Abstract
Diabetes mellitus in pregnancy is associated with impaired endothelium-mediated dilatation of maternal arteries, although the underlying cellular mechanisms remain unknown. In this study, we hypothesized that diabetes during rat gestation attenuates agonist-induced uterine vasodilation through reduced endothelial cell (EC) Ca2+ elevations and impaired smooth muscle cell (SMC) hyperpolarization and SMC intracellular Ca2+ concentration ([Ca2+]i) responses. Diabetes was induced by an injection of streptozotocin to second-day pregnant rats and confirmed by the development of maternal hyperglycemia. Control rats were injected with a citrate buffer. Fura-2-based measurements of SMC [Ca2+]i or microelectrode recordings of SMC membrane potential were performed concurrently with dilator responses to ACh in uteroplacental arteries from control and diabetic pregnant rats. Basal levels of EC [Ca2+]i and ACh-induced EC [Ca2+]i elevations in pressurized vessels and small EC sheets were studied as well. Diabetes reduced ACh-induced vasodilation due to a markedly impaired EDHF-mediated response. Diminished vasodilation to ACh was associated with attenuated SMC hyperpolarization and [Ca2+]i responses. Basal levels of EC [Ca2+]i and ACh-induced EC [Ca2+]i elevations were significantly reduced by diabetes. In conclusion, these data demonstrate that reduced endothelium-mediated hyperpolarization contributes to attenuated uteroplacental vasodilation and SMC [Ca2+]i responses to ACh in diabetic pregnancy. Impaired endothelial Ca2+ signaling is in part responsible for endothelial dysfunction in the uterine resistance vasculature of diabetic rats. Pharmacological improvement of EC Ca2+ handling may provide an important strategy for the restoration of endothelial function and enhancement of maternal blood flow in human pregnancies complicated by diabetes.
Keywords: endothelial dysfunction, acetylcholine-induced hyperpolarization, fura-2, calcium signaling, pressurized arteries
diabetes mellitus is one of the most common medical complications of pregnancy that significantly contributes to maternal and perinatal morbidity and mortality (5, 7, 11, 13, 44). The worldwide rise in obesity among women of childbearing age is in part responsible for the increased prevalence of diabetes during pregnancy (44, 47). Pregestational and gestational diabetes are serious complications with short- and long-term consequences for both offspring and mothers. Despite the improvement in perinatal outcome in well-controlled diabetic pregnancies, problems related to abnormal fetal growth, stillbirth, and congenital malformations persist (13). Women with gestational and especially pregestational diabetes are also at higher risk for the development of hypertension and preeclampsia during pregnancy (5, 10, 60, 61, 68).
Human diabetic pregnancy is associated with an increased vascular resistance in the maternal uteroplacental circulation. The prevalence of an abnormal uterine artery Doppler velocity waveform is much higher in diabetic than nondiabetic populations (6, 9, 39, 59). The strongest correlation between abnormal uterine artery Doppler waveform and adverse pregnancy outcome has been reported in diabetic pregnant women with preexisting vascular complications (59). Impaired uteroplacental blood flow was also documented in a rodent model of streptozotocin (STZ)-induced diabetes and a mouse model of gestational diabetes (14, 25, 63, 64).
Normal pregnancy is characterized by a marked increase in uterine blood flow due to growth and remodeling of the uterine vasculature as well as enhanced endothelium-dependent uterine vasodilation (8, 33, 52, 55). Therefore, multiple causes might be responsible for the reduced uteroplacental blood flow in diabetic pregnancies, including functional abnormalities in the maternal uteroplacental vasculature. Recent studies (4, 45, 57) have indicated that human diabetic pregnancy is associated with abnormal regulation of peripheral vascular tone resulting in part from vascular endothelial dysfunction. Impaired endothelial function was also found in the maternal peripheral vasculature of women with a previous history of gestational diabetes (2, 36). Experimental data on the functional behavior of maternal uterine arteries in diabetic pregnancy are exceptionally limited. Endothelial dysfunction has been reported in the main uterine artery of diabetic rats and mice and myometrial arteries of diabetic pregnant women, although the underlying cellular mechanisms were not defined (16, 63, 64). In human and rodent pregnancy, maternal uteroplacental vascular resistance is mainly regulated by the vascular tone of uterine radial arteries. These vessels connect large main and arcuate uterine arteries with spiral arteries; the latter vessels are characterized by their significant enlargement and loss of contractility due to trophoblast invasion (12, 52, 55). The effect of experimental diabetes on endothelium-mediated vasodilation of uteroplacental radial arteries remains unexplored and was the major objective of the present study.
We hypothesized that diabetes during rat pregnancy impairs agonist-induced uterine vasodilation due to reduced smooth muscle cell (SMC) hyperpolarization and SMC intracellular Ca2+ concentration ([Ca2+]i) responses. Previously, we (32) demonstrated the key role of an endothelial cell (EC) [Ca2+]i rise in agonist-stimulated uterine vasodilation. Therefore, impaired Ca2+ signaling may be one of the essential mechanisms underlying the reduced endothelium-dependent vasodilation in the diabetic maternal vasculature. To test our hypotheses, we performed direct measurements of changes in SMC [Ca2+]i or SMC membrane potential concurrently with vasodilator responses to ACh in uteroplacental arteries from control and diabetic pregnant rats. In addition, basal levels of EC [Ca2+]i and ACh-induced EC [Ca2+]i elevations in pressurized vessels and small EC sheets were studied in two experimental groups.
METHODS
Animals and preparation of arteries.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub No. 85-23, Revised 1996). Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Virgin Sprague-Dawley rats (10–11 wk of age) were purchased from Charles River Laboratories (St. Constant, QC, Canada) and housed in the animal care facility at the University of Vermont. The estrus cycle for female rats was determined by an examination of vaginal smears, and rats in proestrus were used for breeding. Female rats were bred with Sprague-Dawley male rats overnight in isolated pairs using metabolic cages. If copulative plugs were observed the following morning, that day was designated day 1 of pregnancy. On day 2 of pregnancy, female rats were anesthetized with 4% isoflurane, and 50–55 mg/kg STZ in 1 ml citrate buffer was injected intraperitoneally. Control pregnant rats were injected with 1 ml citrate buffer. Both STZ- and citrate buffer-injected rats were weighed every 2 days. Maternal blood glucose levels were determined from a tail nick using a Freestyle glucometer every other day.
On day 20 of pregnancy, rats were euthanized with 4% isoflurane followed by decapitation. The abdominal wall was transected, and the entire uterus and uterine vasculature were rapidly removed and pinned in a dissecting dish filled with aerated cold physiological salt solution (see Solutions and drugs for the composition of PSS). Second-order uterine radial arteries were identified within the mesometrial arcade and dissected free of connective tissue. Only radial arteries feeding the placenta (uteroplacental arteries) were used for this study. Arterial segments were cannulated from both ends in the arteriograph and continuously superfused at 3 ml/min with aerated (10% O2-5% CO2-85% N2) PSS at 37°C. Cannulated arteries were initially pressurized to 10 mmHg using the servo pressure system (Living System Instrumentation, Burlington, VT). After a 1-h equilibration period, intraluminal pressure was elevated to 50 mmHg. All experiments were performed at 50 mmHg and under no intraluminal flow conditions. Uteroplacental radial arteries from late pregnant rats can develop vasoconstriction (myogenic tone) in response to elevations of pressure exceeding 50 mmHg (32). To avoid the development of myogenic tone and its interference with phenylephrine (PE)-induced constriction, arteries were pressurized at 50 mmHg. Radial arteries are located between the main uterine artery and placenta. Physiological levels of pressure measured in these vessels in vivo are 1/3 to 2/3 of central arterial pressure that approximate 40–70 mmHg (52).
Fetuses and their placentas were carefully dissected from each uterine horn and individually weighed without membranes and umbilical cords. The litter size and number of fetal resorptions were recorded for each control or diabetic rat.
Selective loading of ECs or SMCs with fura-2 and measurement of [Ca2+]i.
A detailed description of the procedure for selective loading of ECs or SMCs of uterine arteries with the Ca2+-sensitive dye fura-2 has been previously published (32). Heat-polished glass cannulas were used in all experiments to prevent accidental damage of the endothelial layer during the cannulation procedure and to avoid diffusion of fura-2 to the SMC layer. ECs were loaded with fura-2 at room temperature by an intraluminal perfusion of pressurized arteries with fura-2 AM-containing solution (5 μM) for 5 min followed by 10 min of washout with regular PSS. A similar protocol was used in our previous study (32), where preferential loading of ECs with fura-2 was confirmed by the nearly complete disappearance of fluorescent signals after arterial denudation. SMC loading with fura-2 was performed by an extraluminal incubation of arteries in fura-2 AM (5 μM)-containing solution at room temperature in the dark for 60 min. Fura-2-loaded arteries were washed two to three times and then continuously superfused with aerated PSS at 37°C. Ratiometric measurements of fura-2 fluorescence from ECs or SMCs were performed with a photomultiplier system (IonOptix, Milton, MA). Experimental ratios were corrected for background fluorescence taken from each artery before they were loaded with fura-2. Background-corrected ratios of 510-nm emission were obtained at a sampling rate of 5 Hz from arteries alternately excited at 340 and 380 nm. All experimental protocols were started after an additional 15-min equilibration period at 10 mmHg to allow the intracellular deesterification of fura-2 AM.
Measurements of SMC membrane potential from pressurized arteries.
For intracellular measurements of SMC membrane potential from pressurized arteries, we used short arterial segments (400–500 μm) that had been carefully cleaned of any residual connective tissue. Glass microelectrodes were filled with 0.5M KCl and had tip resistances of 110–150 MΩ; an Ag-AgCl pellet was used as an indifferent electrode. A microelectrode was connected to a motorized micromanipulator (World Precision Instruments), and membrane potential was recorded using a high-input impedance amplifier (Electro 705, World Precision Instruments). Changes in membrane potential and arterial diameter were simultaneously displayed and recorded on a desktop computer using a data-acquisition program (IonOptix). The following criteria were used for the acceptance of membrane potential recordings: 1) an abrupt negative change in voltage upon impalement of the cells, 2) a sharp return to zero voltage after the withdrawal of the microelectrode tip, 3) a tip potential of <7 mV, and 4) unchanged resistance of microelectrodes after impalement. A stable membrane potential recording for at least 1 min was accepted for data collection.
Enzymatic isolation of ECs and fura-2-based measurements of [Ca2+]i from small sheets of ECs.
Our protocol for the isolation of sheets of ECs from radial uteroplacental arteries was adopted from Hannah et al. (35). After the dissection and careful cleaning from perivascular connective tissue, arterial segments were cut open and placed in dissociation media (see composition in Solutions and drugs) for 10 min at 4–6°C. Vessels were digested in dissociation media containing neutral protease [dispase (0.5 mg/ml)] and elastase (0.5 mg/ml) for 60 min at 37°C; 0.5 mg/ml collagenase type 1 was included for the final minute. Gentle trituration of arteries with a glass pipette yielded small sheets of ECs (EC sheets) as well as single ECs. To study Ca2+ responses from ECs, a suspension of EC sheets was placed into an experimental chamber. After a 15-min equilibration period and attachment of EC sheets to the glass bottom, the chamber was superfused with aerated PSS at 37°C for 20 min. Loading of ECs with fura-2 was then performed during 7–8 min at room temperature (no-flow condition) followed by washout for the next 15 min to allow the intracellular deesterification of fura-2 AM. For each experiment, fura-2 fluorescence was recorded from one individual sheet of ECs containing 21.8 ± 3.2 cells (n = 12). ECs were slightly elongated, with diameters of 9.6 ± 0.2 μm (n = 49 cells; measurements were taken from microphotographs of six EC sheets).
Protocols for testing the endothelial function of uteroplacental arteries.
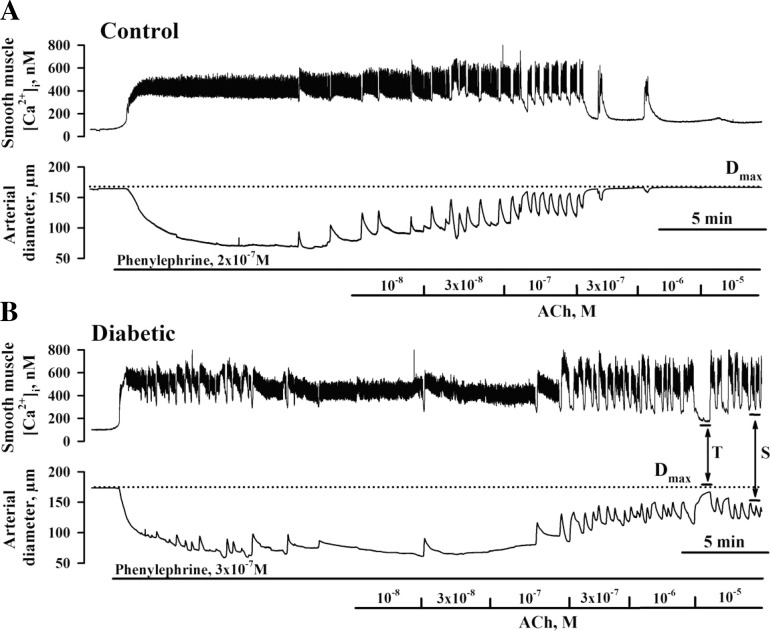
The effects of diabetic pregnancy on uteroplacental endothelial function were evaluated based on responses of pressurized arteries to ACh. Arteries were preconstricted with PE to 50–70% of the initial diameter. After the stabilization of vasoconstriction, ACh was applied in increasing concentrations. For each artery, dose-dependent effects of ACh were studied only once. A combination of papaverine (100 μM, a phosphodiesterase inhibitor) and diltiazem (10 μM, a Ca2+ channel blocker) was added at the end of the experiment to obtain the diameter under maximally dilated conditions (passive diameters). ACh-induced maximal and sustained changes in lumen diameters were calculated for each tested artery [as indicated by maximal (T) and sustained (S) values in Fig. 1]. The transient change in lumen diameter was defined as the transient change in the response and in the majority of records was calculated at the end of the first minute of ACh application. The sustained change in lumen diameter was determined at the last 15–20 s of the 3-min application of each concentration of ACh. Each calculated mean value for maximal and sustained change in lumen diameter represents averaged data points over a time period of 15–20 s obtained using IonOptix software and includes two to three oscillations when present.
Fig. 1.
Smooth muscle cell (SMC) intracellular Ca2+ concentration ([Ca2+]i) and dilator responses of uteroplacental radial arteries to ACh are impaired in diabetic pregnancy. A and B: representative changes in SMC [Ca2+]i and diameter of arteries from control (A) and diabetic (B) rats induced by cumulative application of ACh. A: application of phenylephrine (PE) resulted in an elevation of SMC [Ca2+]i from 66 to 332 nM and in a reduction of arterial diameter from 164 to 84 μm (control). Administration of ACh reduced the SMC [Ca2+]i response and dilated the artery in a concentration-dependent manner. B: application of PE increased SMC [Ca2+]i from 101 to 401 nM and reduced the diameter of the diabetic artery from 174 to 75 μm. Dotted lines show the diameters of maximally dilated control (168 μm) and diabetic (175 μm) arteries in response to 10 μM diltiazem and 100 μM papaverine. Solid horizontal lines depict the time of exposure of arteries to PE and ACh. “T” and “S” indicate transient and sustained decreases in SMC [Ca2+]i responses and vasodilation, respectively. T is the maximal change in the ACh response calculated during 15–20 s at the end of first min of ACh application. S was calculated during the last 15–20 s of the 3-min application of each concentration of ACh. ACh-induced vasodilation was expressed as the percentage of maximal dilatation induced by papaverine and diltiazem (Dmax).
Vasodilation to ACh was expressed as the percentage of maximal dilator responses to papaverine and diltiazem (Dmax) as well as the percentage of the maximal response to ACh. PE-induced constrictions were expressed as the percentage of Dmax. In an additional set of experiments, vasodilator responses were studied in combination with recordings of changes in SMC [Ca2+]i. Maximal [Ca2+]i responses to each tested concentration of ACh were compared between experimental groups.
Protocols for studying EDHF-mediated arterial responses.
After an equilibration period, arteries were incubated with 200 μM N-nitro-l-arginine [l-NNA; a nitric oxide (NO) synthase inhibitor] and 10 μM indomethacin (cyclooxygenase inhibitor) for 20 min to abolish the production of NO and prostacyclin, respectively. A subset of arteries was loaded with fura-2 for simultaneous measurements of changes in arterial diameter and SMC [Ca2+]i. PE was added in increasing concentrations (1–3 doses) to produce a constriction of 50–70% of the initial diameter, and ACh was then applied in increasing concentrations. ACh-induced vasodilation was expressed as Dmax as well as a percentage of the maximal response to ACh.
ACh-induced hyperpolarization.
In our electrophysiological experiments, each artery was initially pressurized to 10 mmHg and equilibrated during 1 h. Resting membrane potential was measured from two to three different cells, and intraluminal pressure was then elevated to 50 mmHg. After membrane potential was recorded at 50 mmHg, PE was added to preconstrict arteries to 50–70%. After stabilization of the PE-induced constriction, microelectrode impalement of SMCs was performed, and membrane potential was recorded for 2–3 min. ACh was applied in concentrations of 3 × 10−7 and 10−6 M during the next 15–20 min. SMC membrane potential and arterial diameter were measured at 5–8 min of ACh application. A combination of papaverine and diltiazem was added at the end of each experiment to maximally dilate the artery.
Protocols for measurement of EC [Ca2+]i responses to ACh in pressurized arteries and EC sheets.
After arteries had been loaded with fura-2, intraluminal pressure was elevated from 10 to 50 mmHg, and EC [Ca2+]i transients were recorded in response to increasing concentrations of ACh (3 min for each dose). These experiments were performed without preconstriction of the arteries with PE as recent studies (38, 43, 53) have indicated that Ca2+ and/or inositol 1,4,5-trisphosphate (IP3) elevated in response to agonist stimulation of SMCs can diffuse to ECs through myoendothelial gap junctions and modulate endothelial Ca2+ signaling. In separate experiments, ACh-induced EC [Ca2+]i responses were also studied using small EC sheets enzymatically isolated from uteroplacental arteries of control and diabetic rats.
Solutions and drugs.
PSS (in mM) contained 119 NaCl, 4.7 KCl, 24.0 NaHCO3, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, and 11.0 glucose (pH 7.4). For the fura-2 calibration procedure, we used a solution of the following composition: 140 mM KCl, 20 mM NaCl, 5 mM HEPES, 5 mM EGTA, 1 mM MgCl2, 5 μM nigericin, and 10 μM ionomycin (pH 7.1). The dissociation media for enzymatic isolation of endothelial cells contained (in mM) 55 NaCl, 80 Na glutamate, 5.9 KCl, 2 MgCl2, 0.1 CaCl2, 5 glucose, and 10 HEPES (pH was adjusted to 7.3).
The majority of chemicals was purchased from Sigma Chemical (St. Louis, MO) with the exception of ionomycin and nigericin, which were obtained from Calbiochem (La Jolla, CA). Fura-2 AM and pluronic acid were purchased from Invitrogen (Carlsbad, CA). Protease, elastase, and collagenase were purchase from Worthington Biochemical (Lakewood, NJ). Fura-2 AM was dissolved in dehydrated DMSO as a 1 mM stock solution, refrigerated in small aliquots, and used within 1 wk of preparation. PE, ACh, and papaverine were dissolved in deionized water on the experimental day. Diltiazem and indomethacin were prepared as 10 mM stock solutions in deionized water and alcohol, respectively, and kept refrigerated until use. Ionomycin and nigericin were dissolved in ethanol (10 mM) and kept at −20°C.
Calculations and statistical analysis.
EC or SMC [Ca2+]i was calculated using the following equation (34): [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where R is the experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is the ratio in the absence of [Ca2+]i, Rmax is the ratio at Ca2+-saturated fura-2 conditions, and β is the ratio of the fluorescence intensities at 380-nm excitation wavelength at Rmin and Rmax. Rmin, Rmax, and β were determined by an in situ calibration procedure from arteries treated with ionomycin (10 μM) and nigericin (5 μM). Calibration was performed for two separate sets of pressurized vessels loaded intraluminally or extraluminally with fura-2. In addition, a similar calibration procedure was performed on a subset of EC sheets loaded with fura-2. These values were then pooled and used to convert the ratio values into [Ca2+]i. Kd for fura-2 was 282 nM, as determined by the in situ titration of Ca2+ in fura-2-loaded small arteries (46). Kd for fura-2 is known to be sensitive to pH. It has been reported that after 3 wk of STZ-induced diabetes, only minor changes in basal intracellular pH (from 7.12 to 7.14) were detected in mesenteric arteries (40). pH variations over a reasonable range of intracellular values (7.05 to 6.75) did not affect either the spectra of the Ca2+-free or Ca2+-bound species or effective Ca2+ Kd for fura-2 (34). Therefore, we used the same Kd value for the conversion of experimental ratios into [Ca2+]i concentrations for both control and diabetic vessels. Arterial diameter and intraluminal pressure in combination with membrane potential or ratios were simultaneously recorded using an IonOptix data-acquisition program and imported into the SigmaPlot program for graphical representation, calculations, and statistical analysis. In view of the significant oscillatory activity in uterine arteries, all measurements were made by averaging records of arterial diameters, membrane potential, or SMC [Ca2+]i during 15–20 s. Data are expressed as means ± SE, where each n is the number of arterial segments studied. One or two arteries from the same animal were used on each experimental day with 1 vessel/animal used for a particular protocol. A paired or unpaired Student's t-test or two-way repeated-measures ANOVA were used to determine the significance of differences between sets of data, with P value of <0.05 considered significant. The concentration of ACh required to produce half-maximal vasodilation (EC50) was determined for each tested artery using standard curve analysis from data imported into the SigmaPlot program.
RESULTS
Maternal and fetal pregnancy outcome in a rat model of diabetic pregnancy.
Maternal and fetal pregnancy outcome in a rat model of diabetic pregnancy are shown in Table 1. STZ injection produced sustained hyperglycemia from day 4–5 of pregnancy, with average nonfasting glucose levels at day 20 of pregnancy of 428.2 ± 10.0 mg/dl. Control rats injected with citrate buffer remain normoglycemic throughout their whole pregnancy. Hyperglycemic rats gained significantly less weight during gestation than control rats. Diabetic pregnancy resulted in significantly reduced fetal weights. In contrast, placental weights and placental surface areas were significantly larger in hyperglycemic animals. There were no differences in the number of fetal resorptions (0.3 ± 0.17 vs. 0.2 ± 0.08) or litter size in diabetic versus control rats, respectively (P > 0.05).
Table 1.
Effect of experimental diabetes on maternal and fetal pregnancy outcome of 20-day pregnant rats
| Control Rats | Diabetic Rats | P Value | |
|---|---|---|---|
| Maternal weight gain, g | 123.3 ± 3.7 | 85.4 ± 4.5 | <0.001 |
| Maternal blood glucose, mg/dl | 80.1 ± 1.4 | 423.3 ± 9.9 | <0.001 |
| Fetal weight, g | 2.26 ± 0.03 | 1.90 ± 0.03 | <0.001 |
| Placental weight, g | 0.455 ± 0.011 | 0.525 ± 0.011 | <0.001 |
| Placental surface area, mm2 | 145.3 ± 4.4 | 168.6 ± 3.6 | <0.001 |
| Litter size, number of fetuses | 14.9 ± 0.4 | 14.6 ± 0.3 | >0.05 |
Values are means ± SE; n = 39 control rats and 51 diabetic rats.
Diabetes impairs endothelial function of uteroplacental arteries.
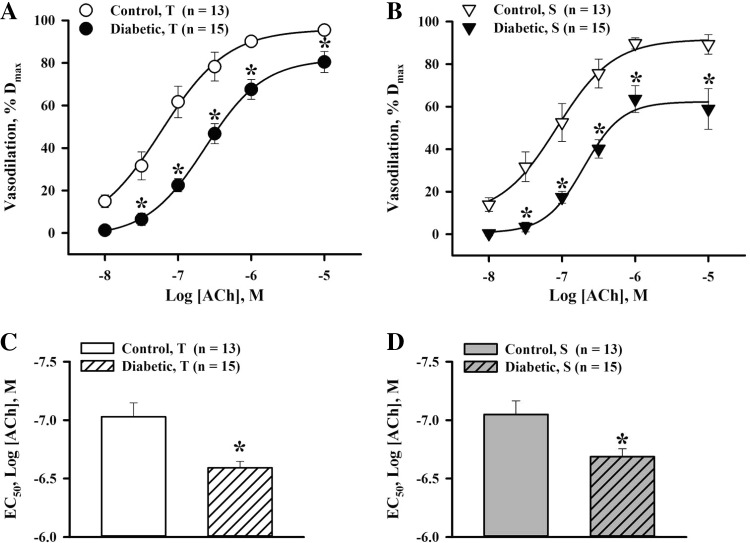
Next, we evaluated the effect of experimental diabetes on ACh-induced responses of arteries of control and diabetic rats. In all experiments, passive lumen diameters of uteroplacental arteries at 50 mmHg were not significantly different from initial vessel diameters before PE application in control (179.3 ± 8.6 vs. 174.5 ± 8.5 μm, n = 40) and diabetic (197.7 ± 8.1 vs. 194.2 ± 7.9 μm, n = 44) rats. These data indicate that uteroplacental arteries did not develop pressure-induced vasoconstriction at 50 mmHg. Passive arterial diameters were not significantly different between control and diabetic rats. Levels of PE-induced preconstriction were comparable between the two studied groups (61.7 ± 2.9% in control rats vs. 63.1 ± 1.7% in diabetic rats). PE-induced constriction of both control and diabetic vessels was associated with significant SMC [Ca2+]i elevation and the appearance of frequent SMC [Ca2+]i transients (Fig. 1, A and B). ACh application resulted in a transient interruption of Ca2+ oscillations or abolished them in a concentration-dependent manner. Oscillatory changes in [Ca2+]i were followed by transient changes in arterial diameter, and maximal changes in SMC [Ca2+]i were associated with maximal vasodilation (Fig. 1A). ACh-induced reductions in SMC [Ca2+]i and dilator responses were significantly blunted in arteries of diabetic rats (Fig. 1B). Figure 2, A and B, shows the effects of diabetes on transient and sustained components of ACh-induced vasodilation. EC50 values for ACh were significantly higher in diabetic compared with control vessels, demonstrating their decreased sensitivity to ACh (Fig. 2, C and D).
Fig. 2.
Decreased vasodilator responsiveness and sensitivity to ACh in uteroplacental arteries of diabetic pregnant rats. A and B: summary graphs showing the degree of T and S vasodilation as a function of ACh concentrations in arteries from control and diabetic rats. ACh-induced vasodilation is expressed as Dmax. *Significantly different compared with the respective control group at P < 0.05 (by two-way repeated-measures ANOVA). C and D: bar graphs showing the significant increase in the concentration of ACh required for half-maximal T and S dilatation (EC50) of uterine arteries of diabetic rats [−6.69 ± 0.05 and −6.67 ± 0.07 log(ACh); in M] compared with control rats [−7.03 ± 0.12 and −7.05 ± 0.12 log(ACh), in M]. *Significantly different compared with the respective control group at P < 0.05 (by unpaired Student's t-test). Numbers in parentheses indicate the numbers of arteries tested.
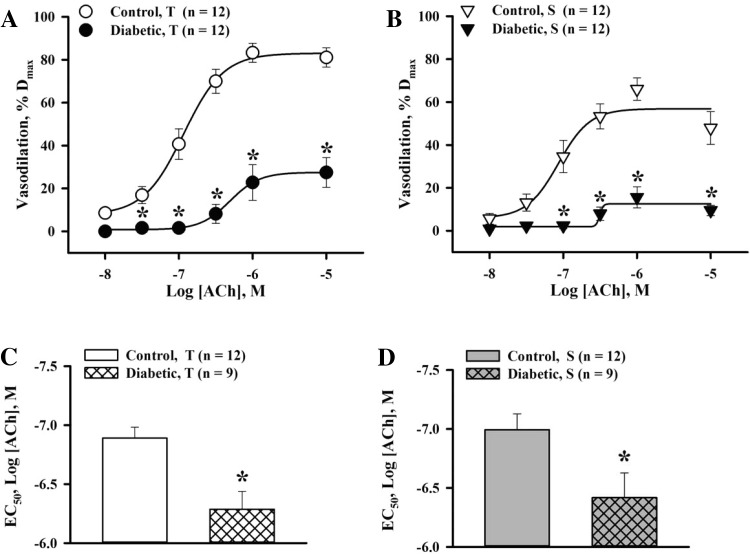
To evaluate the role of EDHF in the diabetes-induced impairment of endothelium-dependent vasodilation, comparative experiments were performed in vessels pretreated with l-NNA and indomethacin. Application of ACh resulted in significant concentration-dependent EDHF-mediated vasodilation of control arteries. The transient vasodilation to ACh was markedly reduced and sustained ACh-induced responses were almost abolished in diabetic vessels, demonstrating the transient nature of EDHF-mediated responses (Fig. 3, A and B). EC50 values for ACh were significantly increased in diabetic pregnancy (Fig. 3, C and D).
Fig. 3.
Diabetes in rat pregnancy markedly impairs EDHF-mediated uteroplacental vasodilation to ACh. A and B: summary graphs showing the degree of T and S EDHF-mediated vasodilation as a function of ACh concentrations in arteries from control and diabetic rats pretreated with N-nitro-l-arginine (l-NNA; 200 μM) and indomethacin (10 μM). ACh-induced vasodilation is expressed as Dmax. *Significantly different compared with the respective control group at P < 0.05 (by two-way repeated-measures ANOVA). C and D: bar graphs showing the marked increase in ACh EC50 concentrations for T and S vasodilation in uterine arteries of diabetic [−6.29 ± 0.15 and −6.42 ± 0.21 log(ACh), in M] versus control [−6.89 ± 0.09 and −6.99 ± 0.13 log(ACh), in M] rats. *Significantly different compared with the respective control group at P < 0.05 (by unpaired Student's t-test). Numbers in parentheses indicate the numbers of arteries tested.
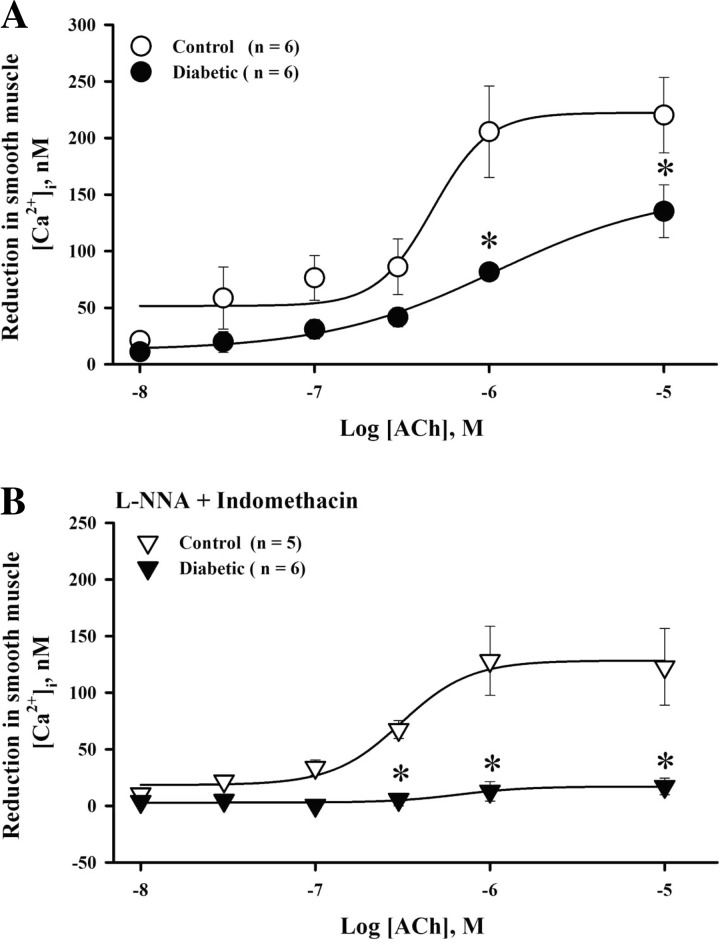
Diabetes-reduced vasodilation to ACh was associated with diminished SMC [Ca2+]i responses in untreated arteries as well as in vessels treated with l-NNA and indomethacin (Fig. 4, A and B). These data suggest that the mechanisms responsible for the ACh-induced reduction in SMC [Ca2+]i are significantly impaired by diabetes.
Fig. 4.
Diabetic pregnancy is associated with reduced SMC [Ca2+]i responses to ACh. A and B: graphs showing diabetes-induced effects on SMC [Ca2+]i responses to ACh in intact (A) and l-NNA- and indomethacin-treated (B) uteroplacental arteries. *Significantly different compared with the respective control group at P < 0.05 (by two-way repeated-measures ANOVA).
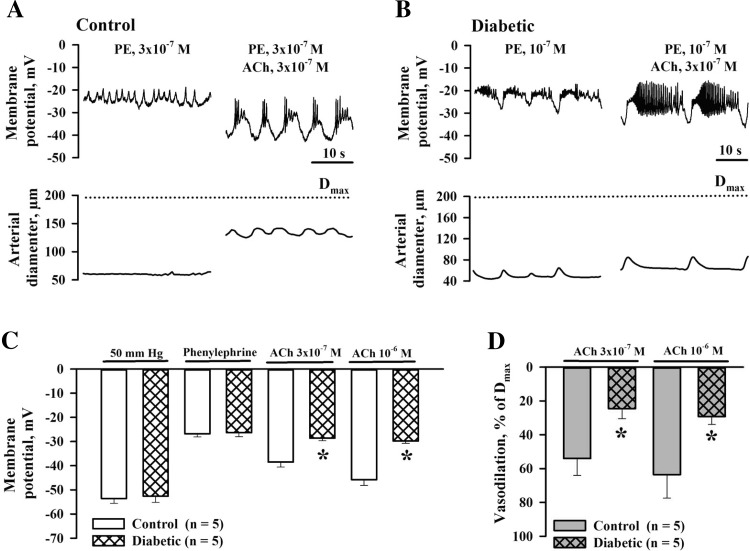
Experimental diabetes impairs ACh-induced hyperpolarization.
Recently, we demonstrated that ACh-induced vasodilation of maternal uteroplacental arteries is associated with vascular SMC hyperpolarization (33). Therefore, impaired ACh-induced SMC hyperpolarization may be the mechanism underlying attenuated dilator and SMC [Ca2+]i responses in diabetic pregnancy. Measurements of SMC membrane potential were performed in pressurized arteries from control and diabetic rats. The resting membrane potential of SMCs at 10 mmHg was not different between two groups of vessels (−55.1 ± 1.7 mV, n = 9, in control rats vs. −53.6 ± 2.1 mV, n = 8, in diabetic rats, P = 0.295). Elevation of intraluminal pressure from 10 to 50 mmHg produced no significant changes in the membrane potential of control (−54.7 ± 4.2 mV, P = 0.821) or diabetic (−47.9 ± 11.9 mV, P = 0.074) vessels. Application of PE depolarized SMCs to −26.8 ± 1.3 mV (n = 5) in control and to −26.3 ± 1.7 mV (n = 5) in diabetic arteries, which was associated with vasoconstriction of 66.4 ± 1.3% and 68.7 ± 1.8%, respectively. Subsequent administration of ACh in concentrations of 3 × 10−7 and 10−6 M resulted in SMC hyperpolarization to −38.5 ± 2.1 and −45.8 ± 2.4 mV in control (n = 5) and to −28.6 ± 1.1 and −29.8 ± 1.0 mV in diabetic vessels (n = 5). Representative recordings of ACh-induced changes in SMC membrane potential and diameters of arteries from control and diabetic rats are shown in Fig. 5, A and B. As shown by the summary graphs in Fig. 5, C and D, ACh-induced hyperpolarization was significantly reduced in arteries of diabetic rats and was associated with diminished vasodilation. In l-NNA- and indomethacin-treated and PE-preconstricted arteries of control rats, administration of 3 × 10−7 and 10−6 M ACh hyperpolarized SMCs from −26.0 ± 0.3 to −35.2 ± 2.2 and −39.2 ± 2.1 mV and resulted in vasodilation of 37.9 ± 6.9% and 53.2 ± 7.6%, respectively (n = 5). In similar experiments on vessels of diabetic rats, ACh-induced hyperpolarization was significantly reduced (P < 0.05). Application of ACh in concentrations of 3 × 10−7 and 10−6 M hyperpolarized SMCs of diabetic arteries from −25.8 ± 2.6 to −27.0 ± 2.8 and −28.8 ± 1.0 mV. These responses were associated with vasodilation of 2.7 ± 1.0% and 14.9 ± 11% (n = 4). No significant differences were detected between ACh-induced hyperpolarization of intact and l-NNA- and indomethacin-treated arteries from both groups of rats.
Fig. 5.
Impairment of SMC hyperpolarization and associated dilation of uteroplacental arteries to ACh in rat diabetic pregnancy. A: original tracings showing membrane potential with oscillatory activity and diameter changes recorded from a pressurized artery of a control rat before and on minutes 8–9 of application of ACh. B: original tracings of membrane potential and lumen diameter changes before and on minutes 6–7 of ACh administration in an artery from a diabetic pregnant rat. Arteries were preconstricted with PE before ACh was tested. Membrane potential and arterial diameter axes are scaled to show actual values for tracings in A and B. C: bar graphs showing resting membrane potential values, PE-induced depolarization, and hyperpolarizing effects of 3 × 10−7M and 10−6M ACh in arteries of control and diabetic rats. D: bar graphs showing vasodilator responses of arteries from control and diabetic rats associated with the ACh-induced hyperpolarization shown in C. Vasodilation is expressed as Dmax. *Significantly different compared with the respective control group at P < 0.05 (by two-way repeated-measures ANOVA).
Diabetes in pregnancy reduced EC [Ca2+]i responses to ACh.
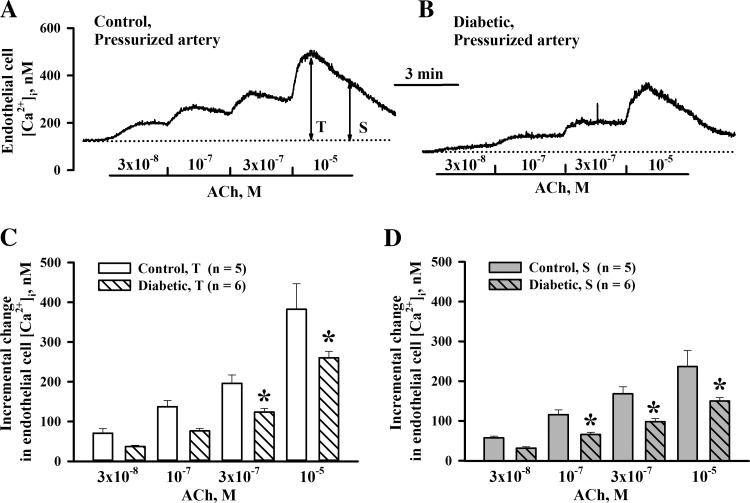
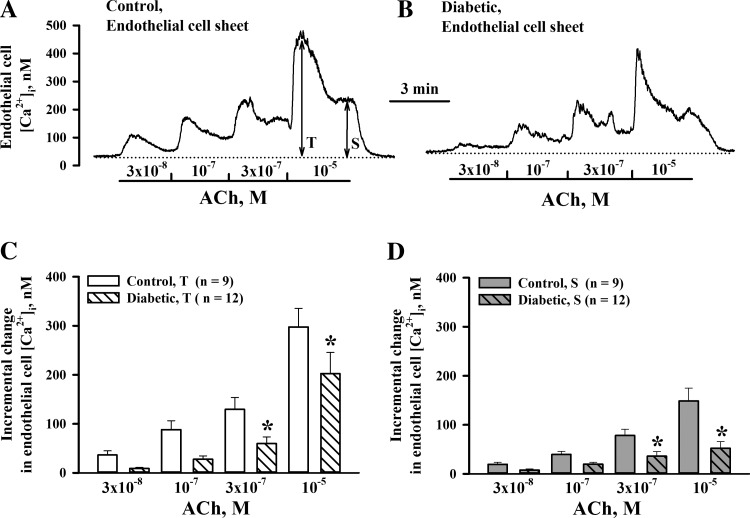
To explore the contribution of altered Ca2+ signaling in diabetes-induced endothelial dysfunction, we next compared EC [Ca2+]i responses to ACh in pressurized vessels of control and diabetic rats. Levels of basal EC [Ca2+]i measured at 50 mmHg before ACh application were significantly lower in the diabetic group compared with the control group (105.9 ± 8.6 nM, n = 6, vs. 149.9 ± 10.8 nM, n = 5, P < 0.05). Representative recordings of the EC [Ca2+]i rise to ACh are shown in Fig. 6, A and B. As shown in the summary graphs in Fig. 6, C and D, both transient and sustained EC [Ca2+]i elevations were reduced in vessels of diabetic rats. Similar experiments were performed on small sheets of ECs enzymatically isolated from uteroplacental arteries. At 37°C, basal levels of EC [Ca2+]i were similar in cells from control (41.8 ± 4.2 nM, n = 9) and diabetic (47.8 ± 5.1 nM, n = 12) rats. These levels were significantly lower compared with those in pressurized vessels. ACh-induced EC [Ca2+]i responses were blunted in EC sheets from diabetic rats (Fig. 7, A and B). The summary graphs shown in Fig. 7, C and D, demonstrate the significant decrease in both transient and sustained elevations in [Ca2+]i in response to application of ACh in EC sheets of diabetic rats.
Fig. 6.
Attenuation of ACh-induced endothelial cell (EC) [Ca2+]i elevations in pressurized uteroplacental arteries of diabetic rats. A and B: representative changes in EC [Ca2+]i in response to increasing concentrations of ACh in arteries from a control rat (A) and a diabetic rat (B). Mean values for T and S responses were calculated by averaging all data points over 5–10 s of the peak response (T) and over last 15–20 s of the sustained response (S) to each concentration of ACh. C and D: bar graphs showing diabetes-induced impairment of T and S EC [Ca2+]i responses to ACh. Incremental changes in EC [Ca2+]i were calculated after the subtraction of basal [Ca2+]i levels measured before the administration of ACh. *Significantly different compared with the respective control group at P < 0.05 (by two-way repeated-measures ANOVA).
Fig. 7.
Experimental diabetes in pregnancy impairs ACh-induced EC [Ca2+]i responses in uteroplacental sheets of ECs. A and B: original recordings of EC [Ca2+]i elevations in response to increasing concentrations of ACh in endothelial sheets dissociated from arteries of a control rat (A) and a diabetic rat (B). Mean values for T and S responses were calculated by averaging all data points over 5–10 s of the peak response (T) and over last 15–20 s of the sustained response (S) to each concentration of ACh. C and D: bar graphs showing diabetes-induced impairment of T and S EC [Ca2+]i responses to ACh. Incremental changes in EC [Ca2+]i were calculated after the subtraction of basal [Ca2+]i levels measured before the administration of ACh. *Significantly different compared with the respective control group at P < 0.05 (by two-way repeated-measures ANOVA).
DISCUSSION
This study aimed to explore the effects of experimental diabetes in rat pregnancy on the endothelium-dependent vasodilation of maternal uteroplacental radial arteries and to determine the underlying cellular mechanisms with a specific focus on EC Ca2+ signaling. The main findings of this study were 1) experimental diabetes during rat pregnancy is associated with intrauterine growth restriction; 2) ACh-induced vasodilation, SMC [Ca2+]i responses, and hyperpolarization are significantly attenuated in arteries of diabetic rats; 3) l-NNA- and indomethacin-resistant EDHF-mediated uterine vascular responses are severely impaired in diabetic pregnancy; and 4) basal levels of EC [Ca2+]i and [Ca2+]i responses to ACh are significantly reduced by experimental diabetes, supporting the essential role of impaired endothelial Ca2+ signaling in uteroplacental vascular dysfunction.
In this study, the induction of diabetes during pregnancy was achieved by STZ-induced damage of pancreatic β-cells to mimic type 1 diabetes in human pregnancy. The manifestation of diabetes was confirmed by the development of marked maternal hyperglycemia during the last 2 wk of rat pregnancy. This model of diabetes aimed to mimic the development of hyperglycemia during untreated diabetic pregnancy in humans and has been previously used to explore the effects of maternal diabetes on pregnancy outcome in rodents (41). Although this model of diabetes is short lasting, it covers most of the duration of rat pregnancy and allows us to study the effect of hyperglycemia, the most damaging factor of diabetic pregnancy, on maternal uteroplacental vascular function. In agreement with previously published findings, fetal weights were significantly decreased demonstrating fetal growth restriction in association with maternal hyperglycemia during rodent pregnancy (14, 25, 58, 63). Placental weights and placental surface areas were significantly increased in our diabetic rat model. Similar placental changes have been described in poorly controlled human diabetic pregnancy and may represent compensatory adaptations to ensure adequate nutrient and O2 supply to the growing fetus (21).
Both animal and human studies (14, 25, 59, 64) have provided evidence for decreased maternal uterine blood flow in pregnancies complicated by diabetes. Normal pregnancy is associated with a significant increase in maternal uterine blood flow mainly due to remodeling of the uterine vasculature and enhanced uterine vasodilation (8, 12, 52, 55). In our recent study (58), we found no changes in growth of maternal uterine vasculature in the rat model of STZ-induced diabetic pregnancy. This suggests that the decreased maternal uterine blood flow previously described in rat diabetic pregnancy (14, 25) may be due to abnormal functional behavior of the uteroplacental resistance vasculature.
Endothelial dysfunction is considered a key mechanism for diabetic vascular disease. There is experimental and clinical evidence for the presence of impaired endothelium-dependent vasodilation in various vascular beds of animal models of diabetes or in humans with type 1 and type 2 diabetes (18, 20, 22, 27). The results of the present study clearly demonstrate the significant attenuation of ACh-induced vasodilation of small radial uteroplacental arteries from diabetic pregnant rats. In contrast, both maximal responses and sensitivity to ACh were unchanged in the main uterine artery from pregnant mice with STZ-induced diabetes (63). Inhibition of NO and prostacyclin production in this vessel revealed a significantly blunted EDHF-dependent relaxation. These findings suggest that in the main uterine artery of diabetic pregnant mice, NO production can effectively compensate for reduced EDHF mechanism (63). Available experimental data provide evidence that in large systemic arteries, endothelium-mediated vasodilation mostly relies on NO production. For example, NO is the major mediator of endothelium-dependent dilation in the femoral artery (66). Endothelial function of these vessels was mostly preserved in diabetic male rats. In contrast, EDHF-mediated vasodilation is prominent in the mesenteric resistance vasculature, and it was markedly reduced in the same diabetic model (66). Previously, we (33) have shown a predominance of the EDHF mechanism in endothelium-dependent vasodilation of radial uteroplacental arteries. In the present study, EDHF-mediated vasodilation was almost abolished by diabetes (Fig. 3). We concluded that EDHF is exceptionally sensitive to diabetic damage and that impaired EDHF is a key mechanism responsible for uteroplacental endothelial dysfunction in rat diabetic pregnancy. An important implication of these findings is that endothelial function of small resistance uterine arteries may be more vulnerable to the damaging effects of diabetes compared with large uterine vessels.
A decrease in SMC [Ca2+]i and reduction in sensitivity to intracellular Ca2+ are two major mechanisms that account for the vascular dilatation in the macro- and microcirculation. Both mechanisms are importantly involved in the regulation of vascular tone and can be significantly altered under various pathological states (19). Our data demonstrate that impaired ACh-induced vasodilation in diabetes was associated with significantly blunted SMC [Ca2+]i responses (Fig. 1). ACh-induced SMC hyperpolarization was also decreased in diabetic vessels. As evident from the representative records shown in Figs. 1 and 5, the pattern of Ca2+ transients (Ca2+ waves with superimposed fast Ca2+ spikes) was identical to that of electrical activity (waves of membrane potential with superimposed fast spikes), revealing a close association between SMC electrical activity and Ca2+ handling. Our findings demonstrate that inhibition of ACh-induced hyperpolarization is one of the essential mechanisms of impaired uterine vasodilation in diabetic pregnancy. Similarly, ACh-induced hyperpolarization was significantly diminished in mesenteric and carotid arteries of diabetic rats (29, 42, 66). Therefore, impaired agonist-induced hyperpolarization may be a common mechanism contributing to diabetes-induced endothelial dysfunction in various vascular beds.
In maternal uterine arteries, diabetes may impair ACh-induced SMC hyperpolarization via reduced production of endothelium-derived relaxing factors as well as alterations of cellular pathways leading to EC and/or SMC hyperpolarization. An increase in [Ca2+]i is a specific mechanism that translates the effects of mechanical or agonist stimulation of ECs into cellular responses. In maternal uterine arteries, intracellular chelation of endothelial [Ca2+]i with BAPTA abolished ACh-induced vasodilation, implicating [Ca2+]i rise as a critical step in the generation of both NO and EDHF (32). Despite the key role of EC [Ca2+]i in the production of endothelium-derived factors, the importance of altered Ca2+ signaling in endothelial dysfunction during diabetes remains unknown. Here, we demonstrated, for the first time, that experimental diabetes during rat pregnancy significantly attenuates ACh-induced EC [Ca2+]i responses in pressurized uteroplacental arteries. Similar effects were observed in isolated EC sheets, minimizing the possible role of altered vascular SMC Ca2+ handling in abnormal EC [Ca2+]i responses of diabetic vessels. Previously, we (32) demonstrated increased basal levels of EC [Ca2+]i in rat uterine radial arteries in the pregnant state compared with nonpregnant state. Elevated basal EC [Ca2+]i is one of the essential mechanisms of pregnancy-enhanced basal production of endothelium-derived relaxing factors (8). In the present study, we found that basal levels of EC [Ca2+]i are significantly reduced by diabetes and may contribute to the attenuated endothelial influence on maternal uterine vascular tone in diabetic pregnancy. Collectively, our data demonstrate that impaired endothelial Ca2+ signaling is an essential mechanism underlying endothelial dysfunction in the maternal uterine resistance vasculature during experimental diabetes.
We also noticed that basal levels of [Ca2+]i in EC sheets were significantly lower compared with those in pressurized vessels. ECs in the walls of blood vessels are subjected to mechanical forces, such as pressure-induced distension and shear stress, that can stimulate Ca2+ influx through Ca2+-permeable channels, playing an important role in the production and release of endothelium-derived autacoids (3, 26, 69). Mechanical load in isolated EC sheets is minimal, providing a potential explanation for the low basal levels of EC [Ca2+]i. On the other hand, two-way communications between ECs and SMCs in the walls of blood vessels are well documented (49, 53). IP3 generated in SMCs of pressurized arteries can diffuse to ECs through gap junctions of myoendothelial projections, resulting in Ca2+ release from the endoplasmic reticulum (ER) and hyperpolarization of ECs due to the activation of intermediate-conductance Ca2+-actived K+ (IKCa) channels (43, 53). The colocalization of the endothelial ER and IKCa channels in myoendothelial projections of the mesenteric vasculature has been recently reported (48). Hyperpolarization of ECs results in an increased driving force for Ca2+ influx and may contribute to higher basal levels of EC [Ca2+]i in arteries versus sheets. Therefore, the absence of background SMC influence on Ca2+ signaling may also account for the lower basal levels of [Ca2+]i in EC sheets compared with those in pressurized arteries.
Currently, the cellular mechanisms of abnormal EC Ca2+ signaling in maternal uterine vasculature of diabetic pregnant rats remain unknown. IP3-induced Ca2+ release from the ER and Ca2+ entry from the extracellular space are two principal mechanisms that contribute to the Ca2+ elevation in response to stimulation of ECs (54, 69). At present, pathways of Ca2+ influx into ECs of uterine vessels remain largely unexplored. Diverse transient receptor potential (TRP) channels play a critical role in regulating Ca2+ entry in different cell types, including the vascular endothelium. Recent studies have demonstrated that TRPV4 channels can provide a Ca2+ entry pathway in the systemic vascular endothelium. These channels can be activated by mechanical (shear stress, pressure, and stretch) or chemical (ACh and bradykinin) stimulation and products of arachidonic acid [epoxyeicosatrienoic acids (EETs)]; they are sensitive to temperature and are regulated by caveolin (23, 54, 62, 67, 69). TRPV4 channels are also key regulators of endothelial small-conductance Ca2+-activated K+ (SKCa)/IKCa channel function and, as a consequence, endothelium-mediated vasodilation (62). Another class of Ca2+ entry pathway that is activated by store depletion, store-operated Ca2+ channels, has been documented in ECs (28). The exact role of different Ca2+ entry pathways in diabetes-induced dysregulation of endothelial Ca2+ signaling in the maternal uterine vasculature remains unknown and is an important area for future studies.
Hyperglycemia is considered as a major causal factor of diabetes-associated endothelial dysfunction in animals and humans (20, 22, 30, 31). However, experimental studies of hyperglycemia-evoked vascular effects have produced conflicting results. Acute treatment of cerebral or uterine arteries with high glucose was associated with enhanced endothelium-dependent vasodilation (15, 17). Short-term (24–48 h) chronic treatment of cultured ECs from large conduit vessels resulted in increased or unchanged EC [Ca2+]i responses to endothelium-dependent agonists (22). However, prolonged (5 and 10 days) exposure of cultured bovine aortic ECs to high glucose resulted in a significant attenuation of the bradykinin-induced [Ca2+]i rise and NO production (65). Our present findings demonstrate that hyperglycemia during 2 wk of pregnancy is associated with significant reduction in basal levels of EC [Ca2+]i and ACh-induced EC [Ca2+]i responses. Collectively, these data implicate long-term hyperglycemia as a potential factor of impaired Ca2+ signaling and vascular dysfunction in diabetic pregnancy.
Accumulating data have indicated that hyperglycemia-induced endothelial mitochondrial overproduction of ROS in microvasculature activates a number of intracellular pathways resulting in impaired endothelial function (31). One of these mechanisms is enhanced generation of diacylglycerol (DAG) and excessive PKC activation, which is a well-characterized cause of endothelial dysfunction in humans and animals with diabetes (30, 31). A significant increase in DAG production and PKC activity in the placenta and decidua has been demonstrated in STZ-induced diabetic or transiently hyperglycemic pregnant mice. Although the cell type responsible for this phenomenon was not determined in this study, vascular cells in the decidua or placenta may be a source of increased DAG-PKC signaling (37). PKC is a key second messenger generated in response to receptor stimulation in ECs and plays a diverse role in the regulation of EC Ca2+ signaling. Recent data have demonstrated that PKC-α mediates ACh-induced activation of TRPV4-dependent Ca2+ influx in vascular ECs (1). On the other hand, chronic hypoxia enhances PKC-dependent inhibition of Ca2+ entry in ECs of rat intrapulmonary arteries (56). The role of enhanced PKC activity in impaired EC Ca2+ signaling in uteroplacental arteries of diabetic pregnant rats requires further investigation.
In our study, EDHF-mediated vasodilation was almost abolished despite the partial preservation of EC [Ca2+]i responses to ACh. These results suggest that, in addition to impaired endothelial Ca2+ signaling, some other mechanisms contribute to the inhibition of EDHF-mediated vasodilation. EDHF was originally defined as a factor that hyperpolarizes vascular SMCs independently of NO and prostacyclin production (24). Extensive in vivo and in vitro studies during the last decade have demonstrated an essential role of EDHF in regulating the tone of resistance arteries and arterioles in a variety of vascular beds, including uterine circulation (33, 51). The nature of EDHF remains the matter of substantial controversy, and EDHF is no longer considered as a single endothelium-derived factor. Several diffusible substances (K+, EETs, H2O2, and H2S) or the mechanism of myoendothelial electrical coupling have been proposed for the role of EDHF in different vascular beds. These multiple mechanisms can work separately or in a combination depending on the type of EC stimulation as well as the origin of blood vessels (for a review, see Ref. 24).
Agonist-induced activation of endothelial SKCa and IKCa channels is a key mechanism of EDHF-mediated vasodilation of rat uteroplacental and human myometrial arteries (33, 51). Consequent electrotonic spreading of hyperpolarization from ECs to adjacent SMCs and K+ efflux through opened SKCa/IKCa channels are two potential mechanisms contributing to the hyperpolarization of SMCs. Several recent studies have tested the role of altered expression of SKca/IKCa channels in diabetes-induced vascular dysfunction. In STZ-induced experimental diabetes, expression of these channels was reduced in cavernous tissue (70). In contrast, impaired EDHF-mediated dilatation was associated with increased SKCa/IKCa channel expression in mesenteric arteries of diabetic rats (50). These data suggest that diabetes may modulate Ca2+ sensitivity/activation and/or cellular trafficking of SKCa/IKCa channels in blood vessels independently of channel expression. In addition, impaired expression and/or function of connexins of myoendothelial gap junctions may play a role in the reduced EDHF-mediated responses of uterine arteries in diabetic pregnancy (22). Further studies are needed to clarify the contribution of SKCa/IKCa channels and connexins to endothelial dysfunction in the maternal uterine vasculature of diabetic rats.
In conclusion, the findings of the present study demonstrate that experimental diabetes during rat pregnancy results in uteroplacental endothelial dysfunction with severely impaired EDHF-mediated vasodilation. Reduced vascular SMC hyperpolarization is one of the essential mechanisms of attenuated SMC [Ca2+]i and dilator responses of diabetic arteries to ACh. Impaired endothelial Ca2+ signaling is in part responsible for endothelial dysfunction of the uterine resistance vasculature and could contribute to reduced uteroplacental blood flow in rat diabetic pregnancy. Pharmacological improvement of EC Ca2+ handling may provide an important strategy for the restoration of endothelial function and enhancement of maternal uterine blood flow in human pregnancies complicated by diabetes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-088245.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.I.G. and A.D.B. conception and design of research; N.I.G., A.P.G., and G.G. performed experiments; N.I.G. and G.G. analyzed data; N.I.G., A.D.B., and G.G. interpreted results of experiments; N.I.G. prepared figures; N.I.G. drafted manuscript; N.I.G., A.D.B., A.P.G., and G.G. edited and revised manuscript; N.I.G., A.D.B., A.P.G., and G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Erika Linder and Olga Kuzina for excellent technical assistance and Lia Venner and Serena Parnau for help with editing of the manuscript.
REFERENCES
- 1. Adapala RK, Talasila PK, Bratz IN, Zhang DX, Suzuki M, Meszaros JG, Thodeti CK. PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol 301: H757–H765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, Stamatelopoulos SF. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care 21: 2111–2115, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal 15: 1389–1403, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Ang C, Lumsden MA. Diabetes and the maternal resistance vasculature. Clin Sci (Lond) 101: 719–729, 2001 [PubMed] [Google Scholar]
- 5. Barden A, Singh R, Walters BN, Ritchie J, Roberman B, Beilin LJ. Factors predisposing to pre-eclampsia in women with gestational diabetes. J Hypertens 22: 2371–2378, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Barth WH, Jr, Genest DR, Riley LE, Frigoletto FD, Jr, Benacerraf BR, Greene MF. Uterine arcuate artery Doppler and decidual microvascular pathology in pregnancies complicated by type I diabetes mellitus. Ultrasound Obstet Gynecol 8: 98–103, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med 21: 103–113, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol 284: R245–R258, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Bracero LA, Schulman H. Doppler studies of the uteroplacental circulation in pregnancies complicated by diabetes. Ultrasound Obstet Gynecol 1: 391–394, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 158: 1148–1153, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 115: 485–491, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473–482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr 133: 1674S–1683S, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Chartrel NC, Clabaut MT, Boismare FA, Schrub JC. Uteroplacental hemodynamic disturbances in establishment of fetal growth retardation in streptozocin-induced diabetic rats. Diabetes 39: 743–746, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Chirayath HH, Wareing M, Taggart MJ, Baker PN. Acute hyperglycemia in uterine arteries from pregnant, but not non-pregnant mice, enhances endothelium-dependent relaxation. Vasc Pharmacol 46: 137–143, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Chirayath HH, Wareing M, Taggart MJ, Baker PN. Endothelial dysfunction in myometrial arteries of women with gestational diabetes. Diabetes Res Clin Pract 89: 134–140, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Cipolla MJ, Porter JM, Osol G. High glucose concentrations dilate cerebral arteries and diminish myogenic tone through an endothelial mechanism. Stroke 28: 401–410, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Cohen RA. ATVB in focus: Diabetic vascular disease: pathophysiological mechanisms in the diabetic milieu and therapeutic implications. Arterioscler Thromb Vasc Biol 24: 1340–1341, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys 510: 160–173, 2011 [DOI] [PubMed] [Google Scholar]
- 20. De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 30, Suppl 2: S120–S126, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflügers Arch 459: 977–994, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 148–153, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflügers Arch 459: 863–879, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Eriksson UJ, Jansson L. Diabetes in pregnancy: decreased placental blood flow and disturbed fetal development in the rat. Pediatr Res 18: 735–738, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103: 2–17, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Fitzgerald SM, Kemp-Harper BK, Tare M, Parkington HC. Role of endothelium-derived hyperpolarizing factor in endothelial dysfunction during diabetes. Clin Exp Pharmacol Physiol 32: 482–487, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol 3: 121–127, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol 121: 1383–1391, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol 290: H2124–H2135, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 35. Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hannemann MM, Liddell WG, Shore AC, Clark PM, Tooke JE. Vascular function in women with previous gestational diabetes mellitus. J Vasc Res 39: 311–319, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Hiramatsu Y, Sekiguchi N, Hayashi M, Isshiki K, Yokota T, King GL, Loeken MR. Diacylglycerol production and protein kinase C activity are increased in a mouse model of diabetic embryopathy. Diabetes 51: 2804–2810, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Jaffe R, Friedman Z. Changes in uterine artery doppler velocimetry in pregnant patients undergoing glucose tolerance test may predict adverse outcome in later pregnancy: a preliminary study. Fetal Diagn Ther 13: 241–243, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Jandeleit-Dahm K, Hannan KM, Farrelly CA, Allen TJ, Rumble JR, Gilbert RE, Cooper ME, Little PJ. Diabetes-induced vascular hypertrophy is accompanied by activation of Na+-H+ exchange and prevented by Na+-H+ exchange inhibition. Circ Res 87: 1133–1140, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev 31: 680–701, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Kamata K, Ohuchi K, Kirisawa H. Altered endothelium-dependent and -independent hyperpolarization and endothelium-dependent relaxation in carotid arteries isolated from streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 362: 52–59, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, Welsh DG, Plane F. Endothelial feedback and the myoendothelial projection. Microcirculation 19: 416–422, 2012 [DOI] [PubMed] [Google Scholar]
- 44. King H. Epidemiology of glucose intolerance and gestational diabetes in women of childbearing age. Diabetes Care 21, Suppl 2: B9–B13, 1998 [PubMed] [Google Scholar]
- 45. Knock GA, McCarthy AL, Lowy C, Poston L. Association of gestational diabetes with abnormal maternal vascular endothelial function. Br J Obstet Gynaecol 104: 229–234, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langer O, Yogev Y, Xenakis EM, Brustman L. Overweight and obese in gestational diabetes: the impact on pregnancy outcome. Am J Obstet Gynecol 192: 1768–1776, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Leo CH, Hart JL, Woodman OL. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol 162: 365–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luksha L, Luksha N, Kublickas M, Nisell H, Kublickiene K. Diverse mechanisms of endothelium-derived hyperpolarizing factor-mediated dilatation in small myometrial arteries in normal human pregnancy and preeclampsia. Biol Reprod 83: 728–735, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Moll W. Structure adaptation and blood flow control in the uterine arterial system after hemochorial placentation. Eur J Obstet Gynecol Reprod Biol 110, Suppl 1: S19–S27, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 302: H594–H602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium 10: 5–15, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paffett ML, Riddle MA, Kanagy NL, Resta TC, Walker BR. Altered protein kinase C regulation of pulmonary endothelial store- and receptor-operated Ca2+ entry after chronic hypoxia. J Pharmacol Exp Ther 334: 753–760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paradisi G, Biaggi A, Ferrazzani S, De Carolis S, Caruso A. Abnormal carbohydrate metabolism during pregnancy : association with endothelial dysfunction. Diabetes Care 25: 560–564, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Phillips JK, Vance AM, Raj RS, Mandala M, Linder EA, Gokina NI. Impact of experimental diabetes on the maternal uterine vascular remodeling during rat pregnancy. Reprod Sci 19: 322–331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pietryga M, Brazert J, Wender-Ozegowska E, Biczysko R, Dubiel M, Gudmundsson S. Abnormal uterine Doppler is related to vasculopathy in pregestational diabetes mellitus. Circulation 112: 2496–2500, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 308: 1592–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis 175: 189–202, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stanley JL, Ashton N, Taggart MJ, Davidge ST, Baker PN. Uterine artery function in a mouse model of pregnancy complicated by diabetes. Vascul Pharmacol 50: 8–13, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Stanley JL, Cheung CC, Rueda-Clausen CF, Sankaralingam S, Baker PN, Davidge ST. Effect of gestational diabetes on maternal artery function. Reprod Sci 18: 342–352, 2011 [DOI] [PubMed] [Google Scholar]
- 65. Tang Y, Li GD. Chronic exposure to high glucose impairs bradykinin-stimulated nitric oxide production by interfering with the phospholipase-C-implicated signalling pathway in endothelial cells: evidence for the involvement of protein kinase C. Diabetologia 47: 2093–2104, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol Heart Circ Physiol 281: H232–H240, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Wong CO, Yao X. TRP channels in vascular endothelial cells. Adv Exp Med Biol 704: 759–780, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol 191: 1655–1660, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 57: 133–139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu JH, Jia RP, Xu LW, Wu JP, Wang ZZ, Wang SK, Bo CJ. Reduced expression of SK3 and IK1 channel proteins in the cavernous tissue of diabetic rats. Asian J Androl 12: 599–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]