Abstract
Exosomes, which are 50- to 100-nm-diameter lipid vesicles, have been implicated in intercellular communication, including transmitting malignancy, and as a way for viral particles to evade detection while spreading to new cells. Previously, we demonstrated that adult cardiac myocytes release heat shock protein (HSP)60 in exosomes. Extracellular HSP60, when not in exosomes, causes cardiac myocyte apoptosis via the activation of Toll-like receptor 4. Thus, release of HSP60 from exosomes would be damaging to the surrounding cardiac myocytes. We hypothesized that 1) pathological changes in the environment, such as fever, change in pH, or ethanol consumption, would increase exosome permeability; 2) different exosome inducers would result in different exosomal protein content; 3) ethanol at “physiological” concentrations would cause exosome release; and 4) ROS production is an underlying mechanism of increased exosome production. We found the following: first, exosomes retained their protein cargo under different physiological/pathological conditions, based on Western blot analyses. Second, mass spectrometry demonstrated that the protein content of cardiac exosomes differed significantly from other types of exosomes in the literature and contained cytosolic, sarcomeric, and mitochondrial proteins. Third, ethanol did not affect exosome stability but greatly increased the production of exosomes by cardiac myocytes. Fourth, ethanol- and hypoxia/reoxygenation-derived exosomes had different protein content. Finally, ROS inhibition reduced exosome production but did not completely inhibit it. In conclusion, exosomal protein content is influenced by the cell source and stimulus for exosome formation. ROS stimulate exosome production. The functions of exosomes remain to be fully elucidated.
Keywords: exosome, proteomics, ethanol, valosin-containing protein, heat shock protein 60, alcoholic cardiomyopathy, reactive oxygen species
studies of exosomes, which are 50- to 100-nm-diameter lipid vesicles produced by a variety of cell types, and the cardiovascular system have been very limited. Most work on exosomes has focused on cancer, and this work suggests that exosomes have a role in intercellular signaling (5, 43). Previously, we (12) found that heat shock protein (HSP)60 was released in exosomes. We (19) have also shown that extracellular (ex)HSP60 can activate the innate immune system via Toll-like receptor (TLR)4, leading to cardiac myocyte apoptosis. ExHSP60 has also been shown to contribute to myocardial injury during ischemia (25). HSP60 has been found in human and rat plasma and serum samples in studies of both cardiovascular disease and diabetes (3, 11, 24, 26, 35). We reasoned that if HSP60 was contained within the exosome, it would not be toxic. However, if HSP60 was released from the exosome, this would promote cardiac myocyte death. The stability of exosomes and their ability to retain their cargo have not been investigated. In addition, the underlying mechanisms regulating exosome formation are not well understood and have not been studied in cardiac cells. Understanding the function and fate of cardiac exosomes can provide new insights into cardiovascular disease.
We investigated the stability of exosomes under physiological and pathological conditions, including changes in temperature and pH. We hypothesized that pathological conditions, such as fever and acidosis, would increase the leakiness of exosomes and release HSP60. We also examined whether ethanol, an organic solvent, at levels seen with moderate and heavy consumption of alcoholic beverages would increase the leakiness of exosomes. To better understand the function of cardiac myocyte-derived exosomes, we studied their protein content by mass spectrometry (MS) and compared exosomes produced after two different treatments: mild hypoxia and ethanol. Finally, we hypothesized that ROS production was a common factor between hypoxia/reoxygenation and ethanol treatment leading to exosome release. The results of these experiments, which demonstrated that exosomes are quite stable, exosomal protein content varies depending on the stimulus for production, and ROS have an important role in exosome generation, are reported here.
METHODS
Isolated Adult Cardiac Myocytes
Isolated adult cardiac myocytes were prepared from the hearts of 3-mo-old male Sprague-Dawley rats, as previously described (48). The animal protocol was approved by the University of California-Davis Research Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All research adhered to the American Physiological Society's “Guiding Principles in the Care and Use of Vertebrate Animals in Research.” Rats were anesthetized with an intraperitoneal injection of ketamine(75 mg/kg) and xylazine (3.9 mg/kg). Before removal of the heart, a terminal event, for the preparation of isolated cardiac myocytes, deep anesthesia was confirmed by the absence of corneal and toe pinch reflexes. Adult cardiac myocytes were cultured in media containing human serum albumin. As serum samples can contain exosomes, this human serum albumin was first centrifuged at 164,000 g for 2 h to remove all exosomes, as previously described (12).
Exosome Isolation
Untreated adult cardiac myocytes in culture produce a very minimal amount of exosomes. Exosome production by cardiac myocytes was increased by hypoxia (2 h) followed by 1 h of reoxygenation, as previously described (12). In later experiments, treatment with cell culture grade ethanol, at concentrations found in humans consuming alcoholic beverages, was done for 2 h followed by 1 h of recovery, matching the timing of the hypoxia protocol, after we found that ethanol treatment greatly increased exosome production.
Two different approaches were used to purify exosomes from the media. The first was a method adhering to current good manufacturing processes that generates exosomes that can be used clinically, which was tried so that exosomes could then be used for in vivo experiments (21). Alternatively, ExoQuick (System Biosciences) was used as a second method to prepare exosomes. This approach involves less labor than the serial centrifugation we used previously (12). ExoQuick was used following the directions of the manufacturer. When exosomes were prepared from media, the media was first concentrated from 50 ml to 130 μl with Amicon Ultra filter (Millipore, Billerica, MA) with a 100,000 molecular weight cutoff before ExoQuick treatment.
Exosome Quality
As exosomes are too small to readily visualize, the quality of exosome preparations was confirmed by measuring the hydrodynamic radius with a particle sizing system (NICOMP 380 zls, PSS, Port Richey, FL). In addition, acetyl choline esterase activity, which reflects the amount of cell membrane present, was used to indirectly follow the quantity of exosomes isolated along with measurement of total protein by the BCA assay (Pierce, Rockford, IL), as previously described (12). Highly consistent preparations of exosomes were obtained with Exoquick.
Electron Microscopy
Electron microscopy was done in the core Electron Microscopy Laboratory (Department of Pathology and Laboratory Medicine, School of Medicine, University of California, Davis, CA) following the approach of Thery et al. (50). Exosomes were ultracentrifuged to generate a pellet as part of the final step of isolation. A drop of this resuspended pellet was allowed to settle on a gold-coated grid, blotted, fixed in 1% glutaraldehyde, washed for 2 min in double-distilled water, incubated in uranyl oxylate for 5 min, incubated in three separate drops of methyl cellulose with uranyl acetate with 5 min in the first two drops and 10 min in the last drop, and finally removed from methyl cellulose-uranyl acetate by slow-drag on edge on filter paper. Exosomes were visualized by standard transmission electron microscopy with a Philips CM120 microscope.
Exosome Treatments
Exosomes were placed in standard PBS for all incubations. Protein concentrations were measured by the BCA assay (Pierce). After incubations under different conditions, exosomes were isolated by centrifugation at 164,000 g for 2 h. Protein in the supernatant was concentrated in an Amicon Ultra filter (Millipore) with a 100,000 molecular weight cutoff cutoff. Exosomal (pellet) and released (supernatant) proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose, and then analyzed for HSP60 expression using our previously published methods, as detailed below.
Temperature incubations were done using precision water baths set at appropriate temperature and a 4°C refrigerator. pH was adjusted with concentrated HCl. Ethanol (100%, tissue culture grade) was added to the buffer to achieve concentrations of 21.7 and 65.1 mM. These ethanol levels correspond to legally intoxicated levels and levels found with the consumption of multiple alcoholic drinks (15). Similar concentrations have been studied using in vivo rodent models of ethanol ingestion (16).
Cell Injury
Apoptosis was assessed by quantifying DNA fragmentation with the cell death detection assay (Roche) as previously described (19). Lactate dehydrogenase (LDH) release was measured using a standard assay, as previously reported (45).
ROS Levels
ROS levels were measured by a fluorescent method with confocal microscopy. CellROX deep red (Invitrogen) was used as an indicator for cell ROS, as previously described (4). Cardiac myocytes were treated with high-dose ethanol for 2 h and changed to standard media, at which time CellROX deep red was added. Cells were incubated with CellROX deep red for 30 min, fixed with 4% paraformaldehyde in PBS, and mounted with 4′,6-diamidino-2-phenylindole for counterstaining. The intensity of CellROX fluorescence was calculated and analyzed to quantify the ROS level, as previously described (4).
MS
Experiments of exosomal protein content were done in the University of California-Davis Proteomics Core. Samples were briefly separated using standard 10% SDS-PAGE and visualized with Instant Blue Protein Stain (Fisher Scientific). Gel pieces, containing all the proteins in the sample, were cut out and digested overnight with trypsin according to a standard protocol. LC-MS/MS analysis was performed using the standard top 15 method using a Thermo Scientific QExactive orbitrap mass spectrometer in conjunction with Paradigm MG4 HPLC (Michrom Bio Resources, Auburn, CA). Digested peptides were loaded onto a Michrom C18 trap and desalted before being separated using a Michrom 200 × 150-mm Magic C18AQ reverse-phase column. A flow rate of 2 ml/min was used. Peptides were eluted using a 60-min gradient with 2% solvent B to 35% solvent B over 40 min, 35% solvent B to 80% solvent B for 3 min, 80% solvent B for 2 min, and then a decrease from 80% to 5% solvent B in 1 min (where solvent A = 0.1% formic acid and solvent B = 100% acetonitrile). A spray voltage of 2.2 kV was used with a transfer capillary temperature of 200°C.
Data Analysis
Database searching.
Tandem mass spectra were extracted, charge state deconvoluted, and deisotoped. All MS/MS samples were analyzed using X! Tandem [version TORNADO (2010.01.01.4), The GPM; thegpm.org]. X! Tandem was set up to search the Uniprot complete rat proteome database (unknown version, 71,443 entries) plus an equal number of reverse sequences and 115 common laboratory contaminant proteins (www.gpm.org/crap/), assuming the digestion enzyme trypsin. X! Tandem was searched with a fragment ion mass tolerance of 20 ppm and a parent ion tolerance of 20 ppm. The iodoacetamide derivative of cysteine was specified in X! Tandem as a fixed modification. Deamidation of asparagine and glutamine, oxidation of methionine and tryptophan, sulphone of methionine, tryptophan oxidation to formylkynurenin of tryptophan, and acetylation of the NH2-terminus were specified in X! Tandem as variable modifications.
Criteria for protein identification.
Scaffold (version Scaffold_3.2.0, Proteome Software, Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at >90.0% probability as specified by the Peptide Prophet algorithm (17a). Protein identifications were accepted if they could be established at >90.0% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (33). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Using these parameters, the false discovery rate was calculated as 3.7% on the peptide level and 0.2% on the protein level for samples searched against the rat database.
Western Blot Analysis
Western blot analysis to analyze protein expression was performed as previously described (32). Anti-HSP60 (Enzo Life Sciences, Farmingdale, NY) was used at 1:1,000, and anti-GAPDH (Fitzgerald Industries, Acton, MA) was used at 1:30,000. Antibodies to myomesin and myosin-binding protein C were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a 1:1,000 dilution. Antibodies to valosin-containing protein (VCP), tropomyosin, and α-crystallin were obtained from ABCAM (Cambridge, MA) and used at a 1:1,000 dilution. Appropriate secondary antibodies with horseradish peroxidase were used at a 1:1,000 concentration. Western blots were developed with a chemiluminescent system (Pierce), and the subsequent images were analyzed as previously described (46). We attempted to identify a loading control, which could be used in addition to Ponceau staining of the blots to confirm equivalent loading of blots. Exosomes are not cells but the products of cells subjected to different stresses, and their protein content may not be the same. GAPDH varied between ethanol-treated and hypoxia groups. Na+-K+-ATPase (Santa Cruz Biotechnology, 1:5,000), which was tested as a membrane protein, could have been more consistently present among different exosome preparations but varied among different exosome preparations and thus was not satisfactory as a loading control (data not shown). β-Actin (Millipore, 1:500), a common loading control, was not found either by MS or Western blot analysis (data not shown). Therefore, Western blot analysis was done without normalization to density of another protein, relying instead on the confirmation of equal protein loading by staining the membrane with Ponceau red before the development with antibody.
Statistics
All data are expressed as means ± SE. Data were compared by ANOVA followed by the Student-Neuman-Keuls test. Normalized data were compared by ANOVA on ranks followed by a Dunnett's test. Two-way ANOVA was used to compare data on acetylcholine esterase activity. A Student's t-test was used when there were only two sets of data to be compared, such as for Western blot and ROS data. P values of <0.05 were considered to be significant.
RESULTS
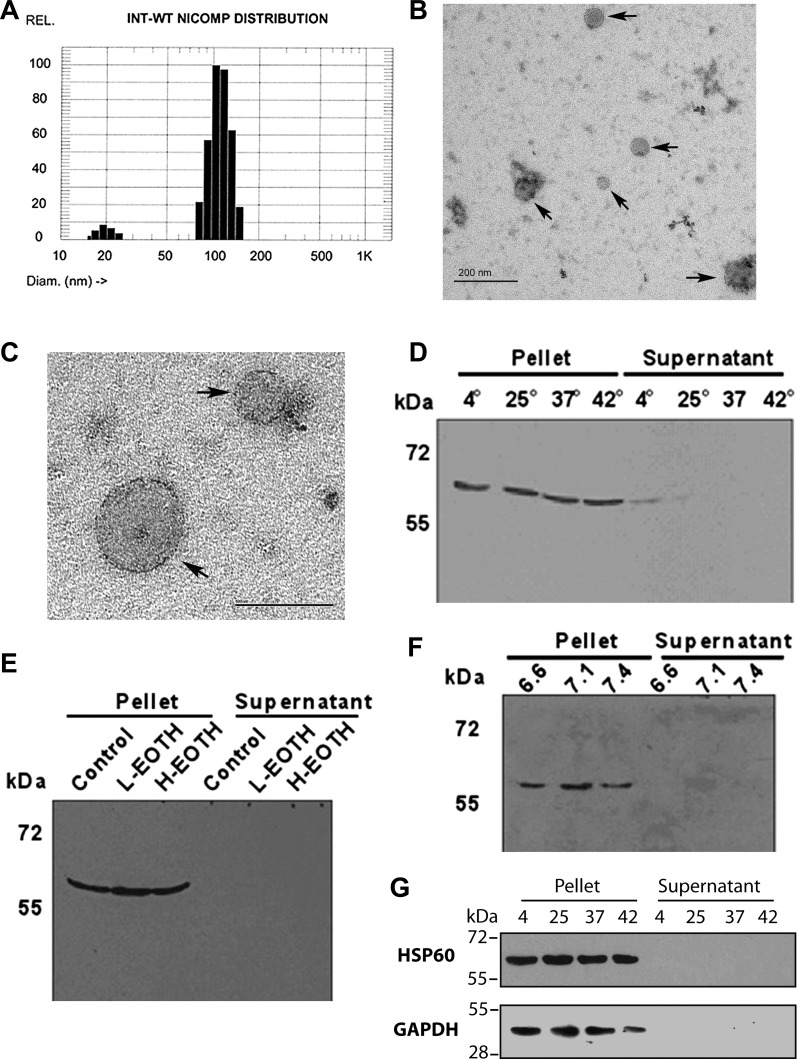
Purified exosomes were prepared from the media of adult cardiac myocytes after brief hypoxia (2 h) and reoxygenation (1 h), as previously described (12). Exosome size was analyzed with a Nanotrak particle sizing system (NICOMP 380 zls). A representative sizing graph is shown in Fig. 1A. Exosomes were, on average, 100 nm in diameter. Electron microscopy with negative staining was used to visualize the exosomes and confirm that 50- to 100-nm membranous spheres were isolated, as shown in Fig. 1, B and C.
Fig. 1.
Exosome isolation and stability. A: size distribution of exosomes, as measured by hydrodynamic radius using a particle sizing systems (NICOMP 380 zls). B and C: electron microscopy with negative staining to visualize exosomes. B: low-magnification image showing a field of exosomes (arrows) of 50–100 nm in size. Bar = 200 nm. C: higher-magnification image of two exosomes with sizes of 50–100 nm. Bar = 100 nm. D: exosomes were incubated for up to 18 h at varying temperatures, and high-speed centrifugation was used to pellet exosomes, generating pellet (exosomes) and supernatant fractions. As shown, little to no heat shock protein (HSP)60 was present in the supernatant. E: ethanol (EtOH) and exosome stability. Fractions are as described in D. F: effect of pH on exosome stability. Fractions are as described in D. Little or no release of HSP60 was seen with any treatment. G: HSP60 is tightly associated with the membrane (see text), and this might interfere with release from the exosome. The effect of temperature on GAPDH release was compared with HSP60 at 18 h.
Exosome Integrity
As discussed above, exHSP60 causes cardiac myocyte apoptosis, and HSP60 is released from cardiac myocytes in exosomes. If HSP60 is sequestered in the exosomes, this should prevent toxicity; however, if exosomes release HSP60, it would be able to bind to TLR4 and cause apoptosis. Therefore, whether exosomes release proteins under varying conditions was assessed with three different sets of physiological/pathological conditions for as long as 18 h. A temperature range of 4–42°C had no adverse effects on exosome stability, as assessed by the release of HSP60 (Fig. 1D). The temperature of 4°C was tested not as a pathophysiological state but with regard to the issue of stability during temporary storage. Ethanol is an organic solvent, and we reasoned that consumption of ethanol might increase exosome leakiness. As shown in Fig. 1E, there was no release of HSP60 under conditions of low ethanol (21.7 mM) or high ethanol (65.1 mM), levels that correlate with legally intoxicated in most states and with the consumption of a high number of alcoholic drinks (15). Over time, the ethanol would be expected to have some evaporation, but in vivo it would decrease secondary to metabolism. Similarly, pH variation was tested, and again there was no release of HSP60 from exosomes with acidosis, which would be seen with disease states such as ischemia, sepsis, and diabetic ketoacidosis (Fig. 1F).
Previously, we (12) have found that HSP60 is predominantly associated with the exosome membrane and tightly bound to the membrane but not the transmembrane. If HSP60 is tightly bound, it might not be released by exosomes in response to changing conditions. However, GAPDH, a cytosolic protein, is also found in exosomes, as discussed below, and GAPDH might more readily be released by exosomes. As shown in Fig. 1G, after an 18-h incubation at different temperatures, neither HSP60 nor GAPDH were released from exosomes.
Clinical Grade Exosomes
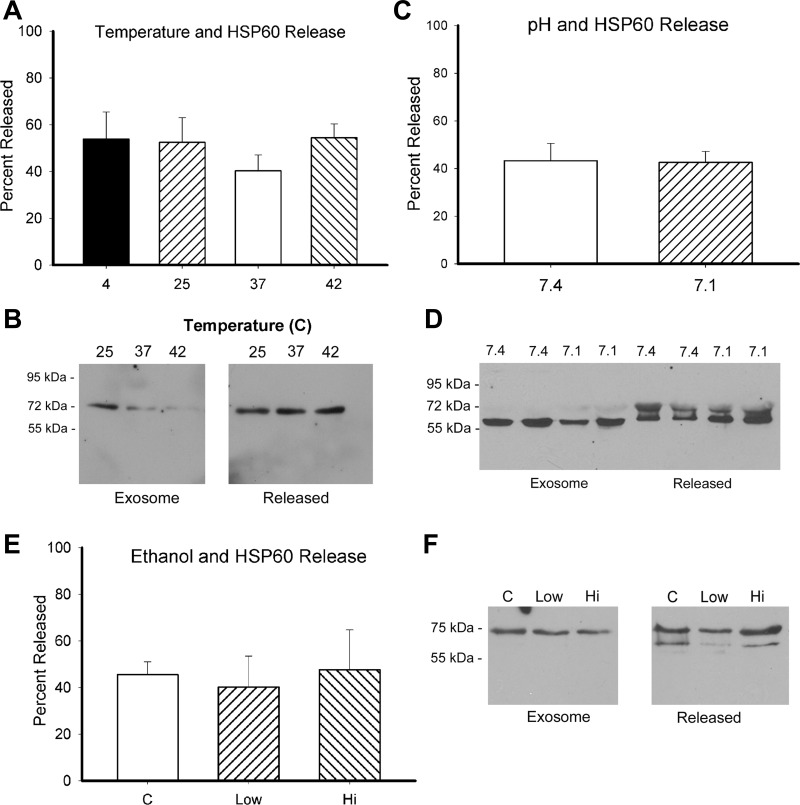
Clinical grade exosomes are desirable for potential in vivo studies of exosome function. Clinical grade exosomes were isolated following the method of Lamparski et al. (21). At 18 h, almost 50% of HSP60 had been released by exosomes over a range of temperatures (Fig. 2, A and B). Similarly, ∼40% of HSP60 was released with exposure to either pH 7.4 or 7.1 (Fig. 2, C and D). Interestingly, HSP60 was present as a doublet in the protein released into the supernatant. Treatment with 21.7 and 65.1 mM ethanol did not change the release of HSP60 (Fig. 2, E and F). Further investigation showed that at as early as 2 h, half of the HSP60 had been released by exosomes regardless of treatment. With Exoquick, as shown in Fig. 1, there was little or no release of HSP60 from exosomes, but the clinical grade method, which involves multiple steps with concentrating and isolating the exosomes with high-speed centrifugation followed by filtration steps, was consistently associated with the release of HSP60 from exosomes in our hands.
Fig. 2.
Exosome stability for exosomes prepared by the clinical grade method (method of Lamparski et al.; see text for details). Exosomes were treated for 18 h, at which time ultracentrifugation was used to generate the supernatant and pellet (exosomes). A: graph showing the percentage of HSP60 released from exosomes over a range of temperatures. Results summarize 6 experiments with 5–6 data points/group. B: representative Western blot for temperature treatment. Both images are from the same blot. Marks for molecular weights, which were between lanes 3 and 4, were removed. The same is true for F. C: graph showing the effect of pH on exosome stability. There were 5 experiments, with a total of 9–11 experiments/group. D: representative Western blot showing the effect of pH change on HSP60 release from exosomes. Not infrequently, released HSP60 ran as doublet. E: graph showing the results of 5 experiments on ethanol and exosome stability. n = 4–5 per group. F: representative Western blot showing exosomal and supernatant HSP60 content at 18 h.
Ethanol and Exosome Generation
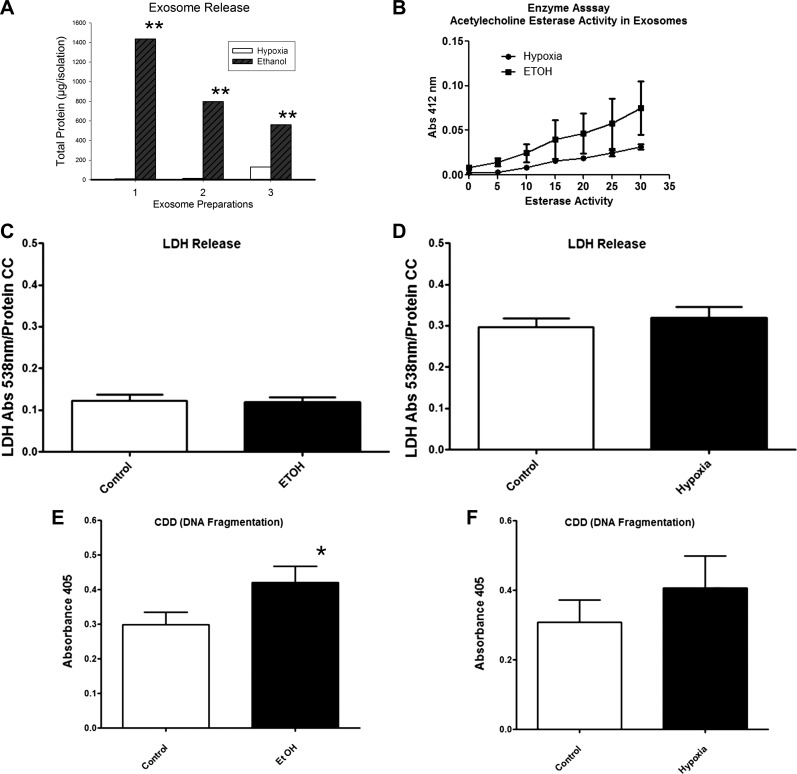
We were surprised that ethanol did not increase exosome permeability. Even if ethanol did not effect exosome stability, it could change the production of exosomes, possibly through increased protein denaturation or other effects on the cell. Thus, we hypothesized that ethanol levels seen with alcohol consumption would increase the production of exosomes. Adult cardiac myocytes were treated with either 21.7 or 65.1 mM cell culture grade ethanol. Both treatments resulted in exosome production. The particle sizing profile for the exosomes was similar to that seen with hypoxia/reoxygenation-derived exosomes. Ethanol treatment resulted in much higher production of exosomes than hypoxia/reoxygenation. As shown in Fig. 3A, three different cardiac myocyte isolations were split, and half of the cells were treated with hypoxia/reoxygenation and half were treated with 65.1 mM ethanol. Ethanol treatment consistently generated far more exosomes than hypoxia/reoxygenation based on the protein content of the exosome preparations (P < 0.05). Furthermore, acetylcholine esterase activity in ethanol-derived exosomes tended to be higher than that in exosomes isolated after brief hypoxia/reoxygenation, but this did not reach significance (Fig. 3B). Thus, the differences in protein content were more marked than any difference in acetylcholine esterase activity, as shown in Fig. 3.
Fig. 3.
A: exosome production after ethanol (65.1 mM) versus hypoxia/reoxygenation (H/R) was compared for 3 different cardiac myocyte isolations. After exosome purification, total protein was measured as an index of the amount of exosomes present. B: acetylcholine esterase activity, which reflects the presence of membranes, in ethanol- and hypoxia/reoxygenation-derived exosomes (n = 3 per group). C and D: LDH release by cardiac myocytes measured as an index of cell injury after 2 h of treatment with 65.1 mM ethanol (n = 5 per group; C) or after 2 h of hypoxia followed by reoxygenation (n = 8/group; D). E and F: DNA fragmentation in cardiac myocytes was measured as an index of apoptosis after ethanol treatment (n = 17 per group; E) and after hypoxia/reoxygenation (n = 9 per group; F). *P < 0.05 vs. control (C); **P < 0.05, ethanol-derived exosomes vs. hypoxia/reoxygenation total protein. CC, concentration.
Does Exosome Generation Reflect Cardiac Myocyte Injury?
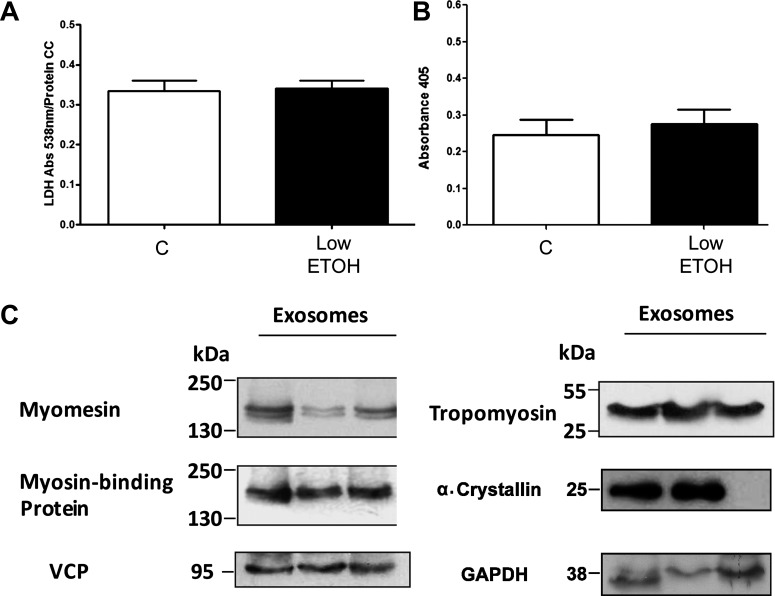
Previously, we have shown that the brief hypoxia/reoxygenation treatment used to generate exosomes does not cause LDH release, a sign of necrosis. Ethanol treatment (65.1 mM) and hypoxia/reoxygenation were each tested for evidence of cardiac myocyte necrosis (LDH release) and apoptosis (DNA fragmentation). As shown in Fig. 3, C and D, there was no increase in LDH release from cardiac myocytes with either treatment. Ethanol treatment caused a small but significant increase in DNA fragmentation, whereas hypoxia/reoxygenation had no effect on DNA fragmentation (Fig. 3, E and F). Given that the higher concentration of ethanol caused mild DNA fragmentation, the effect of low-dose ethanol (21.7 mM) on cardiac myocytes was also assessed. Low-dose ethanol caused no increase in LDH release (Fig. 4A) or DNA fragmentation (Fig. 4B).
Fig. 4.
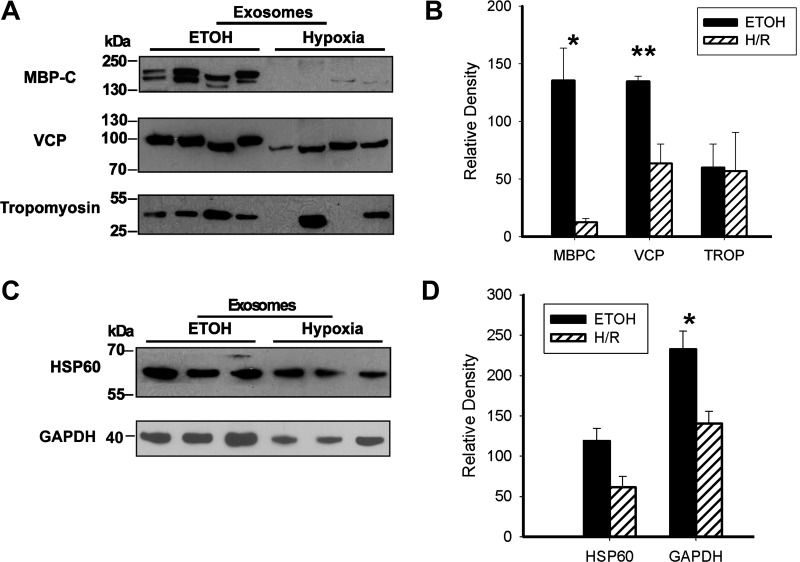
A: LDH levels after treatment of cardiac myocytes with low-dose ethanol. B: DNA fragmentation assessed by cell death detection assay after treatment of cardiac myocytes with low-dose ethanol. C: Western blots confirming the key proteins present in ethanol-derived exosomes. All proteins were studied on same membrane. n = 9 per group. VCP, valosin-containing protein.
Exosome Proteomics
Ethanol.
To gain insight into the function of exosomes, the protein content of three different exosome preparations after ethanol treatment (65.1 mM) of cardiac myocytes was analyzed by MS. Fifty-seven proteins were identified. Of these, four proteins were clearly contaminants from cell culture, sample preparation, and protein digestion with trypsin (human serum albumin, trypsin, keratin, and human hemoglobin). Two proteins, haptoglobin and ferritin, are common serum proteins and may also have been contaminants from the cell culture media. These six proteins were excluded from analysis. As shown in Table 1, 51 different exosomal proteins were identified, ranging from membrane to cytosolic and mitochondrial proteins. Eleven proteins were only found in one of the three exosome preparations, and five proteins were identified as uncharacterized proteins. The remaining 35 proteins included tropomyosin-α1, myomesin 2 (M-band), α-crystallin B, cardiac α-actin, GAPDH, and long-chain specific acyl-CoA dehydrogenase, a mitochondrial protein. Western blot analysis was used to confirm proteins of interest. As shown in Fig. 4C, myomesin, myosin-binding protein C, VCP (also known as p97 AAA-ATPase, Cdc48, and transitional endoplasmic reticulum ATPase), and tropomyosin were all found in multiple exosome preparations, confirming the MS results. α-Crystallin was found in two of three Western blot samples examined (Fig. 4C).
Table 1.
Protein content of ethanol-derived exosomes by mass spectrometry
| Gene Name | Accession Number |
|---|---|
| 2-Oxoglutarate dehydrogenase, mitochondrial | Q5XI78 |
| 60-kDa HSP, mitochondrial | P63039 |
| α-Actin, cardiac muscle 1 | P68035 |
| α-Crystallin B chain | P23928 |
| Aspartate aminotransferase, cytoplasmic | P13221 |
| ATP synthase subunit β, mitochondrial | P10719 |
| Creatine kinase, mitochondrial 2, protein | B0BNC0 |
| Creatine kinase, M type | P00564 |
| Δ(3,5)-Δ(2,4)-dienoyl-CoA isomerase, mitochondrial | Q62651 |
| Desmin (Rattus norvegicus) | P48675 |
| Fructose-bisphosphate aldolase A | P05065 |
| Glucose-6-phosphate isomerase | Q6P6V0 |
| GAPDH | P04797 |
| Glycogenin-1 | O08730 |
| Heat shock cognate 71-kDa protein | P63018 |
| HSP β-6 | P97541 |
| Isocitrate dehydrogenase (NADP), mitochondrial | P56574 |
| l-LDH, A chain | P04642 |
| l-LDH, B chain | P42123 |
| Long-chain specific acyl-CoA dehydrogenase, mitochondrial | P15650 |
| Malate dehydrogenase, cytoplasmic | O88989 |
| Malate dehydrogenase, mitochondrial | P04636 |
| Myomesin 2 | G3V7K1 |
| Myosin light chain 3 | P16409 |
| Myosin-binding protein C, cardiac type | P56741 |
| Nucleoside diphosphate kinase B | P19804 |
| Phosphorylase | B1WBU9 |
| Proteasome subunit α, type 6 | P60901 |
| Proteasome subunit β, type 6 | P28073 |
| Pyruvate kinase isozymes M1/M2 | P11980 |
| RCG23609, isoform CRA_a | G3V885 |
| RCG30552 | D3ZCV0 |
| VCP | P46462 |
| Tropomyosin α1-chain | P04692 |
| Vinculin | P85972 |
| In one sample only | |
| Aconitate hydratase, mitochondrial | Q9ER34 |
| Aspartyl aminopeptidase | Q4V8H5 |
| δ-Aminolevulinic acid dehydratase | P06214 |
| Enoyl-CoA hydratase, mitochondrial | P14604 |
| Heat shock 70-kDa protein 4 | O88600 |
| Hemopexin | G5BBR0 |
| Propionyl-CoA carboxylase β-chain, mitochondrial | P07633 |
| Proteasome subunit α, type 3 | P18422 |
| Proteasome subunit α, type 4 | P21670 |
| Proteasome subunit β type | G3V7Q6 |
| Proteasome subunit β, type 4 | P34067 |
| Uncharacterized | |
| Uncharacterized protein | F1M614 |
| Uncharacterized protein | E9PTA1 |
| Uncharacterized protein | F1MAA7 |
| Uncharacterized protein | F1LNI6 |
| Uncharacterized protein | F1LN88 |
There were 51 proteins in total. HSP, heat shock protein; VCP, valosin-containing protein.
Hypoxia/reoxygenation.
We generated exosomes by two distinct treatments, which cause at most minimal harm based on LDH and DNA fragmentation. Whether these exosomes have similar protein content has a bearing on their potential function. Therefore, MS analysis of three different sets of hypoxia/reoxygenation exosomes was also done. As shown in Table 2, 33 proteins were identified in hypoxia/reoxygenation-derived exosomes, excluding serum contaminants. Hypoxia/reoxygenation-derived exosomes contained HSP27 and HSP90, neither of which was found in ethanol-derived exosomes by MS. The 2 sets of exosomes had 17 proteins in common, including HSP60, GAPDH, tropomyosin-α, myomesin, myosin-binding protein C, α-crystallin B chain, and VCP. Tables 3 and 4 show proteins only found in ethanol-derived exosomes (Table 3) or hypoxia/reoxygenation-derived exosomes (Table 4). Figure 5 shows a comparison of the expression of key proteins from the two different types of exosomes. Ethanol-derived exosomes contained more myosin-binding protein C than hypoxia/reoxygenation-derived exosomes (P < 0.05; Fig. 5, A and B). Similarly, VCP levels were higher in ethanol-generated exosomes than those released after hypoxia/reoxygenation (P < 0.01; Fig. 5, A and B). Tropomyosin levels, while fairly constant in the ethanol-treated group, varied in the hypoxia/reoxygenation-treated group and overall did not differ. HSP60 levels were somewhat higher in the ethanol-treated group, but this was not significant (Fig. 5, C and D). GAPDH levels were lower in hypoxia-treated exosomes (P < 0.05; Fig. 5, C and D).
Table 2.
Protein content of mild hypoxia/reoxygenation-derived exosomes
| Gene Name | Accession Number |
|---|---|
| αβ-Crystallin | P23928 |
| fructose-biphosphate aldolase | P05065 |
| pyruvate kinase isoenzymes M1/M2 | P11980 |
| HSP60 | P63039 |
| LDH B chain | P42123 |
| Creatine kinase, mitochondrial 2 | BOBNCO |
| GAPDH | PO4797 |
| Myosin-6 | P02563 |
| VCP | P46462 |
| Tropomyosin-α1 | P04692 |
| Actin, cardiac muscle | p68035 |
| HSP β-6 | P97541 |
| Hemopexin | P20059 |
| Myosin-binding protein C | P56741 |
| RCG30552 (rat) | D3ZCVO |
| Phosphorylase | B1WBU9 |
| LDH, A chain | P04642 |
| Myosin light chain 3 | P16409 |
| HSP90 β | P34058 |
| Long-chain specific acyl-CoA dehydrogenase | P15650 |
| Nucleosome assembly protein 1 | Q5U2Z3 |
| Nucleoside diphosphate kinase B | P19804 |
| Myomesin 2 | G3V7K1 |
| HSP27 protein 1 | G3V913 |
| In one sample only | |
| Proteasome subunit α, type 7 | P48004 |
| Glycogen phosphorylase, brain form | P53534 |
| UTP-glucose-1-phosphate uridylyltransferase | G5C8M4 |
| Tubulin β-2A chain | P85108 |
| Proteasome subunit α, type 6 | P60901 |
| Uncharacterized | |
| Uncharacterized protein | F1MAA7 |
| Uncharacterized protein | D3ZQN7 |
| Uncharacterized protein | F1M614 |
| Uncharacterized protein | F1LM84 |
There were 33 proteins total.
Table 3.
Proteins found in ethanol-derived exosomes but not in hypoxia/reoxygenation-derived exosomes by mass spectrometry
| Gene Name | Accession Number |
|---|---|
| 2-Oxoglutarate dehydrogenase, mitochondrial | Q5X178 |
| Aspartate aminotransferase, cytoplasmic | P13221 |
| ATP synthase subunit β, mitochondrial | P10719 |
| Creatine kinase, M type | P00564 |
| Δ(3,5)-Δ(2,4)-dienoyl-CoA isomerase, mitochondrial | Q62651 |
| Desmin (Rattus norvegicus) | P48675 |
| Glucose-6-phosphate isomerase | Q6P6V0 |
| Glycogenin 1 | O08730 |
| Heat shock cognate 71-kDa protein | P63018 |
| Isocitrate dehydrogenase (NADP), mitochondrial | P56574 |
| Malate dehydrogenase, cytoplasmic | O88989 |
| Malate dehydrogenase, mitochondrial | P04636 |
| RCG23609, isoform CRA_a | G3V885 |
| Vinculin | P85972 |
| In one sample only | |
| Aconitate hydratase, mitochondrial | Q9ER34 |
| Aspartyl aminopeptidase | Q4V8H5 |
| δ-Aminolevulinic acid dehydratase | P06214 |
| Enoyl-CoA hydratase, mitochondrial | P14604 |
| Heat shock 70-kDa protein 4 | O88600 |
| Propionyl-CoA carboxylase β chain, mitochondrial | P07633 |
| Proteasome subunit α, type 3 | P18422 |
| Proteasome subunit α,type-4 | P21670 |
| Proteasome subunit β type | G3V7Q6 |
| Proteasome subunit β, type 4 | P34067 |
| Uncharacterized | |
| Uncharacterized protein | E9PTA1 |
| Uncharacterized protein | F1LNI6 |
| Uncharacterized protein | F1LN88 |
Table 4.
Proteins found only in exosomes generated after mild hypoxia/reoxygenation and not ethanol treatment
| Gene Name | Accession Number |
|---|---|
| Myosin 6 | P02563 |
| HSP90 β | P34058 |
| Nucleosome assembly protein 1 | Q5U2Z3 |
| HSP27 | G3V913 |
| In one sample only | |
| Proteasome subunit α, type 7 | P48004 |
| Glycogen phosphorylase, brain form | P53534 |
| UTP-glucose-1-phosphate uridylytransferase | G5C8M4 |
| Tubulin β-2A chain | P85108 |
| Uncharacterized | |
| Uncharacterized protein | D3ZQN7 |
| Uncharacterized protein | F1LM84 |
Fig. 5.
A: direct comparison of proteins in ethanol-derived exosomes with proteins in mild hypoxia-derived exosomes. Four different exosome isolations were compared for each treatment. The same blot was developed for the three proteins. B: graph showing Western blot results. Myosin-binding protein C (MBPC) and VCP were increased in ethanol-derived exosomes. TROP, tropomyosin. C: Western blots from the same sample sets showing the expression of HSP60 and GAPDH in ethanol- versus hypoxia-derived exosomes. D: graph showing Western blot results. *P < 0.05; **P < 0.01.
Proteomics Control
To control for nonspecific proteins, we prepared exosomes from the media of untreated control cardiac myocytes. Such cells would produce very few exosomes, and this “exosome isolation” (blank) control was to determine what proteins were nonspecifically isolated. SDS-PAGE on this control sample showed only a single faint band, consistent with albumin (data not shown). The typical volume used for MS in the ethanol and hypoxia experiments was sent for MS analysis. As expected, human albumin was the predominant protein present followed by trypsin. LDH B chain and human keratin were both present. Finally, very small amounts of pyruvate kinase isozymes M1/M2 and cardiac α-actin were present compared with the robust amounts found in ethanol- and hypoxia/reoxygenation-derived exosomes.
ROS
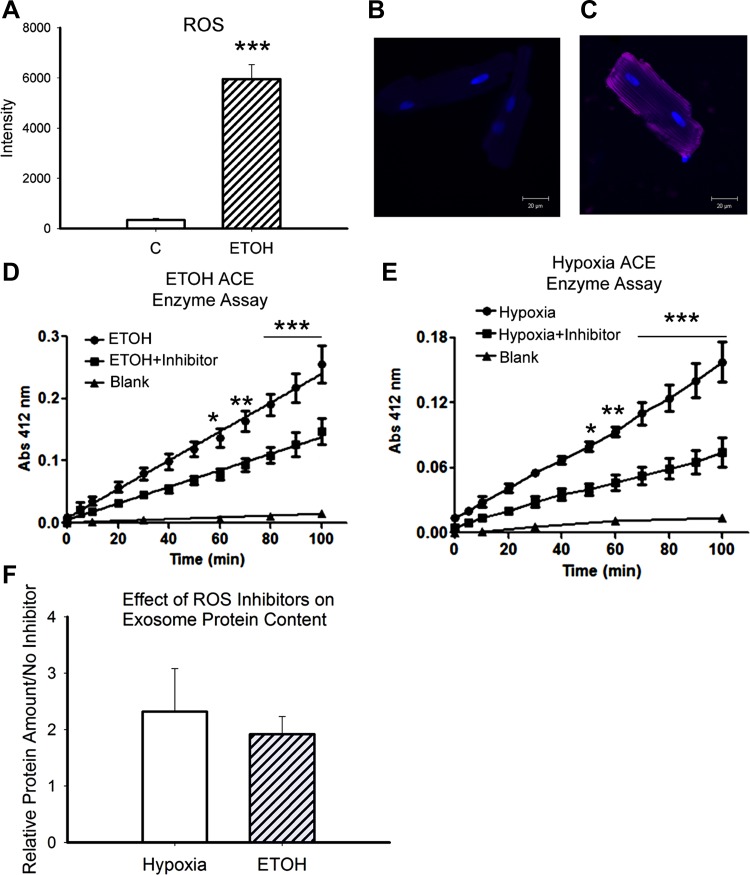
Hypoxia/reoxygenation is well established as a source of ROS. We hypothesized that ROS might be a common denominator for stimulation of exosome production. Ethanol has previously been reported to increase ROS in the heart, as discussed below. CellRox red (Invitrogen) was used to stain for ROS at the end of the 2-h ethanol treatment. As shown in Fig. 6, A–C, ROS production was barely detectable in control untreated cardiac myocytes but markedly increased after ethanol treatment (P < 0.001). The mitochondria are a major source of ROS, and ethanol-generated exosomes contained a large number of mitochondrial proteins. Cells were treated with a combination of antioxidants: 10 mM N-acetyl-l-cysteine, 1 mM ascorbate, and 100 μM Trolox, based on the literature (13, 36). Treatment was begun 30 min before the initiation of ethanol treatment or hypoxia to allow equilibration. As shown in Fig. 6, D and E, antioxidant treatment significantly decreased acetylcholine esterase activity, a marker for the amount of exosomes present (Fig. 6, D and E). However, total protein/exosomes increased in groups treated with inhibitor compared with hypoxia/reoxygenation or ethanol alone, but this increase was not significant (Fig. 6F).
Fig. 6.
A: Cell RoxRed was used to analyze ROS production by cardiac myocytes treated with ethanol. The average cell intensity was compared between ethanol treatment and controls. B: representative image of a control cell developed with Cell RoxRed. C: representative image of an ethanol-treated cell developed with CellRox Red. D: results of the acetylcholine esterase activity assay (ACE), an index of exosome quantity. Results of ethanol treatment (65.1 mM) compared with ethanol + antioxidant treatment are shown (see text). E: acetylcholine esterase activity assay for hypoxia/reoxygenation-treated cells with and without antioxidants. F: comparison of total protein for exosomes from ethanol treatment with and without antioxidants and hypoxia/reoxygenation with and without antioxidants. Each cardiac myocyte isolation was split in two, and half of the cardiac myocytes were treated with ethanol or hypoxia/reoxygenation and half were treated the same plus antioxidant inhibitors. The protein content of exosomes derived from these matching cell sets was compared. n = 3–4 per group; for CellRox Red, n = 11–12. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
Although exosomes have been recognized for 15–20 yr, their actual function(s) remains elusive. Exosomes were originally described as the mechanism by which reticulocytes shed unneeded receptors and protein complexes as they matured to erythrocytes (14). One function of exosomes is thought to be the removal of denatured proteins and organelle fragments. A number of studies (20, 43) have implicated exosomes in transmitting/transferring the malignancy of cancer to other cells. Cancer cells have been found to manipulate the environment and evade immunity via exosomes (5). Exosomes released by a medulloblastoma-derived cell line promoted cell proliferation and tumor cell migration and had immune modulatory effects on lymphocytes (7). Exosomes have also been identified as allowing virus transmission from cell to cell while evading the immune system (28, 30). Thus, exosomes promote disease progression and are considered to be immunosuppressive in cancer (49). However, little is known about exosomes in the cardiovascular system.
HL-1 cells have been studied as a model of cardiac cells. Microvesicles ranging in size from 40 to 300 nm have been isolated from the media of HL-1 cells and were found to change gene expression in fibroblasts after 48 h of coincubation (52). The broad size range of these vesicles indicates a mixed population, making results difficult to interpret with respect to the specific vesicle type. Yu et al. (54) reported that neonatal cardiac myocytes released TNF-α in exosomes after prolonged hypoxia. Thus, very limited evidence supports that cardiac-derived exosomes may have important downstream signaling effects.
ExHSP60
ExHSP60 is toxic to cardiac cells through the activation of TLR4 triggering late cardiac myocyte apoptosis (19). HSP60 is released from cardiac myocyte in exosomes, where potentially it is safely sequestered. In the present study, exosomes under a range of physiological, pathophysiological, and extreme conditions did not release HSP60. As exosomal HSP60 is tightly bound to the exosomal membrane, we tested whether GAPDH would be released from exosomes. Like HSP60, GAPDH remained within the exosome. Thus, HSP60 is stable within the exosome and not released under a range of physiological conditions.
Plasma/Serum HSP60 and Exosomes
There is a substantial literature reporting HSP60 in the plasma in various disease states (11, 24, 35). One study (41) found very high levels of plasma HSP60, 10 μg/ml or more, rivaling albumin levels in some patients. On the other hand, in a study of Italian diabetics (3), we found relatively low levels of HSP60 in serum. Similar low serum levels correlated with the development of lung problems after motor vehicle accidents (34). Most studies are done on frozen plasma or serum samples, which would rupture any exosomes. Although we have shown that HSP60 is released by cardiac myocytes via exosomes in the absence of necrosis, this does not eliminate other routes for release of HSP60 by other cell types. Plasma/serum HSP60 reflects the sum of HSP60 released by all organs and tissues. Many questions remain to be answered about the source, amount, and toxicity (or lack thereof) of plasma/serum HSP60. We and others are pursuing investigations in this area, and further work will be needed to define the role of exosomes versus other cellular mechanism in the release of HSP60 into the bloodstream. However, the results of the present study supports that HSP60 released in exosomes remains in exosomes.
Exosome Protein Content
As shown in Table 5, comparison of reported content of exosomes from other cell types shows that our exosomes shared only a handful of common proteins with previous studies. Of the 51 ethanol exosomal proteins identified, there were only a few proteins in common with previous studies, including actin, heat shock cognate 71-kDa protein, and GAPDH (2, 6, 8, 18, 29, 37, 42, 51, 53). A number of other proteins that are commonly associated with exosomes, such as HSP90, major histocompatibility complex I/II, and tetraspanins (such as CD63), were not seen in our MS analysis; however, HSP90 was found in hypoxia/reoxygenation-derived exosomes (data not shown). Although these proteins may be considered common exosome proteins, there was still a sizeable variance among proteomic studies of exosome composition (29). In addition, it should be noted that the majority of proteomic studies were taken from human cells, commonly cancer cells. These data give credence to the possibility that the composition of exosomes can vary greatly based on their origins; thus, exosomes from different cell types may serve different purposes based on their composition.
Table 5.
Proteins in common with previous proteomic studies
| Protein | Accession Number | Reference(s) |
|---|---|---|
| α-Actin, cardiac muscle | P68035 | 2, 8, 18, 29, 37, 51, 53 |
| Heat shock cognate 71-kDa protein | P63018 | 2, 6, 8, 29, 44, 51 |
| GAPDH | P04797 | 18, 29, 37, 37, 42, 53 |
| Heat shock 70-kDa protein 4 | O88600 | 6, 9, 22, 42 |
| Pyruvate kinase isozymes M1/M2 | P11980 | 8, 18, 29, 37 |
| HSP60 | P63039 | 6, 29, 44 |
| Fructose-bisphosphate aldolase A | P05065 | 29 |
| l-LDH, A chain | P04642 | 37 |
| VCP | P46462 | 37 |
| l-LDH, B chain | P42123 | 37 |
| Myosin light chain 3 | P16409 | 42 |
Of the 51 proteins identified by mass spectrometry from exosomes isolated from rat cardiomyocytes, 11 proteins were seen in previous proteomic studies but only 6 proteins were found in multiple studies. The corresponding protein accession number and gene name are provided along with the relevant literature.
VCP
VCP is an abundant cellular protein known by several names, including p97, p97 AAA-ATPase, TER94, and CDc48 (the latter in yeast only). VCP is widely distributed throughout the cell and was originally identified for its role in ubiquitin-mediated protein degradation (1). However, results from a recent study (31) have led to an increasing list of cellular functions and processes in which VCP is involved. Besides its role in protein processing for degradation, VCP has been found to be involved in autophagy, regulation of proteins in the outer mitochondrial membrane through degradation, and endosomal sorting (17). VCP localizes to the endosomal membrane, where it binds early endosome antigen 1 (38). Inhibition of VCP or downregulation with small interfering RNA delayed trafficking of the endocytic cargo and resulted in an increase in size and clustering of the endosomes (38). The presence of VCP in cardiac myocyte-derived exosomes raises the question of whether VCP is involved in exosome formation or exosomal protein trafficking. We identified only one other study where VCP was localized in exosomes (37). VCP's role in the formation of the Golgi stacks as well as its association with the endosomal membrane certainly suggest that this protein is involved in organelle and vesicle dynamics.
Ethanol and Exosomes
An unexpected finding was that ethanol, at concentrations seen with the consumption of alcoholic beverages, greatly increased the production of exosomes by cardiac myocytes. With high-dose ethanol there was a slight increase in apoptosis, but there was none with the low dose. Neither ethanol treatment nor hypoxia/reoxygenation resulted in LDH release. There was also no evidence of apoptosis with hypoxia/reoxygenation. Ethanol has not previously been found to stimulate exosome production. The particle sizing profile for ethanol-derived exosomes compared with hypoxia/reoxygenation-derived exosomes did not differ. Ethanol-induced exosomes appeared to have increased protein content compared with hypoxia/reoxygenation-induced exosomes, based on similar acetylcholine esterase activity, which reflects the amount of membrane present, but a greatly increased exosomal protein content.
Ethanol is a hydroxyl radical scavenger leading to the production of several radicals, of which the 1-hydroxy-ethyl radical is the most abundant (80%) (39). ROS generation in the heart was detected in vivo after the ingestion of alcohol for 2 wk in a rat model (40). Others (27) have found ROS-related cardiac damage within hours of ethanol exposure, and this could be ameliorated by pretreatment with the antioxidant N-acetyl-l-cysteine. The increase in ROS would be exacerbated by a drop in mitochondrial glutathione (cytosolic levels unchanged) observed after one treatment of oral ethanol in a rodent model (16). In the present study, we found that 2 h of ethanol exposure resulted in a marked increase in ROS in cardiac myocytes. Many of the proteins in ethanol-derived exosomes were mitochondrial. We treated cardiac myocytes with a combination of potent antioxidants to reduce ROS production. Exosome quantity, based on the acetylcholine esterase assay, was decreased with both ethanol treatment and hypoxia/reoxygenation. Thus, antioxidants decreased exosome formation but did not completely inhibit it.
In the present study, we found that exosomes are quite stable under physiological as well as pathological conditions. In addition, exosome formation is greatly enhanced after brief exposure to ethanol at concentrations that would be seen after the consumption of multiple alcoholic drinks. Alcohol consumption can lead to cardiomyopathy, and some have proposed that 21–36% of dilated, nonischemic cardiomyopathy is caused by excessive ethanol consumption (10, 23). The enhanced release of exosomes after ethanol treatment raises the possibility that this increase in exosome release might have a role in the eventual development of cardiomyopathy. The increase in exosomes and their protein content after brief ethanol treatment are likely secondary to denatured proteins and cellular damage, in part from increased ROS. This is the first report, to our knowledge, identifying ethanol at levels seen in the general public as stimulating exosome production. Proteomic analysis established that the exosomes contained a wide assortment of proteins, including proteins from the mitochondria, cytosol, and plasma membrane. The protein content of ethanol- versus hypoxia/reoxygenation-derived exosomes differed, suggesting that different stimuli will result in different proteins being released in exosomes from the same cell type. Furthermore, there was a greater amount of protein present in ethanol-induced exosomes. Exosomes form in the multivesicular body, which either traffics to the lysosome or fuses with the plasma membrane, emptying its contents of exosomes into the extracellular space. The regulation of the gating of this process, lysosome versus extracellular space, remains to be understood, but given VPC's known functions, it is possible that it may have a role here, too.
Exosomes are released in the extracellular space and distributed throughout the body. The fate of exosomes remains to be elucidated. One study (43) has suggested that exosomes from cancer cells may convey malignancy to noncancerous cells. Exosomes have also been reported in the urine, and these may arise from the kidney or represent one method of clearing these vesicles. Work to date suggests that exosomes may have a role in intercellular signaling, as cancer studies would suggest (8, 9, 29, 44). Exosomes have also been suggested as a way for viruses to move between cells without being detected (47). More work is need to understand the function and fate of these small vesicles.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-077281 and HL079071 and a Merit Award from the Department of Veterans Affairs (all to A. A. Knowlton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.A.M., K.S.K., and A.A.K. conception and design of research; Z.A.M., K.S.K., A.J.P., T.K., L.C., and K.W.F. performed experiments; Z.A.M., K.S.K., A.J.P., T.K., L.C., and A.A.K. analyzed data; Z.A.M., K.S.K., A.J.P., T.K., L.C., and A.A.K. interpreted results of experiments; Z.A.M., A.J.P., T.K., and A.A.K. prepared figures; Z.A.M. and A.A.K. edited and revised manuscript; Z.A.M., K.S.K., A.J.P., L.C., K.W.F., and A.A.K. approved final version of manuscript; A.J.P. and A.A.K. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank James Heiserman and Alice Tran for excellent technical assistance and Dr. Brett S. Phinney and Darren Weber (Proteomics Core Facility, University of California-Davis Genome Center; http://www.proteomics.ucdavis.edu/) for the help with LC-MS/MS.
REFERENCES
- 1. Bandau S, Knebel A, Gage ZO, Wood NT, Alexandru G. UBXN7 docks on neddylated cullin complexes using its UIM motif and causes HIF1α accumulation. BMC Biol 10: 36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bastos-Amador P, Royo F, Gonzalez E, Conde-Vancells J, Palomo-Diez L, Borras FE, Falcon-Perez JM. Proteomic analysis of microvesicles from plasma of healthy donors reveals high individual variability. J Proteomics 75: 3574–3584, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Blasi C, Kim E, Knowlton AA. Improved metabolic control in diabetes, HSP60, and proinflammatory mediators. Autoimmune Dis 2012: 346501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen L, Liu TT, Tran AL, Lu X, Tomilov AA, Davies VJ, Cortopassi G, Bers DM, Votruba M, Knowlton AA. OPA1 mutation and late onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc 1: e003012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clayton A. Cancer cells use exosomes as tools to manipulate immunity and the microenvironment. Oncoimmunology 1: 78–80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci 118: 3631–3638, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Epple LM, Griffiths SG, Dechkovskaia AM, Dusto NL, White J, Ouellette RJ, Anchordoquy TJ, Bemis LT, Graner MW. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLos One 7: e42064, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16: 415–421, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65: 5238–5247, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George A, Figueredo VM. Alcoholic cardiomyopathy: a review. J Card Fail 17: 844–849, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Giannessi D, Colotti C, Maltinti M, Del Ry S, Prontera C, Turchi S, Labbate A, Neglia D. Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones 12: 265–274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292: H3052–H3056, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Heberlein W, Wodopia R, Bartsch P, Mairbaurl H. Possible role of ROS as mediators of hypoxia-induced ion transport inhibition of alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L640–L648, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Johnstone RM. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis 34: 214–219, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Jones AW. Evidence-based survey of the elimination rates of ethanol from blood with applications in forensic casework. Forensic Sci Int 200: 1–20, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Kannan M, Wang L, Kang YJ. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med (Maywood) 229: 553–559, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Karbowski M, Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol 23: 476–482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a. Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O'Neal W, Pickles RJ, Sheehan JK. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J 23: 1858–1868, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes and apoptosis. Circ Res 105: 1186–1195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLos One 6: e24234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 270: 211–226, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Lancaster G, Febbraio MA. Exosome-dependent trafficking of HSP70. J Biol Chem 280: 23349–23355, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail 11: 453–462, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Lewthwaite J, Owen N, Coates A, Henderson B, Steptoe A. Circulating human heat shock protein 60 in the plasma of british civil servants: relationship to physiological and psychosocial stress. Circulation 106: 196–201, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem 286: 31308–31319, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon S, Terre-Aminone G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 293: H2238–H2247, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Mansura A, Demelliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther 298: 737–743, 2001 [PubMed] [Google Scholar]
- 28. Marleau AM, Chen C, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 10: 134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73: 1907–1920, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Meckes DG, Raab-Traub N. Microvesicles and viral infection. J Virol 85: 12844–12854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol 14: 117–123, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Nakano M, Mann DL, Knowlton AA. Blocking the endogenous increase in HSP72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation 95: 1523–1531, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Pespeni M, Mackersie RC, Lee H, Morabito D, Hodnett M, Howard M, Pittet JF. Serum levels of Hsp60 correlate with the development of acute lung injury after trauma. J Surg Res 126: 41–47, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Pockley AG, de Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 20: 1815–1820, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Rafii B, Tanswell AK, Otulakowski G, Pitkannen O, Belcastro-Taylor R, O'Brodovich H. O2-induced ENaC expression is associated with NF-κB activation and blocked by superoxide scavenger. Am J Physiol Lung Cell Mol Physiol 275: L764–L770, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Raimondo F, Morosi L, Chinello C, Magni F, Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 11: 709–720, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Ramanathan HN, Ye Y. The p97 ATPase associates with EEA1 to regulate the size of early endosomes. Cell Res 22: 346–359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reinke LA. Spin trapping evidence for alcohol-associated oxidative stress. Free Radic Biol Med 32: 953–957, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Reinke LA, Lai EK, DuBose CM, McCay PB. Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: Correlation with radical formation in vitro. Proc Natl Acad Sci USA 84: 9223–9227, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shamaei-Tousi A, Stephens JW, Bin R, Cooper JA, Steptoe A, Coates ARM, Henderson B, Humphries SE. Association between plasma levels of heat shock protein 60 and cardiovascular disease in patients with diabetes mellitus. Eur Heart J 27: 1565–1570, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics 8: 4083–4099, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170: 3037–3045, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Stice JP, Chen L, Kim SC, Chen L, Tran AL, Liu TT, Knowlton AA. 17β-Estradiol, aging, inflammation and the stress response in the female heart. Endocrinology 152: 1589–1598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stice JP, Mbai FN, Chen L, Knowlton AA. Rapid activation of nuclear factor-κB by 17β-estradiol and selective estrogen receptor modulators: pathways mediating cellular protection. Shock 38: 128–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 80: 205–212, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Sun L, Chang J, Kirchhoff SR, Knowlton AA. Activation of HSF and selective increase in heat shock proteins by acute dexamethasone treatment. Am J Physiol Heart Circ Physiol 278: H1091–H1096, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive environments. Semin Immunopathol 33: 441–454, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Thery C, Amigorena S, Raposa G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Prot Cell Biol 30: 3.22.1–3.22.29, 2012 [DOI] [PubMed] [Google Scholar]
- 51. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2: 569–579, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLos One 7: e34653, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics 9: 1324–1338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α, presented by exosomes. J Mol Cell Cardiol 53: 848–857, 2012 [DOI] [PubMed] [Google Scholar]