Abstract
Muscle metaboreflex activation (MMA) during dynamic exercise increases cardiac work and myocardial O2 demand via increases in heart rate, ventricular contractility, and afterload. This increase in cardiac work should lead to metabolic coronary vasodilation; however, no change in coronary vascular conductance occurs. This indicates that the MMA-induced increase in sympathetic activity to the heart, which raises heart rate, ventricular contractility, and cardiac output, also elicits coronary vasoconstriction. In heart failure, cardiac output does not increase with MMA presumably due to impaired ability to improve left ventricular contractility. In this setting actual coronary vasoconstriction is observed. We tested whether this coronary vasoconstriction could explain, in part, the reduced ability to increase cardiac performance during MMA. In conscious, chronically instrumented dogs before and after pacing-induced heart failure, MMA responses during mild exercise were observed before and after α1-adrenergic blockade (prazosin 20–50 μg/kg). During MMA, the increases in coronary vascular conductance, coronary blood flow, maximal rate of left ventricular pressure change, and cardiac output were significantly greater after α1-adrenergic blockade. We conclude that in subjects with heart failure, coronary vasoconstriction during MMA limits the ability to increase left ventricular contractility.
during exercise, metabolite-sensitive afferent neurons within the skeletal muscle may be stimulated and evoke a reflex increase in sympathetic nerve activity to the heart and vasculature, known as the muscle metaboreflex (1, 2, 5, 28, 34, 40, 44, 55, 61–64, 69). In normal subjects during submaximal dynamic exercise, the metaboreflex elicits an increase in blood pressure mainly via a marked increase in cardiac output (CO), which occurs via tachycardia coupled with sustained or slightly increased stroke volume (SV) (5, 14, 28, 56, 61, 70). This increase in total systemic blood flow serves to partially restore flow and oxygen delivery to the ischemic muscle (48, 51).
However, in heart failure this reflex increase in blood pressure occurs mainly due to peripheral vasoconstriction, since little or no increase in CO occurs (2, 13, 28, 49, 58). Despite the tachycardia, the metaboreflex does not increase CO because a decrease in SV occurs (13, 28, 58). This fall in SV is likely due to increased ventricular afterload sensitivity coupled with an impaired ability to increase contractility (47, 49, 59). In addition to the structural/anatomical ventricular pathology, which impairs ventricular performance (20, 25, 60, 65, 66), limitations in oxygen supply to the myocardium during exercise may also contribute to poor cardiac function in heart failure. Reduced coronary vasodilator reserve as well as enhanced neurogenic coronary vasoconstriction may contribute to the restraint of coronary blood flow (CBF) during high oxygen demand situations such as exercise (2, 46). Even in normal subjects during exercise the left ventricle is functionally vasoconstricted inasmuch as coronary vasodilation increases with α1-adrenergic blockade (12, 26, 30), and with the increase in blood flow a significant rise in left ventricular contractility occurs (12, 26).
In normal subjects, muscle metaboreflex activation markedly increases ventricular work: increased heart rate (HR) and CO pumped against a much higher afterload. However, coronary vasodilation is markedly restrained and virtually all of the increase in CBF occurs via the substantial increase in arterial pressure (3, 12, 45, 50). With metaboreflex activation in heart failure, frank coronary vasoconstriction occurs and the increases in CBF are much smaller (2). To what extent this functional metaboreflex-induced coronary vasoconstriction in heart failure limits the ability to improve ventricular function and therefore ultimately limits the ability to increase CO is unknown.
MATERIALS AND METHODS
All of the methods and procedures were reviewed and approved by the Wayne State University Institutional Animal Care and Use Committee. The experiments were conducted on mongrel dogs (N = 7), weighing 22.7 (± 2.02) kg. The dogs were selected for their willingness to exercise on a motor-driven treadmill. No intended selection was made for sex; however, by random-source availability all animals were female. Previously this laboratory has shown that sex has little or no effect on metaboreflex responses in dogs (37).
The medications and surgical preparations used have been described in detail previously (2, 3, 12, 52, 59). Briefly, a 20-mm flow transducer was placed around the aortic root to measure CO. Hydraulic vascular occluders were placed around the superior and inferior vena cavae to manipulate preload. Two pairs of sonomicrometry crystals were implanted in the endocardium of the left ventricle on the short axis (SA) and long axis (LA) to estimate ventricular volume. A catheter was placed in the left ventricle for left ventricular pressure and its telemeter-pressure transducer was implanted subcutaneously. A 3-mm flow transducer was placed around the circumflex artery to assess CBF. Three ventricular pacing wires (0-Flexon) were sutured to the free wall of the right ventricle for subsequent ventricular pacing to induce heart failure. The animal was allowed at least 10 days for recovery. In a second surgical session, a 10-mm flow transducer was placed around the terminal aorta proximal to the iliac arteries to measure hindlimb blood flow (HLBF). Just distal to the flow probe a vascular occluder was placed about the terminal aorta. Arterial and central venous catheters were placed to measure systemic blood pressures.
Experimental protocol.
Each dog was directed to stand on the treadmill for 10–15 min while all equipment was connected and adequacy of the signals verified. All data were recorded on digital recording systems.
We obtained 1 min of steady-state resting data with the dog standing on the treadmill. Steady-state data and data during transient vena caval occlusions (for variably loaded pressure-volume loops) were recorded during the conditions of rest, mild exercise (3.2 km/h), and mild exercise with muscle metaboreflex activation. The reflex was activated by partially inflating the vascular occluder on the terminal aorta to reduce HLBF to ∼50% of the normal value during mild exercise. The experiments were performed with and without α1-adrenergic blockade (prazosin; 20–50 μg/kg iv 30 min before exercise). In each experiment, the dose of prazosin was sufficient to abolish any pressor response to 4 μg/kg of phenylephrine for the duration of the experiment. After completion of the control and α1-blockade experiments, congestive heart failure was induced via rapid ventricular pacing (28, 29). Briefly, the right ventricular pacing electrodes were connected to a pacemaker set at 200–220 beats/min for ∼30 days, and the experiments were repeated. The pacemaker was disconnected during the experiments.
Data analysis.
We calculated left ventricular volume (LVV) using a modified ellipsoid equation: [LVV = (π/6) × (SA)2 × (LA)]. Left ventricular pressure-volume loops were plotted for each condition. Left ventricular preload recruitable stroke work (PRSW) and maximal rate of left ventricular pressure change (dP/dtmax) were calculated. PRSW is the slope of the relationship between stroke work (integral of the pressure-volume area) and left ventricular end-diastolic volume. Changes in slope directly reflect changes in contractility (21, 32, 39). Cardiac power was calculated as the product of stroke work and HR. The integral of the CO wave was calculated to give SV. LVV data were corrected using the end-diastolic volume obtained from sonomicrometry and stroke volume from the aortic flow signal, as discussed in a previous study (12). Coronary vascular conductance (CVC) was calculated as CBF/[mean arterial pressure (MAP) − central venous pressure (CVP)]. Systemic vascular conductance to all nonischemic areas (e.g., all areas except the hindlimbs) is termed nonischemic vascular conductance (NIVC) and was calculated as (CO − HLBF)/(MAP − CVP). A two-way repeated-measures ANOVA was used for the main effects analyses, and a pairwise comparison was used for post hoc analyses using the Test for Simple Effects. The relationships between indices of contractility (dP/dtmax and PRSW) versus CBF were analyzed via linear regression. Statistical significance was defined as P < 0.05.
RESULTS
In normal animals, prazosin did not affect HLBF at rest or during exercise; however, after induction of heart failure there were significant increases in HLBF in both settings. HLBF was reduced to the same values in all conditions in an attempt to equate the level of metaboreflex activation (Table 1).
Table 1.
Hindlimb blood flow (in l/min ± SE) at rest and during mild exercise and metaboreflex activation in control and heart failure conditions before and after α1-adrenergic blockade
| Rest | Mild Exercise | Mild Exercise With Muscle Metaboreflex Activation | |
|---|---|---|---|
| Control | 0.58 ± 0.05 | 1.00 ± 0.09† | 0.52 ± 0.04 |
| α1-Blockade | 0.61 ± 0.06 | 1.07 ± 0.09† | 0.55 ± 0.04 |
| Heart failure | 0.46 ± 0.05 | 0.86 ± 0.11† | 0.51 ± 0.06 |
| α1-Blockade | 0.57 ± 0.07* | 1.16 ± 0.12*† | 0.51 ± 0.06 |
Significant effect of α-blockade;
significantly different from rest.
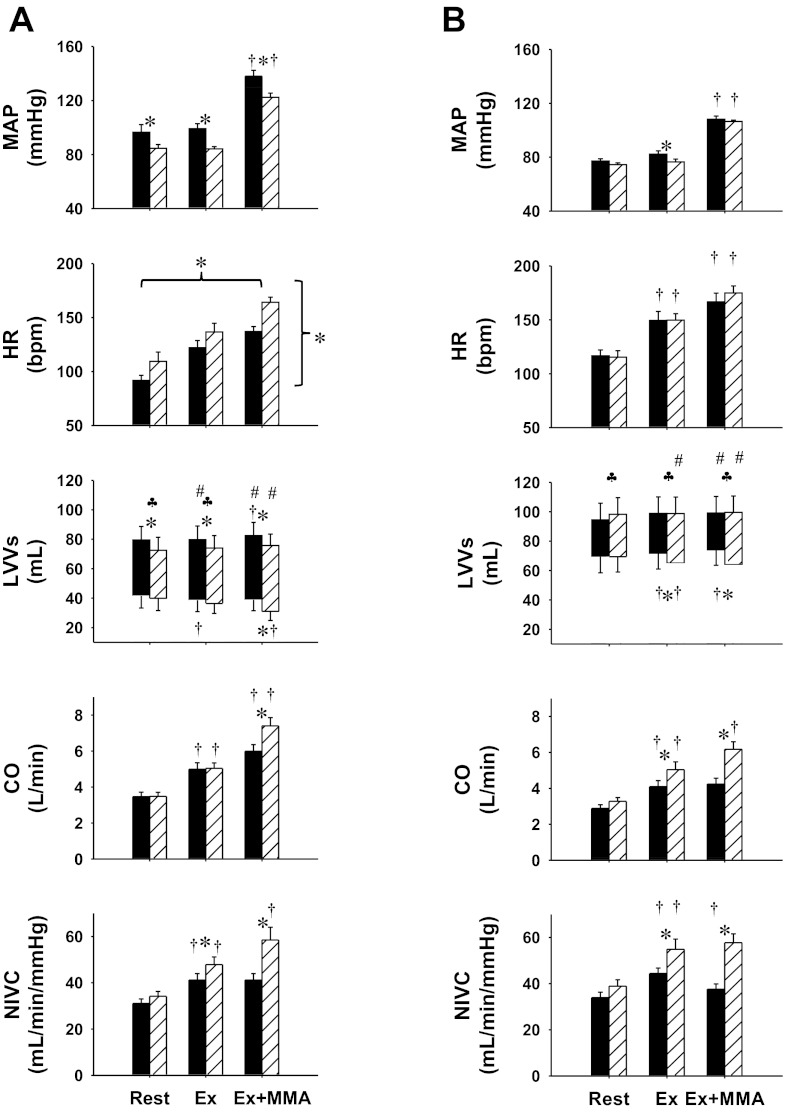
Figure 1 shows the mean values during steady state of MAP, HR, left ventricular end-diastolic and end-systolic volumes, CO, and NIVC at rest, during mild exercise, and during exercise with metaboreflex activation in control and after α1-adrenergic blockade in normal animals and in the same animals after induction of heart failure. In control experiments in normal animals, there was no change in MAP from rest to mild exercise; however, HR, SV, CO, and NIVC increased. Imposed reductions in HLBF caused muscle metaboreflex-induced increases in MAP, HR, SV, and CO. No significant change in NIVC occurred with metaboreflex activation. At rest, α1-adrenergic blockade caused a significant decrease in MAP, tachycardia, and reduced SV likely due to a reduced end-diastolic volume, with no changes in CO and NIVC. Responses to mild exercise were similar to those observed in control experiments. Metaboreflex activation caused a significant increase in MAP, HR, and SV. End-diastolic volume was still reduced compared with control; however, end-systolic volume was also reduced, resulting in comparable SV between control and α1-adrenergic blockade conditions. A greater reflex increase in CO compared with control and a significant increase in NIVC occurred (e.g., ANOVA revealed no significant interaction effect between settings and conditions).
Fig. 1.
Hemodynamic responses: mean arterial pressure (MAP), heart rate (HR), left ventricular volumes (LVVs), cardiac output (CO), and nonischemic vascular conductance (NIVC) and during rest, mild exercise (Ex) and mild exercise with muscle metaboreflex activation (Ex + MMA), in control (A) and heart failure (B) conditions, before (black bars) and after α1-blockade (striped bars). Bpm, beats/min. *Between bars, significant pairwise comparison (P < 0.05); †significant change from the previous setting; ♣significant pairwise comparison in left ventricle stroke volume (P < 0.05); #significant change in stroke volume from the previous setting (P < 0.05); *brackets, significant effect of settings or conditions (horizontal: rest, Ex, MMA; vertical: control, α1-blockade).
After induction of heart failure, there was no change in MAP or SV from rest to mild exercise; however, HR, CO, and NIVC increased. Imposed reductions in HLBF caused muscle metaboreflex-induced increases in MAP and HR, but decreases in SV and NIVC occurred. There was no change in CO with metaboreflex activation. Thus the mechanisms of the reflex shifted from increased CO in the normal animal to increased peripheral vasoconstriction in heart failure. MAP at rest after α1-adrenergic blockade was slightly lower, but this was not statistically significant. There was no change in HR and SV increased, likely due to an increase in end-diastolic volume. During mild exercise MAP was significantly lower. Left ventricular end-systolic volume decreased with exercise, which resulted in an increased SV. CO and NIVC also increased greater than observed before α1-adrenergic blockade. Metaboreflex activation caused a similar increase in MAP as that without α1-adrenergic blockade; however, the mechanisms of the pressor response were markedly different. In contrast with the control experiments in heart failure, after α1-adrenergic blockade, left ventricular end-systolic volume was maintained during muscle metaboreflex activation and, since end-diastolic volume did not change, SV remain at the same levels. This sustained SV coupled with the tachycardia now caused a significant increase in CO with metaboreflex activation in heart failure after α1-blockade. Rather than a decrease in NIVC as normally seen with metaboreflex activation in animals with heart failure, no significant change occurred. Thus, after α1-adrenergic blockade in heart failure, the metaboreflex pressor response returned to a CO-based response as seen before induction of heart failure.
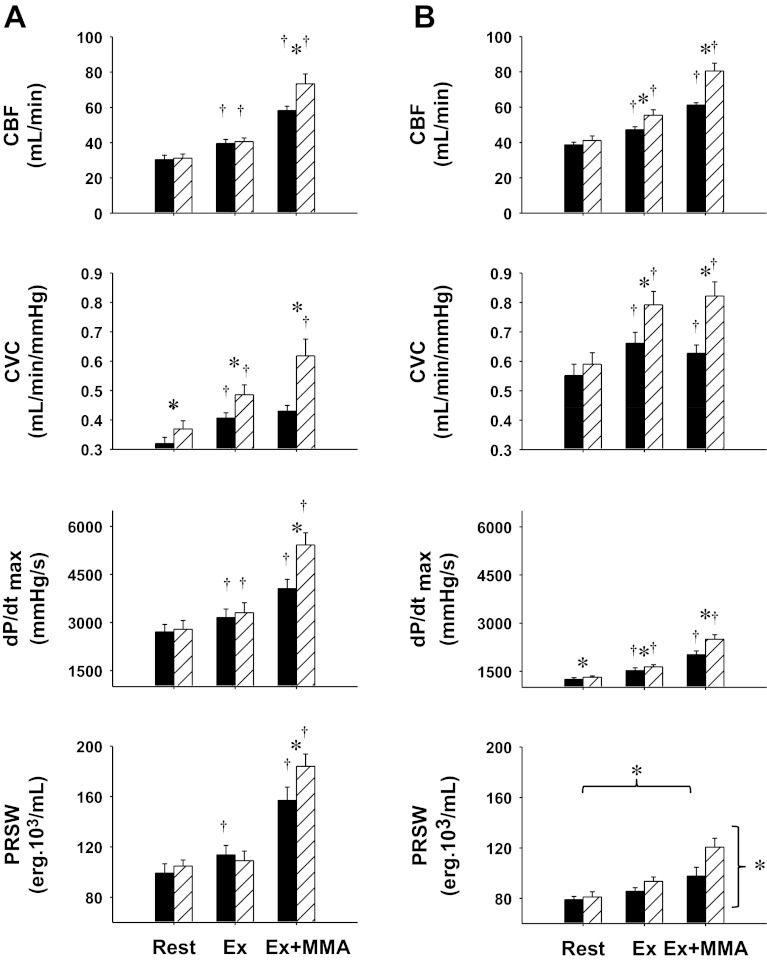
Figure 2 shows the cardiac vascular and ventricular performance responses to mild exercise and metaboreflex activation in normal animals during control and after α1-adrenergic blockade and in the same animals after induction of heart failure. With the transition from rest to exercise in control experiments there were significant increases in CBF, CVC, dP/dtmax, and PRSW. Metaboreflex activation further increased CBF and left ventricular contractility; however, no vasodilation occurred in the coronary circulation since there was no significant increase in CVC. Thus all of the increase in CBF with metaboreflex activation was due to the increase in perfusion pressure. After α1-adrenergic blockade there was an increase in CVC at rest. There were also significant increases in all parameters with the exception of PRSW from rest to mild exercise, and the rise in CVC was significantly greater than that observed in control; however, CBF was not different from control, likely due to the lower perfusion pressure. After α1-adrenergic blockade, activation of the muscle metaboreflex now elicited significantly greater increases in CBF. Although the perfusion pressure was lower, substantial coronary vasodilation occurred as indicated by the large increase in CVC. Metaboreflex activation in this setting caused significantly greater increases in both indexes of myocardial contractility.
Fig. 2.
Left ventricular hemodynamic and function responses: coronary blood flow (CBF), coronary vascular conductance (CVC), maximal rate of left ventricular pressure change (dP/dtmax), and preload recruitable stroke work (PRSW) and during Ex and Ex + MMA in control (A) and heart failure (B) before (black bars) and after α1-blockade conditions (striped bars). Symbols are as defined in Fig. 1.
CBF and CVC were higher at rest in heart failure compared with control, whereas dP/dtmax and PRSW were markedly reduced. CBF, CVC, and dP/dtmax all increased from rest to mild exercise. Metaboreflex activation caused small increases in CBF and dP/dtmax; however, vasoconstriction occurred in the coronary circulation as CVC decreased significantly. After α1-adrenergic blockade there were also significant increases in all illustrated parameters from rest to mild exercise, which were statistically greater than control experiments in heart failure for CBF, CVC, and dP/dtmax. After α1-adrenergic blockade, activation of the muscle metaboreflex now elicited significantly greater increases in CBF. In this setting coronary vasodilation now occurred with metaboreflex activation as CVC significantly increased. With the coronary vasodilation and larger increases in CBF, greater increases in ventricular contractility occurred [dP/dtmax and PRSW; although PRSW was from n = 3 there were still significant differences across settings (rest, exercise, and metaboreflex activation) and conditions (control and α1-adrenergic blockade)].
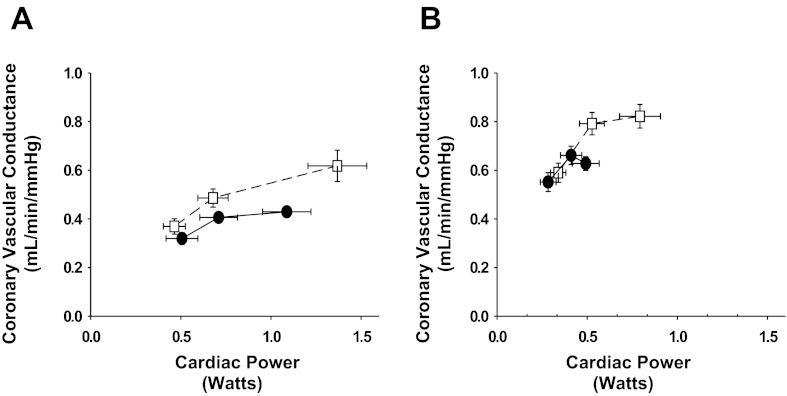
Figure 3 shows the relationship between CVC and cardiac power before and after α1-adrenergic receptor blockade at rest, during exercise, and with muscle metaboreflex activation (data points from left to right, respectively) in control experiments and after induction of heart failure. In control experiments, this relationship was shifted upward after α1-adenergic receptor blockade and extended to greater cardiac power and much more vasodilation with muscle metaboreflex activation. After induction of heart failure, there was little increase in cardiac power from rest to exercise and little further change with muscle metaboreflex activation, and in the latter transition coronary vascular conductance actually decreased. After α1-adrenergic receptor blockade, however, cardiac power and CVC increased much more from rest to exercise and both increased significantly further with muscle metaboreflex activation.
Fig. 3.
Relationship between coronary vasodilation and cardiac power at rest, during exercise and with muscle metaboreflex activation before (A) and after (B) induction of heart failure during control experiments (●) and after α1-adrenergic blockade (□). Coronary vasodilation in response to the increases cardiac power that occurred with exercise and metaboreflex activation was greater after α1-adrenergic blockade in control experiments. In heart failure, α1-adrenergic blockade reversed the coronary vasoconstriction seen with metaboreflex activation into vasodilation and markedly improved the ability to raise cardiac power.
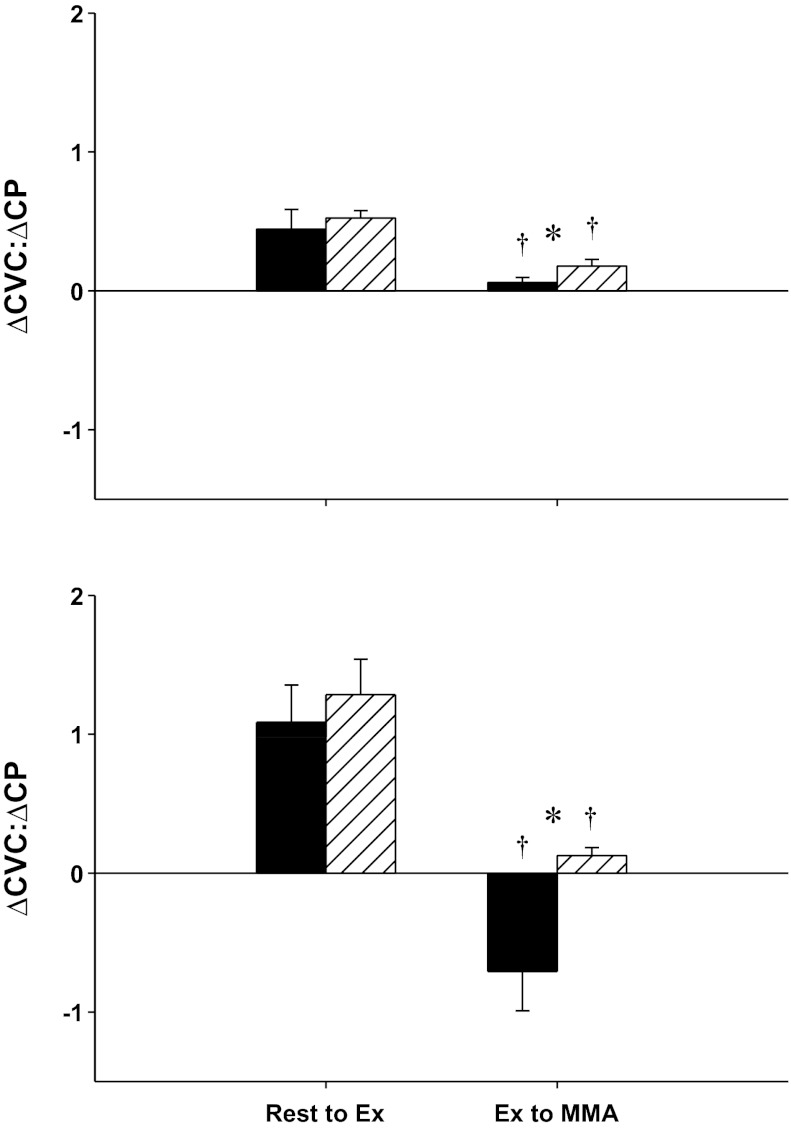
Figure 4 shows the ratio of the changes in CVC with respect to the changes in cardiac power (ΔCVC:ΔCP; e.g., the slope of the lines between each data point shown in Fig. 3), from rest to mild exercise and from mild exercise to metaboreflex activation in control experiments and after α1-adrenergic blockade before and after the induction of heart failure. During control experiments in normal animals, with the transition from rest to mild exercise, there is an increase in cardiac power and a coronary vasodilation; therefore, the ratio of CVC to CP is positive. This ratio was not significantly different after α1-blockade. With metaboreflex activation whereas cardiac power increased, little vasodilation occurred, and this ratio fell. With α1-adrenergic blockade, increases in both CVC and cardiac power occurred with metaboreflex activation, and this ratio increased significantly. After the induction of heart failure, with the transition from rest to exercise, coronary vasodilation and an increase in cardiac power occurred (therefore, there was a positive CVC:CP ratio). In contrast, with metaboreflex activation in heart failure actual coronary vasoconstriction occurred and there was little change in cardiac power; therefore, the CVC:CP ratio reversed to markedly negative. With α1-adrenergic blockade this ratio reversed back to a positive value as now vasodilation did occur with increased cardiac power.
Fig. 4.
Ratio between change in CVC (ΔCVC) and change in cardiac power (ΔCP) in normal (top) and heart failure (bottom). The black bars represent control, and the striped bars represent the corresponding values after α1-blockade. Both are compared across rest to mild exercise (rest to Ex) and mild exercise to muscle metaboreflex activation (Ex to MMA). *Above setting, significant pairwise comparison (P < 0.05); †significant decrease from the previous setting (P < 0.05).
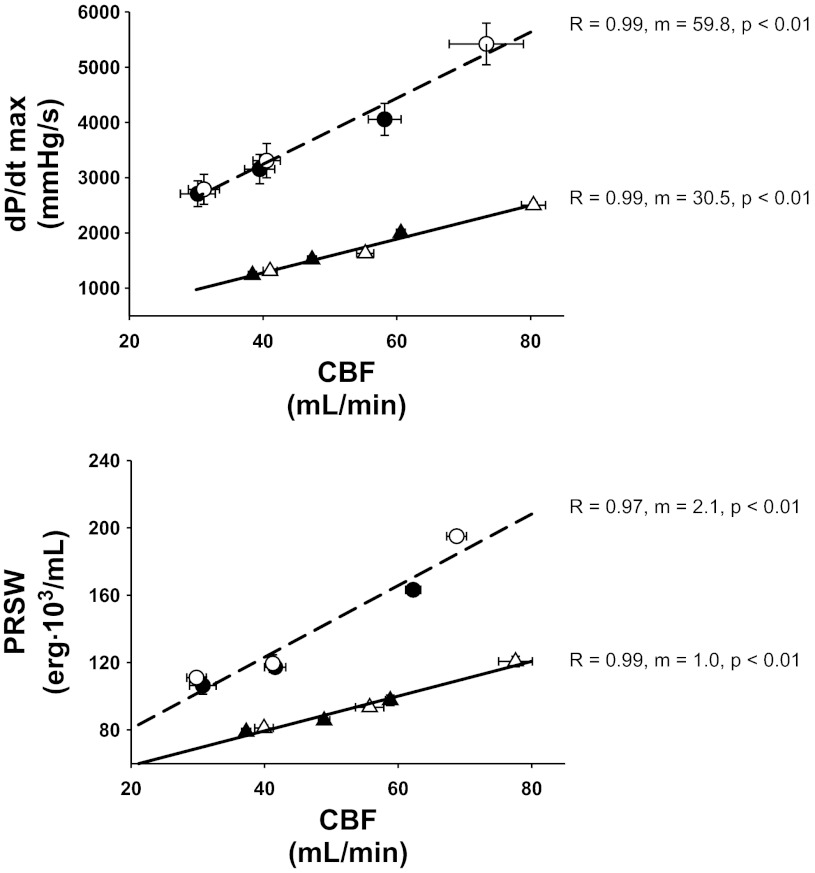
Figure 5 shows the relationship between the two indexes of contractility versus CBF. The data points are the steady state values at rest, during exercise, and during muscle metaboreflex activation (from left to right) with and without α1-adrenergic blockade in normal animals and in the same animals after induction of heart failure. The relationship between either index of contractility and CBF was highly linear and the data from control experiments and those after α1-adrenergic blockade fell along the same line both in normal animals and after induction of heart failure (there were no significant differences in the regression relationships if analyzed separately). In both normal animals and after induction of heart failure, α1-adrenergic blockade extended the relationship to higher flows and greater contractility. Heart failure depressed the contractility-flow relationships such that there were lower levels of contractility at any flow and the slope was significantly lower, e.g., smaller increases in contractility occurred for any given increase in CBF.
Fig. 5.
Relationship between CBF and left ventricular contractility as indexed by dP/dtmax and PRSW before and after the induction of heart failure. Because no significant difference between control and α1-blockade was found (P > 0.05), a single relationship is represented by a single line. Normal, circles; heart failure, triangles; black symbols, control experiments; white symbols, α1-blockade.
DISCUSSION
Our major finding is that the inability to raise ventricular contractility during metaboreflex activation in subjects with heart failure is not solely due to the ventricular dysfunction but is also due to coronary vasoconstriction and the resultant limitation in the ability to increase CBF and O2 delivery. Thus the inotropic incompetence seen during exercise in heart failure stems both from impaired contractile function as well as restrained ability to raise CBF due to α1-adrenergic mediated coronary vasoconstriction.
Previously we have shown in normal subjects that the muscle metaboreflex-induced restraint of coronary vasodilation functionally limits increases in left ventricular contractility (12). Even during moderate to heavy exercise in normal subjects there is a constant push/pull situation between the vasodilatory stimuli of metabolic as well as possible β2-mediated feed forward vasodilation (23), versus the vasoconstricting effects of coronary vascular α1-adrenergic receptor stimulation (26, 27, 45, 50). In heart failure, sympathetic activity is chronically elevated at rest (16, 29, 38). During exercise with muscle metaboreflex activation sympathetic activity is markedly increased, often causing profound vasoconstriction in inactive areas such as the kidney (28). This increased sympathetic drive, coupled with a limited ability to increase metabolic rate, likely shifts the push/pull balance toward vasoconstriction, thereby limiting the increase in CBF and therefore oxygen supply to the heart. (2, 12). This reduced ability to increase O2 delivery significantly contributes to the inability to raise ventricular contractility (Fig. 5). The suppressed increase in left ventricular contractility likely limits the ability to increase cardiac output, which thereby impedes the ability of the reflex to restore blood flow to ischemic working skeletal muscle (51).
Effects of heart failure.
Several structural and functional impairments occur during heart failure including ventricular remodeling as well as extensive cellular damage. The reduced cardiac function results from a myriad of complications including abnormal myosin cross-bridge activity (65, 66), prolonged calcium transients due to dysfunctional calcium channels on the sarcoplasmic reticulum (25), and reduced myocyte β1-adrenergic receptor density as well as a greatly reduced adenylate cyclase activity, indicating that myocardial β1-receptor function is also attenuated (7). The ventricular structural remodeling further attenuates cardiac function (31, 65, 66).
In the present study, we hypothesized that limited blood flow and therefore oxygen delivery to the myocardium may be another important factor contributing to the reduced cardiac performance during metaboreflex activation in heart failure. Canetti et al. (8) showed that the maximal capacity for coronary arteries to dilate is impaired during heart failure, indicating a possible restraint of CBF during high oxygen demand situations such as exercise and metaboreflex activation. We showed that muscle metaboreflex activation during heart failure elicited coronary vasoconstriction, which suppressed increases in blood flow. After α1-adrenergic receptor blockade, coronary vasodilation occurred with metaboreflex activation and increases in contractility and cardiac power were seen.
Coronary hemodynamics and ventricular performance.
Coronary vasomotor tone is determined by a wide number of factors including metabolic vasodilation as well as neurogenic vasoconstriction. Sympathetic activation may cause both an increase in cardiac work (as increases in HR and inotropic state occur) as well as potentially activation of coronary α-adrenergic receptors. Thus one approach is to analyze the coronary vasomotor response as function of myocardial work or O2 consumption (12, 18, 30, 67, 68). In a recent study from this laboratory (12), we used cardiac power (stroke work times heart rate) as an index of the steady-state O2 demands of the heart (19, 35, 42) and showed that in the normal heart there is a linear relationship between coronary vasodilation versus cardiac power. The slope of this relationship is higher after α1-receptor blockade, which indicates that after blockade of the coronary vasoconstrictor effects of the rise in cardiac sympathetic activity during metaboreflex activation, a given increase in myocardial workload causes greater vasodilation. However, this linear model is lost in heart failure. Metaboreflex activation caused minimal increases in cardiac power and frank coronary vasoconstriction occurred. Therefore, to analyze this relationship, the ratio of the changes in coronary vasodilation to the changes in cardiac power was calculated separately for the transitions from rest to exercise and from exercise to metaboreflex activation, e.g., the slope of the CVC:CP relationship between each setting. With the transition from rest to mild exercise in control experiments, there were increases in both CVC and CP, and this ratio was unaffected by α1-adrenergic blockade. With metaboreflex activation, this ratio was significantly reduced as the increases in cardiac power occurred with little vasodilation. In this setting, α1-adrenergic receptor blockade did cause a significant increase in slope. This indicates that neurogenic vasoconstriction attenuates coronary vasodilation. After induction of heart failure, metaboreflex activation actually caused a negative slope; little increase in cardiac power was seen, whereas significant coronary vasoconstriction occurred. This ratio was reversed to a positive value following α1-adrenergic blockade. This marked change in the vasodilation/function relationship with α1-adrenergic blockade underscores the severe consequences of coronary vasoconstriction in heart failure.
There was a linear relationship between indexes of contractility and CBF in both normal subjects and after induction of heart failure (Fig. 5). Within each condition, the values before and after α1-adrenergic blockade fell on the same line; α1-adrenergic blockade extended this relationship to higher levels of CBF and higher levels of inotropic state. Heart failure markedly depressed the slope of this relationship; however, a significant slope still occurred, indicating that the impaired ventricular function is in part due to the restraint of CBF. Whereas this slope is significantly lower in heart failure, ventricular function is already so depressed that relatively small increases in contractile strength may make significant differences in overall cardiovascular function.
In the present study prazosin was used as an α1-adrenergic antagonist. Short-term clinical results have shown that prazosin therapy provides favorable hemodynamic responses such as reduced pulmonary venous congestion, improved end-diastolic and -systolic volumes, increased CBF, CO, and improved New York Heart Association functional class (4, 6, 9, 11, 17, 22, 41, 43, 57). However, these responses were attenuated in the long-term (11, 17), and there was no improvement in mortality (9). Another concern with the long-term clinical use of prazosin is the possible attenuation of ventricular preload below that of optimal filling pressure (6). The systemic effects of prazosin may also complicate its clinical usefulness. If α-receptor blockade could be specifically targeted only to the coronary vasculature, a more beneficial outcome may occur.
Limitations.
Systemic α1-adrenergic blockade can lower arterial pressure, which could elicit baroreflex compensation as well as raise CO via reduced afterload. However, in normal subjects MAP was decreased by α1-adrenergic blockade to a similar extent at rest, during exercise, and with muscle metaboreflex activation. Similarly, only during metaboreflex activation was CO significantly elevated. This supports previous studies that concluded that the arterial baroreflex operates mainly via modulation of peripheral vasoconstriction rather than CO (10, 53, 54). After induction of congestive heart failure, prazosin had no significant effects on MAP in any setting.
We observed systemic vasodilation with muscle metaboreflex after α1-adrenergic blockade. A large portion of this change likely occurs in skeletal muscle (36). In previous experiments from our laboratory, this systemic vasodilation was blocked by propranolol (50), indicating a likely β2-mediated adrenergic vasodilation. It is possible that some of the coronary vasodilation observed is also β2-mediated vasodilation (24).
Due to difficulties in attaining PRSW during heart failure, we were limited to a small sample size (N = 3). However, a clear trend is visible, which supplements the results observed with dP/dtmax. Although dP/dtmax is considered sensitive to changes in loading conditions (33, 39), it is still a widely used index of contractility.
Canines were used as the animal model. Several recent studies have confirmed that many of the observations obtained using dogs readily translate to humans despite the differences in relative heart size, tonic orthostatic challenge, and maximal O2 consumption. Metaboreflex activation in normal humans can elicit significant increases in cardiac output, and, in heart failure patients, this reflex shifts toward peripheral vasoconstriction (13, 15). Furthermore, muscle metaboreflex-induced coronary vasoconstriction is also seen in humans (45). We performed these experiments during mild exercise, a setting wherein substantial cardiac reserve still exists, even in heart failure (29). During heavier exercise metaboreflex mediated coronary vasoconstriction may be even more intense and this may contribute to exercise intolerance seen in heart failure.
In summary, in heart failure muscle metaboreflex-induced increases in cardiac sympathetic activity elicit coronary vasoconstriction, which limits increases in CBF. The restricted increases in CBF in turn limit increases in ventricular function. Thus the inability to effectively raise CO during metaboreflex activation in heart failure is not only due to the ventricular dysfunction but also occurs as a result of coronary vasoconstriction.
GRANTS
This study was supported by National Heart, Lung, and Blood Grant HL-55,473.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C., J.A.S.-M., M.I., E.J.D., and D.S.O'L. conception and design of research; M.C., J.A.S.-M., M.I., Z.L., E.J.D., and D.S.O'L. performed experiments; M.C., J.A.S.-M., M.I., Z.L., and D.S.O'L. analyzed data; M.C., J.A.S.-M., M.I., and D.S.O'L. interpreted results of experiments; M.C., J.A.S.-M., and D.S.O'L. prepared figures; M.C., J.A.S.-M., and D.S.O'L. drafted manuscript; M.C., J.A.S.-M., M.I., and D.S.O'L. edited and revised manuscript; M.C., J.A.S.-M., M.I., Z.L., E.J.D., and D.S.O'L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Erin Welsh-Krengel, Jody Helme-Day, and Janine Mattei for superior technical assistance.
REFERENCES
- 1. Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol 84: 1827–1833, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Ansorge EJ, Augustyniak RA, Perinot RL, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Ansorge EJ, Shah SH, Augustyniak R, Rossi NF, Collins HL, O'Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Aronow WS, Lurie M, Turbow M, Whittaker K, Van CS, Hughes D. Effect of prazosin vs placebo on chronic left ventricular heart failure. Circulation 59: 344–350, 1979 [DOI] [PubMed] [Google Scholar]
- 5. Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Awan NA, Miller RR, DeMaria AN, Maxwell KS, Neumann A, Mason DT. Efficacy of ambulatory systemic vasodilator therapy with oral prazosin in chronic refractory heart failure. Concomitant relief of pulmonary congestion and elevation of pump output demonstrated by improvements in symptomatology, exercise tolerance, hemodynamics and echocardiography. Circulation 56: 346–354, 1977 [DOI] [PubMed] [Google Scholar]
- 7. Calderone A, Bouvier M, Li K, Juneau C, De Champlain J, Rouleau JL. Dysfunction of the beta and alpha adrenergic systems in a model of congestive heart failure. Circ Res 69: 332–343, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Canetti M, Akhter MW, Lerman A, Karaalp IS, Zell JA, Singh H, Mehra A, Elkayam U. Evaluation of myocardial blood flow reserve in patients with chronic congestive heart failure due to idiopathic dilated cardiomyopathy. Am J Cardiol 92: 1246–1249, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 314: 1547–1552, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Collins HL, Augustyniak RA, Ansorge EJ, O'Leary DS. Carotid baroreflex pressor responses at rest and during exercise:cardiac output vs. regional vasoconstriction. Am J Physiol Heart Circ Physiol 280: H642–H648, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Colucci WS, Wynne J, Holman BL, Braunwald E. Long-term therapy of heart failure with prazosin: a randomized double blind trial. Am J Cardiol 45: 337–344, 1980 [DOI] [PubMed] [Google Scholar]
- 12. Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol 109: 271–278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu A. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291: H3035–H3042, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation 93: 1667–1676, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Elkayam U, LeJemtel TH, Mathur M, Ribner HS, Frishman WH, Strom J, Sonnenblick EH. Marked early attenuation of hemodynamic effects of oral prazosin therapy in chronic congestive heart failure. Am J Cardiol 44: 540–545, 1979 [DOI] [PubMed] [Google Scholar]
- 18. Feigl EO. Coronary Physiology. Physiol Rev 63: 1–205, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 44: 340–348, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Fletcher L, Thomas D. Congestive heart failure: understanding the pathophysiology and management. J Am Acad Nurse Pract 13: 249–257, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009, 1985 [DOI] [PubMed] [Google Scholar]
- 22. Goldman SA, Johnson LL, Escala E, Cannon PJ, Weiss MB. Improved exercise ejection fraction with long-term prazosin therapy in patients with heart failure. Am J Med 68: 36–42, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1892–1902, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1903–1911, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res 61: 70–76, 1987 [DOI] [PubMed] [Google Scholar]
- 26. Gwirtz PA, Overn SP, Mass HJ, Jones CE. α1-Adrenergic constriction limits coronary flow and cardiac function in running dogs. Am J Physiol Heart Circ Physiol 250: H1117–H1126, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Gwirtz PA, Stone HL. Coronary blood flow changes following activation of adrenergic receptors in the conscious dog. Am J Physiol Heart Circ Physiol 243: H13–H19, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Kajstura J, Zhang X, Liu Y, Szoke E, Cheng W, Olivetti G, Hintze TH, Anversa P. The cellular basis of pacing-induced dilated cardiomyopathy: myocyte cell loss and myocyte cellular reactive hypertrophy. Circulation 92: 2306–2317, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Karunanithi MK, Michniewicz J, Copeland SE, Feneley MP. Right ventricular preload recruitable stroke work, end-systolic pressure volume, and dP/dtmax-end-diastolic volume relations compared as indexes of right ventricular contractile performance in conscious dogs. Circ Res 70: 1169–1179, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility—experimental and theoretical-analysis based on pressure-volume relationships. Circulation 76: 1422–1436, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–I65, 1987 [PubMed] [Google Scholar]
- 35. Khouri EM, Gregg DE, Rayford CR. Effect of exercise on cardiac output, left coronary flow and myocardial metabolism in the unanesthetized dog. Circ Res 17: 427–437, 1965 [DOI] [PubMed] [Google Scholar]
- 36. Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005 [DOI] [PubMed] [Google Scholar]
- 37. LaPrad SL, Augustyniak RA, Hammond RL, O'Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation 80: 1378–1387, 1989 [DOI] [PubMed] [Google Scholar]
- 40. MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Markham RV, Jr, Corbett JR, Gilmore A, Pettinger WA, Firth BG. Efficacy of prazosin in the management of chronic congestive heart failure: a 6-month randomized, double-blind, placebo-controlled study. Am J Cardiol 51: 1346–1352, 1983 [DOI] [PubMed] [Google Scholar]
- 42. Mendoza DD, Cooper HA, Panza JA. Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am Heart J 153: 366–370, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Mettauer B, Rouleau JL, Bichet D, Kortas C, Manzini C, Tremblay G, Chatterjee K. Differential long-term intrarenal and neurohormonal effects of captopril and prazosin in patients with chronic congestive heart failure: importance of initial plasma renin activity. Circulation 73: 492–502, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Mittelstadt SW, Bell LB, O'Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol 77: 2761–2766, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Momen A, Gahremanpour A, Mansoor A, Kunselman A, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol 102: 735–739, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mundhenke M, Schwartzkopff B, Kostering M, Deska U, Klein RM, Strauer BE. Endogenous plasma endothelin concentrations and coronary circulation in patients with mild dilated cardiomyopathy. Heart 81: 278–284, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998 [DOI] [PubMed] [Google Scholar]
- 48. O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999 [DOI] [PubMed] [Google Scholar]
- 49. O'Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol 287: H2612–H2618, 2004 [DOI] [PubMed] [Google Scholar]
- 50. O'Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol 103: 190–194, 2007 [DOI] [PubMed] [Google Scholar]
- 51. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995 [DOI] [PubMed] [Google Scholar]
- 52. O'Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91: 73–77, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olivier NB, Stephenson RB. Characterization of baroreflex impairment in conscious dogs with pacing-induced heart failure. Am J Physiol Regul Integr Comp Physiol 265: R1132–R1140, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 56. Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Rubin SA, Chatterjee K, Gelberg HJ, Ports TA, Brundage BH, Parmley WW. Paradox of improved exercise but not resting hemodynamics with short-term prazosin in chronic heart failure. Am J Cardiol 43: 810–815, 1979 [DOI] [PubMed] [Google Scholar]
- 58. Sala-Mercado JA, Ichinose M, Hammond RL, Ichinose TK, Pallante M, Stephenson LW, O'Leary DS, Iellamo F. Muscle metaboreflex attenuates spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2867–H2873, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation 83: 504–514, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987 [DOI] [PubMed] [Google Scholar]
- 62. Sinoway LI, Rea RF, Mosher TJ, Smith MB, Mark AL. Hydrogen ion concentration is not the sole determinant of muscle metaboreceptor responses in humans. J Clin Invest 89: 1875–1884, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994 [DOI] [PubMed] [Google Scholar]
- 64. Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992 [DOI] [PubMed] [Google Scholar]
- 65. Spinale FG, Fulbright BM, Mukherjee R, Tanaka R, Hu J, Crawford FA, Zile MR. Relation between ventricular and myocyte function with tachycardia-induced cardiomyopathy. Circ Res 71: 174–187, 1992 [DOI] [PubMed] [Google Scholar]
- 66. Spinale FG, Hendrick DA, Crawford FA, Smith AC, Hamada Y, Carabello BA. Chronic supraventricular tachycardia causes ventricular dysfunction and subendocardial injury. Am J Physiol Heart Circ Physiol 259: H218–H229, 1990 [DOI] [PubMed] [Google Scholar]
- 67. Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol 97: 404–415, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med 227: 238–250, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]