Abstract
α1-Adrenergic receptors (α1-ARs) elicit a negative inotropic effect (NIE) in the mouse right ventricular (RV) myocardium but a positive inotropic effect (PIE) in the left ventricular (LV) myocardium. Effects on myofilament Ca2+ sensitivity play a role, but effects on Ca2+ handling could also contribute. We monitored the effects of α1-AR stimulation on contraction and Ca2+ transients using single myocytes isolated from the RV or LV. Interestingly, for both the RV and LV, we found heterogeneous myocyte inotropic responses. α1-ARs mediated either a PIE or NIE, although RV myocytes had a greater proportion of cells manifesting a NIE (68%) compared with LV myocytes (36%). Stimulation of a single α1-AR subtype (α1A-ARs) with a subtype-selective agonist also elicited heterogeneous inotropic responses, suggesting that the heterogeneity arose from events downstream of the α1A-AR subtype. For RV and LV myocytes, an α1-AR-mediated PIE was associated with an increased Ca2+ transient and a NIE was associated with a decreased Ca2+ transient, suggesting a key role for Ca2+ handling. For RV and LV myocytes, α1-AR-mediated decreases in the Ca2+ transient were associated with increased Ca2+ export from the cell and decreased Ca2+ content of the sarcoplasmic reticulum. In contrast, for myocytes with α1-AR-induced increased Ca2+ transients, sarcoplasmic reticulum Ca2+ content was not increased, suggesting that other mechanisms contributed to the increased Ca2+ transients. This study demonstrates the marked heterogeneity of LV and RV cellular inotropic responses to stimulation of α1-ARs and reveals a new aspect of biological heterogeneity among myocytes in the regulation of contraction.
Keywords: α1-adrenergic, calcium, inotropic, myocyte
previously, we found interventricular differences in inotropic responses to stimulation of α1-adrenergic receptors (α1-ARs), with an overall positive inotropic effect (PIE) in left ventricular (LV) myocardium in contrast to an overall negative inotropic effect (NIE) in right ventricular (RV) myocardium (27, 28). Alterations in myofilament Ca2+ sensitivity played a role in the contrasting α1-AR inotropic responses of the LV versus RV myocardium (27, 28). However, the role of Ca2+ handling in α1-AR inotropic responses remains unclear. Previously, an interventricular difference in Ca2+ handling was reported, with smaller Ca2+ transients noted in RV myocytes versus LV myocytes (15). Here, we investigated the role of Ca2+ handling in the differing α1-AR inotropic responses of the RV versus LV. We used adult mouse ventricular myocytes from the RV or LV free wall and monitored Ca2+ transients and contractile responses in response to stimulation of α1-ARs.
Interestingly, we found that for both RV and LV myocytes, there was considerable cellular heterogeneity in the effects of α1-AR stimulation on inotropic responses. Moreover, compared with the LV, the RV had a significantly greater proportion of cells in which α1-AR stimulation elicited a NIE (68% vs. 36%). Differences in α1-AR inotropic responses appeared to be driven by effects on Ca2+ transients; moreover, α1-AR effects on Ca2+ loading of the sarcoplasmic reticulum (SR) played a role.
This study demonstrates cellular heterogeneity of α1-AR inotropic responses, which is a new aspect of biological heterogeneity among myocytes that potentially might extend to other types of receptors.
METHODS
The Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center approved all procedures.
Previously, we measured α1-AR inotropic responses using multicellular myocardial samples (trabeculae) dissected from the LV or RV (27, 28). The present study used single myocytes isolated from the LV or RV and measured α1-AR inotropic responses and fura-2-assessed Ca2+ transients.
Myocyte isolation.
Twelve-week-old male C57BL/6 mice were deeply anesthetized with pentobarbital (100 mg/kg ip) mixed with heparin (100 units). A midline thoracotomy was performed, and the heart was rapidly removed, immersed in ice-cold arrest solution [containing (in mM) 120 NaCl, 30 KCl, and 0.1 CaCl2], and mounted to a cannula by the aorta. Myocytes were isolated by enzymatic digestion of the heart by retrograde perfusion of collagenase solution through the coronary vasculature (20). After collagenase treatment, the heart was placed in a “stop buffer” to halt enzymatic digestion (20). The free wall of the RV or LV was dissected, placed in 10 ml of stop buffer, and gently teased with fine forceps followed by repeated pipetting to release the cells. Cells from only one ventricle were studied per animal. The cell isolation buffers contained 10 mM 2,3-butanedione monoxime to prevent cell contraction. Cells were used within 4 h of isolation.
The RV yielded ∼350,000 cells. The LV free wall (representing ∼50% of the LV) yielded ∼720,000 cells. These yields suggest a cell yield for the entire heart of ∼1.8 million cells, which is similar to the cell yield obtained in our previous study (17) of mouse hearts. Moreover, the ratio between RV versus LV cell yield is consistent with the ratio of RV versus LV weight for the mouse heart in our previous study (27).
Overall, we studied 163 cells from 64 animals. Few cells (2–3 cells) were studied per heart due to the difficulty of maintaining stable cell contractions for the 20–30 min required for an equilibration period followed by recording the response to an agonist. Technical problems, such as cell movement and arrhythmias, resulted in a success rate ∼50%.
Fura-2 loading.
Myocytes were loaded with fura-2 by exposure to 1 μM fura-2 AM for 20 min. After being washed, myocytes were equilibrated for 20 min to allow for deesterification of the indicator and used within 1.5 h after completion of the loading protocol.
Contractility and fluorescence measures.
Myocytes were superfused in a small glass-floored chamber with Krebs-Henseleit solution containing (in mM) 112 NaCl, 5 KC1, 1.2 MgCl2, 10 glucose, 24 NaHCO3, 1.2 Na2SO4, 2.0 NaH2PO4, and 1 CaCl2. The perfusate was oxygenated with 95% O2-5% CO2 to give a pH of 7.4 at 22°C. The chamber was mounted on an inverted Nikon Diaphot microscope, and cells were visualized at ×40 magnification. Cells were electrically stimulated with 4-ms square-wave pulses at 25 V and at a frequency of 0.5 Hz. Cells were selected based on a rod-shaped appearance with clear striations and contractions (≥2% basal length) in response to electrical stimulation. Cell contraction and fura-2 fluorescence were monitored using an IonOptix Hyperswitch system (Milton, MA). Cell contraction was computed by monitoring changes in muscle sarcomere length measured from the myocyte striation spacing. Fura-2 fluorescence at an emission wavelength of 510 nm was measured, with the excitation alternated between 340 and 380 nm at a frequency of 240 Hz. On each experimental day, for both excitation wavelengths, the background autofluorescence of the instrument plus unloaded cells was assessed (n = 5 cells), and the background autofluorescence at each wavelength was subtracted before the fura-2 fluorescence ratio was computed. Calibration factors were determined to calibrate the fura-2 ratio to units of Ca2+ concentration. Briefly, fura-2 loaded cells (n = 10) were metabolically inhibited, permeablilized, and exposed to calibration solutions of various Ca2+ concentrations, as previously described (18). Fura-2 ratio was converted to Ca2+ concentrations using the formula of Grynkiewicz (12).
Inotropic responses.
Contracting myocytes were equilibrated for 10 min in the presence of the β-AR antagonist timolol (10 μM). Myocytes were then exposed to the α1-AR agonist phenylephrine (PE) at a dose (10 μM) that mediates a near-maximal inotropic response in the mouse myocardium (13, 19, 21, 26). In a subset of experiments, myocytes were exposed to the α1A-AR subtype-selective agonist A-61603 (100 nM). Previously, we noted that myocardium from mice lacking the α1A-AR subtype did not respond to A-61603, indicating that A-61603 did not stimulate other α1-AR subtypes (18). Contraction was monitored for ∼15 min, and fura-2 transients were monitored intermittently during this time (to limit photobleaching).
Caffeine-induced contractures.
In some experiments, rapid application of caffeine was used to assess SR Ca2+ loading. Before or 10 min after exposure to PE, electrical stimulation was stopped, and, 5 s later, cells were rapidly exposed to a stream of superfusate containing 20 mM caffeine passed through a micropipette placed close to the cell (1).
Statistical analysis.
Data are presented as means ± SE. Groups were compared using Students t-test or a χ2-test.
RESULTS
RV cells have smaller Ca2+ transients and slower relaxation than LV cells.
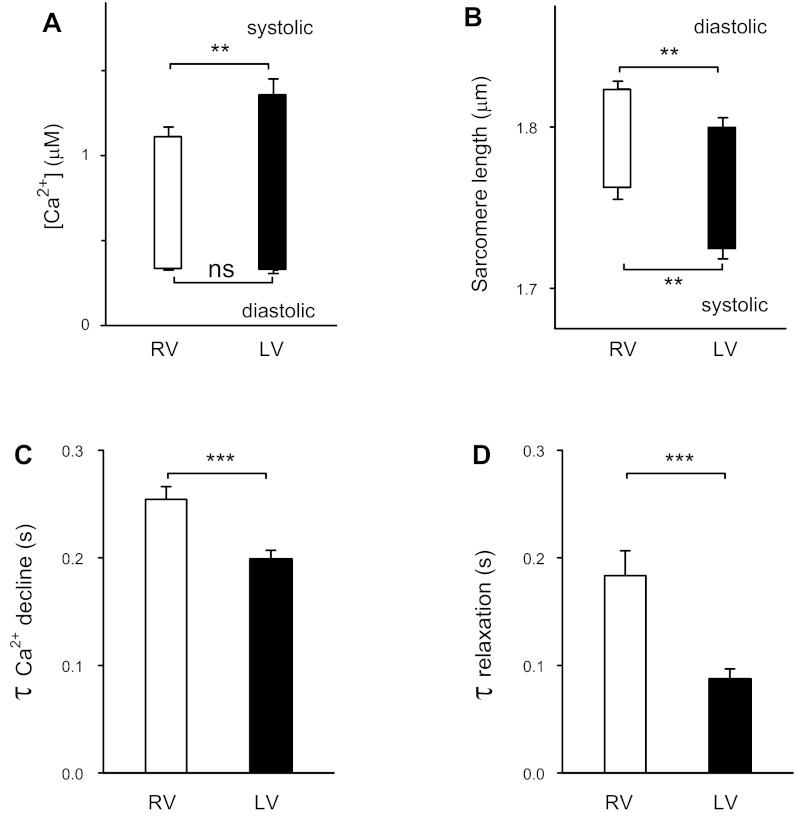
Figure 1 shows the contraction and Ca2+-handling baseline characteristics of LV versus RV cells. Consistent with a previous study (15), RV cells had smaller Ca2+ transients and slower relaxation than LV cells. New findings were that compared with LV cells, the decline phase of the Ca2+ transient was slower in RV cells and the diastolic sarcomere length was longer in RV cells.
Fig. 1.
Baseline recording of Ca2+ transients and contractions of right ventricular (RV) myocytes (n = 31) and left ventricular (LV) myocytes (n = 28). A: diastolic and systolic Ca2+ levels. B: sarcomere length. C: time constant (τ) of Ca2+ decline. D: τ of relaxation of contraction. **P < 0.01; ***P < 0.001.
Consistent with a previous report (15), for electrically stimulated cells, RV cells had significantly lower systolic Ca2+ levels compared with LV cells (1.11 ± 0.06 μM, n = 30, vs. 1.35 ± 0.1 μM, n = 26, P < 0.05; Fig. 1A).
Figure 1B shows that for contracting cells, the diastolic sarcomere length of RV cells was 24 nm longer than for LV cells (1.823 ± 0.005 μm, n = 31, vs. 1.799 ± 0.007 μm, n = 28, P < 0.01). This was not due to differences in diastolic Ca2+ levels, which did not differ between LV and RV cells (Fig. 1A). Furthermore, this difference in sarcomere length was evident in quiescent cells maintained in the cell isolation buffer containing 2,3-butanedione monoxime and without Ca2+ (not shown). Thus, effects of contraction did not play a role in the longer diastolic sarcomere length of RV versus LV cells.
The systolic sarcomere length of RV cells was 37 nm longer than LV cells (1.763 ± 0.008 μm, n = 31, vs. 1.726 ± 0.007 μm, n = 28, P < 0.01). Consistent with smaller Ca2+ transients in RV cells, there was a trend for the mean contraction amplitude (difference between diastolic and systolic sarcomere lengths) for RV cells to be smaller (80%) compared with LV cells, but the difference did not reach statistical significance (P = 0.09).
Quantitation of the relaxation phase used the time constant estimated for the exponential decline of signals for Ca2+ concentration and sarcomere length. Compared with LV cells, RV cells had slower Ca2+ transient decline (greater time constant value; Fig. 1C). Furthermore, RV cells had a slower time course of relaxation than LV cells, consistent with a previous report (15). The slower Ca2+ transient decline might have contributed to the slower relaxation in RV cells compared with LV cells.
In summary, compared with LV myocytes, RV myocytes have smaller Ca2+ transients with slower Ca2+ decline and somewhat smaller contractions with slower relaxation.
Cellular heterogeneity of α1-AR inotropic responses.
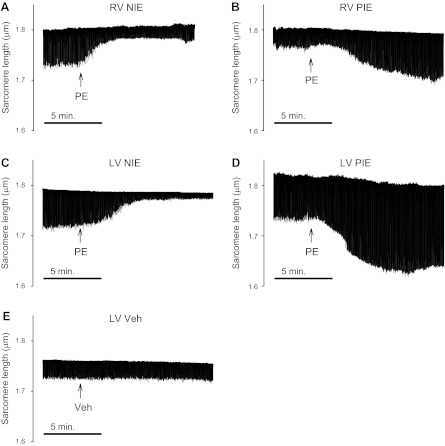
Previously, we found that α1-AR stimulation resulted in a NIE in the RV myocardium but a PIE in the LV myocardium (27, 28). Interestingly, in this study, when examined at the cellular level, considerable heterogeneity of α1-AR inotropic responses was noted. Figure 2 shows that α1-AR stimulation manifested either a NIE or PIE in cells from the RV free wall (A and B) or cells from the LV free wall (C and D). For all cells studied, there was no relation between baseline contractility and the direction of the inotropic response. For example, the cells shown in Fig. 2, C and D, had similar baseline contraction amplitudes but directionally opposite inotropic responses.
Fig. 2.
Slow time-based recordings of electrically stimulated contractions before and after stimulation of α1-adrenergic receptors (α1-ARs) with 10 μM phenylephrine (PE) plus the β-blocker timolol (10 μM). A: addition of PE (arrow) elicited a negative inotropic effect (NIE) in some RV cells. B: PE elicited a positive inotropic effect (PIE) in other RV cells. C and D: LV cells also manifest a NIE or PIE after α1-AR stimulation. E: there was in no inotropic response to treatment with the vehicle (Veh) control.
For RV cells, α1-AR stimulation with PE elicited a NIE in the majority (68%) of cells (21 of 31 cells, P < 0.05; Fig. 3A). This is consistent with the overall NIE of α1-AR stimulation in the intact RV myocardium. In contrast, for LV cells, α1-AR stimulation elicited more of a mixed response, with a NIE in a minority (36%) of cells (10 of 28 cells) and a PIE in a majority (64%) of cells (18 of 28 cells), perhaps reflecting the overall PIE elicited by α1-AR stimulation in the intact LV myocardium.
Fig. 3.
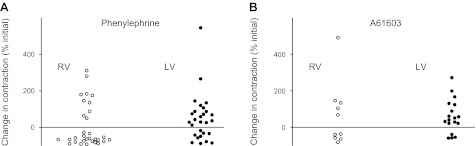
Summary of α1-AR inotropic responses of RV and LV myocytes (expressed as the percent change from the baseline contraction level for each cell). Heterogeneity of inotropic responses was observed using the nonsubtype-selective α1-AR agonist PE (A) and also using the α1A-AR subtype-selective agonist A-61603 (B).
Few cells from either the RV or LV were studied per heart (range: 1–5 cells, average 2.4 cells/heart). Nevertheless, for the majority (66%) of the 21 hearts from which at least 2 cells were studied, α1-AR stimulation with PE elicited both PIE and NIE types of the cellular response. This indicates that the observed cellular heterogeneity of α1-AR responses existed within hearts rather than arising from variability between animals.
Cellular heterogeneity of α1A-AR subtype inotropic responses.
There are two predominant α1-AR subtypes on cardiac myocytes (α1A and α1B), which may have different roles in cardiac inotropy (6, 9, 11, 16, 22). Therefore, in a subgroup of cells, we tested the role of a single α1-AR subtype (α1A-ARs) in cellular inotropic responses. For both RV and LV myocytes, we found that the highly selective α1A-AR agonist A-61603 mediated either a PIE or a NIE (Fig. 3B). Thus, the single α1A-AR subtype could mediate fundamentally different inotropic responses, suggesting that the type of inotropic response (PIE or NIE) for a particular cell depends on events downstream from the receptor. Similar to PE, A-61606 elicited a PIE in the majority (82%) of LV cells (14 of 17 cells).
Cellular heterogeneity of α1-AR effects on Ca2+ handling.
Contraction and relaxation are triggered by the rise and fall of activator Ca2+. Therefore, we determined the role of activator Ca2+ in the heterogeneous α1-AR inotropic responses of LV and RV myocytes.
Figure 4 shows that fura-2-induced Ca2+ transients and sarcomere length transients measured in LV and RV myocytes before and after α1-AR stimulation with PE elicited either a NIE (A and C) or a PIE (B and D). Evidently, the α1-AR-mediated NIE was associated with a marked decrease of the Ca2+ transient, whereas the PIE was associated with an increase of the Ca2+ transient.
Fig. 4.
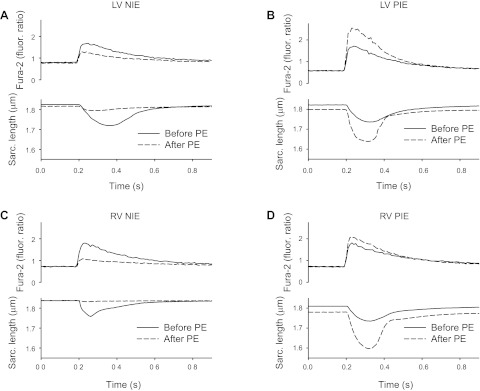
Examples of a NIE and PIE elicited by α1-ARs in LV and RV myocytes. Records of fura-2-induced Ca2+ transients and cell contractions (assessed from sarcomere length measures) are shown. Compared with before the addition of PE, α1-AR stimulation caused decreased Ca2+ transients and decreased contractions in some cells (A and C) or increased Ca2+ transients and contraction in other cells (B and D).
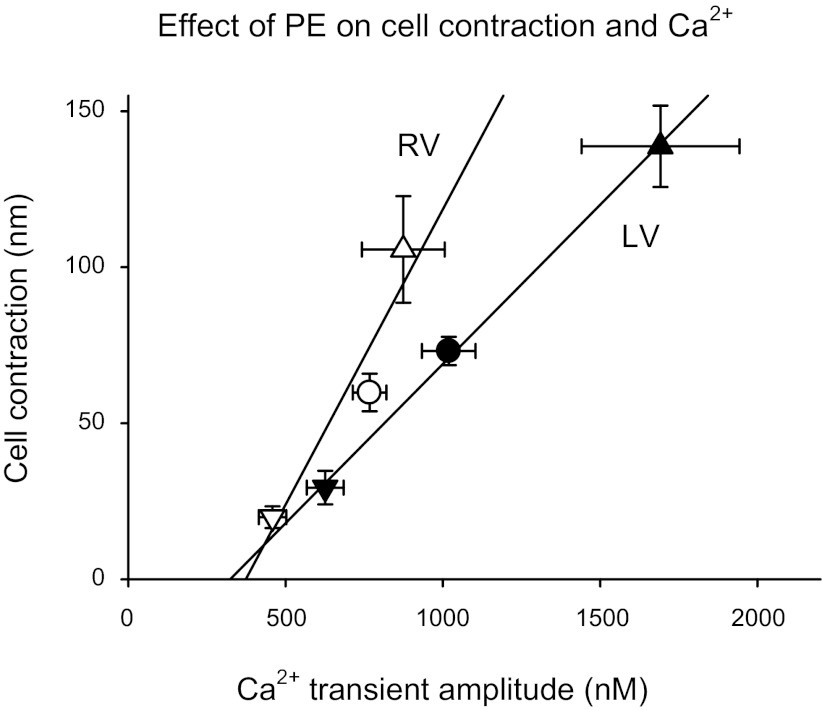
Although changes in Ca2+ and sarcomere length are not in a dynamic equilibrium during myocyte contraction, nevertheless there is a positive correlation between the size of the Ca2+ transient and the corresponding magnitude of contraction (10). The pooled data shown in Fig. 5 demonstrate the relationship between cell contraction amplitude and the corresponding Ca2+ transient amplitude. For LV myocytes, there was a linear relationship between contraction amplitude and Ca2+ transient amplitude for cells before and after α1-AR stimulation with PE. This relationship suggests that the NIE or PIE of α1-AR stimulation was appreciably driven by corresponding decreases or increases of Ca2+ transient amplitude. Figure 5 shows that RV myocytes also manifested a relationship between cell contraction and Ca2+ transient amplitude. This relationship appeared steeper for RV myocytes than for LV myocytes, suggesting that for a PIE elicited by PE, the myofilament Ca2+ responsiveness of RV myocytes was greater than for LV myocytes.
Fig. 5.
Relationship between the amplitude of myocyte contraction and Ca2+ transient amplitude for RV cells (open symbols) and LV cells (solid symbols). Data are shown for cells before the addition of PE (●) and after 10 min in the presence of PE for cells that manifested a PIE (▲) or a NIE (▼).
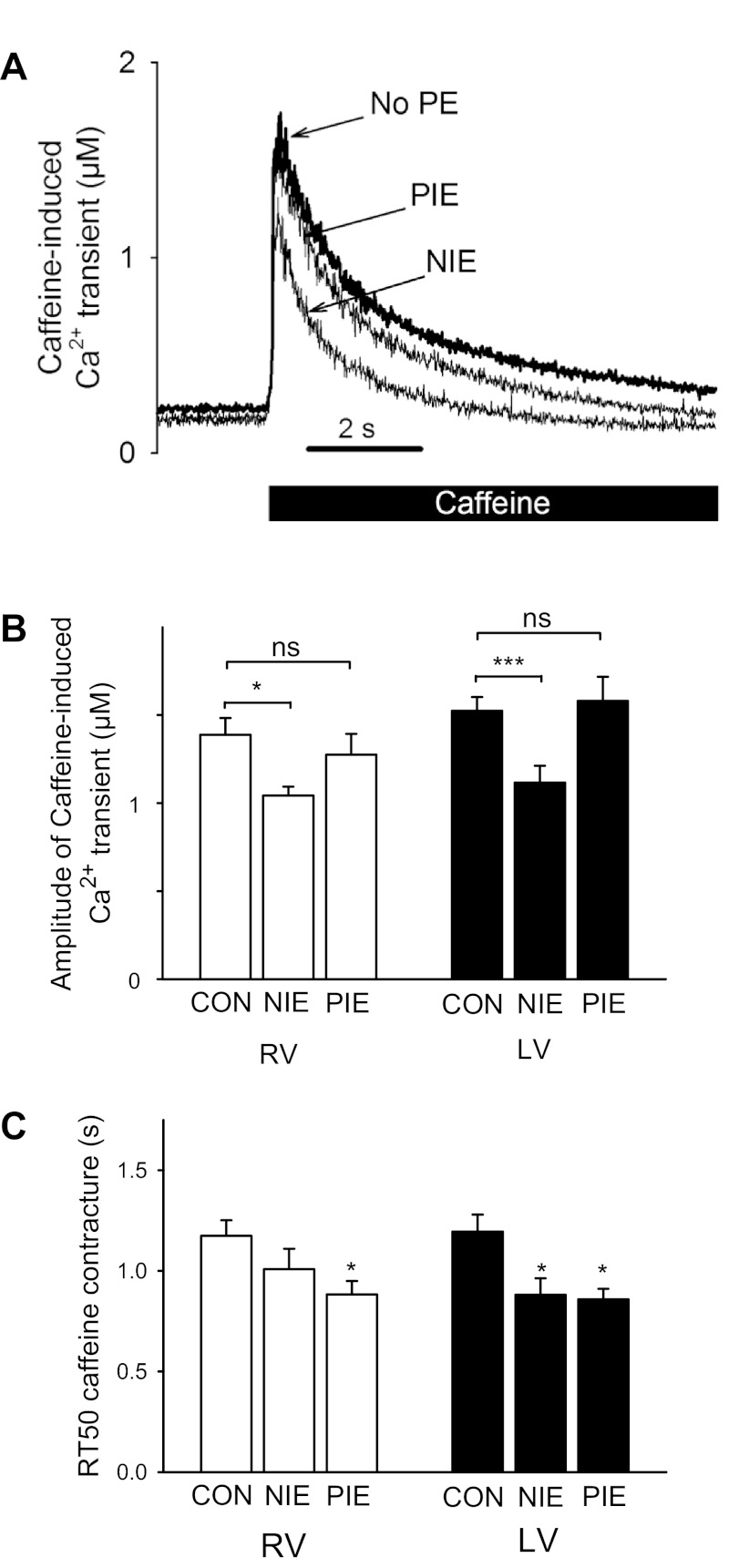
Numerous factors contribute to the amplitude of the Ca2+ transient. We investigated if α1-AR effects on Ca2+ transients were linked to changes in Ca2+ loading of the SR. SR Ca2+ loading was assessed by a rapid application of 20 mM caffeine, which causes rapid unloading of SR Ca2+. Figure 6A shows that caffeine caused a rapid rise of intracellular Ca2+ concentration followed by a slow decline of Ca2+ concentration. Figure 6A shows that for cells manifesting an α1-AR-induced NIE, the peak of the caffeine-induced Ca2+ transient was reduced compared with cells without α1-AR stimulation by PE. In contrast, cells manifesting an α1-AR-induced PIE did not have an increase in the peak of the caffeine-induced Ca2+ transient compared with cells without α1-AR stimulation.
Fig. 6.
Effects on α1-AR stimulation on Ca2+ handling as assessed with caffeine-induced contractures. A: records of caffeine-induced Ca2+ transients in LV cells before the addition of PE and 10 min after PE induced either a NIE or a PIE. B: summary of caffeine-induced Ca2+ transients before (Con) and after α1-AR stimulation induced a NIE or PIE in RV or LV cells. C: summary of the time course of decline of the caffeine-induced Ca2+ transient as assessed from the relaxation half-time (RT50; same groups as in B). RV and LV myocyte numbers were 17 and 14 for Con, 8 and 8 for NIE, and 9 and 7 for PIE, respectively. *P < 0.05; ***P < 0.001.
The pooled data (Fig. 6B) demonstrate that for both RV and LV cells, an α1-AR-induced NIE was associated with a reduced caffeine-induced Ca2+ transient. In contrast, an α1-AR-induced PIE was not associated with a change in the caffeine-induced Ca2+ transient compared with cells without α1-AR stimulation. This suggests that for LV and RV cells, the α1-AR-induced NIE may have been contributed to by a reduction in SR Ca2+ loading. In contrast, for LV and RV cells, the α1-AR-induced PIE did not involve increased SR Ca2+ loading.
The decline of Ca2+ concentration in the presence of caffeine reflects Ca2+ transport from the cytosol by other Ca2+-handling systems besides sarco(endo)plasmice reticulum Ca2+-ATPase, principally, Ca2+ export from the cell by the Na+/Ca2+ exchange (1). Figure 6C shows that for both LV and RV cells, α1-AR stimulation tended to increase the rate of Ca2+ decline by these other Ca2+ transport systems. After α1-AR stimulation, faster Ca2+ export from the cell via Na+/Ca2+ exchange could contribute to decreased SR Ca2+ load and a NIE. In contrast, faster Ca2+ export from the cell would not contribute to an α1-AR-induced PIE.
DISCUSSION
The major finding of this study was that the free walls of both the RV and LV are composed of myocytes that manifest considerable cell to cell heterogeneity in their Ca2+ handling and contractile responses to stimulation of α1-ARs. This finding reveals a new aspect of biological heterogeneity among cardiac myocytes that might encompass other regulatory systems. Moreover, this study illuminates a complexity to the effects of α1-ARs on Ca2+ signaling and contraction that was not suspected from previous studies of the intact myocardium.
Cellular heterogeneity in the heart.
The heart is known to manifest regional heterogeneity, with reports of transmural differences between the endocardium and epicardium in voltage-gated K+ currents (2), action potential duration (14), excitation-contraction coupling (7), myosin light chain kinase abundance (5), and myofilament properties (3, 4, 25).
We have previously reported that α1-ARs mediated fundamentally different inotropic responses in the RV versus LV myocardium (27, 28). This difference was associated with different effects of α1-AR stimulation on myofilament Ca2+ sensitivity of the LV versus RV myocardium (28). However, α1-ARs also influence Ca2+ handling. Moreover, differences in Ca2+ handling between the RV and LV have been reported (15). Therefore, we investigated the effects of α1-AR stimulation on Ca2+ handling in RV and LV myocytes.
Cellular heterogeneity of Ca2+ signaling and inotropic responses to α1-adrenergic stimulation.
At the cellular level, the effects of α1-AR stimulation were complex. We found considerable cell-to-cell variability in α1-AR effects on Ca2+ signaling and contraction. For cells from the RV or LV free wall, α1-ARs elicited a NIE in some cells but a PIE in other cells. The α1-AR-induced NIE was associated with a decreased Ca2+ transient, and the α1-AR-induced PIE was associated with an increased Ca2+ transient. These findings suggest that α1-AR-induced changes in the Ca2+ transient played a key role in the inotropic response.
We found that cells with an α1-AR-induced NIE and reduced Ca2+ transient manifested a reduction in SR Ca2+ loading (as assessed from the caffeine-induced Ca2+ transient). Reduced SR Ca2+ load induced by α1-ARs might contribute to the observed reduction of Ca2+ transients and the NIE. Moreover, with transport of Ca2+ to the SR prevented in the presence of caffeine, the decay of the caffeine-induced Ca2+ transient is thought to reflect other Ca2+ transport systems, predominantly the Na+/Ca2+ exchanger, which exports Ca2+ out of the cell (1). We found that α1-AR stimulation accelerated the decay of the caffeine-induced Ca2+ transient. This is consistent with α1-AR-induced stimulation of Na+/Ca2+ exchange (19). Faster export of Ca2+ from the cell could contribute to a decreased SR Ca2+ load and the NIE elicited by α1-AR stimulation.
In contrast, cells with an α1-AR-induced PIE and increased Ca2+ transients did not manifest an increased SR Ca2+ load, suggesting that other mechanisms contributed to the increased Ca2+ transient [e.g., prolongation of the action potential (8), increased Ca2+ current, and increased gain of Ca2+-induced Ca2+ release from the SR].
Cellular heterogeneity of α1-AR inotropic responses in the RV versus LV.
The inotropic response to α1-AR stimulation has remained uncertain because it has varied considerably among studies and may be influenced by experimental conditions (23, 28). The results of the present study demonstrate that cellular heterogeneity within both the RV and LV is another complexity to α1-AR inotropic responses. Moreover, the fraction of cells manifesting an α1-AR-mediated NIE was significantly greater in the RV (68%) than in the LV (36%). This trend appears consistent with a previous report (28) of α1-ARs mediating a NIE in the RV myocardium. Thus, both intraventricular and interventricular factors may play a role in α1-AR inotropy.
Previous studies (6, 9, 11, 16, 22) have suggested that α1-AR subtypes may have different roles in cardiac inotropy, with the α1A-AR subtype mediating positive inotropy and the α1B-AR playing a negative modulatory role. However, in myocytes, we found that the α1A-AR subtype could mediate either a positive or negative inotropic response. This finding is consistent with our previous report (28), which showed that the α1A-AR agonist A-61603 mediated negative inotropy in the RV myocardium but positive inotropy in the LV myocardium, again indicating that the α1A-AR subtype can mediate a PIE or a NIE. Together, these findings suggest that events downstream from the α1A-AR subtype determine whether a PIE or NIE is elicited. The present study identifies that differential effects of α1-ARs on Ca2+ handling play a critical role.
Limitations.
The clinical disease relevance of the presence of heterogeneous α1-AR responses is unclear. Potentially, the balance of cells manifesting a PIE versus a NIE elicited by α1-ARs may shift in disease and thereby impact the myocardial α1-AR inotropic response. For example, we (27) have previously reported a shift in the myocardial α1-AR inotropic response from a NIE in the nonfailing RV to a PIE in the failing RV.
This study indicates that effects of α1-ARs on Ca2+ handling contribute to the heterogeneous inotropic responses of myocytes. Further studies will be required to identify the specific mechanisms downstream from α1-ARs that determine the inotropic response.
Like most studies of myocytes, only a small number of myocytes per heart were studied. However, the myocytes that survive the isolation process may not be truly representative of the total myocyte populations of the RV or LV. Cells were studied under artificial in vitro conditions, and thus it is not clear how well the in vitro cell behavior reflects that of cells in vivo. We studied mouse cells, which are similar to humans in cardiac α1-AR abundance (24). However, it will be important to study cells from other species. As we studied only a single class of receptors, it will be interesting to determine if there is cellular heterogeneity in responses to other receptor classes.
Conclusions.
This study demonstrates the intraventricular and interventricular heterogeneity of cellular inotropic responses to stimulation of α1-ARs. A single α1-AR subtype (α1A) can manifest either a PIE or a NIE. Multiple mechanisms, including effects on Ca2+ transients, SR Ca2+ loading, and export of Ca2+ from the cell, are involved. This study reveals a new aspect of biological heterogeneity among cardiac myocytes in the regulation of contraction.
GRANTS
This work was supported by Veterans Affairs Merit Awards (to A. J. Baker and to D. H. Lovett), an American Heart Association-Western States grant-in-aid (to A. J. Baker), and National Heart, Lung, and Blood Institute Grant HL-31113 (to P. C. Simpson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.C., K.V.T., K.W.P., P.W., O.M., and D.H.L. performed experiments; C.C., K.V.T., K.W.P., P.W., and A.J.B. analyzed data; D.H.L., P.C.S., and A.J.B. edited and revised manuscript; D.H.L., P.C.S., and A.J.B. approved final version of manuscript; A.J.B. conception and design of research; A.J.B. interpreted results of experiments; A.J.B. prepared figures; A.J.B. drafted manuscript.
REFERENCES
- 1.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol 476: 279–293, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559: 103–120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cazorla O, Lacampagne A. Regional variation in myofilament length-dependent activation. Pflügers Arch 462: 15–28, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Cazorla O, Pascarel C, Garnier D, Le Guennec JY. Resting tension participates in the modulation of active tension in isolated guinea pig ventricular myocytes. J Mol Cell Cardiol 29: 1629–1637, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell 107: 631–641, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Deng XF, Sculptoreanu A, Mulay S, Peri KG, Li JF, Zheng WH, Chemtob S, Varma DR. Crosstalk between alpha-1A and alpha-1B adrenoceptors in neonatal rat myocardium: implications in cardiac hypertrophy. J Pharmacol Exp Ther 286: 489–496, 1998 [PubMed] [Google Scholar]
- 7.Dilly KW, Rossow CF, Votaw VS, Meabon JS, Cabarrus JL, Santana LF. Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall. J Physiol 572: 227–241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedida D, Bouchard RA. Mechanisms for the positive inotropic effect of alpha 1-adrenoceptor stimulation in rat cardiac myocytes. Circ Res 71: 673–688, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Gambassi G, Spurgeon HA, Ziman BD, Lakatta EG, Capogrossi MC. Opposing effects of α1-adrenergic receptor subtypes on Ca2+ and pH homeostasis in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 274: H1152–H1162, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Direct evidence for decreased myofilament Ca2+ responsiveness and altered diastolic function in intact ventricular muscle. Circ Res 76: 1036–1048, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Grupp IL, Lorenz JN, Walsh RA, Boivin GP, Rindt H. Overexpression of α1B-adrenergic receptor induces left ventricular dysfunction in the absence of hypertrophy. Am J Physiol Heart Circ Physiol 275: H1338–H1350, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 13.Hirano S, Kusakari Y, JOU , Morimoto S, Kawai M, Hongo K, Kurihara S. Intracellular mechanism of the negative inotropic effect induced by alpha1-adrenoceptor stimulation in mouse myocardium. J Physiol Sci 56: 297–304, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kim N, Cannell MB, Hunter PJ. Changes in the calcium current among different transmural regions contributes to action potential heterogeneity in rat heart. Prog Biophys Mol Biol 103: 28–34, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, Giles WR. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 571: 131–146, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, Woodcock EA, Feneley MP, Graham RM. Targeted α1A-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res 89: 343–350, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Lopez JE, Myagmar BE, Swigart PM, Montgomery MD, Haynam S, Bigos M, Rodrigo MC, Simpson PC. β-Myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circ Res 109: 629–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCloskey DT, Rokosh DG, O'Connell TD, Keung EC, Simpson PC, Baker AJ. α1-Adrenoceptor subtypes mediate negative inotropy in myocardium from α1A/C-knockout and wild type mice. J Mol Cell Cardiol 34: 1007–1017, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Nishimaru K, Kobayashi M, Matsuda T, Tanaka Y, Tanaka H, Shigenobu K. α-Adrenoceptor stimulation-mediated negative inotropism and enhanced Na+/Ca2+ exchange in mouse ventricle. Am J Physiol Heart Circ Physiol 280: H132–H141, 2001 [DOI] [PubMed] [Google Scholar]
- 20.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Petrashevskaya NN, Bodi I, Koch SE, Akhter SA, Schwartz A. Effects of alpha1-adrenergic stimulation on normal and hypertrophied mouse hearts. Relation to caveolin-3 expression. Cardiovasc Res 63: 561–572, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ross SA, Rorabaugh BR, Chalothorn D, Yun J, Gonzalez-Cabrera PJ, McCune DF, Piascik MT, Perez DM. The α1B-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovasc Res 60: 598–607, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Steinberg SF. α1-Adrenergic receptor subtype function in cardiomyocytes: lessons from genetic models in mice. J Mol Cell Cardiol 34: 1141–1145, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Steinfath M, Chen YY, Lavicky J, Magnussen O, Nose M, Rosswag S, Schmitz W, Scholz H. Cardiac alpha 1-adrenoceptor densities in different mammalian species. Br J Pharmacol 107: 185–188, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Velden J, Merkus D, de Beer V, Hamdani N, Linke WA, Boontje NM, Stienen GJ, Duncker DJ. Transmural heterogeneity of myofilament function and sarcomeric protein phosphorylation in remodeled myocardium of pigs with a recent myocardial infarction. Front Physiol 2: 83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varma DR, Rindt H, Chemtob S, Mulay S. Mechanism of the negative inotropic effects of alpha 1-adrenoceptor agonists on mouse myocardium. Can J Physiol Pharmacol 81: 783–789, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Yeh CC, Jensen BC, Mann MJ, Simpson PC, Baker AJ. Heart failure switches the RV α1-adrenergic inotropic response from negative to positive. Am J Physiol Heart Circ Physiol 298: H913–H920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to α1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol 291: H2013–H2017, 2006 [DOI] [PubMed] [Google Scholar]