Abstract
Diamine oxidase (DAO) is abundantly expressed in mammalian small intestine catalyzing the oxidative breakdown of polyamines and histamine. The aim of this study was to determine the relationship between stimulation of intestinal diamine oxidase secretion with intestinal fat absorption and histamine release. Conscious intestinal lymph fistula rats were used. The mesenteric lymph ducts were cannulated and intraduodenal tubes were installed for the infusion of Liposyn II 20% (an intralipid emulsion). Lymphatic DAO activity and protein secretion were analyzed by radiometric assay and Western blot, respectively. Lymphatic histamine concentration was measured by ELISA. Infusion of Liposyn II (4.43 kcal/3 ml) resulted in a ∼3.5-fold increase in lymphatic DAO protein secretion and DAO activity, peaking at 1 h and lasting for 3 h. Liposyn II infusion also increased the lymphatic histamine release, a substrate for DAO. To determine the relationship of DAO release with histamine release, histamine was administered intraperitoneally (10 mg/kg) in fasting rats and resulted in a significant doubling in lymphatic DAO activity, supporting a link between histamine and DAO. In addition, ip administration of the histamine H4 receptor antagonist JNJ7777120 significantly reduced the Liposyn II-induced DAO output by 65.9%, whereas H1 (pyrilamine maleate), H2 (ranitidine), and H3 (thioperamide maleate) receptor antagonists had little effect. We conclude that DAO secretion may contribute to the catabolism of histamine released during fat absorption and this is probably mediated through the histamine H4 receptor.
Keywords: dietary lipids, intestinal lymph, histamine, histamine receptors
histamine [2-(4-imidazolyl)ethylamine] is widely distributed in peripheral and central tissues including the dermis, small intestine, stomach, lung, and brain (11, 23), It mediates a variety of physiological processes including inflammation and immunity, gastric acid secretion, smooth muscle contraction, tissue growth and repair, and the regulation of appetite and metabolism (8, 11). Histamine is abundant along the whole gastrointestinal (GI) tract, where it is mostly located in intestinal mucosal mast cells (MMC) (23). Also, some histamine is found in enterochromaffin-like cells in the stomach and in neurons (23). Histamine is produced by the decarboxylation of histidine by histidine decarboxylase (11, 23), and it exerts its action by binding to specific histamine receptors (HRs) (11, 23). The four HRs H1R to H4R have been discovered along the GI tract in mammals (11, 23). Through the four types of HRs histamine contributes to the regulation of microvascular dilation and permeability (H1R, H2R) (6, 21), gastric acid production (H2R) (4), intestinal secretion and motility (H1R, H3R) (14), and mucosal recovery and repair after ischemia-reperfusion (H1R) (8). In addition, by triggering immune and inflammatory responses (H4R) (5), histamine plays an important role in a variety of pathophysiological conditions such as anaphylaxis, food allergies (2), and inflammatory bowel diseases (IBD) (35).
The physiological role of histamine in fat absorption is unclear. Fat absorption in the small intestine is associated with a series of physiological reactions including microvascular dilation (1, 6), increased vascular and mucosal permeability (9), and alteration in intestinal motility (15). Fat absorption is associated with a marked increase in protein output in lymph, and this increase can be prevented by both H1R and H2R antagonists (32).
Diamine oxidase (EC 1.4.3.6; DAO) and histamine N-methyltransferase (HNMT) are two major enzymes catabolizing histamine and other polyamines including putrescine and spermidine (17). DAO mainly acts extravascularly whereas HNMT acts mainly intracellularly (26). DAO is unevenly distributed, being abundant in the small intestine, kidney, and placenta of humans and other mammals (18, 28). Intestinal DAO is synthesized continuously by mature enterocytes localized in the upper intestinal villi and is mainly associated with the basolateral aspect of enterocytes (3). DAO is stored in plasma membrane-associated vesicular structures in epithelial cells of intestine (27) and is secreted into the circulation in response to stimuli such as intravenous injection of heparin (25). As a rate-limiting enzyme in the terminal catabolism of histamine and polyamines in vivo, DAO acts as a physiological barrier against gut luminal histamine, putrescine, and cadaverine originating from food and intestinal microbiomes (26). DAO activity has been found to vary among individuals owing to polymorphisms and is potentially linked with food allergies and inflammatory disorders such as IBD (11).
Little information is available on the role of DAO in fat absorption. Our previous studies have shown that continuous duodenal infusion of triolein for 6 h gradually increases DAO activity in the intestinal lymph (34). However, why DAO activity is increased and whether it is linked to histamine release during fat absorption are far from clear. In the present study, we found that histamine is released into intestinal lymph during fat absorption and that it is probably involved in the regulation of the DAO secretion into lymph. This regulatory effect of histamine is mainly mediated through H4R and not through the other histamine receptors such as H1R and H2R. The results demonstrate for the first time the link between histamine and its degrading enzyme DAO released during fat absorption, and this may provide insight into our understanding of the mechanisms of food allergies and IBD.
MATERIALS AND METHODS
Materials
Liposyn II 20% was purchased from Hospira (Lake Forest, IL), and trilinolein from Nu-Chek-Prep (Elysian, MN). Histamine hydrochloride; the HR antagonists H1R-pyrilamine maleate, H2R-ranitidine, H3R-thioperamide maleate, and H4R-JNJ777120; and tricaprylin were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). The radioactive [3H]putrescine was obtained from Amersham Biosciences (Piscataway, NJ). The histamine ELISA kit was purchased from Neogen (product no. 409010, Lexington, KY). The goat anti-mouse DAO polyclonal antibody (sc-67660) was from Santa Cruz Biotechnology (Santa Cruz, CA), and peroxidase-conjugated anti-goat secondary antibody was from Dako (Glostrup, Denmark).
Experiments
Animals.
Adult male Sprague-Dawley rats, weighing 240–350 g (Harlan, Indianapolis, IN), were used. Animals were allowed to acclimate to our animal facility for at least 2 wk prior to the experiment. During this period, the animals were fed rodent chow at libitum and housed in a room with a 12:12-h light-dark cycle. Both the temperature and the humidity of the room were maintained within the range 70–74°F and 40–60%, respectively.
Lymph and duodenal cannulation.
The surgical procedure and the postoperative care have been described previously as described (16). All procedures were approved by the University of Cincinnati Internal Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. Briefly, animals were fasted overnight before surgery. Under isoflurane anesthesia, the superior mesenteric lymph duct was cannulated with soft vinyl tubing (0.8 mm OD) according to the method described (16). A drop of cyanoacrylate glue (Krazy Glue, New York, NY) was used to secure the lymph cannula. Intraduodenal (id) cannulation was performed by inserting a silicone tube (1.6 mm OD) ∼2 cm into the duodenum via a fundal incision of the stomach. The tubing was secured by a transmural suture in the duodenum, and the fundal incision was closed by a purse-string suture. Postoperatively, the animals were kept in Bollman restraining cages. Although the animals were restrained, they had considerable freedom to move forward, backward, and sideways. The restraining was necessary to prevent the animals from chewing and damaging the cannula. The animals were infused id with 5% glucose in saline (145 mM NaCl, 4 mM KCl, and 0.28 M glucose). Beginning 16 h before the nutrient study (which occurred on the following morning), the 5% glucose in saline was switched to saline alone and was infused overnight at a rate of 3 ml/h until the following morning, when the saline solution was replaced with the nutrient infusate described below. Fasting lymph was collected for 1 h before the start of the nutrient infusion. The nutrient infusate was given as a single bolus through the duodenal infusion cannula. Lymph was collected continuously at 30-min intervals during the first hour after the nutrient infusion and hourly thereafter over the remaining 5-h time course.
Preparation of nutrient infusate.
Two groups of animals were tested and infused id with a single bolus of 3 ml of normal saline (control group) or lipid, consisting of 2.215 ml of Liposyn II (20%) + 0.785 ml of saline with the caloric content of 4.43 kcal/3 ml. Following the nutrient bolus infusion, the continuous id infusion of saline was temporarily halted for 30 min to avoid overdistension of the small intestine and saline infusion continued afterward. Liposyn II 20% consists of a 50:50 blend of safflower and soybean oil with a caloric content of 2 kcal/ml. The caloric content of the full dose (4.4 kcal) of fat was equivalent to half of the total daily fat intake of the rat.
To determine the importance of the dose and chain length of fatty acids on lymphatic DAO secretion, the following groups of animals were studied. Four groups of animals were id infused different amounts of Liposyn II (0.55, 1.1, 2.2, and 4.4 kcal/3 ml) to detect the dose effect. In addition, the effect of long-chain triacylglycerol (TG) trilinolein (C18:2, n-6 TG), the major composite (65.8%) of Liposyn II, and medium-chain TG tricaprylin (C8:0) was also studied with id bolus (3 ml) infusions of phosphate-buffered, taurocholate-stabilized emulsions containing 120 μmol trilinolein or 120 μmol tricaprylin. The vehicle control emulsion contained 8.7 μmol egg phosphatidylcholine, 7.8 μmol cholesterol, and 57 μmol sodium taurocholate, sonicated in 3 ml phosphate-buffered saline (pH 6.4) (12).
To determine the role of histamine and the histamine antagonists, 10 mg/kg histamine was intraperitoneally (ip) administered into fasting rats and then lymph was collected every 10 min for 60 min. The dose of histamine (10 mg/kg) was calculated according to the previous reports (33). The four types of potent and specific HR antagonists pyrilamine maleate (H1B, 10 mg/kg), ranitidine (H2B, 8 mg/kg), thioperamide maleate (H3B, 10 mg/kg), or JNJ7777120 (H4B 37 mg/kg) were ip administered into fasting rats 30–45 min before id bolus infusion of 3 ml (4.4 kcal) Liposyn II followed by saline infusion. Then lymph samples were collected at 30-min intervals for 180 min.
DAO activity measurement.
DAO activity was measured by a radiometric assay described by Forget et al. (7) with slight modifications. Briefly, the reaction mixture consisted of a 50-μl enzyme sample and a 50-μl substrate mixture. The substrate mixture was a combination of cold and labeled putrescine prepared in 0.1 M sodium phosphate buffer at pH 7.2 with a ratio of 10 μl of [3H]putrescine per 1,000 μl of 0.9 mM unlabeled putrescine. The final concentration of substrate putrescine was 0.45 mM with the total count of ∼0.8 million dpm. The reaction mixture was then incubated at 37°C for 30 min. The reaction was stopped by the addition of 10 μl of 0.1 mM aminoguanidine, an inhibitor of DAO, followed by the addition of 50 μl sodium carbonate pH 12.2 (187.5 mM final). The reaction product Δ1-pyrroline was extracted twice with 500 μl of toluene. The radioactivity was determined by liquid scintillation counting. Activities were shown to be proportional to time and quantity of putrescine and enzyme. The DAO activities were calculated in milliunits per milliliter of lymph (mU/ml), where 1 unit oxidized 1 μmol of putrescine per hour at 37°C.
Western blot analysis.
The lymph samples (10 μl each well) were loaded onto 4–20% polyacrylamide gradient gel and electrotransferred to polyvinylidene difluoride membranes. Following transfer, the membranes were treated with blocking reagent [5% nonfat milk and 0.1% Tween 20 in Tris-buffered saline (TBS)], after which the membranes were incubated with goat anti-mouse DAO antibody (diluted 1:500) at 4°C overnight. After rinsing with TBS with 0.1% Tween 20, the membranes were further incubated with peroxidase-conjugated anti-goat secondary antibody at 1:2,000 for 1 h and then developed with ECL Western blot detection kit (GE Healthcare) according to the manufacturer's protocol and were quantified with the Total Lab Quant Analysis Software (TL100, FOTODYNE, Hartland, WI).
ELISA analysis of lymphatic histamine.
Lymph histamine levels were measured with histamine ELISA kits. Briefly, 50 μl of lymph was mixed with 50 μl of enzyme conjugate at room temperature for 45 min. After three times rinsing with 300 μl washing buffer, 150 μl of substrate was added to each well of the plate, allowing the plate to incubate at room temperature for 30 min; then the plate was read in a microplate reader with a 650-nm filter. The histamine levels were calculated according to the manufacturer's instruction.
Chemical assays of protein in lymph.
Protein concentration was measured by the Bradford method with bovine serum albumin (BSA) as a standard. Standard curves were constructed, and concentrations of the samples were calculated from the standard curves.
Statistical Analysis
The data shown are mean values ± SE. To compare groups through the 3-h or 6-h infusion, a two-way repeated-measures ANOVA was used. For comparison of data with two independent variables, a two-way ANOVA was used. A t-test was used for the rest of the analyses for comparing only two groups. Differences between treatments were considered significant when P < 0.05.
RESULTS
Effect of Duodenal Feeding of Nutrients on Lymphatic DAO Activity and DAO Output
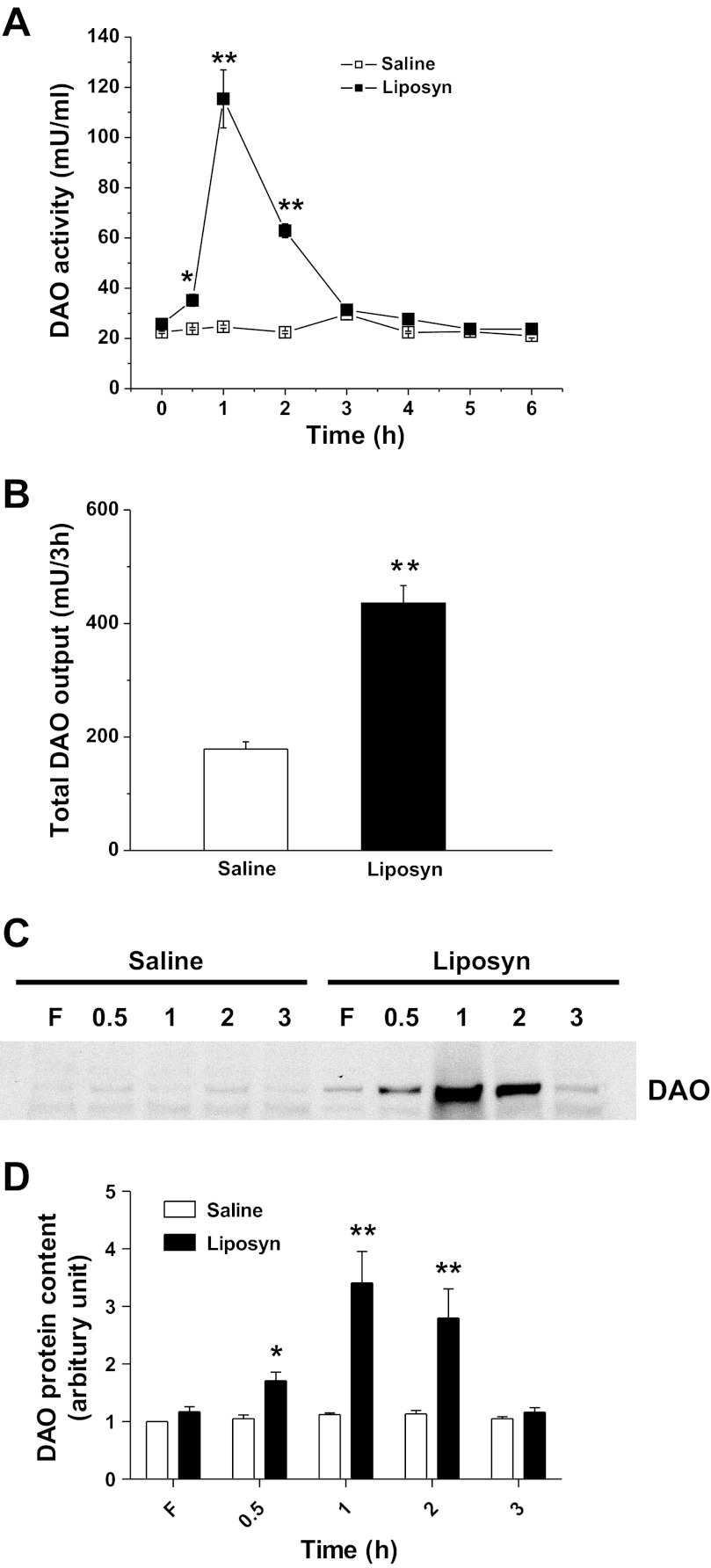
DAO activities in the fasting lymph of lipid or saline-infused rats were comparable (25.7 ± 0.8 and 22.4 ± 0.4 mU/ml, respectively; P > 0.05,). There was, however, a significant increase in DAO activity in lymph from 0.5 to 3 h after id bolus infusion of 3 ml Liposyn II (4.43 kcal) with a peak of 115.4 ± 21.6 mU/ml at 1 h, which is a ∼3.5-fold increase compared with control (n = 6, P < 0.01). Animals that received saline showed no significant alterations in lymph DAO activity throughout the entire 6 h (Fig. 1A).
Fig. 1.
Lymphatic diamine oxidase (DAO) activity (A) and total DAO output (B) after bolus intraduodenal (id) infusion of Liposyn II (4.4 kcal) or saline, n = 6. C: representative blot shows the secretion of DAO protein in lymph at fasting (F) and the time after infusion. D: quantitative data of the DAO content in lymph, n = 3. Data are expressed as means ± SE, *P < 0.05, **P < 0.01 vs. saline.
When taking into account the lymph flow rate, which was not significantly altered relative to the saline control group (data not shown), lymphatic DAO output (the product of DAO activity and lymph flow rate) during 3-h period was significantly increased in the lipid group (435.8 ± 31.0 in lipid, vs. 178.8 ± 12.9 mU in saline group, n = 6, P < 0.01) (Fig. 1B). Our data therefore indicate that a bolus infusion of lipid increases intestinal DAO activity and DAO output in lymph.
To determine whether the increased DAO activity was due to increased DAO protein secretion into lymph, we conducted a Western blot analysis. As shown in Fig. 1C, the lymphatic DAO content increased in Liposyn II-infused rats, starting at 0.5 h, peaking at 1 h, and returning to the fasting level by 3 h postinfusion, whereas no increase in DAO content was observed in the saline group. The calculation of the density of the bands in the Western blot showed a 3.7 ± 0.3-fold increase at the peak DAO secretion at 1 h (Fig. 1D), which was consistent with the extent of the increase in the lymph DAO activity. This result indicates that fat absorption induces DAO secretion into lymph, thereby increasing lymphatic DAO activity.
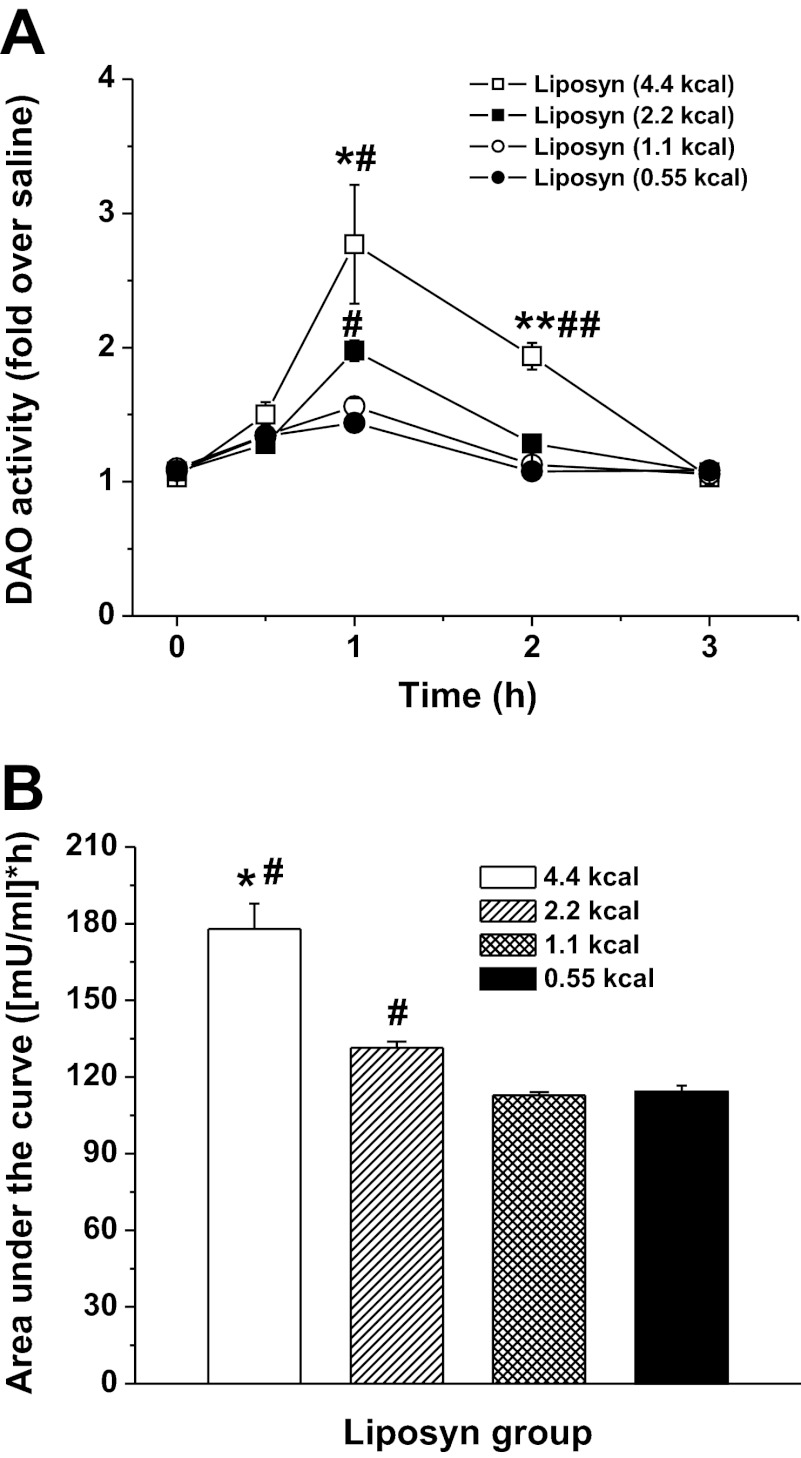
Amount of Fat Affects DAO Activity
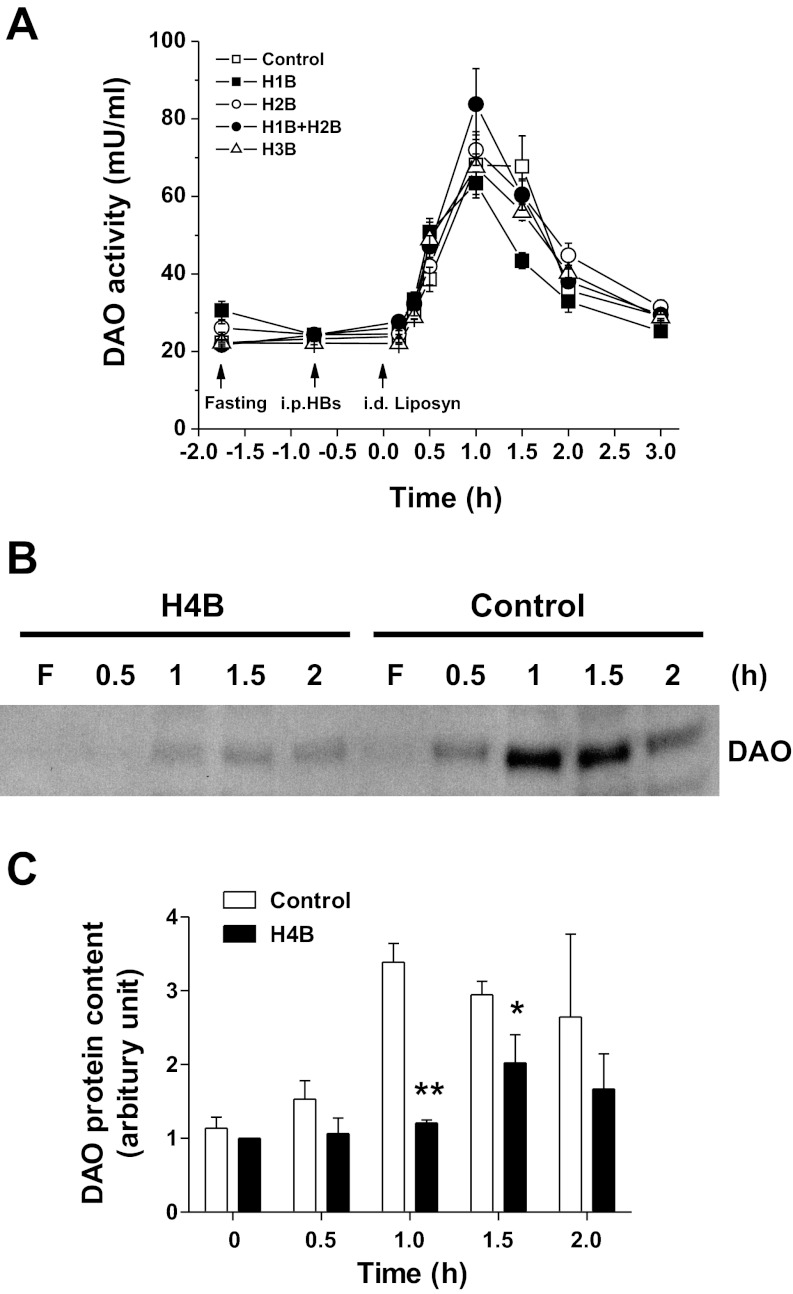
To determine the effect of the amount of fat fed on DAO activity, increasing caloric doses (0.55, 1.1, 2.2, and 4.4 kcal, all in 3 ml) of Liposyn II were infused into the duodenum. As shown in Fig. 2A, the 2.2- and 4.4-kcal doses raised the levels of lymphatic DAO activity above that of the saline control at the 1 h following lipid infusion (1.9 ± 0.2 and 2.8 ± 0.8-fold over saline, respectively). The differences in DAO activity among 4.4- vs. 2.2-, 1.1-, and 0.55-kcal doses as well as 2.2 vs. 1.1- and 0.55-kcal dose groups were all significant (P < 0.05, n = 6), showing a dose-dependent pattern (Fig. 2A). In addition, cumulative DAO secretion, calculated as the area under the curve (AUC) over the 3-h lymph collection period (Fig. 2B), increased in response to larger amounts of dietary lipid: from 114.3 ± 2.4 for the 1.1-kcal lipid dose to 177.9 ± 9.9 for the 4.4-kcal lipid dose (P ≤ 0.05), exhibiting a dose-dependent relationship.
Fig. 2.
Dose-dependent effect of Liposyn II on lymphatic DAO activity. A: responses to 4 infused lipid doses (0.55, 1.1, 2.2, and 4.4 kcal). B: area under the curves (AUC). Values are means ± SE. *P < 0.05, **P < 0.01, 4.4-kcal dose vs. the rest doses of Liposyn II, #P < 0.05, ##P < 0.01 vs. 1.1 and 0.55 kcal.
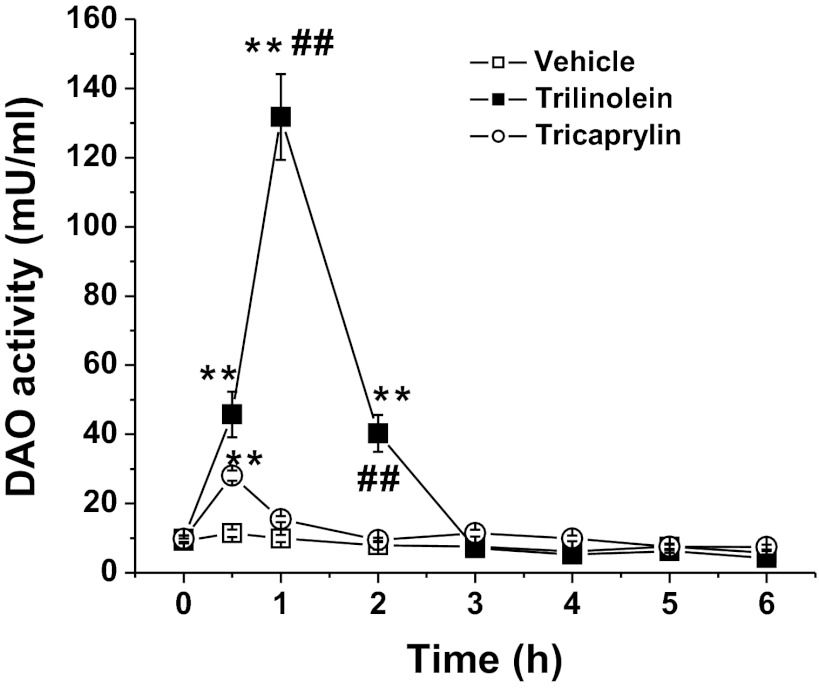
Type of Fat Affects DAO Activity
Our previous study has shown that the 18-carbon chain (C18) triolein more effectively stimulated DAO release into lymph than tricaprylin (C8), when continuously given id in equimolar amounts (34). In the present study we further examined the effect of the long-chain TG trilinolein on DAO activity because linoleic acid (C18:2, n-6) is the major component (∼65.8%) of Liposyn II. Figure 3 shows that after the id bolus infusion of 120 μmol trilinolein emulsion plus vehicle, lymphatic DAO activity significantly increased, with the peak value of 130.6 ± 41.8 mU/ml vs. the vehicle control (lipid emulsion containing phosphatidylcholine and sodium taurocholate) value of 15.5 ± 4.3 mU/ml (n = 6, P < 0.01). The DAO activity returned to fasting level by 3 h. In contrast, the bolus infusion of 120 μmol tricaprylin (C8) emulsion produced only a marginal increase in lymphatic DAO activity with a value of 35.5 ± 11.0 mU/ml (P < 0.05, vs. control, n = 6) at 1 h postinfusion (Fig. 3). The DAO output in lymph was significantly increased in trilinolein-infused rats compared with the tricaprylin group (data not shown). These results confirmed that long-chain TG (both triolein studied previous and trilinolein in this study) is more effective than medium-chain TG in inducing an increase in DAO secretion.
Fig. 3.
Comparison of the effect of long-chain triacylglycerol (TG) trilinolein with medium-chain TG tricaprylin on DAO activity. Values are means ± SE. n = 6. **P < 0.01 vs. vehicle emulsion; ##P < 0.01 vs. tricaprylin.
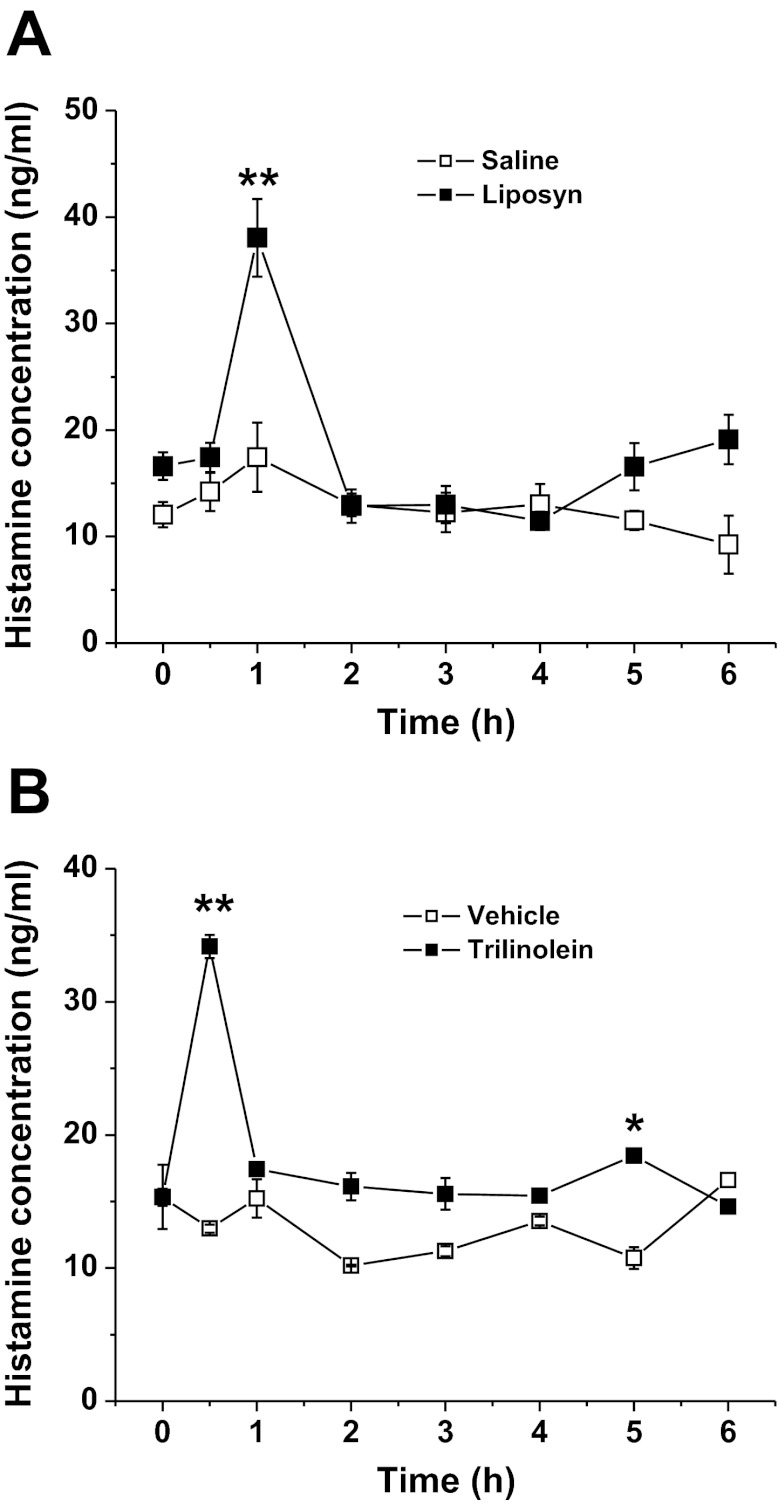
Release of Histamine into Lymph After Nutrient Infusion
We hypothesized that the infusion of a lipid meal causes the secretion of histamine, which in turn stimulate the secretion of DAO to control the unwanted effects of histamine. To test this hypothesis, we measured lymphatic histamine concentration in Liposyn II and trilinolein-infused rats. As shown in Fig. 4, A and B, a significant 1.1-fold and 1.3-fold increase of the peak histamine level was observed after Liposyn II or trilinolein infusion, respectively. The output peaked at 1 h postinfusion in both groups (38.5 ± 1.9 ng/ml in the Liposyn II group vs. 18.5 ± 3.2 ng/ml in saline group, n = 6, P < 0.01, and 35.0 ± 3.6 in the trilinolein group vs. 15.0 ± 1.6 ng/ml in vehicle control rats, P < 0.05). Our data demonstrate that id infusion of Liposyn II containing mainly n-6 long-chain fatty acids (LCFA), or pure trilinolein induces histamine release into lymph. This data is suggestive of the induction of DAO release by histamine, which in turn is tied to active intestinal fat absorption.
Fig. 4.
Lymphatic histamine concentrations after id infusion of Liposyn II (4.4 kcal) (A) or 120 μmol trilinolein (B). *P < 0.05, **P < 0.01 vs. saline or vehicle, n = 6.
Effect of Histamine on Lymphatic DAO Activity
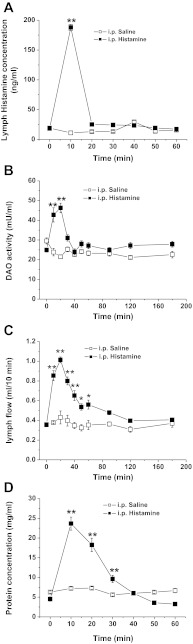
To address the question of whether histamine is a mediator of DAO secretion, we examined the effect of histamine on lymphatic DAO activity by ip injection of histamine (10 mg/kg) into fasting rats. As shown in Fig. 5A, 10 mg/kg ip histamine resulted in a quick increase in the lymphatic histamine level up to ∼200 ng/ml at 10 min and a subsequent decline to baseline by 20 min. At the same time, ip histamine substantially increased the lymphatic DAO activity by 1.1-fold. The increase lasted until 30 min postinjection with a peak at 20 min (Fig. 5B). Our data suggest a close relationship between the increase of lymphatic histamine and DAO activity. It is noteworthy that the effect of ip histamine on DAO activity is transient; after 30 min when the lymph histamine concentration returned to the basal level, lymphatic DAO activity was also restored to the baseline (Fig. 5B).
Fig. 5.
Effect of intraperitoneal (ip) injection of histamine into fasting rats on lymphatic histamine concentration (A), DAO activity (B), lymph flow rate (C), and protein concentration (D). *P < 0.05, **P < 0.01 vs. saline, n = 6.
The effectiveness of ip histamine was confirmed by dramatic elevations in mesenteric lymph flow rate and lymphatic protein transport. As shown in Fig. 5, C and D, the maximal lymph flow rate and the peak lymphatic protein concentration increased by 1.36-fold and 6.43-fold at 20 and 10 min after ip histamine, respectively. Our result confirms the increase in vascular permeability by histamine and suggests that it mediates the secretion of DAO.
Effect of Histamine Receptor Antagonists on Lymphatic DAO Activity
To further examine whether endogenous histamine mediates DAO secretion during fat absorption, four types of specific HR blockers [pyrilamine (H1B), ranitidine (H2B), thioperamide (H3B) or JNJ 7777120 (H4B)] were ip injected at 30–45 min before id infusion of Liposyn II (4.4. kcal/3 ml) into rats, based on the reports that all four types of HRs are distributed along GI tract in rats (5, 11). As shown in Fig. 6A, neither pyrilamine nor ranitidine nor thioperimide, nor pyrilamine plus ranitidine, inhibited the lipid-induced increase of DAO activity. The H4B JNJ 7777120, however, significantly reduced the DAO activity, the AUC over 3 h following lipid infusion was reduced by 65.9% from 827.9 ± 15.6 to 282.1 ± 7.9 mU·ml−1·h (P < 0.01, n = 6). Western blot analysis further confirmed that the ip H4B decreased the lymph DAO secretion by ∼64.3 and 33.3% at 1 and 1.5 h after lipid infusion, respectively (Fig. 6, B and C). These results indicate that histamine, through H4R, is involved in mediating fat-induced DAO secretion into lymph.
Fig. 6.
Effect of ip histamine receptor blockers (HB) on Liposyn II-induced lymph DAO activity. A: lymph DAO activity after ip H1B–H3B; n = 6. B: representative blot shows the DAO content in lymph at fasting and after ip H4B followed by the infusion of Liposyn II. C: quantitative data of the DAO secretion in lymph. Means ± SE, *P < 0.05, **P < 0.01 vs. control.
Effect of Lipid Infusion and Histamine Receptor Antagonists on Lymph Flow Rate and Protein Transport
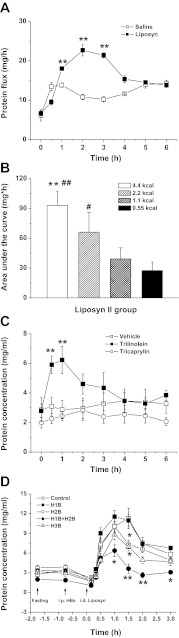
The lymph flow rates were not significantly altered in Liposyn II-infused rats, nor in trilinolein and tricaprylin-infused rats and in the HRs antagonist-administered groups (data not shown), suggesting a complex regulation of lymph flow rate, in which histamine may not be the key factor. On the contrary, id Liposyn II caused a significant increase in lymph protein concentrations as well as protein flux (a product of lymph protein concentration and lymph flow rate), starting at 1 h, peaking at 2 h, and then gradually declining by 4 h following lipid infusion (Fig. 7A). In addition, the lymphatic protein flux over 3 h following lipid infusion showed a significant dose-dependent increase from 27.5 ± 8.6 mg·h in 0.55 kcal to 93.0 ± 13.9 mg·h (P < 0.01, n = 5–6) in the 4.4-kcal lipid-infused group (Fig. 7B). Furthermore, infusion of trilinolein induced a significant increase in lymphatic protein transport, whereas tricaprylin did not (Fig. 7C). These data indicate that the absorption of Liposyn II, mainly containing linoleic acid, increases lymphatic protein transport in a dose-dependent manner.
Fig. 7.
Lymphatic protein flux after id bolus infusion of Liposyn II (4.4 kcal) (A); AUC of different doses of Liposyn II (0.55, 1.1, 2.2, 4.4 kcal) (B). Lymphatic protein concentrations after infusion of long-chain TG trilinolein or medium-chain TG tricaprylin (C) or after ip H1B, H2B, H1B+H2B, and H3B followed by Liposyn II infusion (D). Means ± SE, n = 6, *P < 0.05, **P < 0.01 vs. controls, #P < 0.05, ##P < 0.01 vs. 1.1 and 0.55 kcal.
Histamine, via increasing the intestinal vascular permeability (9, 21), increases lymph protein transport, as shown in Fig. 5D. To determine the participation of histamine in the increased lymph protein transport during fat absorption, HR antagonists were ip administered before id infusion of Liposyn II. As shown in Fig. 7D, ip pyrilamine (H1B) or ranitidine (H2B), or thioperamide (H3B) or JNJ 7777120 (H4B) (data not shown) did not significantly alter the lymph protein concentrations nor the protein flux in response to lipid infusion, except for a 35% (P < 0.05, n = 6) and a 39% (P < 0.05, n = 6) reduction at 90 min after lipid infusion in ranitidine or thioperimide pretreated rats. However, a significant 67.8% (P < 0.01, n = 6) reduction of lymph protein concentrations was observed in pyrilamine (H1B, 10 mg/kg ip) plus ranitidine (H2B, 8 mg/kg) administered animals (Fig. 7D). Our data demonstrate that lymphatic protein transport increases in response to fat absorption; histamine, via increasing the intestinal vascular permeability, may play a role in the regulation of fat-induced lymph protein transport through additive effects on H1R and H2R. These data are consistent with the previous finding showing that histamine H1R antagonists pyrilamine plus H2R antagonists burimamide prevented intestinal lymph protein transport during id olive oil feeding (32). Thus the histamine receptor (H4R) involved in DAO secretion stimulated by fat absorption is different from the histamine receptors (H1R+ H2R) involved in the increase in lymph protein flux.
DISCUSSION
In the present study, using the conscious lymph fistula rat model, we demonstrated that fat absorption induces histamine release as well as DAO secretion in mesenteric lymph. The basal level of plasma DAO is very low (25). The mesenteric lymph collected in our experiment had not entered the circulation. Therefore, the DAO in the mesenteric lymph, which had not been metabolized by the liver, directly reflects the secretion of intestinal DAO. In addition, histamine appears to be rapidly metabolized in the circulation because its half-life is estimated at <1 min (24). The measurement of both molecules using mesenteric lymph provides a more direct way to study the in vivo secretory function of intestinal villi. The released histamine induced by fat absorption in turn stimulates the release of DAO through the H4R. This is a first demonstration of the release of histamine being linked to the release of DAO by fat absorption, but the physiological function of this link is far from clear.
It is well known that fat absorption in the small intestine induces a series of physiological reactions including increases in mesenteric blood flow (1, 6) and vascular permeability (9, 21) as well as alterations in intestinal motility (15). These changes may involve the participation of the whole neural-hormonal-immune network including vagal nerves, enteric neurons, GI hormones such as cholecystokinin, neurotensin, and GLP-1 (9, 22), as well as the intestinal immune system (10, 19). Our present study demonstrated that histamine is released during fat absorption, suggesting that histamine may play a role in this process. Since gut histamine is mostly located in intestinal mast cells (23), the histamine released during fat absorption may originate from the intestinal MMC. This hypothesis is supported by our recent studies in which we demonstrated that the intestinal MMC are activated after id infusion of Liposyn II, causing a peak of ∼20-fold increase in rat MMC protease II (RMCPII), a specific marker of MMC degranulation, in intestinal lymph at 1 h after lipid infusion (10).
The role of histamine during fat absorption is probably related to its vasoactive properties. Histamine increases both mesenteric blood flow and vascular permeability (9, 21, 32). This vasoactive effect of histamine is further confirmed in the present study, in which ip histamine increased both lymph flow rate and lymph protein influx (Fig. 5, C and D). However, in response to the bolus lipid infusion, no significant increase in lymph flow rate was observed, nor was it decreased after pretreatment with ip HR antagonists. This apparent lack of response may be related to our experimental design. Considering that bolus infusion of 3 ml of the emulsion may cause overdistension of the small intestine, we temporarily stopped the saline infusion (3 ml/h) after id bolus for 30 min before resuming. Despite the lack of the effect on lymph flow, the significant increase in lymph protein transport in response to Liposyn II infusion clearly hints the involvement of histamine in regulating vascular permeability. The significant inhibition of the lymph protein flux in response to the administration of H1R and H2R blockers (Fig. 7D) supports this view. The physiological significance of the elevation of the lymphatic protein transport during fat absorption is unclear. It has been reported that the integrity of the intestinal epithelium is compromised during fat absorption (13, 30). In the present study, we showed that it is trilinolein, but not tricaprylin, that increased protein transport in lymph, further indicating that LCFA rather than medium-chain fatty acids (MCFA) induce protein influx into lymph. LCFA are mostly transported as chylomicron (CM) in lymph, whereas the majority of MCFA is transported via the portal circulation (29). Dietary fats (mainly LCFA) are digested and absorbed by the enterocytes and packaged into CM. The LCFA containing CMs are then transported into the expanded intercellular space. The basement membrane of the intestinal villi is obviously a barrier to the passage of the CM from the intercellular space to the lamina propria. We have previously shown that CM travel into the lamina propria by breakages of the basement membrane (30). It is therefore plausible to assume that histamine induces an increase in the hydration (expansion) of the interstitial matrix in the lamina propria by increasing vascular permeability, thus facilitating the diffusion the CM particles into the central lacteals (31). Histamine may also participate in the gut mucosal repair during fat absorption as it does during intestinal ischemia-reperfusion-induced injury (8). Our previous study revealed that the jejunal epithelium is temporarily injured and the “injury” is presumably repaired rapidly during fat absorption (13). It is conceivable that the role of histamine in healing the ischemia-reperfusion-induced mucosal damage may also apply to the temporary mucosal damage induced by fat absorption.
Although histamine certainly has important physiological function during fat absorption, it is equally important to have an efficient system to deactivate it to avoid unwanted side effects. Studies have shown that unwanted or excessive histamine is detrimental and can lead to a number of pathophysiological conditions such as anaphylaxis, food allergy, and IBD (17, 26, 35). Enzymatic inactivation of circulating histamine is exclusively mediated by the degrading enzyme DAO. Our previous and present studies demonstrate that in the rat, id infusion of lipid, either continuously (34) or as a bolus, induces a significant increase of DAO secretion into intestinal lymph. Given the fact that DAO is synthesized and stored in enterocytes (3), it is conceivable that the intestinal mucosa contributes to the large increase in enzyme activity in the intestinal lymph. The rapid DAO release within 1 h in our present study probably reflects the release of the stored DAO rather than an increase in DAO synthesis. In addition, we have found that the lymphatic DAO activity (a measure of the secretion) depends on the amount fed and also types of fat infused: long-chain TG (trilinolein) promotes a significant release of histamine than medium-chain TG (tricaprylin). We propose that CM transport plays a role in DAO release by the intestinal mucosa and the purpose is to maintain optimal histamine concentration to mediate its many roles in intestinal fat absorption.
Lastly, in this study, we found that lymphatic histamine and DAO both peaked at the same time during fat absorption, suggesting a potential close relationship between the two. The present observation (Fig. 5B) that ip histamine (10 mg/kg), similar to id histamine (0.15–1.2 mmol/kg) administration in a previous study (33), induced the release of DAO into intestinal lymph further confirmed this close relationship, suggesting that histamine could be an endogenous mediator in the secretion of DAO to safeguard against deleterious effects of the excessive histamine secretion during fat absorption. Another interesting observation of this study is the particular histamine receptor involved in DAO release. All four classes of HRs (H1R, H2R, H3R, and H4R) are present in the rat small intestine (5, 14), distributing in intestinal musculature, mucosal epithelium, enteric nervous system, and immune/inflammatory cells (5). In this study, we found that only the H4R antagonist, but not the other three HR blockers, inhibited fat-induced DAO secretion. The underlying mechanism of how activation of H4R regulates the fat-induced secretion of DAO is unknown. Recent studies revealed that H4R is primarily expressed on inflammatory/immune cells including eosinophils, mast cells, basophils, dendritic cells, and T cells (5). H4R expression is also present in the myenteric neurons of the rodent GI tract (20), but there is no report on rat enterocytes. The direct connection between H4R and DAO secretion by the enterocytes in response to lipid warrants further studies.
In conclusion, we have reported a number of interesting observations in this study. First, we demonstrated that histamine release is stimulated by fat absorption and this in turn stimulates the release of DAO. Second, this action of histamine is mediated through the H4R, which is different from the role of increasing protein flux mediated by the H1R and H2R. Histamine, thus, may play an important role in the CM trafficking from the intercellular space to the lamina propria and then subsequently into the lacteals during fat absorption. The DAO secreted during fat absorption may act as deamination mechanism to counterbalance an excess of histamine to exert unwanted side effects. It is tempting to speculate that an imbalance between the histamine-DAO system may be involved in intestinal disorders such as inflammatory bowel disorder.
GRANTS
This work was generously supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1-DK-056910 and DK-092138 to P. Tso.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.J. conception and design of research; Y.J., Y.S., X.L., C.Z., Q.Y., and M.X. performed experiments; Y.J., Y.S., X.L., and C.Z. analyzed data; Y.J. and Y.S. interpreted results of experiments; Y.J. and Y.S. prepared figures; Y.J. drafted manuscript; Y.J., A.W., W.L., and P.T. edited and revised manuscript; Y.J., W.L., and P.T. approved final version of manuscript.
REFERENCES
- 1. Aguilar-Nascimento JE. The role of macronutrients in gastrointestinal blood flow. Curr Opin Clin Nutr Metab Care 8: 552–556, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Boden SR, Burks AW. Anaphylaxis: a history with emphasis on food allergy. Immunol Rev 242: 247–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daniele B, Quaroni A. Polarized secretion of diamine oxidase by intestinal epithelial-cells and its stimulation by heparin. Gastroenterology 99: 1675–1687, 1990 [DOI] [PubMed] [Google Scholar]
- 4. DeVault KR, Talley NJ. Insights into the future of gastric acid suppression. Nat Rev Gastroenterol Hepatol 6: 524–532, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Dunford PJ, Varga C, Thurmond RL, Whittle BJ. Histamine H4 receptor antagonism attenuates toll-like receptor signaling and inhibits experimental colitis in the rat. Gastroenterology 130: A689, 2006 [Google Scholar]
- 6. Fara JW, Rubinstein EH, Sonnenschein RR. Intestinal hormones in mesenteric vasodilation after intraduodenal agents. Am J Physiol 223: 1058–1067, 1972 [DOI] [PubMed] [Google Scholar]
- 7. Forget P, Grandfils C, Vancutsem JL, Dandrifosse G. Diamine oxidase and disaccharidase activities in small intestinal biopsies of children. Pediatr Res 18: 647–649, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Fujimoto K, Imamura I, Granger DN, Wada H, Sakata T, Tso P. Histamine and histidine-decarboxylase are correlated with mucosal repair in rat small-intestine after ischemia-reperfusion. J Clin Invest 89: 126–133, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Granger DN, Korthuis RJ, Kvietys PR, Tso P. Intestinal microvascular exchange during lipid absorption. Am J Physiol Gastrointest Liver Physiol 255: G690–G695, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Ji Y, Sakata Y, Yang Q, Li XM, Xu M, Yoder S, Langhans W, Tso P. Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Gastrointest Liver Physiol 302: G1292–G1300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones BL, Kearns GL. Histamine: new thoughts about a familiar mediator. Clin Pharmacol Ther 89: 189–197, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Kalogeris TJ, Fukagawa K, Tso P. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J Lipid Res 35: 1141–1151, 1994 [PubMed] [Google Scholar]
- 13. Kvietys PR, Specian RD, Grisham MB, Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol Gastrointest Liver Physiol 261: G384–G391, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Leurs R, Brozius MM, Smit MJ, Bast A, Timmerman H. Effects of histamine H-1-receptor, H-2-receptor and H-3 receptor selective drugs on the mechanical-activity of guinea-pig small and large-intestine. Br J Pharmacol 102: 179–185, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin HC, Chen JH. Slowing of intestinal transit by fat depends on an ondansetron — sensitive, efferent serotonergic pathway. Neurogastroenterol Motil 15: 317–322, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol 294: G1130–G1138, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr 85: 1185–1196, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Maintz L, Schwarzer V, Bieber T, van der Ven K, Novak N. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Hum Reprod Update 14: 485–495, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Miura S, Tsuzuki Y, Hokari R, Ishii H. Modulation of intestinal immune system by dietary fat intake: relevance to Crohn's disease. J Gastroenterol Hepatol 13: 1183–1190, 1998 [PubMed] [Google Scholar]
- 20. Morini G, Shenton FC, Chazot PL, Grandi D. Immunolocalization of histamine H-4 receptor in the rat gastrointestinal tract. Gastroenterology 132: A226–A227, 2007 [Google Scholar]
- 21. Mortillaro NA, Granger DN, Kvietys PR, Rutili G, Taylor AE. Effects of histamine and histamine-antagonists on intestinal capillary permeability. Am J Physiol Gastrointest Liver Physiol 240: G381–G386, 1981 [DOI] [PubMed] [Google Scholar]
- 22. Qin XF, Shen H, Liu M, Yang Q, Zheng SQ, Sabo M, D'Alessio DA, Tso P. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol 288: G943–G949, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Rangachari PK. Histamine: mercurial messenger in the gut. Am J Physiol Gastrointest Liver Physiol 262: G1–G13, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Rizell M, Naredi P, Lindner P, Hellstrand K, Sarno M, Jansson PA. Histamine pharmacokinetics in tumor and host tissues after bolus-dose administration in the rat. Life Sci 70: 969–976, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Rokkas T, Vaja S, Murphy GM, Dowling RH. Postheparin plasma diamine oxidase in health and intestinal disease. Gastroenterology 98: 1493–1501, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Schwelberger HG. Histamine intolerance: a metabolic disease? Inflamm Res 59: 219–221, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Schwelberger HG, Hittmair A, Kohlwein SD. Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy Inflamm Res 47: S60–S61, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Shaff RE, Beaven MA. Turnover and synthesis of diamine oxidase (Dao) in rat tissues. Studies with heparin and cycloheximide. Biochem Pharmacol 25: 1057–1062, 1976 [DOI] [PubMed] [Google Scholar]
- 29. Sigalet DL, Winkelaar GB, Smith LJ. Determination of the route of medium-chain and long-chain fatty acid absorption by direct measurement in the rat. JPEN J Parenter Enteral Nutr 21: 275–278, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol Gastrointest Liver Physiol 250: G715–G726, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Tso P, Barrowman JA, Granger DN. Importance of interstitial matrix hydration in intestinal chylomicron transport. Am J Physiol Gastrointest Liver Physiol 250: G497–G500, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Wollin A, Jaques LB. Blocking of olive oil induced plasma-protein escape from intestinal circulation by histamine antagonists and by a diamine oxidase releasing agent. Agents Actions 6: 589–592, 1976 [DOI] [PubMed] [Google Scholar]
- 33. Wollin A, Navert H. Release of intestinal diamine oxidase by histamine in rats. Can J Physiol Pharmacol 61: 349–355, 1983 [DOI] [PubMed] [Google Scholar]
- 34. Wollin A, Wang XL, Tso P. Nutrients regulate diamine oxidase release from intestinal mucosa. Am J Physiol Regul Integr Comp Physiol 275: R969–R975, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Xie H, He SH. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J Gastroenterol 11: 2851–2857, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]