficient cells migrated while exerting traction forces at the leading edge (Fig. 4A). The quantification of total traction force reveals that myosin IIA deficient cells exerted significantly reduced detectable traction forces compared to the wild-type and myosin IIB deficient cells (Fig. 4B).
Fig. 4. Myosin IIA deficient cells migrate reduced traction forces at cell periphery.
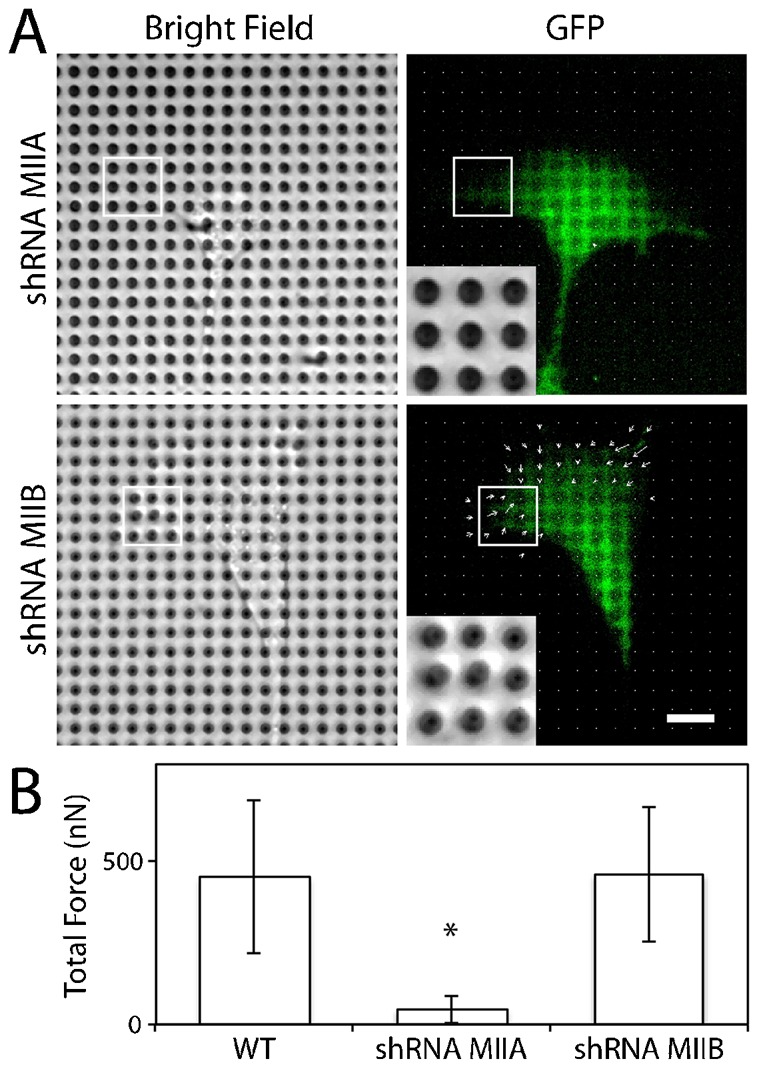
(A) Both MDCK cells expressing myosin IIA (top) or IIB (bottom) shRNA migrate on the pillar array, but myosin IIA deficient cells do not bend pillars while myosin IIB expressing cells bend pillars at similar extent as wild-type cells. The pillar deflection is denoted by the sizes and directions of arrows, which are super-imposed onto the GFP image. Scale bar: 10 µm. (B) Quantification of total traction force exerted by MDCK wild-type (WT, n = 17), myosin IIA knockdown (shRNA MIIA, n = 18) and myosin IIB knockdown (shRNA MIIB, n = 15) cells. *P<0.05. Error bars are standard deviations.
To ensure that the phenotype of myosin IIA deficient cells is solely due to the reduction of myosin IIA, as opposed to off-target shRNA knockdown, we transfected both shRNA specific to myosin IIA and shRNA resistant GFP-tagged mouse myosin IIA. The rescued cell line had minimal levels of endogenous myosin IIA, but expressed a comparable level of shRNA resistant exogenous GFP-tagged myosin IIA (Fig. 5A). Similar to the parental cell line, these myosin IIA rescued cells had epithelial morphology and migrated efficiently (Fig. 5B), and exerted traction forces as they migrated on the pillar array (Fig. 5C). The average magnitude of total traction forces was similar for both the wild-type and myosin IIA rescued cells (Fig. 5C), suggesting that myosin IIA deficiency was solely responsible for the absence of traction forces in the myosin IIA knockdown cells. Although myosin II-dependent force generation is thought to be critical for cell migration, our results show that myosin IIA deficient cells move along a fibronectin-coated surface without exerting significant traction forces at cell periphery.
Fig. 5. Myosin IIA deficient cells are rescued by the exogenous expression of myosin IIA.
(A) (Upper) Western blots of wild-type cells (first lane), myosin IIA-GFP overexpressing cells (second lane) and myosin IIA knockdown cells expressing shRNA resistant myosin IIA-GFP (third lane) using anti-myosin IIA and anti-tubulin antibody (as a loading control). (Lower) Representative bright-field image of myosin IIA rescue (MIIA rescue) cells on a fibronectin-coated glass coverslip. Scale bar: 10 µm. (B) Cell migration speed and persistency of wild-type (WT) and myosin IIA rescue cells on a fibronectin-coated glass coverslip and a pillar array. (C) (Left) The myosin IIA deficient cells with exogenous myosin IIA migrates with traction forces. (Middle) The pillar deflection is denoted by the sizes and directions of arrows, which are super-imposed onto the GFP image. Scale bar: 10 µm. (Right) Quantification of total traction force for wild-type (WT, n = 17) and myosin IIA rescued (MIIA rescue, n = 18) cells. Error bars are standard deviations.
Discussion
Our results demonstrate the remarkable ability of myosin IIA deficient cells to migrate with minimal traction forces at cell periphery. Myosin IIA deficient cells migrate with a broad lamellipodia (Fig. 3A), similar to the migration phenotype of keratocytes. Unlike wild-type MDCK cells that exert significant traction forces at the leading and trailing edges (Fig. 1), keratocytes migrate with minimal traction forces at the leading and trailing edges and the major traction forces are exerted at the sides of cell body (Fournier et al., 2010; Oliver et al., 1999). Since myosin IIA deficient cells extend a broad lamellipodia that is often observed in keratocytes, myosin IIA deficient MDCK cells likely move on the surface using rapid actin polymerization similar to keratocytes. It remains possible, however, that myosin IIA deficient cells exert forces with the magnitudes below our detection limit (<4 nN), and such forces may be distributed over many pillars so that the total traction force may still be significant. Nevertheless, our data demonstrate that the traction force exerted by myosin IIA deficient cells are significantly reduced at cell periphery compared to the wild-type and myosin IIB deficient cells.
For MDCK cells, myosin IIA deficiency leads to cell migration with significantly reduced traction forces at cell periphery, whereas myosin IIB deficiency does not result in any cell motility phenotype. Previous studies have shown that myosin IIB deficient cells showed a lack of polarity and random cell migration (Lo et al., 2004; Vicente-Manzanares et al., 2008). While myosin IIA deficiency leads to faster cell migration in MDCK cells (Fig. 3) and other cell types (Doyle et al., 2012; Even-Ram et al., 2007; Sandquist et al., 2006), myosin IIA deficient breast cancer cells migrate slower than wild-type cells (Betapudi et al., 2006). This experimental discrepancy may be due to the relative levels of myosin II isoforms in the different cell types. While a number of previous studies lacked the quantification of myosin II isoform levels, myosin IIA is often the predominant isoform in various cell lines (supplementary material Fig. S1) (Babbin et al., 2009; Sandquist and Means, 2008; Smutny et al., 2010). The relative levels of myosin II isoforms may explain these phenotype discrepancies in previous studies.
What is the mechanism by which myosin IIA deficient cells migrate with minimal contractile forces? One possibility is that the absence of actin stress fibers in myosin IIA deficient cells (Fig. 2B) may increase the cytoplasmic actin monomer concentration that in turn promotes actin polymerization at the leading edge. This is consistent with broad lamellipodia often observed in myosin IIA deficient cells (Fig. 3A) (Even-Ram et al., 2007; Sandquist et al., 2006; Vicente-Manzanares et al., 2007), and may contribute to the enhanced cell migration (Fig. 3B). Alternatively, myosin IIA deficient cells have reduced cell–extracellular matrix adhesions, which in turn, require less contractile force for de-adhesion, an essential step in cell migration. This is consistent with our observation that myosin IIA deficient cells have minimal paxillin-positive focal adhesions (Fig. 2B). While these possibilities are not mutually exclusive, our analysis demonstrates that the myosin IIA deficiency could be compensated by other mechanisms to enhance cell migration.
Our key finding is that myosin IIA deficient cells migrate with reduced traction forces at cell periphery on a 2D surface. The reduced traction force in migrating myosin IIA deficient cells is consistent with the reduced traction force observed during cell spreading (Cai et al., 2006) or the reduced collagen contraction by myosin IIA deficient cells embedded in the gel (Even-Ram et al., 2007). Interestingly, our previous study found that the myosin IIA deficient MDCK cells do not migrate in a 3D collagen gel (Shih and Yamada, 2010), suggesting that two distinct mechanisms are responsible for 2D and 3D cell migration. While 2D cell migration requires little or no detectable traction force at cell periphery to migrate, the generation of traction force is essential for 3D cell migration. Therefore, our study highlights the unique adaptation of cell migration mechanisms in a myosin II dependent manner, and our observation provides an important first step for a better understanding of how traction forces govern all types of cell migration.
Materials and Methods
Cell lines and reagents
MDCK GII cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA). For Western blot or immunofluorescence analysis, the antibodies used were tubulin (Sigma–Aldrich, St. Louis, MO), myosin IIA (Sigma–Aldrich, St. Louis, MO), myosin IIB (Covance, Princeton, NJ), paxillin (BD Biosciences, Franklin Lakes, NJ) and β1-integrin (Millipore, Billerica, MA). Filamentous actin was labeled with phalloidin (Invitrogen, Carlsbad, CA). For Western blot, the signals on the nitrocellulose membrane were detected by chemiluminescence with an enhanced ECL reagent (Pierce Biotechnology).
Myosin IIA and B knockdown and rescue constructs
All cell lines are described previously (Shih and Yamada, 2010). Briefly, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), co-transfected with GFP-myosin IIB and mCherry-myosin IIA (Addgene, Cambridge, MA). For the myosin II knockdown cell lines, MDCK cells were stably transfected with pSuper-myosin IIA or IIB (a gift from Dr Rick Horwitz). The shRNA target sequence for myosin IIA was GATCTGAACTCCTTCGAGC as described (Vicente-Manzanares et al., 2007), and myosin IIB was altered for the canine specific sequence: GGACCGCTACTATTCGGGA. The rescued cell line was generated by transfecting mCherry containing pSuper-myosin IIA and GFP-myosin IIA.
Live-cell confocal microscopy and cell migration analysis
All samples were imaged on a Zeiss Axio Observer equipped with a Yokogawa spinning disk confocal system, a 10× and 40× objective, 488 and 561 nm solid-state lasers, and a CoolSNAP HQ camera. The microscope system was controlled and automated by Slidebook software (Intelligent Imaging Innovations, Denver, CO, USA). Live cells were imaged on glass bottom dishes (MatTek, Ashland, MA, USA) in a temperature-controlled chamber at 37°C. The average fluorescence intensity profile along the migrating cell was analyzed by taking intensity line scans perpendicular to the migration axis from leading to trailing edges of the cell, and each intensity scan was averaged and plotted (Fig. 1C, top). For cell migration analysis on 2D surfaces, the cells were imaged and analyzed for 12 hours at 10-minute intervals. The position of individual cells was tracked, and the path length and the distance between start and end points of cell trajectories were calculated using Slidebook software. The cell speed is a ratio of the path length and time, and the persistency of cell migration is the ratio of the distance between the start and end points and the total path length. A one-way analysis of variance (ANOVA) with Dunnett's post hoc test was performed to compare the cell migration of myosin IIA deficient cells with that of wild-type or myosin IIB deficient cells. The difference was assumed to be statistically significant when P<0.05.
Micro-fabrication of miniature force sensor
Fabrication of micro-pillar arrays was described previously (Uemura et al., 2011). Briefly, the micro-pillar master was etched with deep reactive ion etcher and consisted of dimensions of 2 µm in pillar diameter, 6 µm in height, and 4 µm in pitch (AdvancedMEMS, Berkeley, CA, USA). Using Polydimethylsiloxane (PDMS), the negative mold was cast, then PDMS pillars were fabricated. A droplet of 100 µg/ml fibronectin (BD Biosciences, San Jose, CA, USA) solution spiked with rhodamine fibronectin (Cytoskeleton, Denver, CO, USA) in 1:5 ratio was deposited on the pillar tips for 10 minutes, then the pillars were wetted in PBS with 1% BSA and 0.05% Triton X-100. The pillars were then washed with PBS and the growth medium. This approach created a fibronectin mesh on the pillar array, making easier for cells to adhere and spread. The traction force was calculated based on the displacement of pillar tips from the original positions and pillar stiffness of 2.5 MPa with the PDMS pillar height of 5.2 µm (Uemura et al., 2011). The numbers of cells analyzed were 17, 18, 15, and 18 for wild-type, myosin IIA deficient, myosin IIB deficient and myosin IIA rescued cells (Fig. 4B, Fig. 5C). The local traction forces analyzed in Fig. 1C are calculated by summation of a set of pillars that are perpendicular to the migrating cell axis. All images are analyzed in ImageJ. See detailed protocol in reference (Uemura et al., 2011). A one-way analysis of variance (ANOVA) with Dunnett's post hoc test was performed to compare the traction force of myosin IIA deficient cells with that of wild-type or myosin IIB deficient cells. The difference was assumed to be statistically significant when P<0.05.
Supplementary Material
Acknowledgments
We thank Dr Rick Horwitz for pSuper and mCherry myosin plasmids. This work was supported by a Beckman Young Investigator Award, a Hellman Family New Faculty Award, a NIH EUREKA GM094798, and the funds from the University of California Cancer Research Coordinating Committee.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Babbin B. A., Koch S., Bachar M., Conti M. A., Parkos C. A., Adelstein R. S., Nusrat A., Ivanov A. I. (2009). Non-muscle myosin IIA differentially regulates intestinal epithelial cell restitution and matrix invasion. Am. J. Pathol. 174, 436–448 10.2353/ajpath.2009.080171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betapudi V., Licate L. S., Egelhoff T. T. (2006). Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 66, 4725–4733 10.1158/0008-5472.CAN-05-4236 [DOI] [PubMed] [Google Scholar]
- Cai Y., Biais N., Giannone G., Tanase M., Jiang G., Hofman J. M., Wiggins C. H., Silberzan P., Buguin A., Ladoux B.et al. (2006). Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 91, 3907–3920 10.1529/biophysj.106.084806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. (2008). Nonmuscle myosin II moves in new directions. J. Cell Sci. 121, 11–18 10.1242/jcs.007112 [DOI] [PubMed] [Google Scholar]
- Doyle A. D., Kutys M. L., Conti M. A., Matsumoto K., Adelstein R. S., Yamada K. M. (2012). Micro-environmental control of cell migration – myosin IIA is required for efficient migration in fibrillar environments through control of cell adhesion dynamics. J. Cell Sci. 125, 2244–2256 10.1242/jcs.098806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even–Ram S., Doyle A. D., Conti M. A., Matsumoto K., Adelstein R. S., Yamada K. M. (2007). Myosin IIA regulates cell motility and actomyosin–microtubule crosstalk. Nat. Cell Biol. 9, 299–309 10.1038/ncb1540 [DOI] [PubMed] [Google Scholar]
- Fournier M. F., Sauser R., Ambrosi D., Meister J. J., Verkhovsky A. B. (2010). Force transmission in migrating cells. J. Cell Biol. 188, 287–297 10.1083/jcb.200906139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. M., Buxton D. B., Chua G. C., Dembo M., Adelstein R. S., Wang Y. L. (2004). Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell 15, 982–989 10.1091/mbc.E03-06-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T., Dembo M., Jacobson K. (1999). Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J. Cell Biol. 145, 589–604 10.1083/jcb.145.3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704–1709 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- Sandquist J. C., Means A. R. (2008). The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol. Biol. Cell 19, 5156–5167 10.1091/mbc.E08-05-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandquist J. C., Swenson K. I., Demali K. A., Burridge K., Means A. R. (2006). Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J. Biol. Chem. 281, 35873–35883 10.1074/jbc.M605343200 [DOI] [PubMed] [Google Scholar]
- Shih W., Yamada S. (2010). Myosin IIA dependent retrograde flow drives 3D cell migration. Biophys. J. 98, L29–L31 10.1016/j.bpj.2010.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M., Cox H. L., Leerberg J. M., Kovacs E. M., Conti M. A., Ferguson C., Hamilton N. A., Parton R. G., Adelstein R. S., Yap A. S. (2010). Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 12, 696–702 10.1038/ncb2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. L., Tien J., Pirone D. M., Gray D. S., Bhadriraju K., Chen C. S. (2003). Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 100, 1484–1489 10.1073/pnas.0235407100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A., Nguyen T. N., Steele A. N., Yamada S. (2011). The LIM domain of zyxin is sufficient for force-induced accumulation of zyxin during cell migration. Biophys. J. 101, 1069–1075 10.1016/j.bpj.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente–Manzanares M., Zareno J., Whitmore L., Choi C. K., Horwitz A. F. (2007). Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 176, 573–580 10.1083/jcb.200612043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente–Manzanares M., Koach M. A., Whitmore L., Lamers M. L., Horwitz A. F. (2008). Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 183, 543–554 10.1083/jcb.200806030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente–Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009). Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 10.1038/nrm2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



