Abstract
Mixed adenoneuroendocrine carcinoma of cecum (MANEC) was first reported by Cardier in 1924. These tumors are thought to arise from multi-potential stem cells, which have differentiated bidirectionally. Location of the tumor influences the treatment and outcome. We report a rare case of MANEC where the patient presented with abdominal pain and distension. Imaging revealed an ileo colic intussusception with the lead point being a MANEC.
Keywords: Cecum, composite tumor, mixed adenoneuroendocrine carcinoma
INTRODUCTION

Aditi Jain
Gastrointestinal neuroendocrine tumors that contain additional glandular component are called mixed adeno-neuroendo-carcinoma. This term has replaced the term mixed exocrine-endocrine carcinoma. Mixed adenoneuroendocrine carcinomas of the colon and rectum account for 3-9.6% of colorectal tumors. These tumors have non-specific imaging characteristics and hence are mainly diagnosed on histopathology.
CASE REPORT
A 68-year-old female presented to the Radiology Department with complaints of abdominal pain and vomiting. In spite of conservative treatment her condition worsened. She was unable to pass stools for a week and subsequently developed abdominal distension. There was no history of fever or weight loss and her abdominal examination revealed guarding and rigidity in the right iliac fossa.
Contrast enhanced computed tomography (CT) of abdomen and pelvis done on Asteion Toshiba single-slice spiral CT scanner revealed ileocolic intussusception with the lead point being an intraluminal heterogeneously enhancing mass in the cecum with associated irregular wall thickening [Figures 1 and 2] resulting in small bowel obstruction. The mass measured 3 × 3.5 cm in size. Few of the distal ileal loops showed thickened walls with mesenteric haziness in the right iliac fossa and minimal ascites, multiple enlarged mesenteric nodes ranging from 10 to 14 mm were seen in the right lumbar and the right iliac fossa with few of them showing central necrosis [Figure 1].
Figure 1.
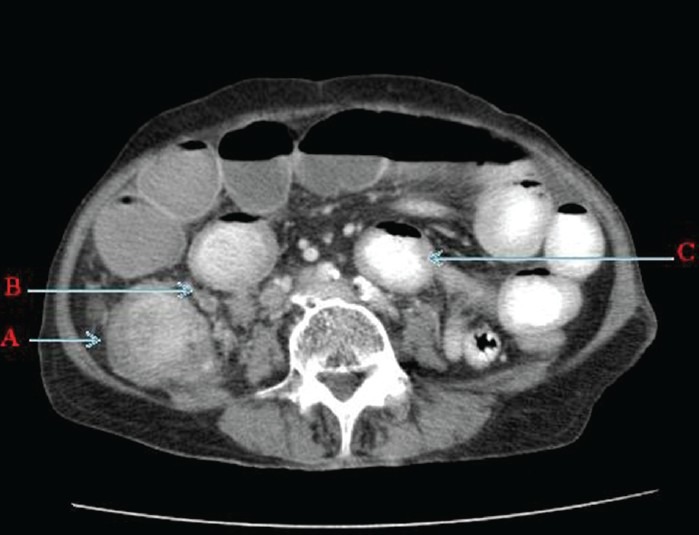
Contrast enhanced computed tomography of abdomen and pelvis (axial section) shows A. Enhancing Intraluminal caecal mass with caecal wall thickening. B. Adjacent necrotic mesentric nodes and C. Dilated small bowel loops with few of them having thickened walls.
Figure 2.
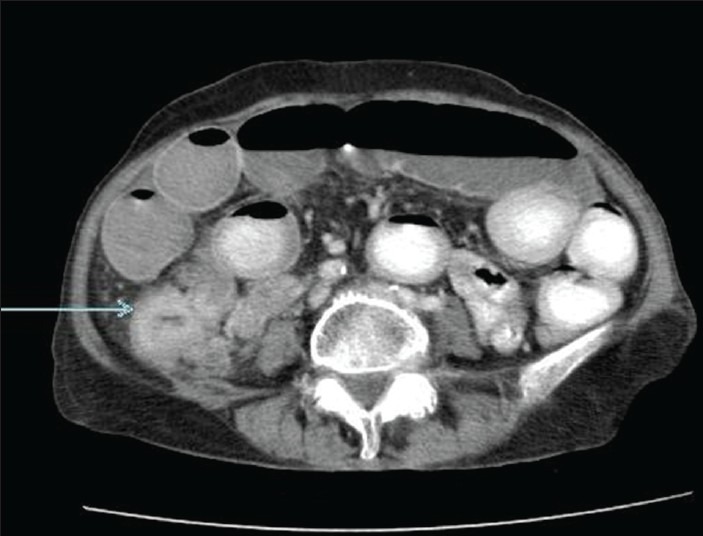
Contrast enhanced computed tomography of abdomen and pelvis (axial section) reveals thickened ileum loop extending into the cecum (arrow).
Patient underwent right hemicolectomy. Cut surface revealed a polypoidal growth in the cecum measuring 3.5 × 3.0 × 2.5 cm. Macroscopic tumor perforation was present and microscopically tumor was adherent to other organs and was infiltrating the peritoneum, pericolic fat, and appendicular wall. Serosal margin was infiltrated by the tumor. The proximal and distal resected margins of the specimen were free from tumor involvement. Thirteen out of 14 lymph nodes were positive.
Histopathology examination of the cecal mass revealed sheets, cords, and islands of monomorphic large cells with large round nucleus and abundant cytoplasm [Figure 3a and b] infiltrating the layers of cecum up to the peritoneum. Nests and islands of tumor with vague glandular structures amounting to more than 30% of the tumor [Figure 4a and b] were seen originating from cecal mucosa and infiltrating the wall amidst pools of extracellular mucin. Few signet ring cells were seen interspersed between the large tumor cells. Focal aggregate of lymphocytes was also seen [Figure 5]. The final histopathological diagnosis was MANEC of the cecum, poorly differentiated to undifferentiated, having >20 mitotic count per 10 high-power field, nodal metastases, tumor Stage T4 N2 M0.
Figure 3.
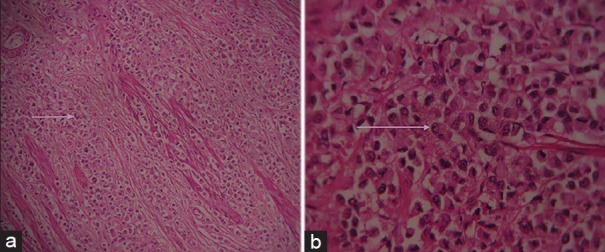
Hematoxylin and eosin stained sample (a) at ×10 and (b) at ×40 demonstrate large monomorphic tumor cells having large round cell nucleus and abundant eosinophilic cytoplasm (seen arranged in cords and nests in Figure 3a).
Figure 4.
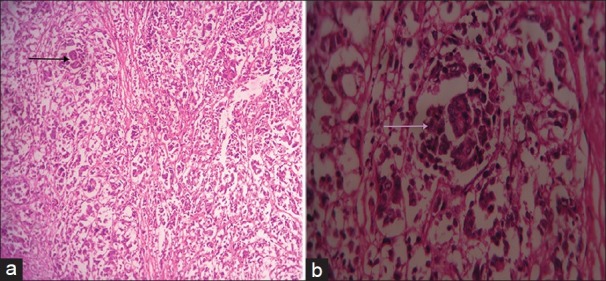
Hematoxylin and eosin stained tissue (a) at ×10 and (b) at ×40 show glandular formation (arrow) amounting to >30% of the tumor.
Figure 5.
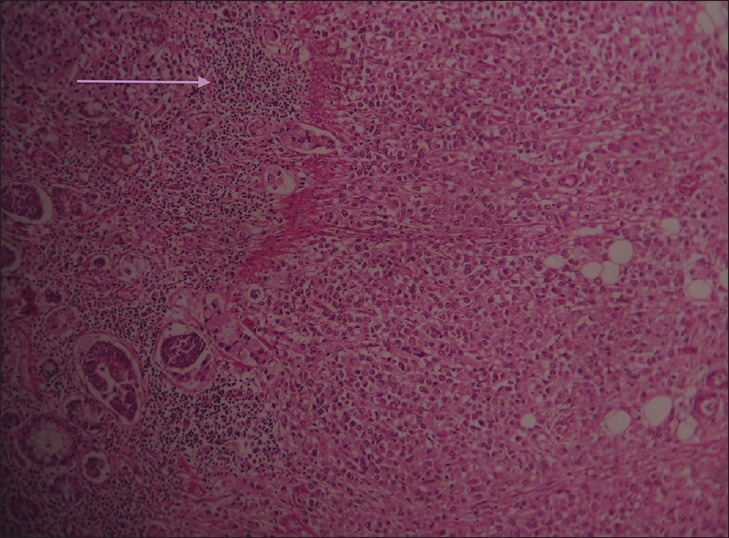
Hematoxylin and eosin stained tissue at ×10 shows tumor cells surrounded by focal lymphocyte response.
Immunohistochemical examination revealed caudal-related homebox transcription factor 2 (CDX2), cytokeratin 20, synaptophysin, and carcino-embryonic antigen were strongly positive. There was focal positivity for chromogranin. While Ki-67 showed high proliferative index amounting to more than 20% of the tumor tissue.
DISCUSSION
Neuroendocrine neoplasms (NENs) arise from endodermal cells These cells share common features, such as looking similar, producing biogenic amines and polypeptide hormones.[1] Majority occur within the gastroenteropancreatic axis and are classified according to site of origin-foregut, midgut, and hind gut. They range from well-differentiated carcinoids to poorly differentiated neuroendocrine carcinomas (NECs). Staging of NENs depends on size, lymphovascular invasion, invasion of adjacent organs, and presence of metastases.[2] Grading depends upon mitotic count and Ki67 index . Sometimes NEN include an additional significant proportion of malignant exocrine glandular cells and then it is termed as Mixed adenoneuroendocrine carcinoma of cecum (MANEC) when each component represents at least 30% of the lesion.[3] Previously, these were termed as mixed exocrine-endocrine carcinomas. They are further classified into collision and composite types. Collision tumors are where two different histologic patterns are in close contact, and mixed or composite tumors where the endocrine and exocrine cells are intermixed within the same tumor. Composite tumors arise through multi-directional differentiation of a single neoplasm whereas collision tumors result from biclonal transformation of two separate but adjacent neoplasms.[4] Our case was a composite tumor.
These tumors are often small, located in submucosa or intramural area and can involve the mesentery and can be firm due to desmoplastic reaction. Overlying mucosa can be intact or ulcerated. Macroscopically, these appear as polypoid masses or ulcerating stenotic lesions measuring 0.5 cm - 14 cm in diameter, with mean size of about 5 cm.
Histologically, the NEC component is morphologically similar to small cell or large cell NEC of the lung (2010 WHO classification). Immunohistochemically, pure neuroendocrine component of the mixed tumor are positive for synaptophysin and chromogranin A.[5]
NENs of the gastrointestinal tract are small and difficult to detect on CT scans. Small tumors are dramatically enhancing submucosal lesions on arterial phase and its metastases to the liver enhance brightly on arterial phase imaging because of their increased vascularity,[6] Mesenteric extension appear as ill-defined mesenteric mass containing calcification or spiculated mass with a stellate pattern. Mesenteric vessels may be involved due to desmoplastic reaction, which can lead to thickening and ischemia of the involved small-bowel as was seen in our case.
These tumors present with non-specific symptoms and are clinically occult, revealed only on exploratory surgery. They usually occur in the fifth or sixth decade of life. In many cases, diagnosis is revealed only with development of metastases. Atypical varieties show higher rate of metastasis.[4] Any component of the mixed tumor can spread regardless of its allocation.
Because of their rarity and unusual presentation, the optimal strategy for management of mixed endocrine tumor is variable. The most contentious component of the mixed endocrine tumors must be borne in mind for alleviation of symptoms. In our case, as there were no metastases from either component at the time of scan, surgery was performed, and follow-up was advised.
CONCLUSION
MANECs are rare occult tumors, more frequently detected due to intussuception and hepatic metastases that can be viewed by newer and faster CT scanners. They should be considered as one of the differential diagnosis for adult intussupception. Cross-sectional imaging plays an important role in detection of mixed adenoneuroendocrine tumors. As imaging features are non-specific, histopathology is necessary to confirm the diagnosis.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/10/107995
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 2.Ramage JK, Davies AH, Ardill J, Bax N, Caplin M, Grossman A, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumors. Gut. 2005;54:iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Rosa S, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinoma (MANECs) of the gastrointestinal tract: An update. Cancers. 2012;4:11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suttmann H. A mixed adenocarcinoma and neuroendocrine carcinoma of the rectum associated with an adenocarcinoma of the prostate as a second primary malignancy: Report of a case. Electron J Pathol Histol. 2003;9:32–8. [Google Scholar]
- 5.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: WHO, International Agency for Research on Cancer; 2010. Nomenclature and classification of neuroendocrine neoplasms of the digestive system; p. 13. [Google Scholar]
- 6.Horton KM, Kamel I, Hofmann L, Fishman EK. Carcinoid tumors of the small bowel: A multitechnique imaging approach. AJR Am J Roentgenol. 2004;182:559–67. doi: 10.2214/ajr.182.3.1820559. [DOI] [PubMed] [Google Scholar]


