Abstract
We identified α-smooth muscle actin (α-SMA)- and vimentin-expressing spindle-shaped esophageal mesenchymal cells in the adult and neonate murine esophageal lamina propria. We hypothesized that these esophageal mesenchymal cells express and secrete signaling and inflammatory mediators in response to injury. We established primary cultures of esophageal mesenchymal cells using mechanical and enzymatic digestion. We demonstrate that these primary cultures are nonhematopoietic, nonendothelial, stromal cells with myofibroblast-like features. These cells increase secretion of IL-6 in response to treatment with acidified media and IL-1β. They also increase bone morphogenetic protein (Bmp)-4 secretion in response to sonic hedgehog. The location of these cells and their biological functions demonstrate their potential role in regulating esophageal epithelial responses to injury and repair.
Keywords: myofibroblast, bone morphogenetic protein-4, interleukin-6, interleukin-1β
the mucosal complications of gastroesophageal reflux range from ulcerations and strictures, to Barrett's esophagus (BE) and adenocarcinoma (4, 18, 23). Gastric acid, pepsins, and bile acids are among the primary injurious components of gastroesophageal refluxate (13, 15). Esophageal mucosal injury is thought to be at least partially mediated by loss of epithelial integrity in response to these injurious luminal agents and the subsequent infiltration of immune cells and nonspecific release of inflammatory mediators (9, 18). Cytokines elaborated by epithelial keratinocytes also contribute to the inflammatory process (9). Once the defensive barrier of the esophageal squamous epithelium is breached, it is postulated that the underlying lamina propria is exposed and that stromal cells are directly exposed to injurious luminal agents (7). In addition to inflammatory cytokines, the reactivation of developmental pathways [e.g., bone morphogenetic protein (Bmp) and Hedgehog pathways] has also been implicated in the pathogenesis of esophagitis and BE (14, 26). Ultimately, however, the cellular and molecular mechanisms underlying esophageal mucosal injury and in particular the contribution of stromal cells to the process remain incompletely understood (10, 19).
Mesenchymal cells such as the myofibroblast are well-characterized in the intestine, and their participation in tissue injury, inflammation, and repair has been described extensively (24). We have previously demonstrated that modulation of colonic myofibroblast secretion of soluble mediators of inflammation and injury may result in protection from colitis and colitis-associated cancer (21). Based on these findings, we hypothesized that mesenchymal/stromal cells with morphological and functional similarities to intestinal myofibroblasts may be present in the esophageal mucosa and that they may participate in the esophageal mucosal response to injury. In the setting of a breach of the squamous epithelium, these cells are potential responders to agents typically first encountered by the squamous epithelium (e.g., acid) as well to mediators elaborated by stimulated squamous epithelium [e.g., IL-1β (18), sonic hedgehog (SHH, see Ref. 26)] or surrounding immune cells. We hypothesized that these stromal cells contribute to the production of cytokines and signaling mediators implicated in the pathogenesis of esophagitis.
We examined whole murine esophagus and identified spindle-shaped stromal cells scattered throughout the lamina propria subjacent to the squamous epithelium. We demonstrate that a subset of these cells coexpress the proteins α-smooth muscle actin (α-SMA) and vimentin. To further define the role of these cells, we isolated them using methods previously established for our colonic myofibroblasts (21). Characterization of primary cultures of these cells demonstrates α-SMA and vimentin coexpression and absence of expression of hematopoietic and endothelial markers. Evaluation of stimulated cells demonstrates IL-6 and Bmp-4 secretion. These findings suggest that esophageal stromal cells are participants in the esophageal mucosal response to injury.
MATERIALS AND METHODS
Animals.
C57Bl/6J mice were housed in a 12:12-h light-dark cycle with free access to food and water. Mice were fed a standard rodent chow diet (PicoLab 20; Purina). All animal experimentation was approved by the Animal Studies Committee of Washington University School of Medicine.
Histology.
Esophagi were removed in total from 10-day-old (neonate) and 4- to 6-mo-old C57Bl/6J male mice and processed for histology.
Immunohistochemistry.
α-SMA was detected using a polyclonal rabbit anti-α-SMA (1:100 dilution, ab5694; Abcam). Antigen retrieval for α-SMA was performed with Diva Decloaker reagent (BIOCARE) on formalin-fixed, paraffin-embedded tissues. Antigen-antibody complexes were detected with biotinylated goat anti-rabbit IgG for SMA, streptavidin-horseradish peroxidase (1:1,600 dilution; Jackson Laboratories), and diaminobenzidine.
Immunofluorescence of paraffin-embedded sections.
Immunofluorescence costaining for α-SMA and vimentin was also performed on paraffin-embedded sections of murine esophagus. Slides were deparaffinized and rehydrated. Antigen retrieval was done with Diva Decloaker (Biocare) in a pressure cooker (18 psi for 3 min at 99°F). Polyclonal rabbit α-SMA and polyclonal goat vimentin were applied at a cocktail overnight at 4°C. An anti-rabbit TritC 1:200 (Jackson labs) and anti-goat FitC 1:200 (Jackson Labs) cocktail were applied for 1 h at room temperature. Mounting media containing DAPI were used (Vector).
Generation of primary cultures of esophageal stromal cells.
Esophageal stromal cell primary cultures were established from wild-type 8- to 12-day-old C57BL/6 mice via modification of the procedure previously established and described (21) for colonic myofibroblast isolation. The entire esophagus was placed in Hanks' balanced salt solution (HBSS; Invitrogen), washed briefly, transferred into fresh HBSS, and minced into 2- to 3-mm pieces. Tissue was washed eight times with HBSS by vigorous shaking, allowing for fragments of esophagus to sediment out. Tissue was then incubated with 300 U/ml collagenase XI (Sigma-Aldrich) and 0.1 mg/ml dispase (GIBCO, Invitrogen) for 25 min at room temperature with gentle shaking. After 25 min, tissue was further minced and then centrifuged at low speed (200 g) for 10 min. Supernatant was discarded. The pellet, consisting of a mixed cell suspension, was transferred to a 1.7-ml tube and washed five times in DMEM (GIBCO, Invitrogen) with 2% sorbitol (Sigma-Aldrich) to eliminate nonviable cells and debris. Cells were seeded in six-well plates and cultured in DMEM with 10% FBS (U.S. Biotechnologies), 10 mg/ml insulin (Sigma-Aldrich), 10 μg/ml transferrin (Roche), 10 μg/ml gentamicin (Calbiochem EMD Chemicals), and 2 ng/ml epidermal growth factor (Sigma-Aldrich). The media described above were used in our previous studies with colonic myofibroblasts and are therefore called myofibroblast media. Once wells were 80% confluent, adherent cells were passaged to T25 flasks using 0.05% trypsin in 0.53 mM EDTA (Cellgro, Mediatech). In our experience with colonic myofibroblasts, epithelial cells do not survive under these culture or passage conditions. Morphology was examined with an inverted microscope to ensure spindle-shaped morphology. Cells used for characterization and in the studies described below were between passages 5 and 15.
Immunocytochemistry.
Primary cultures of esophageal stromal cells were grown on chamber slides (Nalge Nunc International) and fixed in cold methanol for 5 min, then stored in cold PBS at −20°C. After incubation in blocking solution containing 5% BSA and 3% milk in Tris buffer (20 mM Tris·HCl; 150 mM NaCl, pH 7.4; and 0.05% Tween 30) for 60 min, slides were incubated with primary antibody diluted in blocking solution at 4°C overnight. To confirm the stromal cell phenotype, rabbit polyclonal antibodies to α-SMA (ab5694, 1:200 dilution; Abcam), desmin (D8281, 1:100 dilution; Sigma-Aldrich), cytokeratin 18 (1:100 dilution; Millipore), and vimentin (V4630, 1:100 dilution; Sigma-Aldrich) were used. Secondary antibody labeled with fluorescein diluted in blocking solution was applied for 1 h at room temperature. Cells were mounted in Vectashield Mounting Medium with DAPI and examined under a fluorescence microscope (Axioshop 2; Carl Zeiss Jena).
Flow cytometric analysis.
Spleens were removed from mice and disrupted by mechanical dissociation. Single cell suspensions of spleen and primary cultures of esophageal stromal cells were used for flow cytometric analysis. Antibodies used for analysis were anti-mouse anti-α-SMA antibody (Abcam), anti-mouse CD-45 (clone 30.F11; eBioscience), and appropriate isotype controls. Data acquisition was performed as previously described (25) on a FACScan cytometer (BD Biosciences) retrofitted with a second laser using CellQuest (BD Biosciences) and Rainbow (Cytek) software. A Macintosh G4 computer running FlowJo software (Tree Star) was used to perform data analysis. Forward and side light scatter and staining with 7-amino-actinomycin (eBioscience, San Diego, CA) were used to exclude dead cells and to distinguish live cellular populations. Gates for positive staining were defined such that 1% of the analyzed population stained positive with the appropriate isotype control antibodies.
RT-PCR: RNA analysis by qRT-PCR.
qRT-PCR was performed as described previously (19) for α-SMA, vimentin, desmin, cytokeratin, von Willebrand factor (vWF), Bmp-4, Bmp-2, cyclooxygenase-2 (Cox-2), KC (murine homolog of IL-8), and IL-6. SuperScript II Reverse Transcriptase (Invitrogen) was used for synthesis of cDNA. Real-time RT-PCR analysis using SYBR green PCR Master Mix (Applied Biosystems) was performed in an ABI Prism 7700 sequence detection system (Applied Biosystems). All primers were obtained from Integrated DNA Technologies. Primer sequences are listed in Table 1.
Table 1.
•••
| Gene | Forward | Reverse |
|---|---|---|
| 18S | TCG AGG CCC TGT AAT TGG AA | CCC TCC AAT GGA TCC TCG TT |
| α-sma | GTC CCA GAC ATC AGG GAG TAA | TCG GAT ACT TCA GCG TCA GGA |
| Vimentin | CGG CTG CGA GAG AAA TTG C | CCA CTT TCC GTT CAA GGT CAA G |
| Desmin | GTG GAT GCA GCC ACT CTA GC | TTA GCC GCG ATG GTC TCA TAC |
| Cytokeratin 8 | TGT CTA CTC GGT CGG ACT TCT | GCT GCT ACC TAG CTG ACA TGC |
| Bmp4 | TGG GCT GGA ATG ATT GGA TT | CAG TCC CCA TGG CAG TAG AAG |
| Bmp2 | CCA AAA TCC CTA AGG CAT GCT | TTC ATT TTC ATC TAG GTA CAA CAT GGA |
| Follistatin | CCT AGG GAA AGT GTA TCA CAA AG | TGG AAT CCC ATA GGC ATT TTT T |
| Noggin | GCC AGC ACT ATC TAC ACA TCC | GCG TCT CGT TC GAT CCT TCT C |
| Chordin-1 | CAA TAA CAA GCA CAA ACA TGG ACA A | TAG ATT TGG GTG CCA GGA CTC T |
| Gremlin-2 | GCT TCC ATC TGC TCA TTG CA | GTT CTT CCG TGT TTC AGC TAC CTT |
| Cox-2 | AAG GAA CTC AGC ACT GCA TCC | ACA GGG ATT GGA ACA GCA AGG A |
| KC | CAC TAG GTT TGC CGA GTA GAT CTC | TTC TCT GTG CAG CGC TGC TG |
| IL-6 | CCA GAA ACC GCT ATG AAG TTC CT | CAC CAG CAT CAG TCC CAA GA |
| vWF | ATC CGC GTG GCA GTG GTA | CGC TTC CGG GCC TTG |
α-sma, α-Smooth muscle actin; Bmp, bone morphogenetic protein; Cox-2, cyclooxygenase-2; vWF, von Willebrand factor.
Treatment of esophageal stromal cells with acidified media.
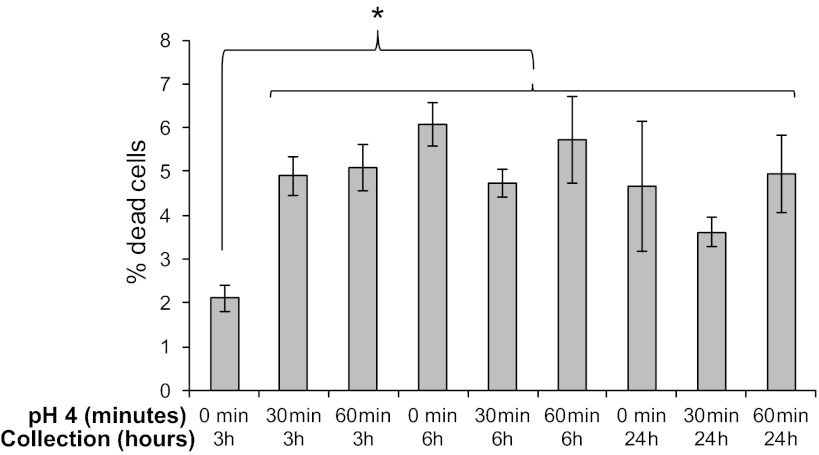
Stromal cells were seeded at the same density in six-well plates (100,000/well) and grown to 85% confluence. Cultured stromal cells were incubated with myofibroblast media acidified to pH 4 by addition of 0.1 N HCl (11) for 3, 10, 30, and 60 min, and RNA expression of inflammatory cytokines was assessed after 3, 6, and 24 h. Based on these experiments, it was determined that cells responded maximally after 30 and 60 min of treatment. Cell viability after acid treatment was assessed by aspirating the media and counting cells shed into the medium. After treatment with acidic media for 30 and 60 min, cells remained adherent to culture dishes at 3, 6, and 24 h. Cells treated with acidified media for 30 and 60 min, along with their untreated controls, were also evaluated for cell death by Trypan blue exclusion after 3, 6, and 24 h in culture. The average percentage of dead cells in untreated cells after 3 h in culture was 2.1 vs. 5.0% after treatment with acidified media for 30 and 60 min (P < 0.05). The percentage of observed dead cells was not significantly different in untreated and treated cells after 6 and 24 h in culture. After treatment with acidified media, cells were washed with PBS, and fresh media were added. Cells were cultured for an additional 3, 6, and 24 h in serum-free media, and supernatants were collected for IL-6 and Bmp-4 measurement. Each time point had a serum-free nonacidified myofibroblast media control on the same plate. Supernatant was collected for enzyme-linked immunosorbent assay (ELISA) after 3, 6, and 24 h. Cells were harvested for RNA and qRT-PCR for IL-6, IL-8, and Cox-2 and for protein using parallel cultures. Acidified media induced secretion of IL-6, and Bmp-4 was determined with ELISA. qRT-PCR data were expressed as fold induction relative to untreated samples.
Treatment of esophageal stromal cells with IL-β.
Esophageal stromal cells plated as described above were treated with 100, 5,000, and 10,000 U/ml IL-β (R&D) in serum-free myofibroblast media for 24 h. Media were collected for evaluation of IL-6 and Bmp-4 by ELISA.
Treatment of esophageal stromal cell primary cultures with SHH.
Stromal cells were seeded in 12-well plates (100,000 cells/well) and grown to 70% confluence. Cultured stromal cells were incubated with recombinant mouse SHH NH2-terminus (1.5 μg/ml; R&D) diluted in serum-free media for 24 h. SHH-containing media was replaced with fresh media, and conditioned media were collected at 24 and 48 h to evaluate for Bmp-4 with ELISA (Bmp-4 duoset; R&D).
Statistics.
Data comparing groups are presented as means ± SE, and significance was analyzed by two-tailed Student's t-test (Microsoft Excel; Microsoft). P values ≤0.05 were considered significant.
RESULTS
Spindle-shaped stromal cells in the lamina propria of the adult and neonate murine esophagus express α-SMA and vimentin.
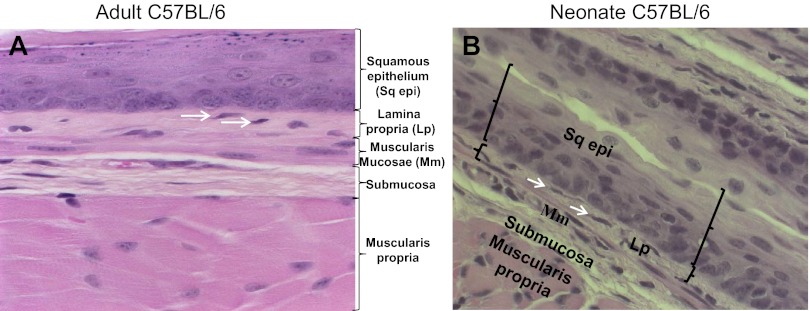
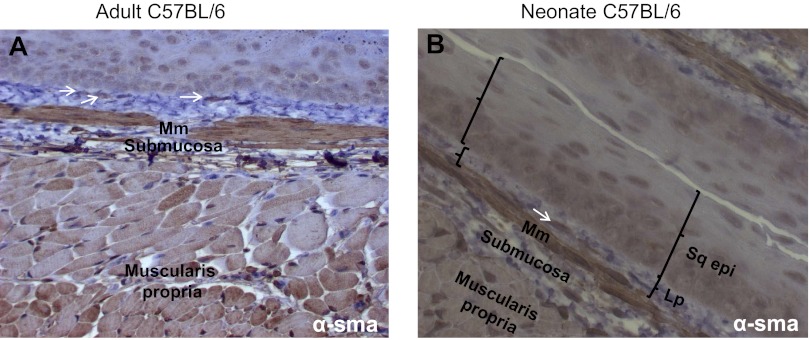
Hematoxylin and eosin staining of adult and neonatal esophageal sections revealed a stratified squamous epithelium with a heterogeneous cellular lamina propria. A discontinuous muscularis mucosa was also evident. Occasional spindle-shaped lamina propria cells distinct from the muscularis mucosa (Fig. 1, A and B) were evident. The morphology and location of these cells suggested they were myofibroblast-like cells and were of interest to us. To further characterize this population of cells, we performed immunohistochemical staining for the myofibroblast markers α-SMA and vimentin. A few of the spindle-shaped cells in the adult and neonate esophageal lamina propria expressed α-SMA (Fig. 2, A and B). Vimentin is an intermediate filament protein that is expressed by fibroblasts but absent in muscularis mucosa (1). To further distinguish these cells from smooth muscle cells of the muscularis mucosa, we performed immunofluorescence costaining for α-SMA and vimentin on paraffin-embedded sections. A few scattered α-SMA-positive (Fig. 3A) and vimentin-positive (Fig. 3B) cells were observed in the lamina propria. These cells coexpressed these myofibroblast markers and were distinct from the muscularis mucosa (Fig. 3, C and D).
Fig. 1.
Adult and neonate murine esophagus lamina propria contains spindle-shaped cells of interest. Hematoxylin and eosin staining was performed on longitudinal sections of adult (A) and neonate (B) murine esophagus. Representative sections (×200 magnification) demonstrate keratinized stratified squamous epithelium (Sq epi) overlying a heterogeneous cellular lamina propria (Lp). The lamina propria is adjacent to a discontinuous muscularis mucosa (Mm). Spindle-shaped cells of interest (white arrows) distinct from the muscularis mucosa are present in the lamina propria. The submucosa and muscularis propria are labeled for orientation.
Fig. 2.
Adult and neonate murine esophagus stroma contains α-smooth muscle actin (α-SMA)-expressing spindle-shaped cells. α-SMA immune staining was performed on longitudinal sections of adult (A) and neonate (B) esophagus (×200 magnification). α-SMA expression is observed most strongly in the muscularis mucosa and in scattered lamina propria cells (white arrows), distinct from the muscularis mucosa. α-SMA is also detected to a lesser extent in the muscularis propria. Sections of esophagus were incubated with rabbit anti-α-SMA antibody (1:100 dilution).
Fig. 3.
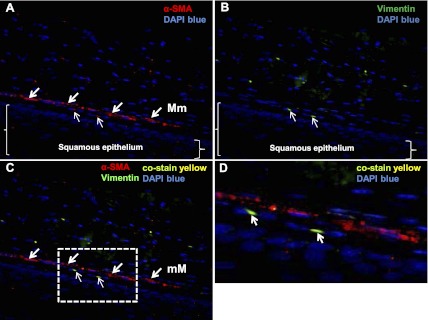
Murine esophageal lamina propria contains cells that coexpress α-SMA and vimentin. Paraffin-embedded sections of murine esophagus immune stained with α-SMA and vimentin are shown (×400 magnification). Representative longitudinal sections demonstrate α-SMA [A, arrows, red, tetramethylrhodamine isothiocyanate (TRITC)] expression in the muscularis mucosa (thick arrows) and in occasional cells in the lamina propria (thin arrows). Vimentin [B, arrows, green, fluorescein isothiocyanate (FITC)] expression is observed in scattered cells in the lamina propria (thin arrows). Squamous epithelium in the section is delineated by brackets. Spindle-shaped cells that coexpress α-SMA and vimentin (C, thin arrows, yellow-green) are present in the lamina propria. The dotted line box marks the region of interest (D, arrows), demonstrating yellow esophageal stromal cells that express α-SMA and vimentin. Blue depicts DAPI nuclear stain.
Primary cultures of esophageal stromal cells demonstrate myofibroblast morphology and express myofibroblast markers.
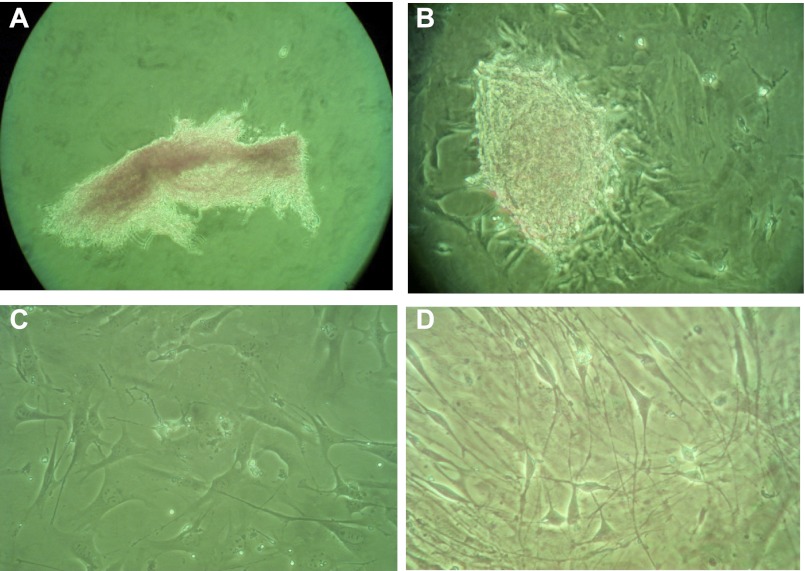
Based on our experience with the colonic myofibroblast (21), we hypothesized that the α-SMA- and vimentin-expressing cells we detected in the esophageal lamina propria could be isolated, grown in primary culture, and further studied. We therefore used a technique previously established for colonic myofibroblast isolation (21) to isolate esophageal stromal cells. Examination of cells immediately after the isolation procedure and plating demonstrated a mixed cell suspension aggregate isolated from the whole esophagus (Fig. 4A). Examination with an inverted microscope within 24 h after plating demonstrated formation of organoid-type structures loosely attached to the plate bottom with sprouting spindle-shaped cells (Fig. 4B). These sprouting cells did not have the cuboidal appearance of epithelial cells. Five to seven days after initial plating and after passage to a larger flask, examination with an inverted microscope revealed cells with spindle-shaped morphology similar to previously described colonic myofibroblasts (Fig. 4C). Cells nearing 80% confluence retained adherence to the plate bottom and maintained spindle-shaped morphology at passages 5–15 (Fig. 4D).
Fig. 4.
Primary cultures of esophageal stromal cells. Esophageal stromal cells were isolated using techniques previously established for colonic myofibroblast isolation, grown in primary culture and examined with an inverted microscope. On initial plating, mixed cell suspensions isolated from whole esophagus are observed partially floating and loosely attached to the plate (A). Within 24 h of plating, organoid-type structures attached to the plate bottom, with sprouting spindle-shaped cells identified (B). These sprouting cells covered the entire well within 5 days and were passaged successfully. Representative images of primary cultures between passage 5 and 15 are shown at low density (C) and when near-confluent (D).
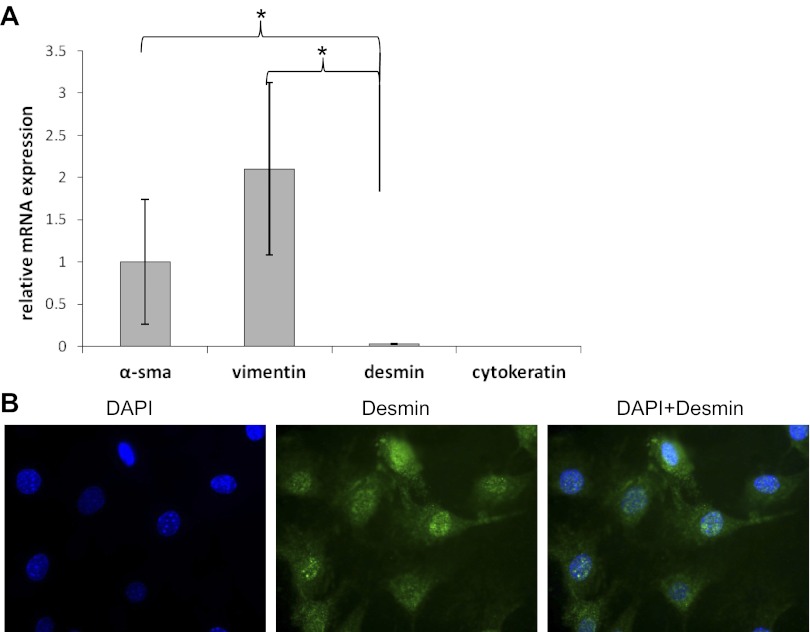
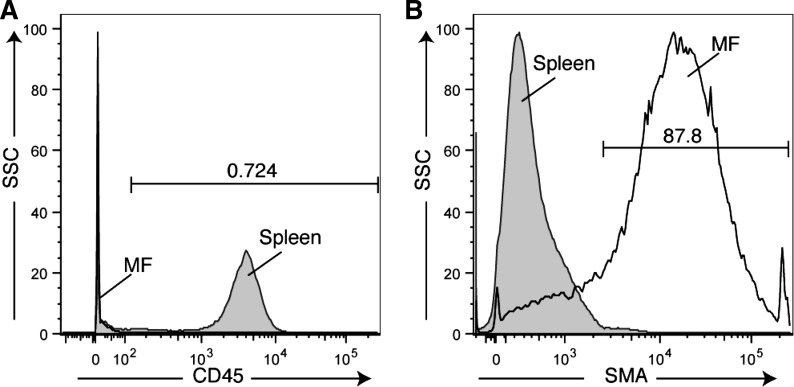
We next evaluated protein expression of myofibroblast markers (α-SMA, vimentin) in esophageal stromal cells harvested at passages 5–15. α-SMA and vimentin were coexpressed in these cells (Fig. 5). Protein isolation from harvested cells and immunoblot also demonstrated α-SMA expression (data not shown). We observed abundant α-SMA and vimentin mRNA and absence of cytokeratin mRNA expression (Fig. 6A). Desmin mRNA and protein expression were minimal (Fig. 6, A and B). These spindle-shaped esophageal stromal cells in primary culture had the protein expression pattern of cells traditionally termed myofibroblasts (16). To evaluate for the presence of endothelial cells in our primary cultures, we assessed expression of the endothelial marker vWF. vWF mRNA was also undetectable (positive control was RNA isolated from whole intestine; data not shown). Flow cytometric analysis performed on cells harvested from primary cultures between passage 5 and 15 demonstrated lack of expression of the hematopoietic marker CD45. The majority of cells in the culture expressed abundant α-SMA (Fig. 7). Overall, these findings suggested our primary cultures consisted predominantly of nonhematopoietic, nonendothelial cells of a myofibroblast/fibroblast nature.
Fig. 5.
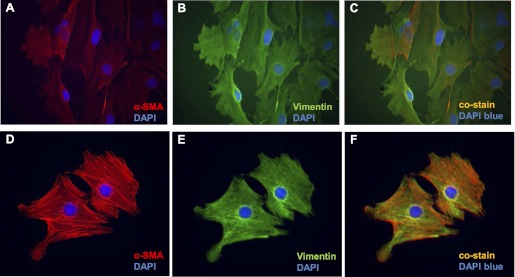
Primary cultures of esophageal stromal cells express α-SMA and vimentin. Primary cultures of esophageal stromal cells at passage 5–15 were grown on chamber slides and costained with α-SMA (ab5694, 1:200 dilution; Abcam) and vimentin (V4630, 1:100 dilution; Sigma-Aldrich). A–C: immunostaining of cells at moderate confluence. D–F: immunostaining of cells at low confluence. Immunocytochemistry demonstrates expression of α-SMA (A and D; red TRITC) and vimentin (B and E, green FITC) expression in cells harvested from primary cultures. Harvested stromal cells coexpress α-SMA and vimentin (C and F, orange-yellow). Blue depicts DAPI nuclear stain.
Fig. 6.
Esophageal stromal cell expression of myofibroblast markers. A: primary cultures of esophageal stromal cells established from C57BL/6 mice at passage 5–15 were harvested, and mRNA expression of α-SMA, vimentin, desmin, and cytokeratin was assessed. B: primary cultures of esophageal stromal cells at passage 5–15 were grown on chamber slides and were stained with desmin (D8281, 1:20; Sigma-Aldrich). Immunocytochemistry demonstrates a speckled, predominantly perinuclear staining along with lower-intensity cytoplasmic staining. *P < 0.05.
Fig. 7.
Primary cultures of esophageal α-SMA stromal cells lack CD45. Analysis of primary cultures of stromal cells by flow cytometry using antibodies for cell surface protein CD45 (A) demonstrates that these cells lack expression of this hematopoietic cell marker. Cells harvested from the spleen were used as the positive control. Analysis using antibodies for the cytoskeletal protein α-SMA (B) demonstrates that the majority of the cells in the population express α-SMA. SSC, side scatter; MF, myofibroblast.
Esophageal stromal cells express and secrete IL-6 in response to acid and IL-1β.
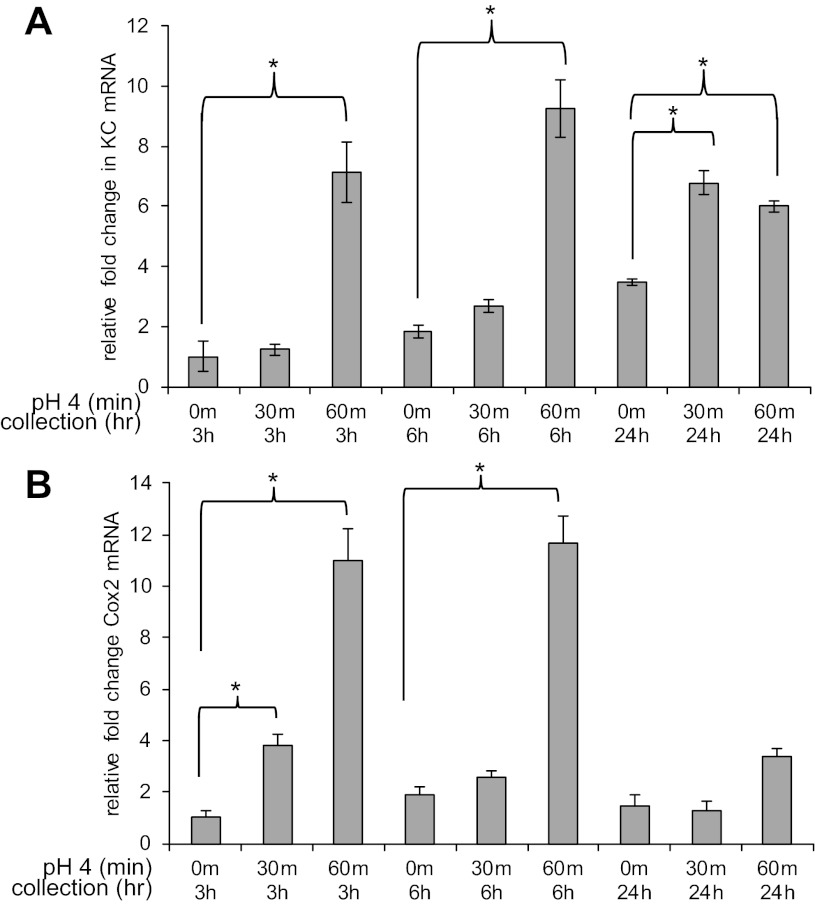
Increased expression and secretion of the inflammatory cytokine IL-6 has been reported in BE (3) and in Barrett's cell lines (28). In addition, elevated protein levels of IL-8 have been reported in reflux esophagitis (8). We were interested, therefore, in the behavior of these esophageal stromal cells in response to acid, one of the known mediators of esophageal injury (23). Pilot studies indicated that treatment with acidified media pH 4 [chosen based on published literature (26)] for 3 or 10 min resulted in minimal to no increase in inflammatory mediators, whereas 30 and 60 min exposure to acidified media maximized IL-6 mRNA expression. Cells appeared normal and remained adherent to tissue culture plates following acid exposure at 3, 6, and 24 h. At 3 h, there was a 3% increase in cell death as assessed by the Trypan blue exclusion method. However, at 6 and 24 h, the percentage of dead cells observed in untreated and acid-treated cells was similar (see materials and methods; Fig. 8). These findings suggested that the duration of treatment and acidity of the media, while sufficient to induce an inflammatory response, would not confound our results as a result of cell death-related cytokine spillage. We therefore treated esophageal stromal cells with myofibroblast media acidified to pH 4 for 30 and 60 min and then replaced the media with fresh serum-free myofibroblast media. We examined mRNA expression of inflammatory mediators Cox-2, KC (murine homolog of IL-8), and IL-6 at 3, 6, and 24 h. Esophageal stromal cells treated for 30 min had a twofold increase in KC mRNA at 24 h. Stromal cells treated with acidified media for 60 min responded with a sevenfold increase in KC mRNA expression at 3 h, a fivefold increase at 6 h, and a twofold increase at 24 h (Fig. 9A). Stromal cells treated for 30 min had a fourfold increase in Cox-2 mRNA at 3 h that dissipated by 6 h. Esophageal stromal cells demonstrated a more profound increase in Cox-2 mRNA after 60 min of treatment. An 11-fold increase in Cox-2 mRNA was detectable at 3 h and a 6-fold increase at 6 h. The increase in Cox-2 mRNA did not persist at 24 h with either 30 or 60 min of treatment (Fig. 9B).
Fig. 8.
Viability of cultures treated with pH 4 acidified media assessed by Trypan blue exclusion. Cells were assessed for cell death by Trypan blue exclusion after 3, 6, and 24 h in culture with and without treatment with acidified media. The average percentage of dead cells observed in untreated cells after 3 h in culture was 2.1 vs. 5.0% after treatment with acidified media for 30 and 60 min (P < 0.05). The percentage of observed dead cells was not significantly different in untreated and treated cells after 6 and 24 h in culture.
Fig. 9.
Esophageal stromal cells increase inflammatory marker mRNA expression in response to acid. Primary cultures of esophageal stromal cells were established from C57BL/6 mice, and mRNA expression of inflammatory markers (KC, a murine homolog of IL-8) and cyclooxygenase-2 (Cox-2) in response to treatment with media acidified to pH 4 was evaluated. Cells were treated with media acidified to pH 4 for 30 or 60 min, and mRNA expression of KC and Cox-2 was evaluated after 3, 6, and 24 h. A: esophageal stromal cells treated for 30 min have a 2-fold increase in KC mRNA at 24 h. Cells treated for 60 min have a 7-fold increase in KC mRNA expression at 3 h, a 5-fold increase at 6 h, and a 2-fold at 24 h. B: esophageal stromal cells treated for 30 min have an 4-fold increase in Cox-2 mRNA at 3 h. Cells treated for 60 min have an 11-fold increase in Cox-2 mRNA at 3 h and a 6-fold increase at 6 h. *P < 0.05 vs. control.
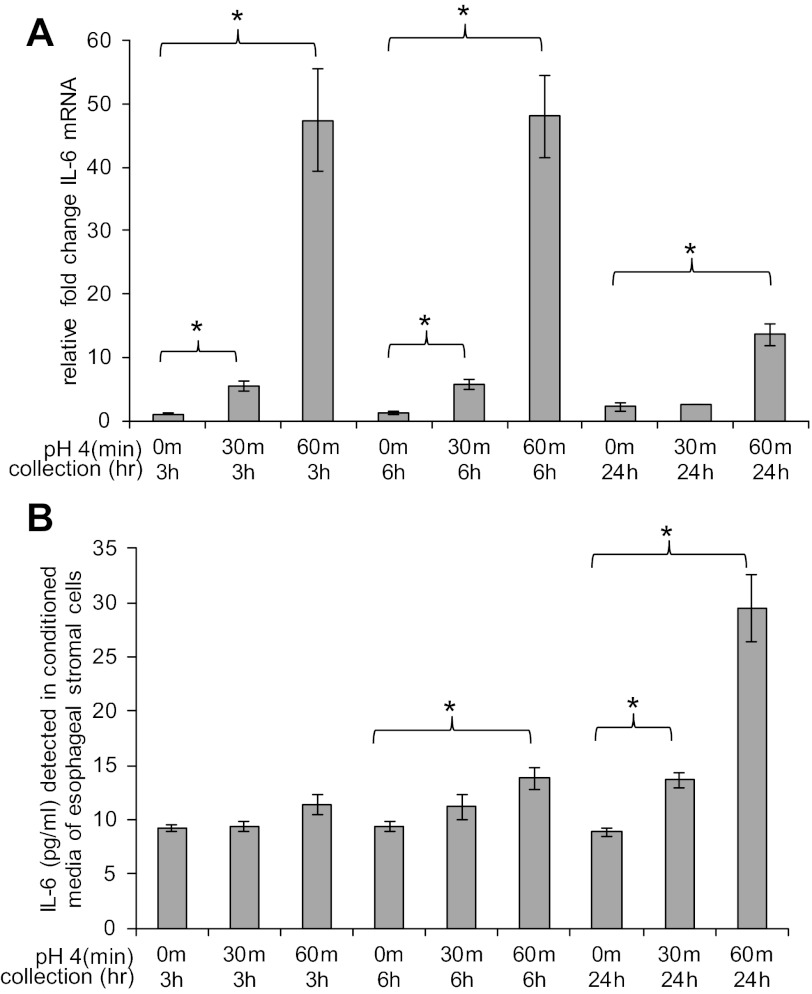
Stromal cells treated with acidified media also had an increase in IL-6 mRNA expression (Fig. 10A). There was a fivefold increase in IL-6 mRNA at 3 h and a sixfold increase at 6 h in response to 30 min of treatment with acidified media. At 24 h, IL-6 mRNA expression in cells treated for 30 min had returned to baseline. The increase in IL-6 mRNA expression in response to treatment with acidified media for 60 min was more profound. There was a 47-fold increase in IL-6 mRNA at 3 h and 48-fold increase at 6 h. At 24 h, there was a persistent 13-fold increase in IL-6 mRNA. Compared with KC and Cox-2, the increase in IL-6 mRNA expression was more substantial.
Fig. 10.
Esophageal stromal cells increase IL-6 mRNA expression and protein secretion in response to acid. A: esophageal stromal cells have an increase in IL-6 mRNA expression in response to treatment with pH 4 acidified media. In response to 30 min of treatment with acidic media, there is a 5-fold increase in IL-6 mRNA at 3 h and a 6-fold increase at 6 h. In response to 60 min of treatment with acidic media, there is a 47-, 48-, and 13-fold increase in IL-6 mRNA at 3, 6, and 24 h, respectively. B: IL-6 secretion is increased from esophageal stromal cells treated with pH 4 acidified media for 30 and 60 min. Constitutive secretion of IL-6 was detected at the lower enzyme-linked immunosorbent assay (ELISA) detection limit (9 pg/ml) from untreated esophageal stromal cells. Cells treated with acidified media for 30 min secrete 14 pg/ml IL-6 at 24 h (*P < 0.05 vs. control). Stromal cells treated for 60 min secrete 14 and 29 pg/ml IL-6 at 6 and 24 h, respectively. IL-6 secretion after allotted incubation times was quantified in media by ELISA as described in materials and methods.
We next examined whether the increase in IL-6 mRNA was reflected by an increase in IL-6 secretion. We observed a low level of constitutive IL-6 secretion (9 pg/ml, the lower limits of detection by ELISA). We observed a small but significant increase in stromal IL-6 secretion at 24 h in response to treatment with acidified media for 30 min (14 pg/ml of IL-6 vs. 9 pg/ml, P < 0.05). In response to 60 min of treatment, we detected an earlier increase in IL-6 secretion at 6 and 24 h (14 and 29 pg/ml at 6 and 24 h, respectively, vs. 9 pg/ml, P < 0.05) (Fig. 10B).
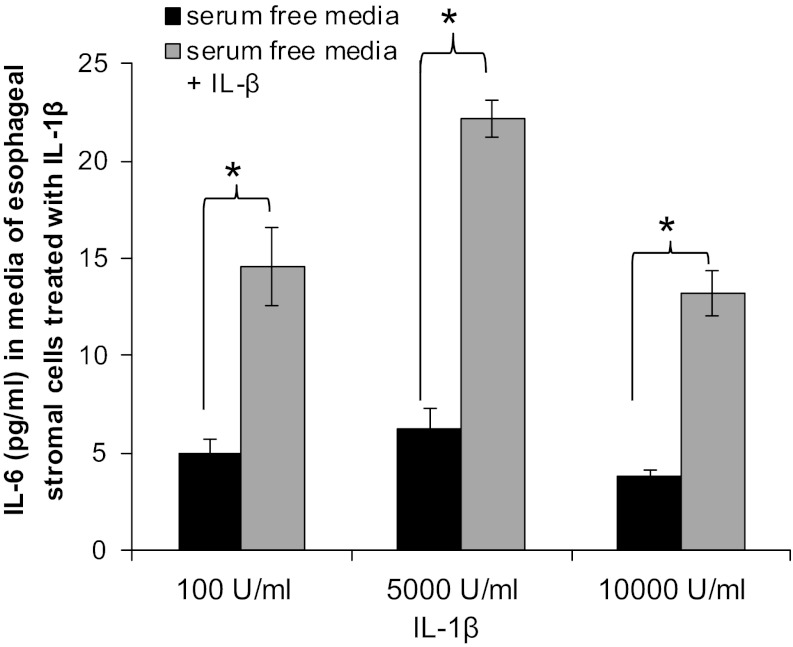
IL-1β is a proinflammatory cyotokine that has also been consistently implicated in the pathogenesis of gastroesophageal reflux disease (GERD) esophagitis, in humans and in animal models (9, 18). We were interested, therefore, in whether esophageal myofibroblasts secreted IL-6 in response to IL-β. Esophageal stromal cells were incubated for 24 h in serum-free media with or without IL-1β (100, 5,000, and 10,000 U/ml). In response to 100, 5,000, and 10,000 U/ml IL-β, esophageal stromal cells secreted 80, 142, and 62 pg/ml IL-6, respectively (P < 0.05 compared with control) (Fig. 11).
Fig. 11.
Esophageal stromal cells secrete IL-6 in response to IL-1β. Primary cultures of esophageal stromal cells were treated with IL-1β (100, 5,000, and 10,000 U/ml) for 24 h (R&D), and IL-6 secretion was evaluated by ELISA. IL-6 secretion (142 pg/ml) was maximal in response to 5,000 U/ml IL-1β (*P < 0.05).
Esophageal stromal cells do not increase Bmp-4 secretion in response to acid or IL-1β.
BMPs are multifunctional proteins that are members of the transforming growth factor family-β family (14). Bmp inhibitor expression is critical to murine esophageal embryonic development (20), and stromal expression of Bmp-4 has been implicated in the pathogenesis of reflux esophagitis and BE (14). We were interested in whether our primary cultured stromal cells represented a source of secreted Bmp-4 in response to injury with acid or stimulation with IL-1β.
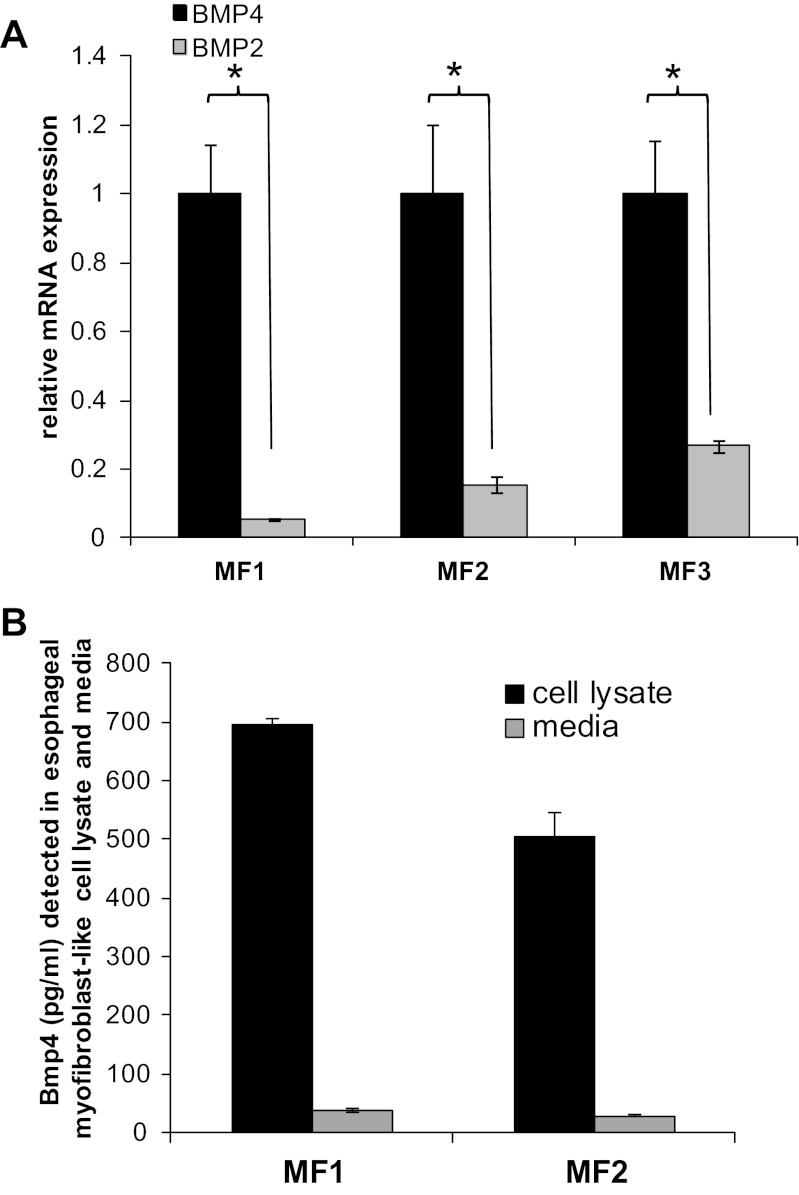
We began by evaluating Bmp-4 mRNA and protein expression in our primary cultures. Expression of BMP-4 (4- to 200-fold) and BMP-2 (2- to 50-fold) mRNA was variable across primary stromal cell cultures established from different mice. In all examined cultures, however, BMP-4 mRNA expression was greater than BMP-2 (4- to 11-fold, P < 0.05) (Fig. 12A). As expected, based on mRNA expression of Bmp-4, Bmp-4 protein was readily and consistently detected by ELISA in cell lysates isolated from multiple primary cultures of esophageal stromal cells (Fig. 12B). Evaluation of Bmp-4 secretion in conditioned media demonstrated negligible to low levels of constitutive Bmp-4 secretion from esophageal stromal cells. We were then interested in whether the conditions that had elicited an increase in IL-6 secretion (acidified media, IL-1β) would also increase Bmp-4 secretion. We did not observe a significant increase in either Bmp-4 mRNA expression or protein secretion in esophageal stromal cells treated with pH 4 acidified media for up to 60 min (data not shown). We also did not observe a significant increase in Bmp-4 secretion in response to treatment with IL-1β (data not shown) after 24 h.
Fig. 12.
Esophageal stromal cells express and secrete low levels of bone morphogenetic protein (Bmp)-4. A: detection of BMP-4 mRNA was greater than BMP-2 (4- to 11-fold) in all examined primary cultures. Representative results from myofibroblast primary cultures (n = 3) established from three mice (MF1, MF2, and MF3) are shown. Relative BMP-4 and BMP-2 mRNA levels were quantified by qRT-PCR as described in materials and methods, *P < 0.05. B: esophageal stromal cells express abundant Bmp-4, and certain subsets constitutively secrete low levels of Bmp-4 protein. Bmp-4 protein was detected in total protein (25 μg) isolated from cell lysates and in serum-free media of esophageal myofibroblast primary cultures. Bmp-4 levels were quantified by ELISA as described in materials and methods. Cells were plated at equivalent density and grown to confluence. Media were then replaced with fresh non-growth hormone-containing media and collected at 3 h. Results shown are from one experiment performed on primary stromal cultures established from two mice (MF1 and MF2), which is representative of at least three others performed.
Esophageal stromal cells increase Bmp-4 secretion in response to SHH.
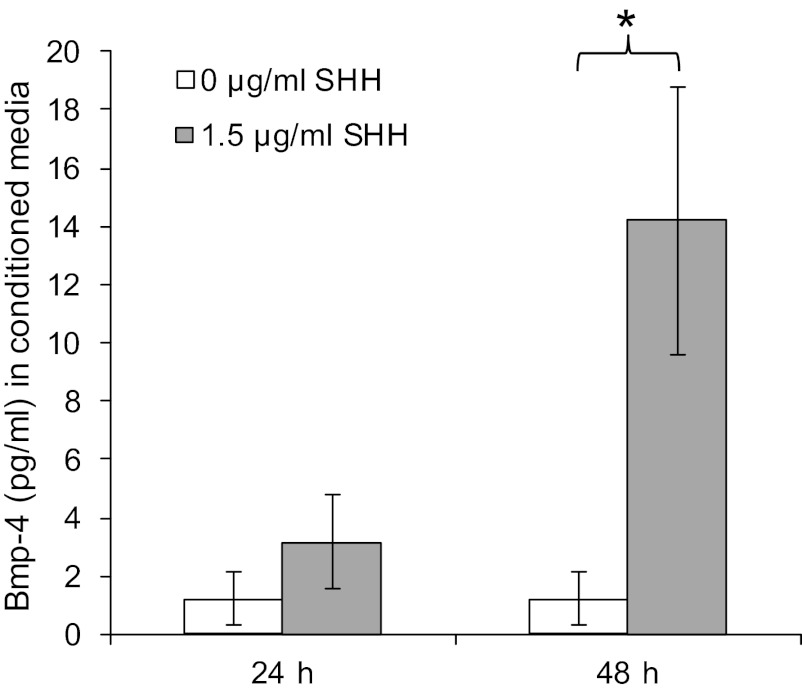
Finally, in a study evaluating Barrett's metaplasia, treatment of HET-1A squamous epithelial cells with pH 4 acidified media resulted in an increase in mRNA expression of SHH, and transgenic expression of SHH resulted in an increase in stromal expression of Bmp-4 (26). These findings suggested that treatment with SHH could induce secretion of Bmp-4 in our primary cultures of esophageal stromal cells. In response to treatment with 1.5 μg/ml SHH for 24 h, a small but significant increase in Bmp-4 secretion was detected after 48 h (Fig. 13).
Fig. 13.
Treatment of esophageal stromal cells with sonic hedgehog (SHH) increases Bmp-4 secretion. Esophageal stromal cells seeded in 12-well plates were grown to 70% confluence and then incubated with recombinant mouse SHH NH2-terminus (1.5 μg/ml; R&D) diluted in serum-free media for 24 h. Media were then replaced, and conditioned media were collected at 24 and 48 h. Bmp-4 levels were quantified by ELISA (Bmp-4 duoset; R&D). At 48 h, 14.2 pg/ml Bmp-4 (*P < 0.05) were detected in conditioned media.
DISCUSSION
We have established primary cultures of nonhematopoietic, nonendothelial esophageal stromal cells that have characteristics often ascribed to myofibroblasts: spindle-shaped morphology, abundant α-SMA and vimentin expression, minimal desmin, and absent cytokeratin expression. Immunostaining of these esophageal primary cultures demonstrates α-SMA and vimentin coexpression, suggesting isolation and culture of a relatively homogeneous-appearing population. Given that our isolation methods consisted of mechanical and enzymatic digestion of the whole esophagus, however, it is more likely that our primary cultures are not homogeneous populations but rather a somewhat more heterogeneous population consisting of myofibroblasts, fibroblasts, and smooth muscle cells. The variability in absolute mRNA levels of BMP-4 and -2 across esophageal primary stromal cell cultures likely reflects the heterogenous nature inherent to primary cultures vs. clonal cell lines (6). The lack of cell surface-specific markers of myofibroblasts and fibroblasts limits the use of techniques such as fluorescence-activated cell sorter analysis often used to sort pure cell populations. Optimal characterization of the subsets of nonhematopoietic, nonepithelial esophageal stromal cells may ultimately be best accomplished by high-throughput RNA transcriptional profiling of clonally derived cell lines. Our in vitro characterization suggests, however, that the cells established in primary culture at least partially reflect the α-SMA- and vimentin-expressing spindle-shaped stromal cells observed in histological sections of murine esophagus. Furthermore, compared with clonally derived cell lines, primary cultures may better reflect the in vivo function of these cells (6). We elected therefore to continue our studies with these primary cultures.
We observed that primary cultures of these esophageal stromal cells have an increase in mRNA expression of KC and Cox-2 and an increase in IL-6 expression and secretion in response to treatment with injurious luminal agents (acidified media) and cytokines elaborated by squamous epithelium as well as neighboring immune cells (IL-1β). The IL-6 secretion patterns observed in cells treated with acidified media were consistent with the observed increase in IL-6 mRNA expression. The significant increase in IL-6 secretion at 24 h in response to treatment with 30 min of acidified media reflects the increase in IL-6 mRNA at the 3- and 6-h time points. Similarly, the more profound increase in IL-6 secretion in response to 60 min of acidified media at 6 and 24 h reflects the greater fold increase in IL-6 mRNA expression at all time points (Fig. 10, A and B). Our results suggest that the esophageal stromal cells respond to acidic injury acutely with an increase in expression of inflammatory mediators as early as 3 h. This response is sustained, with an increase in KC and IL-6 mRNA and IL-6 secretion observed up to 24 h after exposure to acidified media (Figs. 9A and 10). The lack of persistent increase in Cox-2 (Fig. 9B) reflects the known instability of Cox-2 mRNA (5).
The participation of cytokine-stimulated human esophageal nonepithelial cells in mediating esophageal motor abnormalities via IL-1β and IL-6 secretion has been reported previously (19). Cells used in these previous studies, however, were termed fibroblasts based on isolation technique and morphological appearance in culture, and functional studies were limited because of the number of available cells. Our findings demonstrate that stromal cells with myofibroblast-like characteristics in the esophagus are sources of inflammatory mediators, and therefore likely participants in acid-induced injury.
Reactivation of the Bmp and hedgehog developmental pathways have also been implicated in the pathogenesis of esophagitis and BE. During ontogeny, Bmp signaling is necessary for conversion of the simple columnar epithelium of the murine embryo to a stratified squamous epithelium and for suprabasal cell differentiation (17, 20). Bmp-4 expression has been shown to be increased in reflux esophagitis (14) as well as in BE (27). Studies have suggested a stromal source of Bmp-4 in ontogeny (20), in esophagitis (14), and BE (27) although the cellular source(s) of Bmp-4 has not been delineated. The Bmp pathway has also been implicated in the transformation of normal esophageal squamous cells into columnar cells (14), possibly through Cdx2 activation (2). In addition, treatment of HET-1A cells, an SV-40 immortalized human esophageal squamous epithelial cell line, with pH 4 acidified media for 48 h resulted in an increase in SHH mRNA expression (26). Although Bmps in the murine esophagus are likely expressed in multiple cell types, we have demonstrated that esophageal stromal cells with myofibroblast-like features express Bmp-4 (Fig. 12). Despite abundant quantities of Bmp-4 in esophageal myofibroblast cell lysate, Bmp-4 secretion was not stimulated by treatment with either acidified media or IL-β. Primary cultures of esophageal stromal cells demonstrated a slight increase Bmp-4 secretion in response to SHH, however (Fig. 13).
Overall, our data support the role for esophageal stromal cells in the pathogenesis of esophageal mucosal disorders. Traditionally, it has been suggested that prolonged mucosal contact with injurious luminal agents results in a direct chemical injury to the surface squamous epithelium. Injury then progresses into the lamina propria and ultimately results in surface cell death, and necrosis that is followed by basal cell proliferation (12). Both human and animal models have demonstrated alterations in apical junctional complexes in the esophageal epithelium along with dilated intercellular spaces (15), a sensitive but nonspecific marker of GERD. In the setting of chronic reflux, therefore, underlying stromal cells can be exposed to injurious luminal agents and participate in mediating the injury response (15).
There is also evidence, however, that direct acid contact or caustic injury to the squamous epithelium results in stimulation of squamous cell chemokine secretion and recruitment of activated immune cells. Immune cell cytokine secretion is in turn primarily responsible for the inflammatory response and for inflicting further epithelial injury (22). Numerous inflammatory mediators have been implicated in human and animal models of esophagitis, including IL-1β, IL-6, IL-8, and platelet-activating factor (9, 8, 12, 18, 19). IL-6 has also been implicated in the pathogenesis of BE (3, 28). We focused our investigation on secretion of this pleiotropic cytokine from stimulated stromal cells given its relatively greater expression compared with the other examined inflammatory mediators. Broader evaluation of esophageal stromal cell cytokine secretion into the inflammatory mileu in acid-related disorders is warranted.
Our data support the suggestion that esophageal stromal cells with characteristics of myofibroblasts are active participants in the esophageal inflammatory response at least partially via secretion of IL-6 (18, 19). These cells are responsive to luminal sources of injury (acid) as well as to inflammatory cytokines and mediators (IL-1β) thought to originate from the overlying squamous epithelium and surrounding immune cells. Our observation that esophageal stromal cells are a source of Bmp-4 supports further investigation of these cells in the pathogenesis of BE.
The majority of research on the mechanisms underlying gastroesophageal reflux-induced injury and its complications has focused on the epithelium and only more recently on delineating the contribution of traditional immune and mesenchymal cells. Esophageal stromal cells may contribute to the reactivation of developmental and inflammatory pathways implicated in erosive esophagitis and BE, as reviewed previously (23). Furthermore, the location of these cells and their biological functions demonstrate their potential role in regulating esophageal epithelial responses to injury and repair. Current diagnostics and therapeutics are primarily aimed at the epithelial injury response. Understanding the stromal contribution to esophageal epithelial injury offers a new perspective for therapeutic targets in esophageal diseases with major morbidity and mortality.
GRANTS
This work was supported by the AGA-General Mills Bell Institute of Health and Nutrition Research Scholar Award in Gut Physiology and Health, NIH/NCI KO8CA153036-01A1 (to A. Shaker), NIH Grants R01 DK-46122 and DK-61216 (to D. Rubin), and by NIH Grant P30 DK-52574 (to the DDRCC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., R.N., and D.C.R. conception and design of research; A.S., J.B., I.D., E.A.S., and K.M. performed experiments; A.S., J.B., I.D., and K.M. analyzed data; A.S. interpreted results of experiments; A.S. prepared figures; A.S. and D.C.R. drafted manuscript; A.S., R.N., and D.C.R. edited and revised manuscript; A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Kymberli Carter and the Washington University School of Medicine (St. Louis, MO) Digestive Diseases Research Core Center (DDRCC) for assistance with morphology services.
Present address for A. Shaker: 2011 Zonal Ave., HMR 810, Los Angeles, CA 90089.
REFERENCES
- 1. Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 126: 829–836, 2002 [DOI] [PubMed] [Google Scholar]
- 2. di Pietro M, Fitzgerald RC. Barrett's oesophagus: an ideal model to study cancer genetics. Hum Genet 126: 233–246, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Dvorakova K, Payne CM, Ramsey L, Holubec H, Sampliner R, Dominguez J, Dvorak B, Bernstein H, Bernstein C, Prasad A, Fass R, Cui H, Garewal H. Increased expression and secretion of interleukin-6 in patients with Barrett's esophagus. Clin Cancer Res 10: 2020–2028, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Erichsen R, Robertson D, Farkas DK, Pedersen L, Pohl H, Baron JA, Sorensen HT. Erosive reflux disease increases risk for esophageal adenocarcinoma, compared with nonerosive reflux. Clin Gastroenterol Hepatol 10: 475–480, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Faour WH, He Y, He QW, de Ladurantaye M, Quintero M, Mancini A, Di Battista JA. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J Biol Chem 276: 31720–31731, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Furuya S, Furuya K. Subepithelial fibroblasts in intestinal villi: roles in intercellular communication. Int Rev Cytol 264: 165–223, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Hirschowitz BI. Pepsin and the esophagus. Yale J Biol Med 72: 133–143, 1999 [PMC free article] [PubMed] [Google Scholar]
- 8. Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol 98: 551–556, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Kandulski A, Malfertheiner P. Gastroesophageal reflux disease–from reflux episodes to mucosal inflammation. Nat Rev Gastroenterol Hepatol 9: 15–22, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Martin RC., 2nd Reflux injury of esophageal mucosa: experimental studies in animal models of esophagitis, Barrett's esophagus and esophageal adenocarcinoma. Dis Esophagus 20: 372–378, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, Chen X. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis 28: 488–496, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ma J, Altomare A, de la Monte S, Tong M, Rieder F, Fiocchi C, Behar J, Shindou H, Biancani P, Harnett KM. HCl-induced inflammatory mediators in esophageal mucosa increase migration and production of H2O2 by peripheral blood leukocytes. Am J Physiol Gastrointest Liver Physiol 299: G791–G798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Majka J, Rembiasz K, Migaczewski M, Budzynski A, Ptak-Belowska A, Pabianczyk R, Urbanczyk K, Zub-Pokrowiecka A, Matlok M, Brzozowski T. Cyclooxygenase-2 (COX-2) is the key event in pathophysiology of Barrett's esophagus. Lesson from experimental animal model and human subjects. J Physiol Pharmacol 61: 409–418, 2000 [PubMed] [Google Scholar]
- 14. Milano F, van Baal JW, Buttar NS, Rygiel AM, de Kort F, DeMars CJ, Rosmolen WD, Bergman JJ, VAM J, Wang KK, Peppelenbosch MP, Krishnadath KK. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology 132: 2412–2421, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol 24: 873–882, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts II: intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 74: 422–437, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Rieder F, Biancani P, Harnett K, Yerian L, Falk GW. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol 298: G571–G581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rieder F, Cheng L, Harnett KM, Chak A, Cooper GS, Isenberg G, Ray M, Katz JA, Catanzaro A, O'Shea R, Post AB, Wong R, Sivak MV, McCormick T, Phillips M, West GA, Willis JE, Biancani P, Fiocchi C. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology 132: 154–165, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez P, Da Silva S, Oxburgh L, Wang F, Hogan BL, Que J. BMP signaling in the development of the mouse esophagus and forestomach. Development 137: 4171–4176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest 120: 2081–2093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137: 1776–1784, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Souza RF, Schwartz RE, Mashimo H. Esophageal stem cells and 3D-cell culture models. Ann NY Acad Sci 1232: 316–322, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science 324: 1666–1669, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Wang C, McDonough JS, McDonald KG, Huang C, Newberry RD. Alpha4beta7/MAdCAM-1 interactions play an essential role in transitioning cryptopatches into isolated lymphoid follicles and a nonessential role in cryptopatch formation. J Immunol 181: 4052–4061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, Jenkins NA, Copeland NA, Harmon JW, Phillips WA, Watkins DN. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology 138: 1810–1822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, Jenkins NA, Copeland NA, Harmon JW, Phillips WA, Watkins DN. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology 138: 1810–1822, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang HY, Zhang Q, Zhang X, Yu C, Huo X, Cheng E, Wang DH, Spechler SJ, Souza RF. Cancer-related inflammation and Barrett's carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol 300: G454–G460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]