Abstract
Previous studies suggest restoration of angiogenic balance can lower blood pressure and improve vascular endothelium function in models of preeclampsia. Our laboratory has recently reported exercise training mitigates hypertension in an animal model of preeclampsia, but the mechanisms are unknown. AMP-activated protein kinase (AMPK) is stimulated during exercise and has been shown to increase expression of VEGF. Therefore, the purpose of this study was to determine whether AICAR (5-aminoimidazole-4-carboxamide-3-ribonucleoside), a potent AMPK stimulator, would increase circulating VEGF, improve angiogenic potential, decrease oxidative stress, and abrogate placental ischemia-induced hypertension. In rats, reduced uteroplacental perfusion pressure (RUPP) was induced on day 14 of gestation by introducing silver clips on the inferior abdominal aorta and ovarian arteries. AICAR was administered intraperitoneally (50 mg/kg b.i.d.) days 14–18, and blood pressure and tissues were collected on day 19. RUPP-induced hypertension was ameliorated (P < 0.05) with AICAR versus RUPP. AICAR increased (P < 0.05) plasma VEGF and decreased (P < 0.05) plasma soluble VEGF receptor-1 in the RUPP + AICAR versus RUPP. Antioxidant capacity was restored (P < 0.05) by AICAR in RUPP placenta. Renal and placental catalase activity was decreased (P < 0.05) in RUPP + AICAR versus RUPP. Angiogenic potential was increased (P < 0.05) in RUPP + AICAR versus RUPP. Fetal and placental weights were unaffected by AICAR. Placental AMPK phosphorylation was increased (P < 0.05) in RUPP + AICAR versus normal pregnant and RUPP. These findings suggest AICAR may be useful to mitigate angiogenic imbalance, renal, and placental oxidative stress and increase in blood pressure associated with RUPP hypertension. Furthermore, placental AMPK phosphorylation was observed only in the setting of ischemia.
Keywords: preeclampsia, hypertension, angiogenic balance, 5-aminoimidazole-4-carboxamide-3-ribonucleoside
preeclampsia, a pregnancy-specific syndrome, is the leading cause of fetal and maternal morbidity and mortality across the world, and unfortunately the incidence has been increasing in recent decades (32, 35). Currently, there are limited treatments available, and early delivery is often indicated to prevent further progression of the syndrome. Consequently, preeclampsia is the leading cause of premature delivery, which is well known to have numerous deleterious effects on neonatal and life-long health (32, 35).
Recent clinical and experimental studies suggest dysregulation of circulating angiogenic factors results in an angiogenic imbalance that is most notably characterized by an altered ratio of pro-angiogenic (e.g., VEGF) and anti-angiogenic factors (e.g., soluble VEGF receptor-1, or sFlt-1). An imbalance favoring increased anti-angiogenic factors has been strongly linked to the etiology of hypertension during preeclampsia (3, 18, 27, 29). Recent studies also indicate restoration of angiogenic balance can mitigate an increase in blood pressure observed in several rodent models of preeclampsia (17, 18, 27, 28, 37). Moreover, recent experimental and clinical observations of hypertension in pregnancy and preeclampsia have shown exercise can mitigate hypertension and restore angiogenic balance and endothelial function (9, 17). Despite these recent findings, the exact mechanisms by which physical activity improves angiogenic balance and decreases blood pressure in pregnancy remain unclear (15, 17).
Recent studies have revealed some of the metabolic changes observed due to exercise training can be stimulated pharmacologically (22, 30). Of the several pharmacological models of exercise in recent literature, AMP-activated protein kinase (AMPK) has been reported as a major regulator of or contributor to several essential metabolic adaptations observed in exercise (4, 6, 12, 13, 24, 33). Moreover, the pharmacological activation of AMPK through 5-aminoimidazole-4-carboxamide-3-ribonucleoside (AICAR) (22, 30), an adenosine mimetic (8, 23), has been shown to decrease mean arterial pressure (MAP) in hypertensive rats (10). Furthermore, activation of the AMPK pathway has been shown to regulate increases in VEGF expression (39), but whether this occurs with AICAR administration remains unknown. Therefore, we hypothesized AICAR, a potent AMPK stimulator, would stimulate the bioavailability of VEGF in circulation, improved endothelial function, and, most importantly, abrogate the placental ischemia-induced hypertension.
MATERIALS AND METHODS
Animals.
Studies were performed in timed-pregnant Sprague-Dawley rats purchased from Charles River (Wilmington, MA) or Harlan (Indianapolis, IN). Animals were housed in a temperature-controlled room (23°C) with a 12-H:12-H light/dark cycle. All experimental procedures executed were completed in accordance with National Institutes of Health guidelines for use and care of animals and were approved by the Institutional Animal Care and Use Committee at both the University of Minnesota and the University of Oregon. Dams were assigned to normal pregnancy (NP; n = 8) and reduced uteroplacental perfusion pressure (RUPP; n = 8) groups with and without AICAR (Santa Cruz Biotechnology, Santa Cruz, CA) treatment [NP + A (n = 6); RUPP + A (n = 8)]. AICAR treatment was introduced by intraperitoneal injection at 50 mg/kg twice a day (4, 10, 30) mixed in 0.9% sterile saline solution, and controls were treated with a 0.9% sterile saline solution vehicle. Half of the in vivo studies for each group were completed at each institution.
RUPP procedure.
The RUPP procedure is a robust model for studying the link between placental ischemia and hypertension in the pregnant rat and has been described in detail previously (3, 17). In brief, silver clips were placed on the lower abdominal aorta (0.203-mm inner diameter) above the iliac bifurcation and also on branches (0.100 mm inner diameter) of both the right and left ovarian arteries supplying the uterus on day 14 of gestation (term = 21 days). Four NP dams underwent a sham surgery, which included the midline incision and suture. After no differences in the angiogenic factors and blood pressures were observed, these animals were grouped with the NP rats.
Measurement of MAP in chronically instrumented conscious rats.
Animals were instrumented on day 17 of gestation with an indwelling catheter, and arterial pressure was determined in both groups of rats on day 19 of gestation as described previously (17). Briefly, on day 17, V-3 tubing (SCI) catheters were introduced to the carotid artery while the animal was under isoflurane anesthesia. Catheters were exteriorized through the back of the neck after subcutaneous tunneling. On day 19, animals were placed in restraining cages, and direct pressures were monitored using a blood pressure transducer (ADInstruments) for 1 h after a 30-min stabilization period (16). MAP was averaged over the hour time period. Additionally, heart rate was analyzed over the hour period from the arterial pressure measurements using LabChart 7.0.
Conceptus measurements and serum collection.
After the measurement of MAP, the dams were placed under isoflurane anesthesia and a midline ventral incision was made to isolate the abdominal aorta for plasma and serum collection as reported previously (3, 17). Blood was collected for subsequent assays into Corvac sterile serum separator tubes (Sherwood Davis, St. Louis, MO). Blood and amniotic fluid glucose concentrations were measured as reported previously (21). Fetal weight, placental weight, and number of resorptions were recorded in the manner described previously (3, 17). All collected tissues for subsequent analysis were flash frozen in liquid nitrogen and stored at −80°C.
Enzyme-linked immunosorbant assays.
Plasma concentrations of free VEGF and sFlt-1 were measured using commercial enzyme linked immunosorbant assay kits (R&D Systems, Quantikine; Minneapolis, MN) according to the manufacturer's directions as described previously (3, 17).
Oxidative stress assays.
Renal and placental oxidative stress were assessed by measuring total antioxidant capacity and catalase activity, carried out as we have previously reported (21). Total antioxidant capacity was assessed in renal and placental tissue by measuring Trolox-equivalent antioxidant capacity (TEAC) assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's directions. In addition, catalase activity was measured in renal and placental tissue using a commercial assay kit (Cayman Chemical) according to the manufacturer's directions. All values were normalized to total protein concentration in tissue lysate.
Protein extraction and quantitation.
As described previously (15, 17), total soluble protein was extracted from whole placentas and gastrocnemius in radioimmunoprecipitation assay lysis buffer containing PMSF in DMSO, sodium orthovanadate, and a protease inhibitor cocktail (Santa Cruz Biotechnology). Total soluble cellular protein concentration was determined using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL). Renal tissue position (right or left) was chosen at random, and placental samples were carefully selected for middle position on either side of the uterine horns (15, 17).
Western blot.
Western blotting was carried out as previously reported (2, 15). Protein (50 μg) was separated by electrophoresis on 4–20% SDS polyacrylamide separating gels (Life Technologies, Grand Island, NY) and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and Ponceau stained to assess the transfer across each gel. The images of the Ponceau-stained membranes were digitized with a flatbed scanner.
The membranes were then incubated 1 h in casein blocking solution (Bio-Rad). Membranes were incubated in blocking solution containing commercially available antibodies overnight at 4°C [anti-phospho(Thr172)-AMPKα (40H9), 0.1 μg/ml; Anti-AMPKα (23A3), 0.1 μg/ml; Cell Signaling Technology]. Membranes were washed and incubated 1 h with the appropriate horseradish peroxidase-conjugated secondary antibodies (0.01 μg/ml; Cell Signaling Technology, Danvers, MA) and incubated in chemiluminescent substrate (West-Femto; Pierce, Grand Island, NY). The immunoreactive bands were digitized using an Alpha-Innotech digital imaging system. All digitized images were quantified using Un-Scan-It gel 6.1 software (Silk Scientific, Orem, UT). Specificity of primary antibodies (negative controls) was evaluated by imaging membranes with the primary antibody omitted.
Endothelial tube formation assay.
Angiogenic balance was further assessed in the serum of pregnant rats in vitro as previously reported (17) in two separate experiments, and each was performed in duplicate. First, the sera collected from the treatment groups (NP, RUPP, NP +A, RUPP + A) were introduced to human umbilical vascular endothelial cell (HUVEC) solution (plated at 5 × 105 cells/ml) and monitored for 8 h for tubule formation. In addition, the direct effect of AICAR was also assessed by treating cells with serum from NP and RUPP and adding 20 μM AICAR directly to the media. Total number of tubule formations per frame was assessed at 40× optical zoom with a digital inverted compound microscope and ImageJ analysis software (National Institutes of Health, Bethesda, MD). Total tube count was assessed by at least two individual investigators that were blinded to the identity of the experimental groups. Values from each observer were averaged to obtain final counts.
Statistical analysis and calculations.
All data are presented as means ± SE, and statistical significance was accepted when P < 0.05. Data that were not normally distributed (e.g., sFlt-1) were square root transformed before statistical analysis. Comparisons between the groups were made with a two-way between-subjects analysis of variance, and Newman-Keuls post hoc tests were used with groups consisting of NP or RUPP ± AICAR. Statistical calculations were made with GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Effect of AICAR on blood pressure and heart rate.
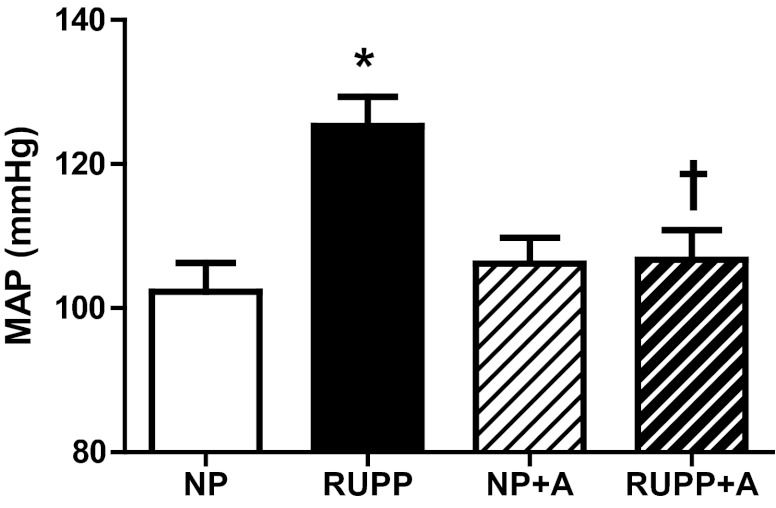
RUPP-induced hypertension was ameliorated with AICAR treatment (P < 0.05) (Fig. 1), and AICAR had no effect on blood pressure in NP rats. There were no differences in resting heart rate observed between or within treatment groups (NP, 394 ± 18; RUPP, 437 ± 22; NP + A, 451 ± 10; RUPP + A, 441 ± 19 beats/min).
Fig. 1.
5-Aminoimidazole-4-carboxamide-3-ribonucleoside (AICAR) mitigates increase in blood pressure in reduced uterine perfusion pressure (RUPP) rats. The RUPP-induced increase in blood pressure was ameliorated with AICAR (50 mg/kg twice a day) treatment. *P < 0.05, different from NP; †P < 0.05, different from RUPP. Data presented as means ± SE; n = 8 RUPP, 8 normal pregnant (NP), 6 NP + AICAR (A), and 8 RUPP + A. MAP, mean arterial pressure.
Effects of AICAR on RUPP-induced angiogenic imbalance.
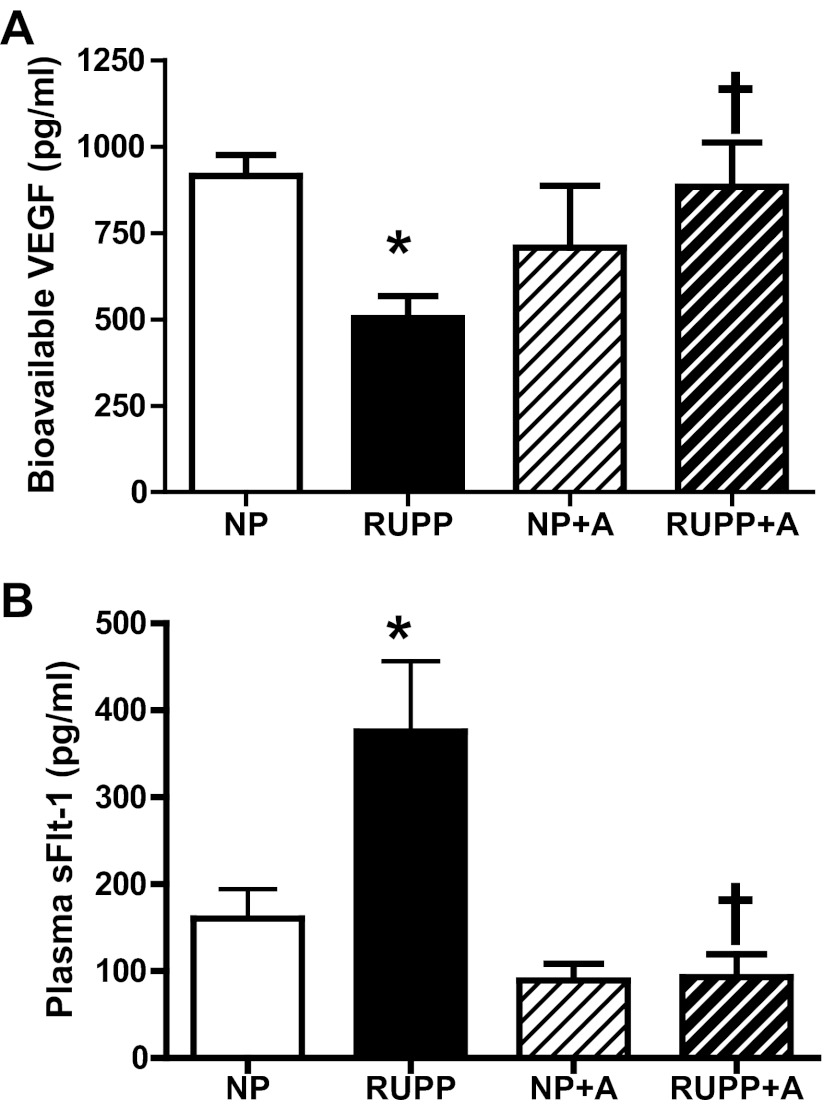
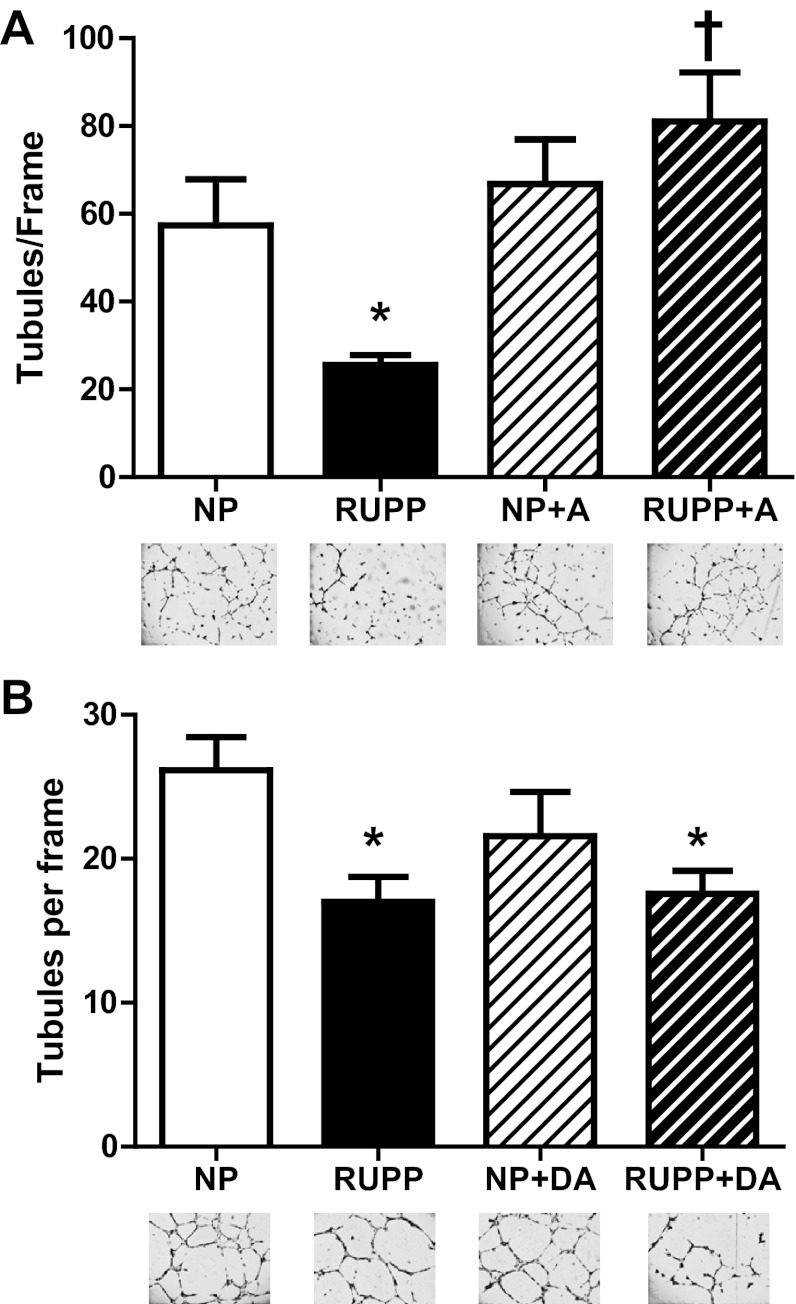
Free VEGF levels in maternal plasma were decreased (P < 0.05) in RUPP versus NP, and AICAR increased (P < 0.05) VEGF in the RUPP compared with RUPP untreated. Plasma sFlt-1 levels were increased (P < 0.05) in RUPP versus NP, and RUPP + AICAR circulating sFlt-1 was decreased (P < 0.05) compared with RUPP (Fig. 2, A and B). Moreover, angiogenic potential was increased (P < 0.05) with AICAR treatment in the RUPP compared with the untreated RUPP (Fig. 3A). Also, there was no difference observed between the NP and NP + A. Additionally, AICAR did not have a direct effect on HUVEC tubule formation in either NP or RUPP sera-treated cells (Fig. 3B).
Fig. 2.
Chronic AICAR administration restores angiogenic balance in RUPP rats. A: free VEGF levels in maternal plasma were restored to NP levels in the RUPP treated with AICAR (50 mg/kg twice a day) and significantly increased (P < 0.05) compared with RUPP controls. B: plasma soluble VEGF receptor-1 (sFlt-1) levels were restored back to NP levels in the RUPP treated with AICAR and significantly decreased (P < 0.05) compared with RUPP controls. *P < 0.05, different from NP; †P < 0.05, different from RUPP. Data presented as means ± SE; n = 8 RUPP, 8 NP, 6 NP + A, and 8 RUPP + A.
Fig. 3.
Angiogenic potential restored by chronic AICAR administration in RUPP rats. A: angiogenic potential was increased (P < 0.05) with chronic AICAR (50 mg/kg twice a day) treatment in the RUPP compared with the untreated RUPP. No statistical difference was observed between NP, NP + A, and RUPP + A. B: direct AICAR (DA) treatment onto human umbilical vascular endothelial cells treated with NP or RUPP serum had no effect on angiogenic potential. *P < 0.05, different from NP; †P < 0.05, different from RUPP. Data presented as means ± SE; n = 8 RUPP, 8 NP, 6 NP + A, and 8 RUPP + A.
Measurement of oxidative stress.
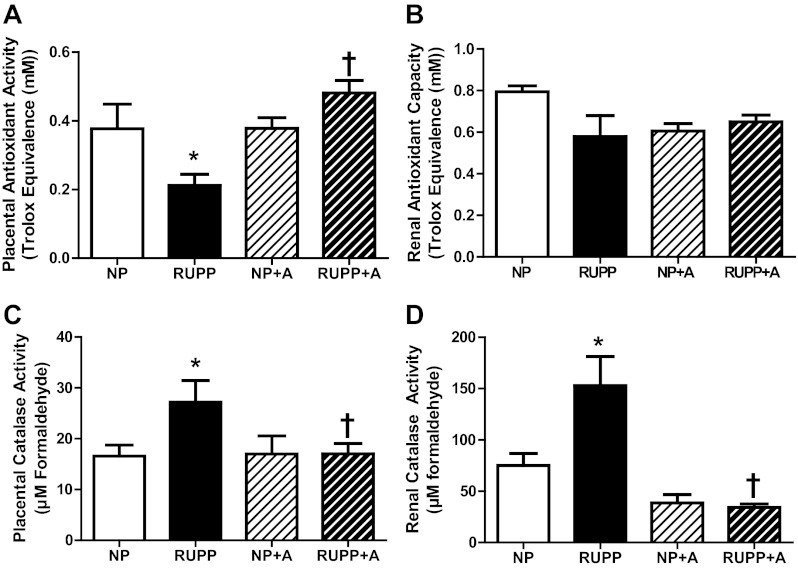
Placental TEAC levels were decreased in the RUPP compared with the NP (P < 0.05), and AICAR treatment obviated the decrease in the RUPP + A (Fig. 4A). No effect on TEAC in the kidney was observed (Fig. 4B). Additionally, placental (Fig. 4C) and renal (Fig. 4D) catalase activity (measured by formaldehyde production) was increased by RUPP and decreased (P < 0.05) in RUPP + A with respect to the RUPP group.
Fig. 4.
Markers of oxidative stress in the RUPP rat were reduced by chronic AICAR administration. A: trolox-equivalent antioxidant capacity (TEAC) levels were decreased in the RUPP placenta compared with the NP (P < 0.05) and AICAR (50 mg/kg twice a day) treatment obviated the decrease in the RUPP + A. B: renal TEAC levels were unaffected by AICAR treatment. C: placental catalase activity (measured by formaldehyde production) was increased (P < 0.05) in the RUPP vs. NP and decreased (P < 0.05) in RUPP + A with respect to RUPP. D: renal catalase activity was increased (P < 0.05) in the RUPP vs. NP and decreased (P < 0.05) in RUPP + A with respect to RUPP. *P < 0.05, different from NP; †P < 0.05, different from RUPP. Data presented as means ± SE; n = 8 RUPP, 8 NP, 6 NP + A, and 8 RUPP + A.
Maternal and fetal morphometric and metabolic data at necropsy.
Maternal weights and fetal weights were decreased (P < 0.05) in the RUPP compared with NP, and AICAR had no significant effect. Placental weight was decreased (P < 0.05) in RUPP versus NP, and AICAR mitigated this decrease. Total litter viability per cent was decreased (P < 0.05) in RUPP versus NP, and AICAR remediated the RUPP resorption. Finally, blood glucose and amniotic fluid glucose were not changed with AICAR treatment in either NP or RUPP. The previous data are summarized in Table 1.
Table 1.
Maternal and conceptus morphometric and metabolic data at necropsy
| Weight, g |
Glucose, mg/dL |
|||||
|---|---|---|---|---|---|---|
| Treatments | Maternal | Fetal | Placental | % Viability | Maternal Blood | Amniotic Fluid |
| NP | 337.8 ± 4.5 | 2.27 ± 0.04 | 0.48 ± 0.01 | 95.6 ± 1.9 | 82.6 ± 7.4 | 137.4 ± 15.1 |
| RUPP | 294.5 ± 10.8* | 2.05 ± 0.06* | 0.42 ± 0.02* | 47.9 ± 8.5* | 101.7 ± 7.7 | 129.8 ± 13.6 |
| NP + A | 342.3 ± 7.7 | 2.34 ± 0.05 | 0.50 ± 0.03 | 98.8 ± 1.2 | 81.0 ± 13.8 | 166.7 ± 33.5 |
| RUPP + A | 305.0 ± 4.7* | 2.08 ± 0.06* | 0.46 ± 0.02† | 69.8 ± 11.1† | 79.0 ± 9.2 | 99.7 ± 31.7 |
Values are means ± SE. NP, normal pregnant; RUPP, reduced uterine perfusion pressure; A, AICAR (5-aminoimidazole-4-carboxamide-3-ribonucleoside).
P < 0.05, different from NP;
P < 0.05, different from RUPP.
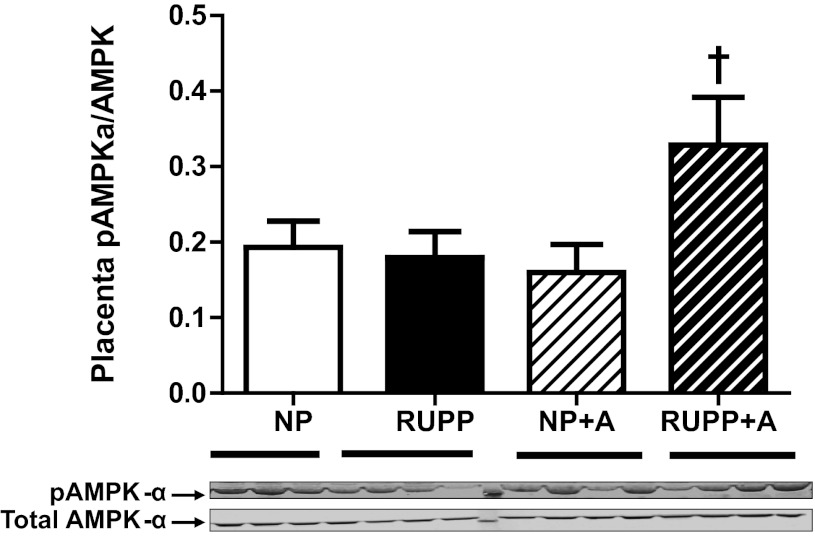
Tissue AMPK activation.
The ratio of phosphorylated AMPK-α (pAMPKα) to total AMPK-α was increased with AICAR treatment in RUPP placenta (P < 0.05) compared with untreated RUPP, NP, and NP + AICAR animals (Fig. 5). Additionally, the ratio of phosphorylated AMPK-α to total AMPK-α in skeletal muscle was not increased in either NP or RUPP treated with AICAR, respective to their untreated controls (NP, 0.20 ± 0.01; RUPP, 0.19 ± 0.01; NP + A, 0.20 ± 0.01; RUPP + A, 0.21 ± 0.01).
Fig. 5.
AICAR administration increased placental tissue AMP-activated protein kinase (AMPK) phosphorylation in RUPP. The ratio of phosphorylated AMPK-α (pAMPK-α) to total AMPK-α was increased with AICAR (50 mg/kg twice a day) treatment in RUPP placenta (P < 0.05) compared with untreated RUPP animals. †P < 0.05, different from RUPP. Data presented as means ± SE; n = 8 RUPP, 8 NP, 6 NP + A, and 8 RUPP + A.
DISCUSSION
The present study is the first to investigate the treatment of placental ischemia-induced hypertension with AICAR, which has revealed novel evidence of restoration of angiogenic balance and abrogation of hypertension in an experimental model of hypertension in preeclampsia. Foremost, the hypertension observed in our RUPP model was abrogated with intraperitoneal 50 mg/kg b.i.d. AICAR treatment. Second, maternal angiogenic balance was restored in the RUPP after AICAR treatment, by both increasing the circulating free VEGF and decreasing circulating sFlt-1. These observations were further supported by the increased microtubule formation in HUVECs treated with serum from the RUPP + AICAR versus RUPP, which indicates an increased angiogenic potential and suggests an increased endothelial function with AICAR in the RUPP. Next, the RUPP-associated increase in oxidative stress markers was attenuated in both placenta and kidney when treated with AICAR. When viewed in concert, these findings suggest AICAR treatment may have several beneficial effects on the hypertensive phenotype of a model of placental ischemia-induced hypertension.
Administration of an adenosine mimetics similar to AICAR, such as metformin, have long been recognized to improve cardiovascular function and lower blood pressure in nonpregnant hypertensives (20, 36), but the mechanisms remain uncharacterized (1, 19, 36, 38). Furthermore, recent studies have shown AICAR can directly improve endothelial function and lower blood pressure in rodent models of hypertension and improved conduit vessel function (10, 11). Therefore, our observation that AICAR treatment lowers blood pressure is in agreement with previous reports in other models of hypertension (4, 6, 10), and we are the first to report these effects in any model of hypertension during pregnancy. It has been noted that the blood pressure effects observed in this model could potentially be directly from AICAR interaction on the vascular endothelial cells, since endothelial-cell-mediated vasorelaxation to AICAR has been shown to be dependent on both nitric oxide and endothelium-derived hyperpolarizing factor production (11). However, the vasodilatory properties observed by Ford and Rush (11) ex vivo are transient, and the mechanisms mediating chronic effects of AICAR remain unclear (6). In the present study blood pressure was recorded more than 12 h after the final intraperitoneal injection of AICAR, suggesting the maternal cardiovascular effects of AICAR in the present study were due to chronic signaling changes such as restoration of angiogenic balance rather than previously reported acute mechanisms (11). In particular, our present data suggest the effects of AICAR administration may be due to chronic changes in factors such as circulating free VEGF and sFlt-1 concentrations that in turn can influence cardiovascular and renal function, rather than direct, acute vasodilatory effects of AICAR administration. These observations are further supported by our tube formation data, which show chronic in vivo treatment alters circulating factors and increases tubule formation while acute treatment with AICAR in the media has no effect. This also suggests that cells other than endothelial cells may be responsible for the changes that promote tube formation. Further studies are underway to identify which cells respond to AICAR in a manner consistent with pro-angiogenic function.
It has been well established that AICAR can mimic many of the metabolic effects observed in moderate physical activity, such as mitochondrial biogenesis (12, 13, 24), decreased lipogenesis (30), increased glucose tolerance (4, 6, 24, 30, 33, 34), and AMPK stimulation (11, 26, 30, 33). Moreover, our laboratory has recently reported exercise training before and during gestation has several beneficial effects on maternal blood pressure and angiogenic balance (17). Although the present study reports that AICAR administration appears to have similar cardiovascular and angiogenic balance effects as exercise in RUPP animals (15, 17), an interesting difference is AICAR did not directly stimulate the production of VEGF in the NP rats (17). When compared with a recent study of adenosine administration and hypoxia-dependent mechanisms in placental explants (14), the current study also suggests these effects on the angiogenic factors may be ischemia or hypoxia dependent. In addition, this study also suggests gestational AICAR treatment may hold promise as a therapeutic independent of its use as a mimetic or model of exercise. Although these findings are intriguing, further studies are required to elucidate the underlying mechanisms by which AICAR lowers blood pressure and restores angiogenic balance.
The effects of AICAR on angiogenic balance present an interesting story since AICAR administration appears to regulate both VEGF and sFlt-1. Indeed, our initial hypothesis only considered effects on VEGF via AMPK activation, since the regulation of sFlt-1 via AMPK is unclear at this point. Also, it is important to note the changes following AICAR administration on the angiogenic balance and blood pressure were only observed in the RUPP, and not NP. This further suggests a possible mechanism that is mediated by the placental ischemia and/or hypoxia. Interestingly, AICAR has been used to improve ischemia-reperfusion injury in cardiac tissue during coronary bypass surgery (7), which, unsurprisingly, may have similar effects in other tissues such as the placenta. Moreover, these specific placental ischemia-dependent mechanisms have been proposed before in in vitro models (14), but we are the first to report this effect of adenosine-mimetic administration in a model of placental ischemia-induced hypertension. These observations are further supported by our placental tissue analysis of AMPK phosphorylation, in which AICAR-induced increases in phosphorylation of AMPK were dependent on the placental ischemia induced by the RUPP procedure and not observed in the NP placenta. Taken together with the MAP and angiogenic balance data, AICAR has no measured effect on the NP dams in this study. We also observed that chronic AICAR did not stimulate AMPK phosphorylation in skeletal muscle in either NP or RUPP groups. Although these findings are interesting, further studies are required to determine whether this is a tissue or pregnancy-specific observation or if the molecular changes are dependent on hypoxia or chronic ischemia.
In addition to the effects on the angiogenic balance, RUPP-associated renal and placental markers of oxidative stress were reduced with AICAR treatment. Previous work has shown that oxidative stress plays an important role in preeclampsia and RUPP hypertension (25, 31), and our present observations suggest that the effects of AICAR may be mediated by a combination of the inhibition of the formation of reactive oxygen species and the stimulation of antioxidant molecules. Similar to our observations with angiogenic balance this effect was only observed in the RUPP groups following AICAR treatment, suggesting a dependence on the presence of placental-ischemia. Although the attenuation of these oxidative stress markers in the RUPP + AICAR coincide with the stimulation of bioavailable VEGF and a concomitant production or maintenance of downstream antioxidative molecules, whether the observed effects are dependent on the VEGF remains unclear. Further studies in direct and indirect antioxidant properties of AICAR are required to identify the exact pathways underlying the present observations.
Although we feel the present results are exciting, it is important to recognize they are not without limitations. Although our current hypothesis focused on the previously reported links between purinergic signaling and VEGF, other angiogenic factors important in preeclampsia such as sEng (5, 37) may also be affected by AICAR, and further studies are planned to evaluate these possibilities. Moreover, this study does not specifically address the exact role of AMPK signaling, and additional studies are planned to evaluate the exact role of that pathway in angiogenic balance. Finally, the source of the changes in VEGF and sFlt-1 in the present study remain unknown, so further studies are required to isolate the source. Nevertheless, studies are planned to further investigate the role of AMPK on the effects of AICAR in vivo. Further studies are currently aimed at the effects of AICAR specific to pregnancy, the cell-type-specific (e.g., placental, vascular endothelial cell, skeletal muscle) effects, and the source of and contribution to VEGF and sFlt-1 control.
In conclusion, the effects of AICAR reveal interesting data on the role of AMPK activation on angiogenic balance and normotension restoration. Also presented are encouraging therapeutic potential for placental ischemia-induced hypertension, by restoring angiogenic balance, abrogating RUPP-induced hypertension, and having no reportable deleterious effects on the fetal-placental unit. Although these results are promising, further pharmacological studies are required to elucidate the exact mechanisms by which AICAR lowers blood pressure and restores angiogenic balance.
GRANTS
This work was supported in part by American Heart Association Grant 10SDG2600040 (to J. S. Gilbert) and the National Heart, Lung, and Blood Institute Grant HL-114096 (to J. S. Gilbert) and HD057332 (to H. C. Dreyer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.T.B., A.J.B., K.M.N., and J.S.G. performed experiments; C.T.B., A.J.B., K.M.N., and J.S.G. analyzed data; C.T.B., A.J.B., H.C.D., and J.S.G. interpreted results of experiments; C.T.B. and J.S.G. prepared figures; C.T.B. drafted manuscript; C.T.B., A.J.B., K.M.N., H.C.D., and J.S.G. edited and revised manuscript; C.T.B., H.C.D., and J.S.G. approved final version of manuscript; J.S.G. conception and design of research.
ACKNOWLEDGMENTS
We thank Susan Capoccia and Haley Gillham for technical assistance in this study.
REFERENCES
- 1. Agarwal N, Rice SPL, Bolusani H, Luzio SD, Dunseath G, Ludgate M, Rees DA. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab 95: 722–730, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Bailey AN, Hocker AD, Vermillion BR, Smolkowski K, Shah SN, Jewett BA, Dreyer HC. MAFbx, MuRF1, and the stress-activated protein kinases are upregulated in muscle cells during total knee arthroplasty. Am J Physiol Regul Integr Comp Physiol 303: R376–R386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banek CT, Bauer AJ, Gingery A, Gilbert JS. Timing of ischemic insult alters fetal growth trajectory, maternal angiogenic balance and markers of renal oxidative stress in the pregnant rat. Am J Physiol Regul Integr Comp Physiol 303: R658–R664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosselaar M, Smits P, van Loon LJC, Tack CJ. Intravenous AICAR during hyperinsulinemia induces systemic hemodynamic changes but has no local metabolic effect. J Clin Pharmacol 51: 1449–1458, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble Fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22: 564–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes 51: 2199–2206, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Dixon R, Gourzis J, McDermott D, Fujitaki J, Dewland P, Gruber H. AICA-riboside: safety, tolerance, and pharmacokinetics of a novel adenosine-regulating agent. J Clin Pharmacol 31: 342–347, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Falcao S, Bisotto S, Michel C, Lacasse AA, Vaillancourt C, Gutkowska J, Lavoie JL. Exercise training can attenuate preeclampsia-like features in an animal model. J Hypertens 28: 1057–1062, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Ford RJ, Teschke SR, Reid EB, Durham KK, Kroetsch JT, Rush JW. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens 30: 725–733, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Ford RJ, Rush JWE. Endothelium-dependent vasorelaxation to the AMPK activator AICAR is enhanced in aorta from hypertensive rats and is NO and EDCF dependent. Am J Physiol Heart Circ Physiol 300: H64–H75, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Frier BC, Hancock CR, Little JP, Fillmore N, Bliss TA, Thomson DM, Wan Z, Wright DC. Reductions in RIP140 are not required for exercise- and AICAR-mediated increases in skeletal muscle mitochondrial content. J Appl Physiol 111: 688–695, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Frier BC, Wan Z, Williams DB, Stefanson AL, Wright DC. Epinephrine and AICAR-induced PGC-1α mRNA expression is intact in skeletal muscle from rats fed a high-fat diet. Am J Physiol Cell Physiol 302: C1772–C1779, 2012 [DOI] [PubMed] [Google Scholar]
- 14. George EM, Cockrell K, Adair TH, Granger JP. Regulation of sFlt-1 and VEGF secretion by adenosine under hypoxic conditions in rat placental villous explants. Am J Physiol Regul Integr Comp Physiol 299: R1629–R1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 303: R520–R526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble Fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham KM. Exercise training attenuates placental ischemia induced hypertension and angiogenic imbalance in the rat. Hypertension 60: 1545–1551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension 55: 380–385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giugliano D, De RN, Di MG, Marfella R, Acampora R, Buoninconti R, D'Onofrio F. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care 16: 1387–1390, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Glueck CJ, Goldenberg N, Pranikoff J, Loftspring M, Sieve L, Wang P. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum Reprod 19: 1323–1330, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Heltemes A, Gingery A, Soldner EL, Bozadjieva N, Jahr KN, Johnson BK, Gilbert JS. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Exp Biol Med (Maywood) 235: 892–899, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol 94: 1373–1379, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Karagounis LG, Hawley JA. The 5′ adenosine monophosphate-activated protein kinase: regulating the ebb and flow of cellular energetics. Int J Biochem Cell Biol 41: 2360–2363, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Lemieux K, Konrad D, Klip A, Marette A. The AMP-activated protein kinase activator AICAR does not induce GLUT4 translocation to transverse tubules but stimulates glucose uptake and p38 mitogen-activated protein kinases alpha and beta in skeletal muscle. FASEB J 17: 1658–1665, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez-Martin N, Blas-Garcia A, Morales JM, Marti-Cabrera M, Monleon D, Apostolova N. Metabolomics of the effect of AMPK activation by AICAR on human umbilical vein endothelial cells. Int J Mol Med 29: 88–94, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 57: 1R–7R, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Ohkuchi A, Hirashima C, Matsubara S, Takahashi K, Matsuda Y, Suzuki M. Threshold of soluble fms-like tyrosine kinase 1/placental growth factor ratio for the imminent onset of preeclampsia. Hypertension 58: 859–866, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54: 928–934, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Poston L, Igosheva N, Mistry HD, Seed PT, Shennan AH, Rana S, Karumanchi SA, Chappell LC. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutr 94: 1980S–1985S, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Russell RR, III, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol Heart Circ Physiol 277: H643–H649, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Salt IP, Connell JM, Gould GW. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3–L1 adipocytes. Diabetes 49: 1649–1656, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365: 785–799, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 43: 647–654, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D′Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Wang XF, Zhang JY, Li L, Zhao XY, Tao HL, Zhang L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol 38: 94–101, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol 586: 6021–6035, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]