Abstract
Mitochondrial damage and dysfunction occur during ischemia and modulate cardiac function and cell survival significantly during reperfusion. We hypothesized that transplantation of autologously derived mitochondria immediately prior to reperfusion would ameliorate these effects. New Zealand White rabbits were used for regional ischemia (RI), which was achieved by temporarily snaring the left anterior descending artery for 30 min. Following 29 min of RI, autologously derived mitochondria (RI-mitochondria; 9.7 ± 1.7 × 106/ml) or vehicle alone (RI-vehicle) were injected directly into the RI zone, and the hearts were allowed to recover for 4 wk. Mitochondrial transplantation decreased (P < 0.05) creatine kinase MB, cardiac troponin-I, and apoptosis significantly in the RI zone. Infarct size following 4 wk of recovery was decreased significantly in RI-mitochondria (7.9 ± 2.9%) compared with RI-vehicle (34.2 ± 3.3%, P < 0.05). Serial echocardiograms showed that RI-mitochondria hearts returned to normal contraction within 10 min after reperfusion was started; however, RI-vehicle hearts showed persistent hypokinesia in the RI zone at 4 wk of recovery. Electrocardiogram and optical mapping studies showed that no arrhythmia was associated with autologously derived mitochondrial transplantation. In vivo and in vitro studies show that the transplanted mitochondria are evident in the interstitial spaces and are internalized by cardiomyocytes 2–8 h after transplantation. The transplanted mitochondria enhanced oxygen consumption, high-energy phosphate synthesis, and the induction of cytokine mediators and proteomic pathways that are important in preserving myocardial energetics, cell viability, and enhanced post-infarct cardiac function. Transplantation of autologously derived mitochondria provides a novel technique to protect the heart from ischemia-reperfusion injury.
Keywords: cardioprotection, ischemia, mitochondria, myocardial infarction
the heart is a highly energetic organ that requires a continuous supply of oxygen to maintain normal function. Under aerobic conditions, the heart derives its energy primarily from the mitochondria, which constitute 30% of the total myocardial cell volume (27). Mitochondria are critical for the synthesis of high-energy phosphates, modulation of calcium stores, and activation of signaling pathways that impact cell fate directly. The maintenance of these processes requires oxygen; therefore, cessation of blood flow causing ischemia can be catastrophic. Previous studies have shown that following the onset of ischemia there is a rapid decline in high-energy phosphate levels with alterations in mitochondrial structure, volume, oxygen consumption, and ATP synthesis (35).
Attempts to lessen myocardial tissue necrosis and improve postischemic function using pharmacological and/or exogenous substrate interventions, either alone or in combination with procedural techniques, have provided only limited cardioprotection (31, 41, 42). Despite these interventions, mitochondrial damage and dysfunction continue to represent major problems following myocardial ischemia and remain significant causes of morbidity and mortality.
Our studies and studies by other investigators have shown that mitochondrial damage occurs mainly during ischemia rather than during reperfusion and that preservation of mitochondrial respiratory function during reperfusion enhances contractile recovery and decreases myocardial infarct size (5, 16, 25). We hypothesized that an alternative approach to modulate myocardial necrosis and decrease postischemic heart function would be to augment the mitochondria damaged during ischemia with autologously derived mitochondria isolated from remote tissue unaffected by ischemia that could then be transplanted into the ischemic area of the heart immediately before reperfusion. This treatment would then allow for reduced necrosis and enhanced myocardial function.
Herein, we present data using the in situ blood-perfused regional ischemic heart model with 4-wk recovery and in vivo and in vitro studies to demonstrate that the transplantation of viable autologously derived mitochondria provides cardioprotection from ischemia-reperfusion injury. We show that the transplanted mitochondria are internalized by cardiomyocytes 2 h after transplantation and provide enhanced oxygen consumption, upregulate chemokines that enhance post-infarct cardiac function, and upregulate the expression of protein pathways that are important in preserving myocardial energetics.
METHODS
Experimental protocol.
The experimental protocol is shown in Fig. 1A. New Zealand White rabbits male (3–4 kg, n = 54; Millbrook Farm, Amherst, MA) were used for the experiments. All experiments were approved by the Institutional Animal Care and Use Committee at Harvard Medical School and conformed to the National Institutes of Health (NIH) guidelines regulating the care and use of laboratory animals (NIH Publication No. 5377-3, 1996). All research was performed in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Fig. 1.
Experimental protocol and mitochondrial isolation. A: New Zealand white rabbits (n = 33, male, 3–4 kg; Millbrook Farm, Amherst, MA) were used for in situ blood-perfused regional ischemia. There were no animal deaths or exclusions. Rabbits were sedated and then anesthetized, and a left mini-thoracotomy was performed. B: the pectoralis major was isolated, and 2 biopsy samples were obtained using a no. 6 biopsy punch and used for autologous mitochondria isolation. Representative tissue samples are shown. The hearts received eight 0.1-ml injections of either respiration buffer (RI-vehicle) or respiration buffer containing mitochondria (RI-mitochondria; 9.7 ± 1.7 × 106 mitochondria) into the ischemic zone. Injections were made obliquely using an insulin syringe with a 28-gauge needle. At 30 min of regional ischemia, the snare was released and the animals were allowed to recover for 2 h or 28 days, and biochemical analysis, histology, and immunology was performed. Serial electrocardiograms, echocardiography, and blood samples were obtained for analysis. C: isolated mitochondria are shown under phase contrast illumination [bright field (BF)] at left and under fluorescence labeled with MitoTracker Red CMXRos (MTRed; middle). The merged image is shown at right. Mitochondrial viability was >99.99%. D: mitochondrial yield per gram tissue wet weight is shown. Correlation coefficient and slope intercept form are shown. E: transmission electron microscopy of isolated mitochondria (scale bars, 500 nm). *Isolated mitochondria were electron dense, with <0.01% of the mitochondria being fractured or damaged. F–H: mitochondrial complex I–V (F), state 3 (active) oxygen consumption (ADP-stimulated respiration; G), and respiratory control index (state 3/state 4) for malate (complex I) and succinate induced (complex II) in energized autologously derived pectoralis major mitochondria (H) are shown. All results are shown as means ± SE for n = 6 analyses. Results demonstrate that isolated mitochondria from pectoralis major are viable and are respiration competent. hs-CRP, high-sensitivity C-reactive protein; cTnI, cardiac troponin-I; CK-MB, creatine kinase MB; ECG, electrocardiogram; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
The rabbits were sedated with intramuscular administration of acepromazine (0.5 mg/kg im). A 22-gauge intravenous (iv) catheter was inserted into the marginal ear vein and secured with tape, and the rabbits were given an injection of 35 mg/kg ketamine and 2.5 mg/kg iv xylazine. This intravenous line was also used intraoperatively to administer heparin and Lactated Ringer's solution at a rate of 10 ml·kg−1·h−1. Heparin was injected (3 mg/kg iv via the intervenous line).
Prior to intubation, the larynx was sprayed with 1% lidocaine to prevent laryngospasm. A cuffed endotracheal tube (pediatric size 3-0 or 3-5 ID) was blindly inserted, and the animals were placed on mechanical ventilation (40% oxygen, tidal volume, 10 ml/kg; ventilation rate, 12–20 breaths/min). Proper endotracheal tube placement was verified by auscultation and observation of condensation of the end of the tube. General anesthesia was maintained with 3.0% isoflurane (Forane; Abbott Laboratories, North Chicago, IL) at the onset of surgical preparation, and the concentration was reduced to 1.0% and maintained at that concentration for the duration of the surgical procedure.
The surgical sites were shaved and prepped with Betadine and 70% isopropyl alcohol, each applied in triplicate and patted dry with sterile gauze pads, and the entire animal (except for the surgical sites) was draped with sterile towels. Core temperature was monitored continually and maintained at >36°C using a heating pad. Intermittent noninvasive blood pressure and continuous pulse oximeter were also monitored.
Under general anesthesia and using sterile techniques, a small left thoracotomy was performed through the fourth intercostal space. The pectoralis major muscle was isolated, and two small muscle samples (∼0.3 g) were removed using a no. 6 biopsy punch (Miltex, York, PA) and placed in sterile saline in a sterile falcon tube and used for mitochondrial isolation under sterile conditions.
Preischemia lasted for 60 min to allow for establishment of equilibrium hemodynamics and mitochondrial isolation and labeling. The pericardium was opened, and the left anterior descending artery (LAD) located, and a Prolene thread (3-0) (Ethicon, Somerville, NJ) was passed around the artery with a taper needle, and both ends of the Prolene tie were threaded through a small vinyl tube to form a snare. The coronary artery was occluded by pulling the snare, which was then fixed by clamping the tube with a mosquito clamp. Regional ischemia was confirmed visually by regional cyanosis of the myocardial surface. Regional ischemia was induced for 30 min under anesthesia. The regional ischemia area (area at risk) was circumscribed in vivo using a Prolene thread (3-0). This region was not used for measurement of infarct size (see below) but only for identification of tissue sites for procurement of tissue samples for histochemical and microscopy studies.
Mitochondrial isolation.
Mitochondria were isolated from pectoralis major muscle tissue and used immediately for injection (24). The isolated mitochondria were suspended in ∼3–4 ml of respiration buffer containing 250 mmol/l sucrose, 2 mmol/l KH2PO4, 10 mmol/l MgCl2, 20 mmol/l K+-HEPES buffer, pH 7.2, 0.5 mmol/l K+-EGTA, pH 8.0, 5 mmol/l glutamate, 5 mmol/l malate, 8 mmol/l succinate, and 1 mmol/l ADP.
Mitochondrial oxygen consumption and complex activity.
Mitochondrial oxygen consumption and complex activities were determined as described previously (24).
Mitochondrial labeling and number.
Mitochondria were labeled with MitoTracker Red CMXRos (Invitrogen, Carlsbad, CA), and the number of mitochondria was determined as described previously (24).
Experimental groups.
Two experimental groups, regional ischemia (RI)-vehicle (vehicle alone) and RI-mitochondria (autologously derived mitochondria), were used for investigation. Following 29 min of regional ischemia, hearts received either 8 × 0.1 ml injections of sterile respiration buffer (RI-Vehicle) into the area at risk or 8 × 0.1 ml injections of sterile respiration buffer containing mitochondria 9.7 × 106 ± 1.7 × 106/ml (RI-mitochondria). Each injection site in RI-mitochondria received 1.2 × 106 of mitochondria. Injections were made using a sterile 1-ml insulin syringe with a 28-gauge needle (24).
Following 30 min of regional ischemia, the snare was released and the animal allowed to recover for 2 h or 28 days. The Prolene thread (3-0) used for the snare was trimmed and loosely tied and left in place to identify the area at risk and infarct size.
For animals recovering for 28 days, the pericardium was closed, and pleural air was evacuated over a needle thoracentesis. The thoracotomy incision was closed with 3-0 Prolene sutures in a simple interrupted pattern. The muscles and subcutaneous layers were closed with 4-0 Vicryl (Ethicon) running sutures. The skin incision was closed with a buried running suture to minimize irritation felt by the animal. All closures were performed under anesthesia.
Following closure of the wound, the animal was weaned from anesthesia and allowed to recover on a warming pad, with laboratory staff continuously monitoring the animals until they were fully recovered (awake and able to walk on their own). A 22-gauge intravenous catheter in the marginal ear vein was left in place and loaded with 0.9% sterile saline.
Thirty minutes prior to the end of the procedure, a first dose of buprenorphine (0.03 mg/kg im) and a fentanyl patch (4 mg/kg, transdermal for 72 h) were administered for pain prophylaxis.
Electrocardiography.
Standard 12-lead electrocardiograms (ECG) (3 limb leads: I, II, and III; 3 computed augmented leads: aVL, aVR, and aVF; and 6 precordial leads: V1, V2, V3, V4, V5, and V6) were recorded at preischemic baseline and at 120 min of reperfusion and serially at 7–28 days of recovery using the Nasiff CardioCard PC-based resting ECG system (Nasiff Associates, Central Square, NY). The recordings were analyzed for heart rate, rhythm, ST segment deviation, and development of infarct-related Q waves and/or conduction system defects such as bundle branch block.
Echocardiography.
Two-dimensional (2D) echocardiograms were obtained during preischemia immediately prior to the induction of regional ischemia and at 120 min of reperfusion and serially at 7–28 days of recovery using a SONOS 5500 with an S12 Pediatric Sector Probe (Philips, Eindhoven, The Netherlands). 2D echocardiograms were acquired from the left parasternal and apical views to assess regional myocardial function. Left ventricular end-diastolic (LVDd)/end-systolic (LVDs) dimensions were measured from a 2D-guided M-mode echocardiogram at maximal circumference of left ventricle. End-diastole and end-systole are defined as the onset of the QRS and the frame with the smallest chamber size, respectively. Left ventricular systolic function was assessed with ejection fraction (EF), calculated by the following formula: EF = (LVDd3 − LVDs3)/LVDd3 (30). Regional ventricular wall contractility in the ischemic zone was assessed from short-axis views of left ventricle and M-mode, with the curser line penetrating the area at risk.
Blood samples.
Blood samples were obtained by intravenous cannula (22-gauge) inserted into the auricular vein for ELISA and autoimmune response analysis at preischemic baseline and at 120 min of reperfusion and serially at 1, 3, 7, 14, and 28 days of recovery. Blood samples were collected in serum-separating tubes (BD Vacutainer SST; BD, Franklin Lakes, NJ), allowed to clot, and then centrifuged (1,000 g, 15 min). Separated serum was dispensed in 1.5-ml microcentrifuge tubes and stored at −80°C until it was used.
Non-survival surgery.
Following 28 days of recovery, rabbits were sedated and anesthetized as described above. The thoracic cavity was opened by medial sternotomy. The pericardial sac was exposed and opened to form a pericardial cradle, and the animals were euthanized under deep anesthesia by exsanguination following removal of the heart.
The extracted heart was placed in a 4°C bath of Krebs-Ringer solution (100 mmol/l NaCl, 4.7 mmol/l KCl, 1.1 mmol/l KH2PO4, 1.2 mmol/l MgSO4, 25 mmol/l NaHCO3, 1.7 mmol/l CaCl2, 11.5 mmol/l glucose, 4.9 mmol/l pyruvic acid, and 5.4 mmol/l fumaric acid). The hearts were then subjected to Langendorff retrograde perfusion for 10 min to wash out blood (24). The area at risk and infarct size were determined, or the left ventricular free wall containing the area at risk as circumscribed by a Prolene stich was dissected out prior to biochemical or proteomic analysis.
Measurement of infarct size.
In a separate group of hearts, infarct size was determined (26). The area at risk and the area of infarct were measured by using planimetry. The ratio of the area at risk to left ventricular weight was calculated. Infarct size was expressed as a percentage of the area at risk for each heart (26).
Perfusion fixation.
To allow for immunohistochemical and fluorescent microscopy, a separate group of hearts was perfusion fixed under pressure with 10% paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO) in PBS for 10 min prior to passive fixation at 4°C overnight in 4% PFA. Transmyocardial samples were dissected from the area at risk in the left ventricular free wall, and after embedding, tissue samples were sectioned completely (5- to 7-μm thickness) and then mounted on glass slides (∼90–100 slides). Slides were stained immunohistochemically with an antibody or mitochondrial marker.
Markers of myocardial injury.
Creatine kinase-MB isoenzyme (CK-MB) was estimated using the CK-MB enzyme immunoassay kit (Biocheck, Foster City, CA) according to the manufacturer's directions. Rabbit cardiac troponin-I (cTnI) was determined using the rabbit cTnI ELISA (Life Diagnostics, West Chester, PA) according to the manufacturer's directions and standards.
Adenosine triphosphate determination.
Adenosine triphosphate (ATP) was determined in frozen tissue sections from the area at risk by fluorometric analysis according to the method as described (21).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed using the ApopTag fluorescein in situ apoptosis detection kit and positive control slides (Millipore, Billerica, MA), as described previously (26). Myocardial cell specificity was determined on opposite adjacent sequential serial slides (n = 5–6 for each sample), using the cardiac-specific monoclonal antibody for troponin I (Spectral Diagnostics, Toronto, ON, Canada) labeled with anti-mouse IgG conjugated to Alexa 350 (Molecular Probes, Eugene, OR) (26).
Caspase-3-like activity.
Total cytoplasmic proteins were isolated in SDS-Nonidet P-40 lysis buffer containing protease (2 μmol/l, Complete; Boehringer Mannheim) (24). Caspase-3-like activity was determined in total cytoplasmic proteins using the caspase-3 colorimetric analysis kit, pNA standards, and inhibitors according to the manufacturer's instructions, (Chemicon International, Temecula, CA) (26).
Detection of anti-mitochondrial antibodies using indirect immunofluorescence.
Serum samples obtained from rabbits following 28 days of recovery were used for detection of antimitochondrial antibody. Indirect immunofluorescence (IIF) was conducted by a blinded independent investigator well experienced with IIF. HEp-2 cells were used as substrate in the IIF assay for the detection of antibodies directed against mitochondrial antigens (AMAs). Hep-2 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM (Sigma-Aldrich) supplemented with 10% fetal calf serum, l-glutamine (2 mmol/l), penicillin (200 U/ml), and streptomycin (200 μg/ml). Hep-2 cells were grown in tissue culture chambers (Nunc, Naperville, IL), fixed with 4% PFA in PBS, and permeabilized with methanol. Human serum from a patient with primary biliary cirrhosis, which contained a high titer of AMA, was used as a positive control. Rabbit sera and human serum were diluted 1:40 and 1:500, respectively, in PBS and incubated with the substrate for 1 h at room temperature. Unbound antibodies were removed by three successive washes with PBS, and bound antibodies were detected with secondary antiserum. Rhodamine-conjugated, species-specific donkey anti-rabbit IgG antiserum and FITC-conjugated, species-specific donkey anti-human IgG antisera were used to detect primary antibodies. Both secondary antisera, obtained from Jackson ImmunoResearch Laboratories (West Grove, PA), were diluted 1:300 in PBS. Cells were examined using a Zeiss Axiophot microscope, and images were processed using Adobe Photoshop 8.0 (Adobe, San Jose, CA) (2).
Fluorescent staining of heart tissue.
Transmyocardial samples were dissected from the area at risk in the left ventricular free wall and after embedding, and tissue samples were sectioned completely (5- to 7-μm thickness) and then mounted on glass slides. The slides were baked overnight at 65°C, deparaffinized in xylenes, rehydrated through a graded ethanol series, and subjected to antigen retrieval by heating three times for 5 min in 1 mmol/l ethylenediaminetetraacetic acid (pH 8.0) using a 700-W microwave oven set to high. Slides were stained immunohistochemically with the following antibodies: anti-human mitochondria antibody MTC02 (Abcam, Cambridge, MA), anti-α-actinin 2 (4), and anti-dystrophin CAP 6–10 (3). All antibodies were used at the manufacturer's or author's suggested dilutions. MitoFluor Green was used at a final concentration of 20 nmol/l (Invitrogen), and 4′,6-diamidino-2-phenylindole (DAPI) dihydrochloride (Invitrogen) was diluted according to the manufacturer's recommendation.
Transmission electron microscopy.
For transmission electron microscopy, heart tissue or cardiomyocyte cultures were fixed in 1.25% formaldehyde, 2.5% grade I glutaraldehyde, and 0.03% picric acid suspended in 100 mmol/l cacodylate buffer overnight (6). Samples were rinsed with buffer, stained with 1% osmium tetroxide-1.5% potassium ferrocyanide and 1% aqueous uranyl acetate, and then dehydrated through a graded ethanol series and propylene oxide. After infiltration and embedding with Epon-Araldite (EMS, Hatfield, PA), sections (60 nm thick) were cut on an Ultracut-S ultramicrotome (Reichert Technologies, Depew, NY) and mounted on copper grids (200 mesh). For immunostaining, cells were fixed in 4% PFA in PBS overnight, infiltrated with 2.3 mol/l sucrose in PBS containing 150 mmol/l glycine, and frozen in liquid nitrogen. Sections were cut at −120°C using the Tokayasu method and incubated with anti-human mitochondrial antibody (MTC02; Abcam), which was detected with an anti-mouse secondary antibody (Jackson ImmunoResearch) and a protein A-gold conjugate (EMS). Transmission electron microscopy was performed on a Jeol 1200EX (80 kV) (42).
HeLa cell culture.
HeLa cells (CRM-CCL-2; American Type Culture Collection) were cultured in DMEM plus GlutaMAX-I (Invitrogen) with 10% fetal bovine serum and penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (250 ng/ml) on tissue culture plates (CytoOne; USA Scientific, Ocala, FL). HeLa cells (104/well) were transferred from a 100-mm plate to a 48-well plate with the serum-free medium AIM-V (Invitrogen) on the day before coculture was started, with mitochondria and disrupted mitochondria.
HeLa cell mitochondria.
HeLa cell mitochondria were isolated from 10 × 100 mm plates at 80% confluence immediately prior to the start of coculture with Hela cells or peripheral blood mononuclear cells (PBMCs), as described above. Mitochondrial number and viability were determined as described above. Mitochondria were suspended in sterile respiration buffer. A subset of mitochondria was homogenized on ice using an Ultrasonic Homogenizer 36260 series (Cole-Palmer, Chicago, IL) with 40 × 1 s pulses with 1-s intervals; output = 60 to disrupt mitochondria. Mitochondria disruption was confirmed by microscopy.
Human PBMCs.
PBMCs were isolated from a healthy male prior to the culture by density gradient centrifugation with Ficoll-Paque according to the instructions of the manufacturer (Pharmacia Biotech, Uppsala, Sweden). Briefly, 15 ml of Ficoll-Paque gradient was pipetted into two 50-ml centrifuge tubes. The heparinized blood (30 ml) was diluted 1:1 in PBS and carefully layered over the Ficoll-Paque gradient in two tubes. The tubes were centrifuged for 20 min at 700 g. The cell interface layer was harvested carefully, and the cells were washed twice in PBS (for 15 min at 340 g, followed by 10 min at 340 g), resuspended, and spread in a 48-well plate with AIM-V medium at 3 × 105/well.
Multiplex assay.
HeLa cells and PBMCs were incubated separately on 48-well plates at 37°C, with CO2 levels of 5 and >95% of humidity for 48 h with stimulations, in the presence of HeLa cell mitochondria (1.5 × 106/ml) in sterile respiration buffer, sonicated HeLa cell mitochondria (1.5 × 106/ml) in sterile respiration buffer, serum-free medium AIM-V, sterile respiration buffer without stimulation, or phytohemagglutinin (10 μg/ml). The final medium volume of each well was 200 μl. Following 48 h of incubation, the supernatants from HeLa cells and the PBMC cocultures were collected separately from each well and stored at −80°C prior to analysis using the Milliplex Human Cytokine 42-plex Discovery Assay (Millipore).
For apoptosis measurements, the HeLa cells cocultured with PBMC were saved following removal of the media and then washed with PBS and lysed with Cell Extraction Buffer (50 μl/well; Invitrogen) containing Complete and Phos stop (1 tab/10 ml each; Roche Diagnostics, Indianapolis, IN). HeLa cell lysates were collected and protein concentrations measured using the Pierce BCA assay kit (Invitrogen). Lysates were normalized with lysis buffer and used for analysis, using the Milliplex MAP Human Apoptosis 3-Plex kit (Millipore). All Multiplex assays were performed by Eve Technologies (Calgary, AB, Canada) using the Luminex suspension array system. All samples were run in triplicate, with standard curves run in duplicate.
Optical mapping.
Lewis rats (200 g, n = 3) were euthanized, and the heart was extracted through a median sternotomy and then perfused with Krebs-Ringer solution at 37°C on a modified Langendorff apparatus (35). Autologous mitochondria were isolated from rat liver tissue, as described above.
Cardiac optical surface mapping of Langendorff-perfused rat hearts was performed as described previously (35). Briefly, three adult Lewis rat hearts were perfused with Krebs-Henseleit solution and paced at 350 beats/min from the right atrial epicardial surface using an insulated mini-coaxial stimulation electrode (Harvard Apparatus, model BS4-73-0181) (6). Perfused hearts were loaded with 5 μmol/l di-8-ANEPPS for 5 min {pyridinium, 4-2-[6-(dioctylamino)-2-naphthalenyl-ethenyl]-1-(3-sulfopropyl)-, inner salt; Invitrogen}, and hearts were rendered motionless with 11 mmol/l 2,3-butanedione monoxime (Sigma-Aldrich). The hearts were illuminated briefly with 475 ± 15 nm of excitation light. During a typical 4-s period of illumination, >605 nm of light emitted from the cardiac preparation was imaged with a high-speed CMOS camera connected to a horizontal macroscope. The left ventricle of each heart was imaged three times for 4 s, with an intervening 5-min recovery period after each of the following treatments: control (i.e., uninjected), injected with sterile respiration buffer at eight sites using a total volume of 800 μl, and injected with respiration buffer containing autologous rat liver mitochondria (3.4 ± 1.7 × 109) at eight additional sites in the same volume. The number of mitochondria injected per site was greater than that used in the in situ heart (4.2 × 108 vs. 1.2 × 106) so that any possible proarrhythmic response could be observed. Simultaneously, bipolar electrographic recordings were acquired and analyzed along with the voltage signals. Optical data were analyzed using software from Redshirt Imaging (Decatur, GA) as described (38), except that five iterations of the low-pass filter set to 3 × 3 mean were applied.
Mitochondrial distribution and uptake.
To clarify the distribution and uptake of injected mitochondria, a separate group of rabbits was used for RI-mitochondria experiments, as described in Fig. 1A, but with the use of xenogeneic human mitochondrial transplantation following 0, 2, 4, 8, and 24 h of recovery. Xenogeneic mitochondria were isolated from HeLa cells (CRM-CCL-2; American Type Culture Collection) as described above. Mitochondria isolation was performed just prior to the mitochondrial transplantation, and the mitochondria were used immediately. Mitochondria viability and number was determined as described above. RI-mitochondria hearts received 1.26 × 106 HeLa mitochondria. Mitochondria were injected into the area at risk, as described above. The area at risk was delineated using 3-0 Prolene, as described above. Following 0, 2, 4, 8, and 24 h of recovery, the hearts were removed and subjected to Langendorff perfusion to remove blood and then perfusion fixed with 10% PFA in PBS. The area at risk was dissected from the hearts and stored in 4% PFA in PBS and then used for immunohistochemical analysis.
Neonatal rat cardiomyocytes.
Neonatal Lewis rat (2 days old) cardiomyocytes were isolated using the neonatal cardiomyocyte isolation system (Worthington Biochemicals). Cells were treated with 2.5 × 107 ± 1 × 106 mitochondria/well on the 3rd or 4th day of culture (7).
Fluorescent staining of cardiomyocyte cultures treated with mitochondria.
Cells were fixed with 4% PFA in PBS for 1 h at 4°C and then permeabilized for 3 min with 0.1% Triton X-100 in PBS. EEA-1 and LAMP-1 (Abcam) polyclonal antibodies diluted in PBS containing 5% FBS were used at 2 and 10 μg/ml, respectively. Anti-human mitochondria MTC02 monoclonal antibody (Abcam) was used at a concentration of 5 μg/ml. Some samples were stained with MitoFluor Red (20 nmol/l), Alexa 488-phalloidin (1:50 dilution), CM-DiI (1 μmol/l), LysoTracker Red DND-99 (50 nmol/l), MitoTracker Red CMX Ros (100 nmol/l), and DAPI (500 nmol/l) (all from Invitrogen). Primary antibodies were detected with highly cross-absorbed goat anti-mouse or anti-rabbit Alexa-conjugated secondary antibodies (Invitrogen) and visualized on an Olympus FSX100 fluorescence microscope.
Oxygen consumption rate and rate of acid effluent.
Oxygen consumption rate was measured in cardiomyocytes cultured in 24-well plates (Seahorse Bioscience, North Billerica, MA) and then treated with either sterile respiration buffer (control) or sterile respiration buffer containing 1.26 × 106 rat liver mitochondria in a volume of 200 μl and allowed to incubate for 2, 4, or 8 h. The plates were washed three times in a plate washer, and oxygen consumption rate (pmol/min) and rate of acid effluent (ECAR; pmol/min) were measured in real-time using the XF24 Extracellular Flux analyzer with all required Seahorse reaction products according to the manufacturer's protocol (Seahorse Bioscience) (37).
Proteomics.
Proteomic analysis was performed using three experimental groups, control (n = 2; perfusion only), RI-vehicle (n = 3), and RI-mitochondria hearts (n = 3), as described previously (24). Mitochondria were isolated from rabbit pectoralis major as described above and used immediately in RI-mitochondria experiments. Hearts were subjected to 30 min of equilibrium, 30 min of RI achieved by the snaring of the LAD for RI-vehicle and RI-mitochondria, or 30 min of sham snaring of the LAD for control. The area at risk or sham area at risk was delineated a using 3-0 Prolene sutures (Ethicon). At 29 min of RI, the hearts received either 8 × 100-μl injections of sterile respiration buffer (RI-Vehicle) or sterile respiration buffer containing autologous mitochondria 9.7 × 106 ± 1.7 × 106/ml (RI-mitochondria). Control hearts received sham injection only. All injections were made into the area at risk using a 1-ml insulin syringe with a 28-gauge needle, as described above. At 30 min of RI the snare was released, and the hearts were allowed to recover for 10 min, and then the area at risk was dissected out and quick-frozen and stored under liquid nitrogen.
Protein for proteomic analysis was obtained from the area at risk. Each sample was isolated separately (23). High-throughput profiling of protein samples was performed at the Mass Spectrometry Core Facility of the Beth Israel Deaconess Medical Center using the Applied Biosystems (Bedford, MA) 8-plex iTRAQ labeling kit with the 4700 MALDI TOF/TOF analyzer (23). All samples were run in duplicate. Control hearts were used to account for constitutive protein expression levels. The comparisons with control and between RI-vehicle and RI-mitochondria were used to determine changes in protein expression levels in RI-vehicle and in RI-mitochondria. Data analysis was performed using Protein Pilot 2.0 software (Applied Biosystems) on SWISS-PROT, TrEMBL (www.ebi.ac.uk/swissprot), and NCBI (www.ncbi.nlm.nih.gov/) nonredundant protein databases. Proteins that were altered significantly between groups were identified on the basis of a significant P value and fold change. The P value was calculated using the Protein Pilot Paragon Algorithm, which allows results to be evaluated on the basis of the certainty of changes in the expression, not just by the magnitude of change.
Statistical analysis.
Statistical analysis was performed using the SAS software package (version 6.12; SAS Institute, Cary, NC). The mean ± SE for all data was calculated for all variables. Statistical significance was assessed using repeated-measures analysis of variance (ANOVA), with group as a between-subjects factor and time as a within-subjects factor. Tukey honestly significant difference test was used for comparisons between control and other groups to adjust for the multiplicity of tests. A one-way ANOVA was used for area at risk, infarct size, markers of ischemic injury, and TUNEL. Statistical significance was claimed at P < 0.05.
RESULTS
To demonstrate the efficacy of mitochondrial transplantation, we used a clinically relevant in situ blood-perfused animal model of ischemia and reperfusion. The model mimics events occurring in human cardiac ischemia-reperfusion with acute coronary artery obstruction followed by surgical intervention to reestablish blood flow to the affected region of the heart (Fig. 1). In this experimental model, a mini-thoracotomy was performed in the anesthetized animal, and the heart was exposed. Two small biopsies from the nonischemic pectoralis major muscle were obtained for mitochondrial isolation (Fig. 1B). These tissue samples weighed ∼0.3 g and provided 5–6 × 109 of mitochondria (Fig. 1D). The mitochondria were isolated in <60 min. Mitochondria viability was >99.99% (Fig. 1C). Transmission electron microscopy of the isolated mitochondria showed that the isolated mitochondria were electron dense, with <0.01% of the mitochondria being fractured or damaged (Fig. 1E). Determination of mitochondrial viability and function demonstrated that mitochondrial complex I–V (Fig. 1F), state 3 (active) oxygen consumption (ADP-stimulated respiration; Fig. 1G), and respiratory control index (state 3/state 4; Fig. 1H) for malate-induced complex I and succinate-induced complex II in energized autologously derived pectoralis major mitochondria were similar to previously reported experimental values (24).
While the mitochondria were being isolated, the LAD was temporarily ligated to induce a regional area of ischemia. This regional ischemic area, the area at risk, was visible as a bloodless light pink area, and ischemia was confirmed by electrocardiogram and echocardiography (Fig. 1A). Following 29 min of regional ischemia, 9.7 ± 1.7 × 106 mitochondria (RI-mitochondria) in 0.8 ml of sterile mitochondria respiration buffer were transplanted into the regional ischemic zone by direct injection using an insulin syringe with a 28-gauge needle. Approximately 1.2 × 106 mitochondria in respiration media were injected into each of eight sites located within the regional ischemic zone in a volume of 0.1 ml each. For comparison, a separate group of animals subjected to regional cardiac ischemia received 0.8 ml of sterile mitochondria respiration buffer only (RI-vehicle). Following 30 min of ischemia, the LAD snare was released and the wound closed, and the animals were weaned from anesthesia and allowed to recover for either 2 h or 28 days. In the groups having 28 days of recovery, there were no postoperative complications or surgical site or respiratory infections, and there was no malnutrition or weight loss. Weight gain over 4 wk for RI-vehicle and RI-mitochondrial rabbits was 0.11 ± 0.14 and 0.05 ± 0.22 kg, respectively.
Autologous mitochondrial transplantation is not proarrhythmic.
Previous reports have shown that following transplantation of skeletal muscle myoblasts, clustering of the cells can occur, resulting in arrhythmia with postoperative episodes of sustained tachycardia. The mechanism by which this occurs is controversial, but it has been speculated that alterations in electrical coupling may be involved (22). We wanted to determine whether mitochondrial transplantation was arrhythmogenic (34). Accordingly, serial 12-lead electrocardiograms were recorded at preischemia (baseline), throughout ischemia, and following 120 min of reperfusion. The electrocardiograms showed no difference between groups prior to ischemia; however, with the onset of regional ischemia, ST-T segment elevations (>1 mm) consistent with myocardial infarction were observed in all hearts (Fig. 2A). Electrocardiographic recordings immediately following the transplantation of autologously derived mitochondria showed no ventricular tachycardia, bradycardia, fibrillation, or conduction system defects or repolarization heterogeneity (Fig. 2A). These observations were unchanged at 120 min of recovery and at serial observations at 7–28 days of recovery. No difference in wet/dry weight ratio between RI-mitochondria and RI-vehicle hearts was observed (Fig. 2C).
Fig. 2.
Autologous mitochondrial transplantation is not proarrhythmic. A: representative ECG at preischemia, ischemia, and 1, 3, and 120 min following mitochondrial transplantation for leads II and aVF are shown. Millivolts and millisecond scales are shown at right. Time points at which ECGs were obtained are shown at bottom. B: regional ischemia was induced by ligating the left anterior descending artery with a snare. The ischemic zone was identified at 29 min of regional ischemia [shown as gray ellipse in heart (inset)]. C: wet/dry weight ratio between RI-mitochondria and RI-vehicle hearts. D and E: QRS duration (ms; D) and corrected QT interval (E) at preischemia and following 28 days of recovery in RI-vehicle (black bars) and RI-mitochondria (open bars). Results are shown as means ± SE; n = 7–8 for each group. F–I: representative sequential isopotential maps from the left ventricles of 3 rat hearts injected with mitochondria are shown. Isopotential maps from 1 cardiac cycle of a representative rat heart are depicted prior to injection (F, far left row), after injection of respiration buffer (G, 2nd row from left), and following mitochondrial transplantation (3.4 ± 1.7 × 109 mitochondria; H, middle row). The color scale from blue to red represents a change in the membrane potential from hyperpolarization to depolarization. I: 2nd row from right shows the corresponding phase of the cardiac cycle for each column on a simultaneously recorded electrogram at the transition point between red and black.
In addition, analysis of QRS duration and corrected QT interval, diagnostic indices for ectopic rhythms originating in the ventricles or ventricular tachycardia, a major cause of sudden cardiac death, showed that there was no difference in QRS duration or corrected QT interval at 28 days of recovery in RI-mitochondria hearts compared with preischemia (Fig. 2, D and E).
These data strongly suggested that the transplantation of mitochondria was not arrhythmogenic; however, to verify these results, optical mapping was performed, using the isolated perfused rat heart model (Fig. 2, F–I). In these experiments, 3.4 ± 1.7 × 109 rat liver mitochondria were injected into each of three isolated perfused adult rat hearts at eight sites approximating an ischemic zone, and recordings were obtained. The number of mitochondria injected per site was significantly greater than that used in the in situ heart (4.2 × 108 vs. 1.2 × 106) so that any acute arrhythmogenic responses could be observed. Sequential isopotential maps from the left ventricles of rat hearts injected with mitochondria showed an excitation wave on the epicardial cardiac surface moving from left to right, with no indication of conduction slowing, impulse block, or ectopic foci (Fig. 2, F–I). Together, these results demonstrate that the transplantation of mitochondria is not arrhythmogenic immediately following mitochondrial transplantation or during recovery for ≤28 days.
Autologous mitochondrial transplantation ameliorates myocardial injury and enhances regional function.
Mitochondrial transplantation into the ischemic zone just prior to reperfusion significantly decreased myocyte necrosis and significantly enhanced postischemic function (Fig. 3A). To quantify the extent of myocardial injury, infarct size was measured biochemically with triphenyl tetrazolium chloride (TTC) staining. Absolute measurement of infarct size by TTC staining revealed that, whereas there was no significant difference in the size of the area at risk (i.e., the region subjected to ischemia by LAD occlusion; Fig. 3B), myocardial infarct size expressed as a percentage of the area at risk was significantly decreased (P < 0.05) in RI-mitochondria hearts when compared with RI-vehicle hearts (Fig. 3B).
Fig. 3.
Autologous mitochondrial transplantation ameliorates myocardial injury and enhances regional function. A: representative photograph of RI-vehicle and RI-mitochondria hearts stained with 1% triphenyltetrazolium chloride (TTC) after 28 days of recovery. White areas show myocardial necrosis. Brick red areas show viable tissue. Scale in mm is shown. B: area at risk, the area subjected to regional ischemia and infarct size as determined by TTC staining in RI-mitochondria and RI-vehicle hearts after 2 h of recovery and after 28 days of recovery. Results are shown as means ± SE; n = 7–8 for each group. **Statistical differences at P < 0.05 vs. RI-vehicle. C and D: representative 2-dimensional (2D) short-axis images of left ventricular diastole and systole at the midportion of the left ventricle are shown for RI-vehicle (C) and RI-mitochondria (D) hearts following 28 days of recovery. ECG wave forms are shown in green at the bottom of each image. The arrows in C indicate regional hypokinesis within the ischemic zone in RI-vehicle heart. Comparative M-mode is indicated with arrows. Hypokinesis was not observed in RI-mitochondria hearts. Representative transthoracic 2D short-axis videos are provided as Supplemental Videos S1 and S2 (available on the AJP-Heart and Circulatory Physiology web site). E and F: CK-MB (ng/ml; E) and cTnI (ng/ml; F) were measured serially on days 1 and 3 following myocardial ischemia in serum from RI-vehicle (black bars) and RI-mitochondria hearts (open bars). Results are shown as means ± SE; n = 7–8 for each group. **Statistical differences at P < 0.05 vs. RI-vehicle. G: total tissue ATP content (μmol/g dry weight) in the area at risk of RI-vehicle and RI-mitochondria hearts at 21 days of recovery. All results are shown as means ± SE; n = 4 for each group. **Significant differences at P < 0.05 vs. RI-vehicle. H and I: TUNEL (positive cell nuclei/1,000 cells; H) and caspase-3 activity (active units: pmol DVED-pNA·μg−1·min−1; I) in RI-vehicle and RI-mitochondria at 28 days of recovery are shown. Results are shown as means ± SE; n = 7–8 for each group. **Statistical differences at P < 0.05 vs. RI-vehicle.
After 2 h of recovery, infarct size in RI-mitochondria hearts was 9.8 ± 4.3% compared with 36.6 ± 5.4% (P < 0.05) in RI-vehicle hearts. In the 28-day recovery groups, infarct size in RI-mitochondria hearts was 7.9 ± 2.9% compared with 34.2 ± 3.3% for RI-vehicle (P < 0.05; Fig. 3B). Examination of tissue sections showed that infarct in RI-vehicle hearts was large and transmural, whereas the infarct in RI-mitochondria hearts was predominantly small, focal, and epicardial (Fig. 3A). Our results also show that infarct size at 28 days of recovery was not significantly greater than that observed at 2 h of reperfusion, indicating that there was no infarct expansion and that the cardioprotection afforded by mitochondrial transplantation was effective for ≥4 wk.
Clinical markers of myocardial infarction, CK-MB and cTnI, determined on days 1–3 of recovery, were elevated in both RI-mitochondria- and RI-vehicle-treated animals, indicating myocardial infarction. However, both CK-MB and cTnI were decreased significantly (P < 0.05) in hearts transplanted with mitochondria (RI-mitochondria) compared with RI-vehicle, suggesting that the extent of myocardial infarction was lessened (Fig. 3, E and F).
The enhanced viability within the area at risk in RI-mitochondria was verified further by measurement of total tissue ATP content (Fig. 3G). Our results show that at 21 days of recovery, total tissue ATP content in the area at risk was increased significantly in RI-mitochondria hearts (19.2 ± 3.3 vs. 10.7 ± 3.7 μmol/g dry weight, P < 0.05).
Myocardial function determined by 2D echocardiography demonstrated that there was no difference between groups prior to ischemia, with baseline echocardiograms showing normal synergetic contraction of the left ventricle. During regional ischemia, regional hypokinesia was observed in the midcavity of the anterior wall in all hearts, consistent with the perfusion area of LAD made ischemic by the snare. This regional hypokinesis remained evident throughout reperfusion and recovery in RI-vehicle hearts but was ameliorated within 10 min after reperfusion was started in RI-mitochondria hearts. There was no alteration in systolic or diastolic noninvasive blood pressure associated with mitochondrial transplantation (Table 1).
Table 1.
Functional data for RI-mitochondria and RI-vehicle
| Reperfusion, min |
||||||
|---|---|---|---|---|---|---|
| Preischemia | Ischemia | 10 | 60 | 120 | Probability vs. RI-Vehicle | |
| Heart rate, min | ||||||
| RI-vehicle | 180.5 ± 28.8 | 176.1 ± 29.3 | 182.0 ± 23.5 | 164.8 ± 23.4 | 162.1 ± 27.6 | |
| RI-mitochondria | 178.8 ± 25.4 | 185.1 ± 29.5 | 183.3 ± 27.2 | 180.9 ± 25.0 | 169.4 ± 11.7 | 0.187 |
| Systolic NIBP, mmHg | ||||||
| RI-vehicle | 74.6 ± 15.0 | 64.0 ± 12.4 | 71.5 ± 12.1 | 71.0 ± 14.3 | 63.6 ± 11.7* | |
| RI-mitochondria | 75.2 ± 10.6 | 72.9 ± 14.2 | 67.0 ± 9.1 | 79.6 ± 10.2 | 69.7 ± 8.3 | 0.21 |
| Diastolic NIBP, mmHg | ||||||
| RI-vehicle | 38.2 ± 10.5 | 31.0 ± 12.7 | 32.6 ± 13.0 | 32.7 ± 9.8 | 27.9 ± 6.8* | |
| RI-mitochondria | 36.4 ± 11.5 | 31.5 ± 8.9 | 31.4 ± 9.9 | 34.9 ± 8.0 | 26.6 ± 5.4 | 0.753 |
All results are shown as means ± SD; n = 12. RI-mitochondria, autologously derived mitochondria; RI-vehicle, vehicle alone; NIBP, noninvasive blood pressure.
Significant differences at P < 0.05 vs. preischemia within each group. The P values are shown for 2-way repeated-measures ANOVA.
In the 28-day recovery groups, followup echocardiograms were performed weekly. In hearts that received mitochondrial transplantation (RI-mitochondria) there was synergetic left ventricular contraction, indicating normal heart function. In contrast, RI-vehicle hearts showed segmental hypokinesis in the anterior wall within the regional ischemic area, indicating continued myocardial dysfunction (Fig. 3, C and D, and Supplemental Videos S1 and S2; Supplemental Material for this article can be found on the AJP-Heart and Circulatory Physiology web site). In addition, the percent systolic wall thickening at the area at risk following 4 wk of recovery, measured from the short-axis view of the left ventricle, was significantly decreased in RI-vehicle compared with RI-mitochondria hearts (RI-mitochondria 44 ± 6% vs. RI-vehicle 8 ± 8%, P < 0.01), confirming continued regional hypokinesis in RI-vehicle hearts.
The effect of mitochondrial transplantation on apoptosis was determined by TUNEL and active caspase-3 determination. At 28 days of recovery, both TUNEL and the level of caspase-3 activity were significantly decreased (P < 0.05) in hearts transplanted with mitochondria (RI-mitochondria) (Fig. 3, H and I). These results were confirmed using the human apoptosis 3-plex assay, demonstrating that the transplantation of mitochondria does not induce apoptosis (Fig. 4). These results demonstrate that autologous mitochondrial transplantation is clinically efficacious and ameliorates myocardial injury and enhances regional function.
Fig. 4.
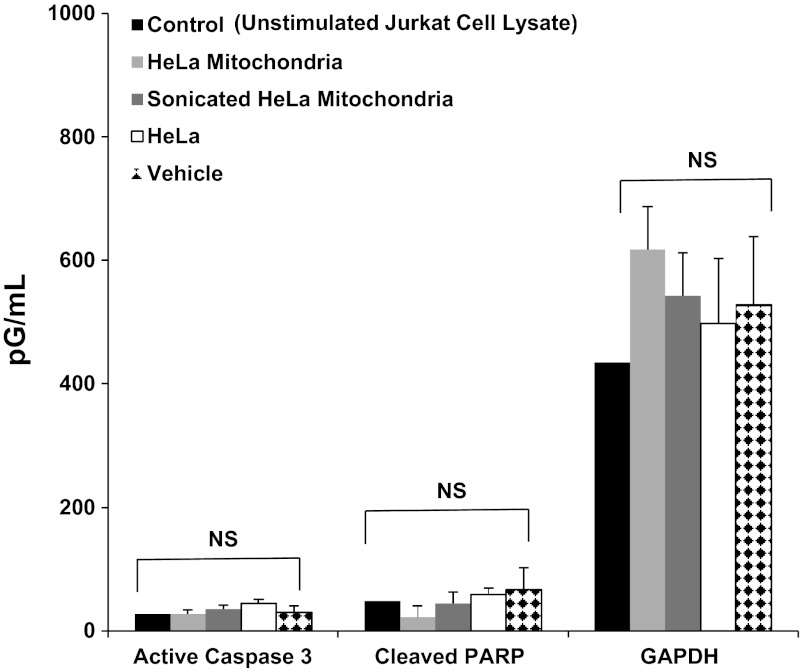
Mitochondrial transplantation and apoptosis activation. The human apoptosis 3-plex assay was performed. Standard curves were run in duplicate. Both intact mitochondria and mitochondrial fragments (sonicated mitochondria) were investigated separately for activation of caspase-3 and poly-ADP-ribose polymerase (PARP) cleavage in HeLa cells following 48-h coculture. GAPDH was used for control. Concentrations are shown in pg/ml. There was no significant difference (P < 0.05) vs. control. All samples were run and assayed in triplicate. NS, not significant.
Autologous mitochondria are internalized by myocardial cells.
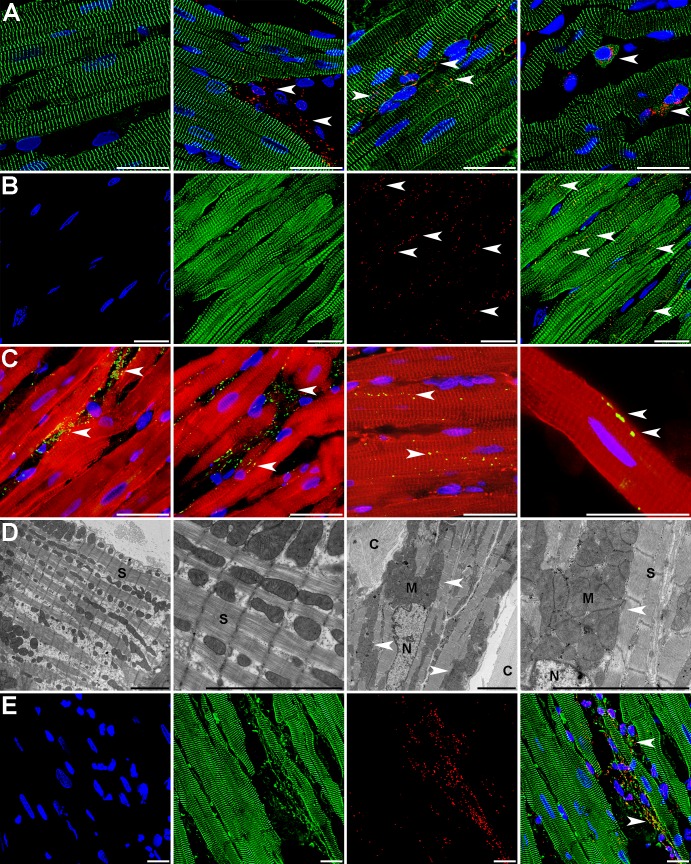
To investigate the tissue distribution of transplanted mitochondria, heart sections from the area at risk in RI-mitochondria hearts injected with autologously derived mitochondria prelabeled with MitoTracker Red CMXRos were fluorescently imaged for 0–24 h (Fig. 5A). The labeled mitochondria were observed in the interstitial spaces surrounding cardiomyocytes at 0, 2, 4, 8, and 24 h following injection, with an extensive epicardial to subendocardial distribution. Careful examination of the sections revealed that a fraction of the labeled mitochondria was localized within cardiomyocytes at 2 h after injection (Fig. 5A). These internalized mitochondria appeared to reside near the sarcolemma between Z-lines of the sarcomeres (Fig. 5B).
Fig. 5.
Localization and uptake of transplanted mitochondria in the rabbit heart. A: control heart left ventricular tissue stained for anti-α-actinin 2 (ACTN2; green) and nuclei (blue; left); RI-mitochondria tissue from the area at risk injected with autologous mitochondria prelabeled prior to injection with MTRed (red) at 2 (left middle), 8 (right middle), and 24 h of recovery (far right). Arrows indicate transplanted mitochondria. B: MTRed CMXRos-labeled autologous mitochondria injected into the area at risk in the in vivo rabbit heart for 4 h. After fixation and sectioning, the tissue was labeled for nuclei [4′,6-diamidino-2-phenylindole (DAPI) shown in blue] and the organelle-specific MitoFluor Green (shown in green) to show the injected mitochondria and in situ mitochondria. The merged image at the right shows the injected autologous mitochondria as yellow (merged red and green). C: RI-mitochondria tissue from the area at risk injected with HeLa cell mitochondria after 8 h of recovery. Hela cell mitochondria were detected using the anti-human mitochondrial antibody (MTC02) and Alexa 488 anti-mouse antibody (green). Myocytes are shown with dystrophin polyclonal antibody (CAP 6–10) detected with Alexa 568 anti-rabbit antibody (shown in red). Nuclei are shown using DAPI (blue). Images left to right show that the majority of transplanted mitochondria remained in the interstitial spaces; however, numerous injected mitochondria did appear to reside within cardiomyocytes. Arrows indicate transplanted mitochondria. D: transmission electron micrographs of RI-vehicle (low and high magnifications on left) and RI-mitochondria tissue from the area at risk injected with autologous mitochondria (right middle and right). C, collagen; M, transplanted mitochondria; N, nuclei; S, sarcomere. Scale bars are 25 and 5 μm in length for fluorescent and electron micrographs, respectively. E: transplanted autologously derived mitochondria were observed in close proximity to cardiomyocytes and internalized in both cardiomyocytes and noncardiomyocytes (arrows). Rabbit heart myocardial cell nuclei stained with DAPI are shown at left, and α-actinin 2 (green) is shown at left middle. Autologously derived mitochondria labeled with MTRed are shown at right middle. The merged images are shown at left. Scale bars, 25 μm.
To verify cardiomyocyte uptake of transplanted mitochondria, a separate group of animals was used for mitochondrial transplantation, using mitochondria isolated from HeLa cells. The use of human mitochondria in a rabbit model allowed for the differentiation between native rabbit mitochondria and transplanted human mitochondria based on immune reactivity to a monoclonal anti-human mitochondria antibody (MTC02). Sections from RI-mitochondria tissue from the area at risk injected with HeLa-derived mitochondria and then allowed to recover for 8 h were used for fluorescent staining with MTC02 and anti-dystrophin CAP 6–10 antibody (Fig. 5C) (3). Dystrophin stains the peripheral sarcolemma and T tubules in longitudinal sections (12). Although the majority of mitochondria remained in the interstitial spaces, many of the injected mitochondria were observed to be localized within cardiomyocytes. To confirm these observations, transmission electron microscopy of the heart tissue injected with autologously derived mitochondria was performed. In ischemic left ventricles that did not receive mitochondrial transplantation, the fragmented contractile apparatus appeared to be separated by rows of single mitochondria after 2 h of reperfusion (Fig. 5D). In contrast, ischemic hearts injected with mitochondria had a tightly packed contractile apparatus with large clusters of mitochondria near the sarcolemma in addition to linear arrays of interfibrillar mitochondria. Transplanted autologously derived mitochondria were observed in close proximity to cardiomyocytes and internalized in both cardiomyocytes and noncardiomyocytes (Fig. 5E).
To further investigate internalization of mitochondria by cardiomyocytes, neonatal rat cardiomyocytes were incubated with Lewis rat liver mitochondria labeled with MitoTracker Red CMXRos for 0, 2, 4, 8, and 24 h. Figure 6A shows that the majority of these syngeneic mitochondria were internalized to a perinuclear location after 24 h of incubation. To confirm these results, a separate series of experiments was performed using cardiomyocytes incubated with unlabeled HeLa cell mitochondria. These xenogeneic mitochondria when incubated with cardiomyocytes showed that the mitochondria were adherent to the cell surface at 2 h and that mitochondria were found primarily within the cardiomyocytes at 8–24 h (Fig. 6B). Cardiomyocytes treated for 4 h with HeLa cell mitochondria and the membrane probe CM-DiI revealed that mitochondria resided inside cardiomyocytes as isolated organelles or in larger clusters (Fig. 6C). Transmission microscopy of cardiomyocytes cocultured with syngeneic and xenogeneic mitochondria (Fig. 6D) showed clusters of spherical mitochondria inside cardiomyocytes 4 h after treatment. To confirm these results, we incubated cardiomyocytes with HeLa cell mitochondria for 24 h and detected these using the MTC02 antibody and a gold-conjugated anti-mouse antibody (Fig. 6D). The results of this experiment confirmed that the transplanted mitochondria represented internalized organelles.
Fig. 6.
Transplantation of autologous mitochondria in rat neonatal cardiomyocytes and high-energy synthesis. A: neonatal rat cardiomyocytes incubated for 24 h with Lewis rat liver mitochondria labeled with MTRed. Nuclei (DAPI, blue; left), F-actin (green; left middle), Lewis rat liver mitochondria (red; right middle), and the merged image (right). Scale bars, 25 μm. B: cardiomyocytes stained for total mitochondria with MitoFluor Red and DNA with DAPI (blue). Control cardiomyocytes (left) and cardiomyocytes cocultured with HeLa cell mitochondria detected with anti-human MTC02 and Alexa 488 anti-mouse antibody (green) for 2 (left middle), 8 (right middle), and 24 h (right). The 2-h time period shows that many mitochondria are extracellular, whereas the 8-h time period shows that most mitochondria are internalized in the cardiomyocytes. The 24-h time period shows a mixture of intra- and extracellular mitochondria. Scale bars, 25 μm. C: cardiomyocytes cocultured with HeLa mitochondria detected using MTC02 and Alexa 488 anti-mouse antibody (green) and CM-DiI for 4 h to reveal the cell membranes. Nuclei are stained blue. Scale bars are 25 μm. D: transmission electron microscopy of syngeneic rat liver mitochondria inside cardiomyocytes in plastic section is shown at left. To detect xenogenic HeLa mitochondria in cardiomyocytes, frozen sections were incubated with anti-human MTC02, which was detected with an anti-mouse secondary antibody and a protein A-gold conjugate (shown as black dots, indicated by white arrows at middle and right). Transplanted mitochondria range in size from 500 to 1,200 nm in diameter. Scale bars, 500 nm. m, Native mitochondria. Arrows represent transplanted mitochondria. E: oxygen consumption rate (OCR) and rate of acid efflux (ECAR) in day 2 neonatal rat cardiomyocytes in control, cocultured with respiration media only (Con), and in cardiomyocytes cocultured with respiration media containing 1.26 × 106 rat liver mitochondria at 2, 4, or 8 h following coculture. Results are means ± SE; n = 4–8 each. *P < 0.05 vs. control. F: costaining with the acidotropic probe LysoTracker (shown in red). G: costaining with the lysosomal protein LAMP-1 (shown in red). H and I (higher magnification): costaining with cadaverine (autophagosome; shown in red). Scale bars, 500 nm. There was no colocalization of mitochondria with any of the lysosomal or autophagosomal markers.
To determine whether internalized mitochondria were associated with lysosomes or autophagosomes, cardiomyocytes were costained with the acidotropic probe LysoTracker (Fig. 6F), the lysosomal protein LAMP-1 (Fig. 6G), and cadaverine (autophagosome; Fig. 6, H and I). There was no colocalization of mitochondria with any of the lysosomal or autophagosomal markers.
Transplanted mitochondria maintain function.
To determine whether autologously derived mitochondria maintained function after internalization, cardiomyocytes were cocultured with either respiration media alone or respiration media containing 1.26 × 106 rat liver mitochondria. After 2, 4, or 8 h of coculture, oxygen consumption rates were measured. This rate was increased significantly in cardiomyocytes containing transplanted mitochondria at 2 and 4 h posttransplantation compared with cardiomyocytes treated with respiration media alone, indicating that the transplanted mitochondria were synthesizing ATP (Fig. 6E). ECAR (rate of acid efflux) was not significantly different between groups (Fig. 6E). These results are in agreement with in vivo data that showed that total tissue ATP content in the area at risk of RI-mitochondria hearts was increased significantly (P < 0.05) at 21 days of recovery compared with RI-vehicle hearts (Fig. 3G).
These results demonstrate that transplanted mitochondria are readily internalized into cardiomyocytes within hours of transplantation and that these organelles maintain viability and function, increasing ATP levels.
Autologous mitochondrial transplantation: immune and autoimmune response.
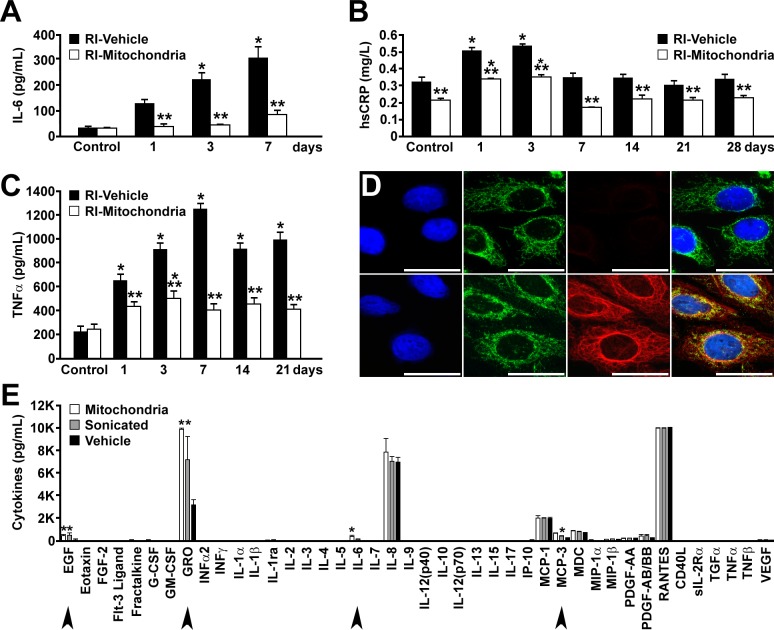
Previous studies have confirmed that nonautologous cell-based therapies trigger cell infiltration and host immune response (15). In our studies, we used mitochondria isolated from the rabbit's own body, and therefore, no immunological response was expected. To confirm these expectations, serial blood samples were obtained, and TNFα, IL-6, and high-sensitivity C-reactive protein (hsCRP), sensitive markers of inflammation, were analyzed by ELISA (13). Our results show that there was an increase in hsCRP in both RI-vehicle and RI-mitochondria hearts compared with levels in preischemia animals; however, the level of hsCRP was decreased significantly in RI-mitochondria compared with RI-vehicle hearts, suggesting that the level of inflammation was ameliorated by mitochondrial transplantation. Similar results were observed for IL-6 and TNFα levels, indicating that the injection of autologous mitochondria was not inflammatory (Fig. 7, A–C). Myeloperoxidase and monocyte chemoattractant protein-1 were below detectable limits in both RI-vehicle and RI-mitochondria animals.
Fig. 7.
Autologous mitochondrial transplantation: immune and autoimmune responses. A–C: serum immune markers of inflammation were determined serially. Control samples were obtained in preischemia. IL-6 (A), hs-CRP (B), and TNFα (C) in RI-mitochondria and RI-vehicle. Time points in days are noted. hs-CRP was within the normal range for both RI-vehicle and RI-mitochondrial hearts at each time point. All results are shown as means ± SE; n = 7–8 for each group at each time point. *Statistical differences at P < 0.05 vs. control; **statistical differences at P < 0.05 vs. RI-vehicle. D: autoimmune response to mitochondrial transplantation was determined at 28 days of recovery. Both RI-mitochondria and RI-vehicle are shown. Serum dilution was 1:40 for each sample. The Left images show Hep-2 cells stained with DAPI (blue). Left middle images show Hep-2 cell mitochondria stained with human serum from a patient with primary biliary cirrhosis, which contained a high titer of anti-mitochondrial antibody (AMA), and FITC-conjugated donkey anti-human IgG antisera. Right middle images show rabbit serum to AMA and rhodamine-conjugated donkey anti-rabbit IgG antisera. No reaction in RI-mitochondria samples is shown. In RI-vehicle, a nonspecific IgG reaction was observed, but it was not AMA. Merged images are at right. Scale bars, 25 μm. Animal no. is n = 4 for each group. E: mitochondrial transplantation and cytokine and chemokine activation. Multiplex (42-plex) analysis of cytokines and chemokines using the Human Cytokine 42-plex Discovery Assay was performed. Standard curves were run in duplicate. Both intact mitochondria and mitochondrial fragments (sonicated mitochondria) were investigated separately for chemokine and cytokine activation in human peripheral blood mononuclear cells following 24 h. Concentration for each cytokine (pg/ml) is shown in log scale. Significantly increased cytokines compared with vehicle (P < 0.05) are indicated with arrows. Epidermal growth factor (EGF), growth-related oncogene (GRO), IL-6, and monocyte chemotactic protein-3 (MCP-3) were elevated in both intact HeLa mitochondria and sonicated HeLa mitochondria. All samples were run in triplicate and assayed in triplicate.
Although an inflammatory reaction was decreased in RI-mitochondria hearts, it is possible that the increased number of mitochondria now present in the myocardium provoked an autoimmune reaction directed against mitochondria. Previous studies have shown that oxidative modification of E2 subunits of mitochondria pyruvate dehydrogenase, branched-chain 2-oxo-acid dehydrogenase, and 2-oxo-glutarate dehydrogenase is a critical step leading to the induction of an autoimmune response in the liver (17). To determine whether mitochondrial transplantation provoked an immune response, blood samples at 28 days of recovery were obtained and tested for the presence of antimitochondrial antibodies (AMA) by indirect immunofluorescence (Fig. 7D). AMAs were not detected in the serum of animals treated with RI-mitochondria, indicating that autologous mitochondrial transplantation did not induce an autoimmune response.
To further analyze possible immune responses to autologous mitochondrial transplantation, multiplex (42-plex) analysis of cytokines and chemokines was performed. In this assay, mitochondria from HeLa cells (ATCC, CRM-CCL-2), both intact and sonicated (used to determine the effects of mitochondria degradation products), were investigated separately for innate chemokine and cytokine activation in human peripheral blood mononuclear cells (Fig. 7E). Results from this investigation showed that there was no upregulation of cytokines associated with the immune response that is seen in patients with acute heart transplantation rejection (IL-1, IL-4, IL-6, IL-12, IL-18, IP-10, and macrophage inflammatory protein-1α and -1β) (30). Cytokines that were upregulated included epidermal growth factor (EGF), growth-related oncogene (GRO), IL-6, and monocyte chemotactic protein-3 (MCP-3). These cytokines have been shown to be associated with enhanced postinfarct cardiac function (36).
Transplantation of autologous mitochondria increases differentially expressed proteins.
The role of autocrine and paracrine factors in cardioprotection has been established (8, 9, 18, 28). To determine whether transplantation of autologous mitochondria altered the proteome ventricular tissue samples from the region at risk, RI-vehicle, RI-mitochondria, and control nonischemic hearts were isolated and used for proteomic analysis. The nonischemic heart was used for constitutive protein expression in these analyses. To account for mitochondrial proteins from transplanted mitochondria, we considered only the proteins for which we identified iTRAQ labeling in all samples from control, RI-vehicle, and RI-mitochondria. The proteins with iTRAQ label in only one group (e.g., mitochondria injection) of proteins were discarded from the analysis. Quality control analysis was performed on the basis of relative expression values of different proteins to identify any outliers. The quality control analysis was performed using pairwise correlation plots, boxplots, and principal component analysis (Fig. 8, A–D).
Fig. 8.
Transplantation of autologous mitochondria increases differentially expressed proteins. A–D: proteomic quality control analysis. A: box plot analysis shows alignment of average iTRAQ intensity values for control (controls 1 and 2), RI-vehicle (RI-vehicle 2 and 3), and RI-mitochondria (RI-mitochondria 1–3). B: dendrogram of proteomic comparisons. C: pairwise correlation plots show highly significant differences in expressed proteins. D: principal component analysis for proteins detected in control (green), RI-vehicle (red), and RI-mitochondria (blue). PC1, principal component 1; PC2, principal component 2. E: Venn diagram for control (green), RI-vehicle (red), and RI-mitochondria (blue). A total of 76 high-confidence proteins were identified as trending toward differential expression. The number of uniquely expressed proteins for each treatment is indicated. F: hierarchical cluster analysis of RI-mitochondria and RI-vehicle. All results are compared with control to account for constitutive protein expression levels. Samples include 2 controls, 2 RI-vehicle, and 3 RI-mitochondria. The differentially expressed proteins were identified by supervised analysis on the basis of P < 0.01 in each group. The log fold change (FC) in protein expression is shown with pseudocolor scale (−3 to 3), with red denoting upregulation and green denoting downregulation. Columns represent FC comparisons, and rows represent the proteins. Dendrograms are found on the left side, and experimental groups are found on the bottom. CTR1 and -2, controls 1 and 2; RI2 and -3, RI-vehicle 2 and 3; MITO1–3, RI-mitochondria 1–3.
Proteomic analysis revealed that there were 76 proteins trending toward differential expression from the three comparisons (control vs. RI-vehicle, control vs. RI-mitochondria, and RI-vehicle vs. RI-mitochondria; Fig. 8E). Hierarchical cluster analysis showed distinct protein expression patterns between RI-mitochondria and RI-vehicle (Fig. 8F). Twenty-six proteins were upregulated (FC > 1.5, P < 0.05; Table 2), and 23 proteins were downregulated (FC < 0.7, P < 0.05; Table 3) in RI-mitochondria compared with RI-vehicle. Functional annotation clustering (P < 0.05, enrichment score >2.0) indicated that the mitochondrion, the generation of precursor metabolites for energy, and cellular respiration were enriched in RI-mitochondria compared with RI-vehicle (Table 4). Functional annotation clustering revealed no downregulated clusters. Results were confirmed by Western blot analysis (results not shown).
Table 2.
Differentially expressed proteins upregulated in RI-mitochondria vs. RI-vehicle
| ID | Name | Fold |
|---|---|---|
| P24752 | Acetyl-CoA acetyltransferase, mitochondrial | 2.861 |
| P08559 | Pyruvate dehydrogenase E1 component subunit-α, somatic form, mitochondrial | 2.698 |
| O77814 | Creatine kinase S-type, mitochondrial | 2.513 |
| P36957 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial | 2.507 |
| Q9TTT8 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | 2.462 |
| P49065 | Serum albumin | 2.358 |
| Q00325 | Phosphate carrier protein, mitochondrial | 2.288 |
| P49411 | Elongation factor Tu, mitochondrial | 2.251 |
| Q09666 | Neuroblast differentiation-associated protein AHNAK | 2.137 |
| P35609 | α-Actinin-2 | 2.12 |
| P40926 | Malate dehydrogenase, mitochondrial | 2.09 |
| P46406 | Glyceraldehyde-3-phosphate dehydrogenase | 1.974 |
| P10809 | 60-kDa heat shock protein, mitochondrial | 1.909 |
| Q99798 | Aconitate hydratase, mitochondrial | 1.88 |
| P02251 | Histone H1.3 | 1.802 |
| P02646 | Troponin I, cardiac muscle | 1.788 |
| Q9NX63 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 3, mitochondrial | 1.756 |
| O46373 | ADP/ATP translocase 1 | 1.712 |
| P08574 | Cytochrome c1, heme protein, mitochondrial | 1.711 |
| P25704 | β-enolase | 1.681 |
| P28331 | NADH-ubiquinone oxidoreductase 75-kDa subunit, mitochondrial | 1.655 |
| Q8NB49 | Probable phospholipid-transporting ATPase IG | 1.624 |
| O75417 | DNA polymerase-θ | 1.573 |
| P15090 | Fatty acid-binding protein, adipocyte | 1.564 |
| P11142 | Heat shock cognate, 71 kDa protein | 1.551 |
| Q71V39 | Elongation factor 1-α2 | 1.528 |
Swiss-Prot accession nos. for each protein are shown under ID. Protein fold change vs. RI-vehicle is shown under fold.
Table 3.
Differentially expressed proteins downregulated in RI-mitochondria vs. RI-vehicle
| ID | Name | Fold |
|---|---|---|
| Q5T4S7 | E3 ubiquitin-protein ligase UBR4 | 0.657 |
| P02645 | Troponin I, slow skeletal muscle | 0.653 |
| P22695 | Cytochrome b-c1 complex subunit 2, mitochondrial | 0.646 |
| P50461 | Cysteine and glycine-rich protein 3 | 0.642 |
| P19404 | NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial | 0.564 |
| P20674 | Cytochrome c oxidase subunit 5A, mitochondrial | 0.559 |
| O43181 | NADH dehydrogenase [ubiquinone] iron sulfur protein 4, mitochondrial | 0.549 |
| O00483 | NADH dehydrogenase [ubiquinone] 1α subcomplex subunit 4 | 0.547 |
| P38646 | Stress-70 protein, mitochondrial | 0.531 |
| P07195 | l-Lactate dehydrogenase B chain | 0.525 |
| O75947 | ATP synthase subunit d, mitochondrial | 0.464 |
| P61604 | 10 kDa heat shock protein, mitochondrial | 0.449 |
| Q9UII2 | ATPase inhibitor, mitochondrial | 0.447 |
| P30049 | ATP synthase subunit-δ, mitochondrial | 0.437 |
| P10916 | Myosin regulatory light chain 2, ventricular/cardiac muscle isoform | 0.419 |
| P00008 | Cytochrome c | 0.386 |
| P09212 | Superoxide dismutase [Cu-Zn] | 0.382 |
| P07919 | Cytochrome b-c1 complex subunit 6, mitochondrial | 0.381 |
| P42765 | 3-Ketoacyl-CoA thiolase, mitochondrial | 0.366 |
| P63316 | Troponin C, slow skeletal and cardiac muscles | 0.325 |
| P08590 | Myosin light chain 3 | 0.252 |
| P58772 | Tropomyosin-α1 chain | 0.244 |
| P05413 | Fatty acid-binding protein, heart | 0.241 |
Swiss-Prot accession nos. for each protein are shown under ID. Protein fold change vs. RI-vehicle is shown under fold.
Table 4.
Functionally enriched pathways upregulated in RI-mitochondria vs. RI-vehicle
| Enrichment Score | No. | P Value | |
|---|---|---|---|
| Annotation cluster 1 | 7.39 | ||
| Mitochondrion | 11 | P < 0.001 | |
| Mitochondrial inner membrane | 7 | P < 0.001 | |
| Annotation cluster 2: mitochondrial matrix | 4.68 | ||
| 7 | P < 0.001 | ||
| Annotation cluster 3: generation of precursor metabolites and energy | 3.59 | ||
| 7 | P < 0.001 | ||
| Annotation cluster 4: cellular respiration | 3.48 | ||
| 4 | P < 0.001 |
Annotation clusters are arranged in order of enrichments score. The number of proteins in each annotation cluster is indicated by number. The P value for each annotation cluster is provided.
DISCUSSION
Herein we present in vivo and in vitro studies demonstrating that the transplantation of autologously derived mitochondria provides cardioprotection intracellularly and extracellularly to significantly decrease myocardial necrosis and significantly enhance postischemic function. These cardioprotective effects are apparent at 10 min of reperfusion and persist for at least 28 days. The transplanted mitochondria decrease inflammatory markers significantly and do not induce autoimmunity or arrhythmia. The transplanted mitochondria are readily internalized by cardiomyocytes 2 h after transplantation and remain viable for at least 24 h, enhancing oxygen consumption rate and high-energy synthesis and inducing cytokine mediators and proteomic pathways, allowing for cardioprotection.
At present, the need for mitochondria to be internalized does not appear to be critical because cardioprotection occurs rapidly within 10 min of reperfusion and suggests that the presence of viable mitochondria in cardiac tissue is all that is required. This would agree with the conclusions of others who have shown that preservation of mitochondrial respiratory function during reperfusion enhances contractile recovery and decreases myocardial infarct size (5, 16).
The need for the transplanted mitochondria to be viable is essential. In previous studies where the isolated perfused rabbit heart was used, we demonstrated that nonviable mitochondria, previously frozen mitochondria, mitochondrial fractions (proteins, complex I–V), mitochondrial DNA and RNA, and exogenous ATP or ADP, when injected directly into the ischemic zone during early reperfusion, did not provide cardioprotection (24). Reactive oxygen species do not appear to play a major role in the cardioprotection provided by mitochondrial transplantation, although there are alterations in cytosolic calcium accumulation with accompanying changes in mitochondrial calcium accumulation during ischemia, and transplanted mitochondria do not act as a calcium sink (1, 24, 25).
The mechanisms through which mitochondrial transplantation provides for cardioprotection clearly involve both enhanced ATP production and alterations in the proteome and cytokine induction. We speculate that these effects act to provide cardioprotection in two phases; first, there is an immediate cardioprotective phase that occurs occurring during the first 10 min of reperfusion, where enhanced ATP production and the enrichment of differentially expressed proteins associated with mitochondrial pathways and those responsible for the generation of precursor metabolites associated with energy and cellular respiration provide cardioprotection. These modulations would allow for cardiac cells to respond to the ischemic insult during the immediate phase of reperfusion, providing needed ATP and needed upregulation of mitochondrial pathways, and as a consequence would decrease necrosis and apoptosis and allow for enhanced postischemic functional recovery. The salvage of the myocytes at this early phase would then act in concert with cytokine induction to enhance long-term viability and function.
The cytokines upregulated by mitochondrial transplantation (EGF, GRO, IL-6, and MCP-3) have been shown to play key roles in angiogenesis, arteriogenesis, immunomodulation, progenitor cell migration, prevention of apoptosis, and enhanced cell salvage and postischemic functional recovery (32). EGF has a key role in ischemic injury protection in the heart by stimulating cell growth, proliferation, and migration (19, 39). After cardiac infarction, GRO participates in the improvement in function and reconstitution of tissue mass and acts with IL-6 as a chemoattractant that allows for enhanced vascularization, protection against cardiomyocyte apoptosis, and improved functional cardiac recovery (14). These chemokines have been shown to act with MCP-3 to enhance postinfarction cardiac function and improve cardiac remodeling independent of cardiac myocyte regeneration (36). These positive actions by cytokines are in agreement with our recent studies demonstrating that cardioprotection is associated with a significant increase in differentially expressed proteins associated with mitochondrial function (1, 23). We speculate that the enhanced total tissue ATP levels in the area at risk and the induction of cardioprotective cytokines play a direct role in enriching differentially expressed proteins associated with mitochondrial pathways and those responsible for the generation of precursor metabolites associated with energy and cellular respiration.
In our studies, we have used both syngeneic and xenogeneic mitochondria to show cellular uptake of transplanted mitochondria. This was done to exclude the possibility that label from the transplanted mitochondria or excess label not bound to the transplanted mitochondria may be labeling endogenous mitochondria and thus suggesting uptake. The use of human mitochondria in a rabbit model allowed for the differentiation between endogenous rabbit mitochondria and transplanted human mitochondria based on immune reactivity to a monoclonal anti-human mitochondria antibody. Using HeLa cell mitochondria, we were able to show definitively using florescent staining in vivo and in vitro and immunogold-conjugated antibody labeling that the transplanted mitochondria were internalized into cells.
Our studies demonstrate that transplanted mitochondria are internalized by myocyte and nonmyocyte cardiac cells 2 h after transplantation and remain viable for at least 24 h, enhancing oxygen consumption rate and high-energy synthesis. The vast majority of transplanted mitochondria appear to remain extracellular and in close proximity to the cells. However, some of the injected mitochondria are integrated as both clusters and individual mitochondria within the cells. In vivo and in vitro data show that the transplanted mitochondria are adherent to the cell surface at 2 h and that at 8–24 h after transplantation some of the syngenic or xenogenic mitochondria are integrated within cardiomyocytes. Visual estimation indicates that ∼3–7% of the transplanted mitochondria are integrated into cardiomyocytes.
The mechanism of internalization of the transplanted mitochondria is as yet unknown. Previous studies have suggested that the involvement of tunneling nanotubes or junctions may be involved in cell-to-cell trafficking of cellular components (10, 20, 29, 40). However, in our studies, isolated mitochondria were either injected in vivo into the myocardium or cocultured in vitro with cardiomyocytes. We observed no extensions from cardiomyocytes or in myocardial sections, suggesting that mitochondrial uptake by tunneling nanotubes, although demonstrated in cell-to-cell transfer, may not play a role in organelle-to-cell transfer.
Although we were unable to identify the mechanisms through which transplanted mitochondria are internalized, our data provide a novel method for cardioprotection and new directions for possible mitochondrial therapy. Mitochondria can be isolated rapidly within the clinical time frame for use in coronary artery bypass grafting and in percutaneous coronary intervention for coronary revascularization for ST segment elevation myocardial infarction. The transplantation of mitochondria with no proarrhythmic response and with no induction of immune response provides an efficacious cardioprotective protocol that could be used as a bridge prior to subsequent cell-based regenerative therapies, which require extended times for cell isolation, purification, and expansion. The internalization of the transplanted mitochondria and enhanced myocardial energetics, with accompanying induction of beneficial cytokine and proteomic pathways, would be expected to enhance such a therapy.
We conclude that transplantation of mitochondria offers a unique therapeutic strategy for cardioprotection that may be used alone or concurrent with, or as an adjunct to, other clinical interventions to ameliorate cell death and enhance function.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-103642 and HL-088206.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M., K.M.B., C.A.P., M.E., R.J.B., C.D., P.S., D.B.B., D.B.C., and J.D.M. performed the experiments; A.M., K.M.B., C.A.P., M.E., R.J.B., P.S., D.B.C., and J.D.M. analyzed the data; A.M., D.B.C., and J.D.M. interpreted the results of the experiments; A.M., K.M.B., C.A.P., M.E., R.J.B., C.D., P.S., D.B.B., S.L., D.B.C., and J.D.M. approved the final version of the manuscript; D.B.C. and J.D.M. prepared the figures; D.B.C. and J.D.M. edited and revised the manuscript; J.D.M. contributed to the conception and design of the research; J.D.M. drafted the manuscript.
Supplementary Material
REFERENCES
- 1. Black KM, Barnett RJ, Bhasin MK, Daly C, Dillon ST, Libermann TA, Levitsky S, McCully JD. Microarray and proteomic analysis of the cardioprotective effects of cold blood cardioplegia in the mature and aged male and female. Physiol Genomics 44: 1027–1041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloch DB, Yu JH, Yang WH, Graeme-Cook F, Lindor KD, Viswanathan A, Bloch KD, Nakajima A. The cytoplasmic dot staining pattern is detected in a subgroup of patients with primary biliary cirrhosis. J Rheumatol 32: 477–483, 2005 [PubMed] [Google Scholar]
- 3. Byers TJ, Kunkel LM, Watkins SC. The subcellular distribution of dystrophin in mouse skeletal, cardiac, and smooth muscle. J Cell Biol 115: 411–421, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan Y, Tong HQ, Beggs AH, Kunkel LM. Human skeletal muscle-specific alpha-actinin-2 and -3 isoforms form homodimers and heterodimers in vitro and in vivo. Biochem Biophys Res Commun 248: 134–139, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319: 1405–1412, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Choi YH, Stamm C, Hammer PE, Kwaku KF, Marler JJ, Friehs I, Jones M, Rader CM, Roy N, Eddy MT, Triedman JK, Walsh EP, McGowan FX, Jr, del Nido PJ, Cowan DB. Cardiac conduction through engineered tissue. Am J Pathol 169: 72–85, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowan DB, Poutias DN, Del Nido PJ, McGowan FX., Jr CD14-independent activation of cardiomyocyte signal transduction by bacterial endotoxin. Am J Physiol Heart Circ Physiol 279: H619–H629, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol 46: 858–866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18: 759–765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy 4: 322–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation 101: 2586–2594, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375: 132–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowski P, Boyle A, Homma S, Wang SF, Itescu S. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol 40: 455–464, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Kofidis T, deBruin JL, Tanaka M, Zwierzchoniewska M, Weissman I, Fedoseyeva E, Haverich A, Robbins RC. They are not stealthy in the heart: embryonic stem cells trigger cell infiltration, humoral and T-lymphocyte-based host immune response. Eur J Cardiothorac Surg 28: 461–466, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tandler B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol 287: H258–H267, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, Miyakawa H, Norman GL, Lee W, Gershwin ME. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology 46: 1436–1442, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Malhotra D, Yeh CC, Tu R, Zhu BQ, Birger N, Wisneski A, Cha J, Karliner JS, Mann MJ. Myocardial survival signaling in response to stem cell transplantation. J Am Coll Surg 208: 607–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorita J, Soley M, Ramirez I. Epidermal growth factor protects the heart against low-flow ischemia-induced injury. J Phys Biochem 66: 55–62, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K, Moore MA. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One 7: e33093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic, 1972, p. 151–156 [Google Scholar]
- 22. Macia E, Boyden PA. Stem cell therapy is proarrhythmic. Circulation 119: 1814–1823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCully JD, Bhasin MK, Daly C, Guerrero MC, Dillon S, Liberman TA, Cowan DB, Mably JD, McGowan FX, Levitsky S. Transcriptomic and proteomic analysis of global ischemia and cardioprotection in the rabbit heart. Physiol Genomics 38: 125–137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296: H94–H105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCully JD, Rousou AJ, Parker RA, Levitsky S. Age- and gender-related differences in mitochondrial oxygen consumption and calcium with cardioplegia and diazoxide. Ann Thorac Surg 83: 1102–1109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 286: H1923–H1935, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Meerson FZ, Zaletayeva TA, Lagutchev SS, Pshennikova MG. Structure and mass of mitochondria in the process of compensatory hyperfunction and hypertrophy of the heart. Exp Cell Res 36: 568–578, 1964 [DOI] [PubMed] [Google Scholar]
- 28. Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, Hagège AA, Aranda P, Andreu EJ, Menasché P, Prósper F. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail 10: 1065–1072, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res 316: 2447–2455, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Pombo JF, Troy BL, Russell RO., Jr Left ventricular volumes and ejection fraction by echocardiography. Circulation 43: 480–490, 1971 [DOI] [PubMed] [Google Scholar]
- 31. Rao V, Borger MA, Weisel RD, Ivanov J, Christakis GT, Cohen G, Yau TM. Insulin cardioplegia for elective coronary bypass surgery. J Thorac Cardiovasc Surg 119: 1176–1184, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Roche S, D'Ippolito G, Gomez LA, Bouckenooghe T, Lehmann S, Montero-Menei CN, Schiller PC. Comparative analysis of protein expression of three stem cell populations: models of cytokine delivery system in vivo. Int J Pharm 440: 72–82, 2013 [DOI] [PubMed] [Google Scholar]
- 33. Rose NR. Critical cytokine pathways to cardiac inflammation. J Interferon Cytokine Res 31: 705–710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rottlaender D, Boengler K, Wolny M, Schwaiger A, Motloch LJ, Ovize M, Schulz R, Heusch G, Hoppe UC. Glycogen synthase kinase 3beta transfers cytoprotective signaling through connexin 43 onto mitochondrial ATP-sensitive K+ channels. Proc Natl Acad Sci USA 109: E242–E251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Opening of mitochondrial KATP channels enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium accumulation, and respiration. Am J Physiol Heart Circ Physiol 287: H1967–H1976, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 25: 245–251, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Seth P, Grant A, Tang J, Vinogradov E, Wang X, Lenkinski R, Sukhatme VP. On-target inhibition of tumor fermentative glycolysis as visualized by hyperpolarized pyruvate. Neoplasia 13: 60–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sill B, Hammer PE, Cowan DB. Optical mapping of Langendorff-perfused rat hearts. J Vis Exp 30: 1138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Somers P, Cornelissen R, Thierens H, Van Nooten G. An optimized growth factor cocktail for ovine mesenchymal stem cells. Growth Factors 30: 37–48, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103: 1283–1288, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ. Myocardial protection in reperfusion with postconditioning. Expert Rev Cardiovasc Ther 3: 1035–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Wakiyama H, Cowan DB, Toyoda Y, Federman M, Levitsky S, McCully JD. Selective opening of mitochondrial ATP-sensitive potassium channels during surgically induced myocardial ischemia decreases necrosis and apoptosis. Eur J Cardiothorac Surg 21: 424–433, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.