Abstract
Endothelial cells in a cultured monolayer change from a “cobblestone” configuration when grown under static conditions to a more elongated shape, aligned with the direction of flow, after exposure to sustained uniform shear stress. Sustained blood flow acts to protect regions of large arteries from injury. We tested the hypothesis that the stable permeability state of individually perfused microvessels is also characteristic of flow conditioning. In individually perfused rat mesenteric venular microvessels, microvascular permeability, measured as hydraulic conductivity (Lp), was stable [mean 1.0 × 10−7 cm/(s × cmH2O)] and independent of shear stress (3–14 dyn/cm2) for up to 3 h. Vessels perfused opposite to the direction of normal blood flow exhibited a delayed Lp increase [ΔLp was 7.6 × 10−7 cm/(s × cmH2O)], but the increase was independent of wall shear stress. Addition of chondroitin sulfate and hyaluronic acid to perfusates increased the shear stress range, but did not modify the asymmetry in response to flow direction. Increased Lp in reverse-perfused vessels was associated with numerous discontinuities of VE-cadherin and occludin, while both proteins were continuous around the periphery of forward-perfused vessels. The results are not consistent with a general mechanism for graded shear-dependent permeability increase, but they are consistent with the idea that a stable Lp under normal flow contributes to prevention of edema formation and also enables physiological regulation of shear-dependent small solute permeabilities (e.g., glucose). The responses during reverse flow are consistent with reports that disturbed flows result in a less stable endothelial barrier in venular microvessels.
Keywords: vascular permeability, shear stress, endothelium
it is recognized that endothelial cells in a cultured monolayer change from a “cobblestone” configuration when grown under static conditions to a more elongated shape, aligned with the direction of flow after exposure to sustained uniform shear stress (10, 12, 36). The endothelial cells also express a phenotype less responsive to inflammatory stimuli and injury after this flow conditioning. There is growing understanding of the action of such sustained uniform blood flow on arterial endothelium to protect regions of large arteries from injury, as well as the corresponding action of disturbed flow (unsteady or locally reversed flow) to predispose such regions to atherosclerosis. Initiation of shear stress has been shown to induce an increased permeability of endothelial monolayers (36). The hypothesis we tested in the present investigations was that the stable permeability state of individually perfused microvessels was also characteristic of a flow-conditioned state. We and others have demonstrated stable permeability in rat and frog mesentery microvessels, measured as a constant permeability to water (hydraulic conductivity, Lp), over a range of perfusion conditions (increased shear stress and perfusion pressure) (1, 28, 31).
To test the hypothesis we monitored microvessel Lp in rat mesentery using established microperfusion methods. In the standard method we perfused the vessel in the direction of blood flow that was seen in the microvessel prior to cannulation. In venular microvessels blood flow is usually toward the larger collecting venules distal to capillary networks. Thus to test the response of vessels to alternate perfusion conditions we also chose microvessels that could be cannulated and perfused for up to 3 h in the direction opposite to normal blood flow. In some of these latter vessels we also punctured the microvessel wall near the arterial end of the microvessel, thereby reducing the microvessel pressure and allowing the vessel to be perfused over a wider range of flow rates than was possible when the pressure was high.
One mechanism that has been proposed to account for increased permeability after flow is imposed on an endothelial monolayer is change in cell-cell adhesion during realignment of all or part of the endothelial cells not previously aligned with the direction of imposed flow. After 1.5 h of exposure to flow, cultured endothelial cells, previously grown under static conditions, developed stress fibers (an indication of activation of the small GTPase Rho, and actin/myosin network modification), and the beginning of changes in cell orientation were seen by 3 h (15). At 5 h there was an extensive reorganization of the peripheral actin band and proteins such as ZO-1 that are part of the adhesion complexes between the peripheral actin band and intercellular adhesion molecules such as VE-cadherin and occludin (38). Rearrangements of cell-matrix adhesion have also been observed (10, 36). In endothelial cells in culture that have already been aligned with flow, changes in junction orientation relative to the direction of flow were observed when the flow direction was reversed. After the initial flow conditioning, regions of overlap between adjacent endothelial cells were aligned with the direction of flow and oriented at shallow angle to the direction of flow. Within 30 min of flow reversal, the regions of overlap of adjacent cells reversed orientation (26).
To evaluate the contribution of the above mechanisms we measured changes in permeability over periods from 10 min to 3.5 h and also examined the distribution of the junction proteins VE-cadherin and occludin in the microvessels in which permeability was directly measured during either forward or reverse flows. The endothelial glycocalyx is also thought to transduce shear stress to the endothelial cells. We therefore tested the effect of stabilizing the glycocalyx on the response to flow by adding chondroitin sulfate and hyaluronic acid to the perfusate (19, 32). All measurements of Lp were made with the Landis-Michel micro-occlusion method, a standard technique in our laboratory for investigations of the modulation of microvessel permeability. We recently demonstrated that the use of erythrocytes as markers of transvascular fluid movement in this method ensures a constant supply of sphingosine-1-phosphate (S1P) at close to normal plasma levels in the perfusate (13). We and others have demonstrated that the presence of S1P is important to establish a stable low baseline permeability state in the microvasculature (9, 29, 37, 42). The stable state maintained by normal S1P levels can be further modified by inflammatory agents (2, 9, 29). Our investigations are the first to investigate flow-dependent changes in permeability under conditions of known S1P concentrations.
METHODS
Animal preparation.
Animal protocols (no. 16158) were approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Experiments were carried out on male rats (Sprague-Dawley, 350–450 g, aged 70–90 days, Hilltop Laboratory Animals) anesthetized with pentobarbital (100 mg/kg body wt sc) and maintained with additional pentobarbital (30 mg/kg sc) as determined using toe pinch reflex. At the end of experiments animals were euthanized with saturated KCl. Rats were placed on a tray with a heating pad to maintain normal body temperature. From a midline abdominal incision (2–3 cm) the mesentery was gently positioned over a cover glass or quartz pillar for microscopic observation. The mesentery was continuously suffused with Ringer's solution (35–37°C).
Hydraulic conductivity measurements.
In situ experiments were performed on straight nonbranched segments of venular microvessels typically of 25- to 35-μm diameter. Experimental vessels had brisk blood flow prior to cannulation and were generally free of leukocytes sticking or rolling. Once cannulated using a refillable micropipette (31) vessels were continuously perfused with Ringer's solution additionally containing bovine serum albumin (10 mg/ml). Because early experiments showed that the increase in Lp occurred after 60–90 min and that perfusions for longer than 3.5 h were difficult to maintain, we chose to analyze vessels perfused for between 2 and 3.5 h. Lp was measured every 15–20 min to characterize vessel wall permeability using the modified Landis technique, which measures the volume flux of water crossing the wall of a microvessel following downstream occlusion of the vessel by following movement of washed red blood cells added to the perfusates (1.3% hematocrit) as flow markers and using established corrections to determine mean fluid velocity from flow marker centerline movement. Assumptions and limitations of the methods have been described (4, 22, 27, 28). The initial transmicrovessel water flow per unit area of the microvessel wall (Jv/S)0 was measured at perfusion pressures of 50–90 cmH2O. Microvessel Lp was calculated as the slope of the relation between (Jv/S)0 and applied hydraulic pressure. Lp was estimated from each occlusion with the assumption that the net effective pressure determining fluid flow was equal to the applied hydraulic pressure minus 3.6 cmH2O, the approximate oncotic pressure contributed by the bovine serum albumin (BSA) in all perfusates. For some of the microvessels that were cannulated and perfused in the direction opposite to normal blood flow we punctured the microvessel wall near the arterial end of the microvessel, thereby reducing the microvessel pressure and allowing the vessel to be perfused over a wider range of flow rates than was possible when the pressure was high. Prior to puncturing, an occluding rod was positioned on the vessel just distal to the site of puncture to prevent significant bleeding from the puncture site.
Solutions and reagents.
Mammalian Ringer's solution was composed (in mM) of 132 NaCl, 4.6 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5.0 NaHCO3, and 20 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and Na-HEPES. The ratio of acid-HEPES to Na-HEPES was adjusted to achieve pH 7.40–7.45. All perfusates were mammalian Ringer's solution additionally containing fatty acid-free BSA (Sigma A0281) at 10 mg/ml and test reagents or vehicle. Chondroitin sulfate (CS) A from bovine trachea (Sigma, C9819) and hyaluronic acid (HA) from rooster comb (Sigma, H5388) were added to perfusates as indicated in results.
Determination of wall shear stress.
At intervals (10–20 min) we recorded the free-flowing red blood cells (RBCs) on videotape using short camera exposure to monitor frame-to-frame movement of individual RBCs. Wall shear stress (τ, tau) was calculated from the velocity of free-flowing RBCs, vessel diameter, assumed cylindrical vessel geometry, assumed centerline flow of RBCs, and published values of viscosity of serum albumin solutions:
where η is viscosity; v̄, the mean fluid velocity, is equal to one-half the centerline RBC velocity; and rw is the vessel radius. The use of RBCs to track centerline flow has been discussed previously (28). Viscosity of Ringer's solution additionally containing BSA at 10 mg/ml was estimated to be 0.0079 (dyn × s)/cm2 (23, 35). The addition of CS and HA (each 0.2 mg/ml, same as in present experiments) to a physiological perfusion media was previously found to yield a viscosity of 0.024 (dyn × s)/cm2, approximately twice the value of Ringer's with BSA (32).
Confocal microscopy of immunolabeled mesenteries.
At the end of perfusion mesenteries were flooded with ice-cold fixative (1% freshly depolymerized paraformaldehyde in PBS, pH 7.2, 5 min). The tissues were labeled with primary antibodies against VE-cadherin (Santa Cruz Biotech, sc6458) and occludin (Zymed Laboratories, 71–1500), labeled with appropriate fluorescent secondary antibodies and then mounted for confocal microscopy. Tissues were mounted whole to retain the three-dimensional structure of the vessels and, when possible, to enable separate collection of either front (near to lens) or rear half of each vessel. From each vessel about six image stacks, typically composed of 10–15 images taken at 0.6-μm steps, were collected (Zeiss LSM510, 40 × 1.4-NA lens) with pinhole settings to achieve 1 μm optical section thickness. Stacks were projected onto a single plane for analysis. To quantitatively test for changes in protein distribution, images were randomized to blind the observer and scored for degree of discontinuity in both the occludin and VE-cadherin channels on a scale of 0 (no breaks), 1 (few breaks per field of 5–6 cells), 2 (1–2 per cell), 3 (>2 per cell), or 4 (severe changes, many or large breaks).
Analysis and statistics.
Because the distributions of Lp had significantly different variances, the nonparametric Mann-Whitney test was used for analysis of these data. Image data were also tested using Mann-Whitney test. The indicated statistical tests were performed assuming significance for probability levels <0.05.
RESULTS
Hydraulic conductivity measured in microvessels with forward and reverse flow.
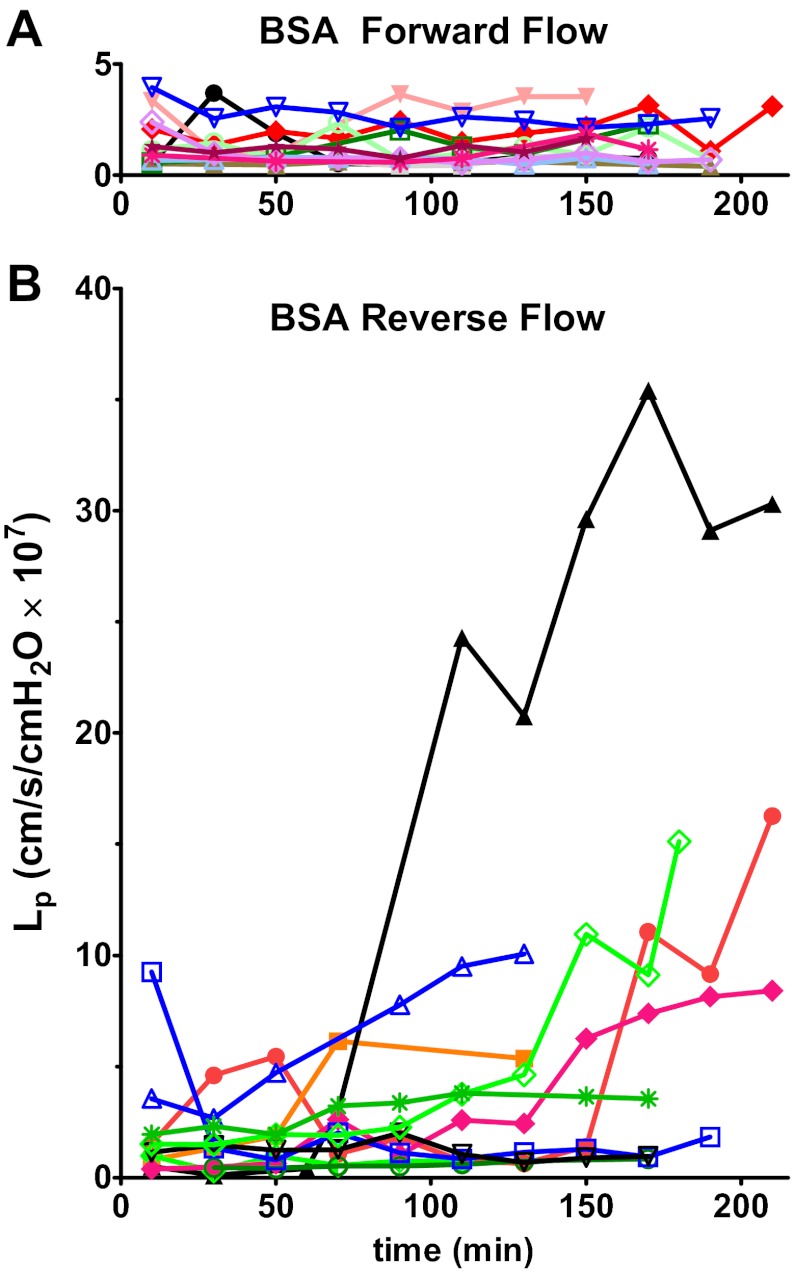
The key result is the striking asymmetry in the magnitude of the increase in vascular permeability measured as a change in Lp depending on the direction of flow. Figure 1 compares the change in Lp in vessels perfused in the direction of normal blood flow (forward; Fig. 1A, n = 12) with vessels perfused against the direction of normal flow (reverse; Fig. 1B, n = 11). The forward-perfusion group had stable Lp for up to 3 h with no significant change in Lp. Baseline Lp was 1.02 ± 0.19 × 10−7 cm/(s × cmH2O) and the change in Lp above baseline (ΔLp) was 0.74 ± 0.22 × 10−7 cm/(s × cmH2O) at 2.9 h (not significant). The reverse-perfusion group was variable. Six of 11 vessels had higher ΔLp than any forward-flow vessels; the Lp of the remaining five vessels in the reverse-flow group changed very little [range 0.3 to 1.3 × 10−7 cm/(s × cmH2O)]. The sustained increases in Lp developed gradually after about 90–120 min of perfusion. Some of reverse-perfusion vessels also had high Lp values early in the perfusion, while none in the forward-perfusion group had early high Lp. The average final increase above baseline (ΔLp) in the reverse-perfusion group [7.6 ± 2.7 × 10−7 cm/(s × cmH2O), n = 11, at t = 2.9 ± 0.2 h] was significantly higher than that of the forward-perfusion group (P < 0.05, Mann-Whitney).
Fig. 1.
Hydraulic conductivity (Lp) plotted over the duration of the experiment for each vessel perfused with BSA/Ringer solution. A: vessels perfused in forward direction have a stable Lp for up to 3 h (n = 12). B: reverse-flow group was more variable; several vessels showed a gradual increase in Lp starting at about 90–120 min of perfusion (n = 11). Individual vessels are identified by a symbol and color.
Wall shear stress was calculated for each vessel from intermittent measurements of marker RBC velocities during free flow. The average value of wall shear stress varied from 2 to 14 dyn/cm2 in different microvessels in the reverse-flow group and from 3 to 12 dyn/cm2 in forward flow. The final ΔLp for each vessel is plotted against the average wall shear stress for that vessel (Fig. 2A). For the forward-flow group ΔLp values were uniformly low across the range of wall shear stress. The reverse-flow group values were scattered and showed no clear relationship between final ΔLp and wall shear stress. Although flow was stopped briefly during the measurement of Lp (<20 s) Lp continued to increase after successive measurements indicating that brief interruptions to flow did not attenuate the permeability increase.
Fig. 2.
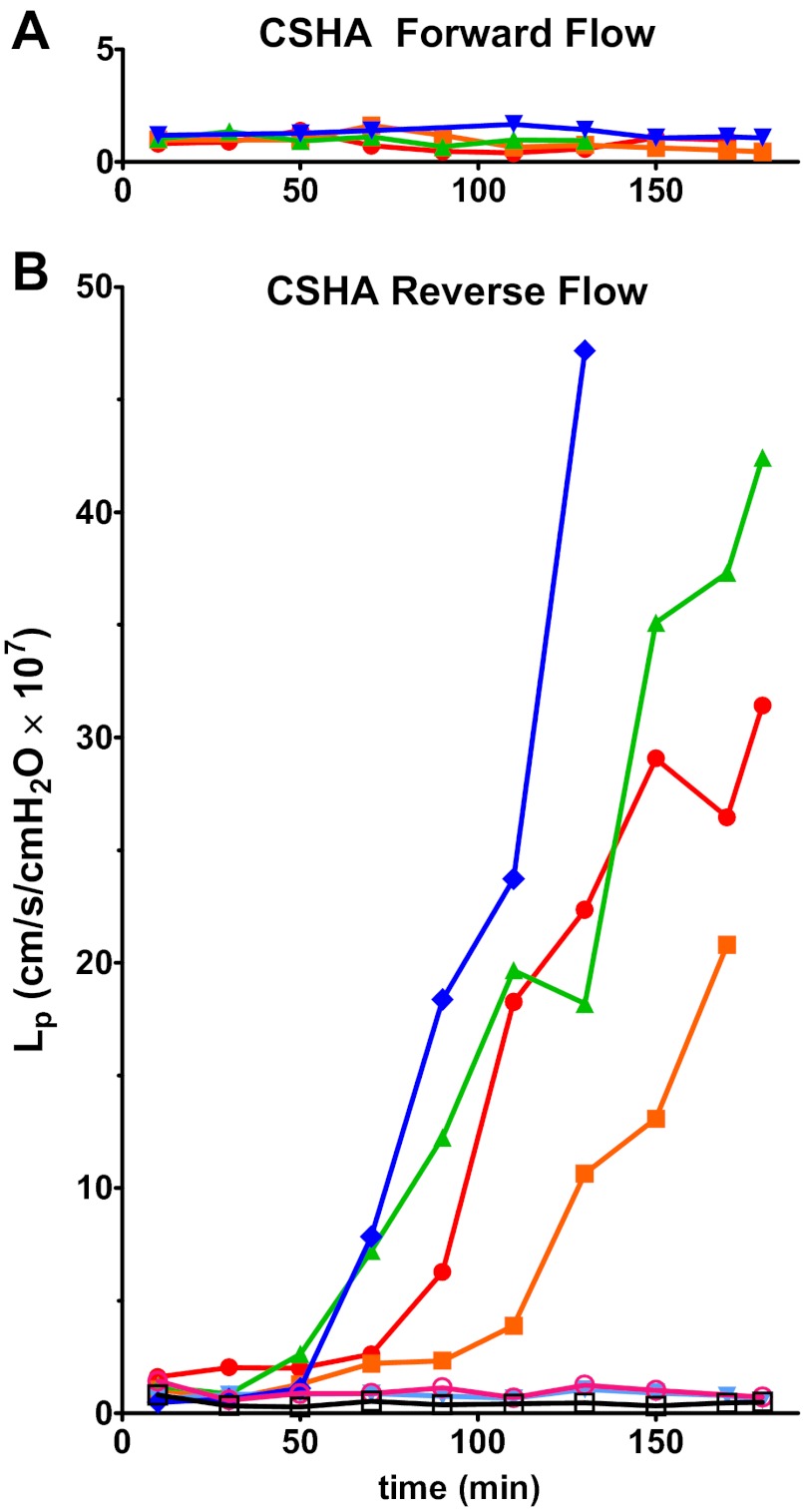
Final ΔLp relative to baseline Lp plotted as a function of the average wall shear stress, τ. A: vessels perfused with BSA solutions. None of the vessels in the forward-flow (open symbols) group had a ΔLp higher than 4. The reverse-flow (closed symbols) group was more variable with five vessels having ΔLp below 4 and six vessels with ΔLp above 4. There was no clear relationship with wall shear stress. B: vessels perfused with CSHA solutions. None of the vessels in the forward-flow group (open symbols) had ΔLp higher than 4. The reverse-flow group (closed symbols) was variable with three vessels having ΔLp below 4 and four vessels with ΔLp above 4. There was no clear relationship with wall shear stress. CS, chondroitin sulfate; HA, hyaluronic acid. See results for description of CSHA solution.
Vessels perfused with CS and HA.
A previous study suggested that addition of CS and HA to perfusion solutions augments the thickness of the hydrodynamically relevant glycocalyx layer of cultured endothelial cells by ∼0.2 μm (32). To test whether the lack of response in the forward direction was dependent on glycocalyx structure, the following series of experiments were performed. Vessels were perfused with solutions containing CS (0.2 mg/ml), HA (0.2 mg/ml), and BSA in Ringer's solution (referred to here as CSHA vessels). Vessels perfused in the forward-flow direction showed a low and normal Lp for up to 3 h (Fig. 3A). In 4 of 7 vessels perfused in the reverse-flow direction the Lp rose rapidly (Fig. 3B); ΔLp reached an average of 15.5 ± 7.8 × 10−7 cm/(s × cmH2O), n = 7, significantly greater than that of the forward-flow CSHA group, which did not increase [ΔLp equaled 0.1 ± 0.1 × 10−7 cm/(s × cmH2O), n = 4, P < 0.05]. Similar to the BSA perfusion group, there was no clear relationship between ΔLp and the mean shear stress (Fig. 2B). For the CSHA group, ΔLp in the reverse-flow perfusion was higher than that of the reverse-flow BSA group. Furthermore, for the microvessels that responded with increased Lp, the rate of rise of Lp in the CSHA reverse perfusion group [0.52 ± 0.18 × 10−7 cm/(s × cmH2O)/min] was higher than the BSA reverse-flow group [0.14 ± 0.06 × 10−7 cm/(s × cmH2O)/min]. When compared with the results for BSA solutions, these increases are likely to represent minimum estimates of the change in porosity of the microvessel wall because the viscosity of the fluid containing CSHA that crosses the microvessel wall in such high permeability states is at least 2 times larger than fluid with only BSA. The increased viscosity would tend to attenuate increases in Lp due to changes in the porosity of the microvessel wall. Detailed statistical comparison between BSA and CSHA groups is also compromised by the presence of nonresponding vessels in both groups, but after accounting for the attenuating action of viscosity there was a clear trend for both the rate of rise of Lp and ΔLp after 2 h to be greater in the responding vessels with CSHA compared with the BSA group. In summary, conditions suggested to improve the integrity of the glycocalyx do not modify the asymmetry in response to direction of flow, although there is a trend for the increase in Lp to be potentiated. Furthermore, there is no clear relation between increased Lp and applied shear stress under the conditions of our experiments. The changes in the barrier are associated with a change in the direction of flow but the magnitude of the shear stress applied to the endothelial barrier is not a significant determinant of increased permeability.
Fig. 3.
Lp plotted over time for vessels perfused with CSHA solutions. A: vessels perfused in forward direction have a stable Lp for up to 3 h (n = 5). B: reverse-flow group was variable; several vessels showed a rapid increase in Lp starting at about 60–90 min of perfusion (n = 7). Individual vessels are identified by a symbol and color.
Structural correlates of increased Lp.
To test for structural correlates of the increases in Lp, three vessels from each group were examined. The vessels chosen from the forward-flow group had no change in Lp [increase in Lp was 0.3 ± 0.1 × 10−7 cm/(s × cmH2O) over 2.8 ± 0.2 h] while the vessels in the reverse-flow group had a definite increase in Lp [11.5 ± 7.0 × 10−7 cm/(s × cmH2O) over 2.9 ± 0.3 h]. The vessels were labeled to reveal the distribution of junctional proteins VE-cadherin and occludin. In the forward-flow group both proteins were continuous around the periphery of endothelial cells. Images from the reverse-flow experiments showed numerous distinct discontinuities in both VE-cadherin and occludin distribution (Fig. 4). The discontinuities in the distributions of fluorescence were quantified by scoring individual images on a scale of 0 (no disruption) to 4 (severe disruption) as described in methods. Images from vessels perfused in the forward direction had a VE-cadherin discontinuity score (0.8 ± 0.2; n = 17 images from 3 vessels) that was significantly lower than that from vessels perfused in the reverse direction (2.6 ± 0.4; n = 18 images from 4 vessels; P < 0.01, Mann-Whitney). Similarly, the occludin discontinuity score for forward-flow (0.8 ± 0.2; n = 17 images from 3 vessels) was significantly less than that for reverse-flow (3.0 ± 0.2; n = 18 images from 4 vessels; P < 0.001, Mann-Whitney). For both VE-cadherin and occludin the low scores in the forward direction corresponded to the observation of very little disruption of the peripheral labeling, while the high scores for the reverse-flow condition reflected substantial discontinuity in the peripheral label.
Fig. 4.
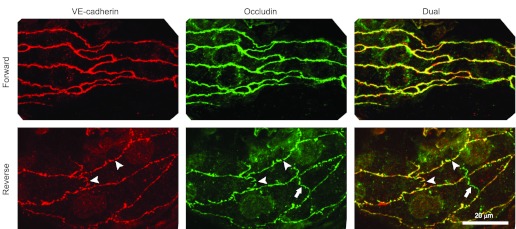
Representative segments of perfused microvessels labeled for VE-cadherin and occludin. The reverse-flow perfusions (bottom panels) showed numerous small gaps in both the occludin and VE-cadherin (arrowheads). Similar gaps were not seen in vessels perfused in the forward direction (top panels). A segment of tight junction occludin in the overlying mesothelium is visible (arrows) as a nonconnecting green shape. Scale bar, 20 μm for all images.
DISCUSSION
Our results conform to the hypothesis that continuous perfusion of microvessels in the direction of normal blood flow maintains permeability of microvessels in a stable, low-permeability state. Consistent with previous results in perfused microvessels with initial normal permeability coefficients, there is no tendency of perfusion over a range of shear stress to increase the hydraulic conductivity. However, perfusion in the direction opposite to blood flow to which the microvessel was conditioned by normal blood flow prior to cannulation induces an unstable state that results in large increases in permeability in 50% of the microvessels. Our results also show that flow conditioning, which is known to be a key determinant of arterial endothelial phenotype, is also a property of venular endothelium even though venular endothelial phenotypes differ in other key characteristics, including baseline permeability and response to inflammatory agents.
A key observation is that there is no graded increase in permeability with increased shear stress with flow in either direction. Rather, sustained increases in permeability occur after the mechanical forces from reverse-flow are present for more than 1 h. The increases in permeability are dependent on the change in direction of flow, not the magnitude of the imposed shear stress. For the CSHA solutions we assumed viscosity was approximately twice that of the Ringer BSA solutions. However, because the reverse- and forward-flow measurements were made under similar conditions, the exact value of viscosity (and therefore wall shear stress) is not important to the observation that reverse flow induced a large Lp increase in some vessels, while no such response was observed in forward-flow vessels. Similar to the Ringer/BSA group, there was no correlation between wall shear stress and degree of response in the CSHA group.
Comparison with other in vitro and in vivo results.
Our results are not consistent with the hypothesis that there is a graded increase in permeability to water and macromolecules with increasing shear stress such as that observed with acute increases in shear stress on endothelial layers grown under static conditions described in vitro (20, 33, 34). These increases are usually observed within tens of minutes of imposing flow and may reflect, in part, less stable adhesion of the cultured cells to the submatrix as well as weaker cell-cell adhesion under culture conditions with no prior exposure to flow (15). The observation that with a normal BSA-Ringer solution there can be increased instability in Lp within minutes of flow may reflect mechanisms involving reversal of the direction of inclination of junctions shown to occur within 30 min (26). However, the fact that the main increases in permeability in the present study did not occur until after 60–90 min indicates that reorientation of the regions of cell overlap was not sufficient to account for the sustained increased permeability. Rather, as discussed in more detail below, the redistribution of VE-cadherin and occludin into a pattern with significant breaks in their continuity within the intercellular junction and still present after 2–3 h is the most likely mechanism to modify the permeability of the junctions.
There have been a few previous tests of the effect of imposed shear stress on in situ permeability measurements, all apparently in the direction of normal blood flow. Using rat mesentery true capillaries a very moderate increase in Lp (maximum doubling of Lp was seen with a 3-fold increase in wall shear stress) was described that was nitric oxide dependent (24). However, the Lp increase was transient, lasting <30 s. It is possible that such changes would have been missed in the present experiments. Whatever the mechanism, such rapid changes in Lp could not represent rearrangements of the vascular adhesion complexes.
A study of Lp in mesenteric microvessels of frog suggests that Lp responds to rapid increases in wall shear stress more than to slow increases (39). A separate report found no increase in Lp related to imposed shear stress in frog mesenteric vessels (31). Using a very different technique, one study reported that permeability to albumin was dependent on flow velocity in isolated coronary venules from pigs, and that this increase in permeability was also nitric oxide dependent (41).
While our present results show no correlation between imposed shear stress and Lp, it is possible that our technique, which relies on stopping the downstream flow at the moment of measuring Lp, would miss very rapidly changing permeability, if such increases were only present during high wall shear stress. We have previously suggested that such rapid changes, possibly representing regulated changes of the tight junction claudins, would be an adaptive mechanism to match solute delivery to tissue metabolism as discussed below (14). However, our principal observation that changes in the direction of flow induce very large changes in permeability in a subpopulation of vessels is not compromised.
Shear-dependent permeability may be modulated by changes in shear stress and not the absolute shear stress. We note that we did not measure blood flow in individual vessels before cannulations, but there was brisk flow in all vessels. However, the range of shear rates (4v̄/rw) investigated in our experiments (250–1700 s−1) is larger than the range (350–700 s−1) over which Zilberberg and Harris reported shear dependent increase in solute permeability in noncannulated rat mesenteric microvessels (43, 44). Further, although we cannot report changes in shear stress in individual vessels, we can estimate, on the basis of the above values that we imposed changes in shear rate from close to zero to up to 1,000 s−1. Thus after 2 h there was no clear relation between ΔLp and changes in shear rate. However, it is interesting to note that Zilberberg and Harris found the presence of attached leukocytes may contribute to shear dependent responses in blood-perfused microvessels (43).
Flow-dependent modulation of permeability vs. other mechanisms to modulate permeability.
There are differences and similarities between the increases in permeability due to reverse flow and those during exposure to an inflammatory agent in this vessel. For example after exposure to an inflammatory agent, such as platelet activating factor, bradykinin, or histamine, there is a transient increase to a peak within 2–10 min, followed by a recovery phase with permeability returning to the nonstimulated baseline within 10–20 min even with the continued presence of the agent (4, 40). An approximation to this trend was seen in the initial instability in Lp observed in BSA-Ringer perfused vessels in reverse flow, and the trend in 2 of 18 (total number of reverse perfused vessels investigated) to return toward control. Thus we cannot eliminate the possibility that a small number of vessels (11%) act in the same way as expected when exposed to an inflammatory agent. However, in vessels where permeability increased significantly after more than 1 h the trend was toward an ever-increasing permeability with no evidence of a systematic return to baseline levels. Even short-term interruptions of flow (to measure Lp by occluding the vessel for periods of up to 20 s) had no effect on the trend to increase permeability. We note however that the magnitude of the increase in permeability after 2–3 h to values in the range of 20–40 × 10−7 cm/(s × cmH2O) is similar to the range of peak values measured within 10 min of an inflammatory response in mesenteric venules. We also note that the redistributions of VE-cadherin and occludin, with breaks in the continuity of the peripheral distribution are similar to those seen at the peak of the inflammatory response (4). This suggests that the increased Lp results from a breakdown of the cell-cell adhesion similar to that seen during an inflammatory episode.
On the other hand it is the persistence of the redistribution of VE-cadherin and occludin in the intercellular junction for 2–3 h that is the most significant difference from acute inflammation. The mechanisms that create such breaks have been shown to be triggered by an increase in intracellular calcium concentration in endothelial cells that is sustained by calcium influx into the endothelial cells (18). Thus it is possible that there are significant differences in calcium influx depending on the direction of flow. Some evidence to support such an asymmetry in calcium responses has been reported in cultured endothelial cells where an increase in intracellular calcium in cells previously conditioned to flow was not observed when measured within seconds of applying forward flow. There was a robust increase in intracellular calcium, however, when cells previously conditioned to flow were exposed to flow in the reverse direction (25, 26). Detailed measurements of calcium concentration over long periods of perfusion were not possible in our experiments due to loss of indicator dyes. Nevertheless, we have previously demonstrated stable intracellular calcium at baseline levels (close to 50 nM) for experiments lasting up to 1 h with forward flow in frog microvessels (18). Measurements in the same rat microvessel preparation used in the present experiments also demonstrated stable baseline calcium levels (near 80 nM) with forward flow (29).
We note that the failure of some vessels to increase permeability during reverse flow is similar to observation that some inflammatory agents (e.g., vascular endothelial growth factor, VEGF) do not increase permeability in all vessels (6). The reason is not well understood but may reflect a nonuniform distribution of sites that initiate calcium entry (e.g., VEGF receptors or other possible shear sensitive membrane structures) (5, 7).
Role of the glycocalyx.
The signal for increased permeability responses appears to be modulated in part by the glycocalyx, but not in a way expected if the glycocalyx transmitted a graded shear-dependent stress to the endothelium. Melchior and Frangos (26) found that the action of cultured endothelial cells to reorient the angle of junction overlap when the direction of flow was reversed was not dependent on the heparin sulfate components of the glycocalyx. If junctions tend to align with the direction of flow, then it is possible that the tips of the projections of endothelial cell membranes that form the intercellular junctions may be regions sensitive to changes in flow direction.
Role of S1P.
We have recently demonstrated that the stable permeability state normally described in these rat mesenteric venular microvessels with perfusion in the direction of normal blood flow requires the continuous presence of sphingosine-1-phosphate (13). This is an agent synthesized and stored in RBCs and released in the plasma where it contributes to the stability of the interendothelial junctions (2, 8, 16, 17). The present result extends this observation by showing that the level of S1P that maintains normal permeability with forward flow is not sufficient to maintain permeability when flow is reversed. The presence of such a threshold was demonstrated previously for inflammatory agents (13, 29). Specifically, the levels of S1P found in circulating plasma or RBC-conditioned perfusates such as those used in the present experiments [300–500 nM (3)] are not sufficient to protect the endothelial barrier from increased permeability when exposed to inflammatory agents. The primary role of a constant supply of S1P is to stabilize the peripheral actin band. It is reasonable to suggest that the reorganization of VE-cadherin and occludin during reverse flow is dependent, at least in part, on reduced stability of the peripheral actin band which results when the action of S1P-dependent mechanisms to maintain its stability is no longer effective. We note that the concentration of S1P in culture media used in experiments on cultured endothelial cells and those in artificial perfusates without RBCs have not been reported previously.
Shear-dependent small-solute delivery in rat mesenteric microvessels.
Our present observations do not rule out subtler shear-dependent changes in permeability in microvessels during perfusion in the direction of normal flow even when the permeability to water remains constant. For example, Michel and colleagues (21, 30) have demonstrated a graded increase of small solute permeability (mol wt < 600) with increased shear stress during forward flow in rat mesenteric microvessels where these responses are regulated by nitric oxide production; these are the same vessels as were investigated in the present experiments. They suggested that there might be a small-pore pathway available to small solutes, but with a very low hydraulic conductivity, in parallel with the main water pathway. This small-pore pathway may lie within the “tight” junctions which are impermeable to larger solutes and most water and are present along 90% of the line of contact between adjacent endothelial cells (1). The tight junction proteins such as occludin and claudin-5 whose molecular arrangements allow ion and small solute penetration might form these small solute pathways (11). Our results for forward flow are consistent with this suggestion. By increasing only small-solute permeability, but maintaining the integrity of the junction pathway when blood flow is increased, such an arrangement would contribute to matching perfusion and small nutrient delivery without compromising fluid balance within the tissue. The observation also suggests that a critical step in restoring normal microvascular function after injury that resulted in disturbed flow condition with components of reverse flow is the restoration of stable uniform forward flow.
In summary we have demonstrated that the magnitude of the permeability of the main transvascular water pathway in intact venular microvessels to applied shear stress depends on the direction of flow and not on the magnitude of the applied shear stress. When flow is in the direction of normal blood flow Lp is stable under normal perfusion conditions including the presence of plasma proteins and RBCs. We suggest this contributes to homeostasis by not only preventing edema formation but also enabling physiological regulation of shear-dependent mechanisms to regulate small solute permeability (e.g., glucose). On the other hand flow in the direction opposite to that to which the vessels have been conditioned causes a delayed increase in permeability in most vessels that shows no sign of attenuation after 2–3 h. The increase in permeability is independent of the magnitude of the applied shear stress but is modulated by the presence of hyaluronic acid and chondroitin sulfate in the perfusate. These responses are consistent with reports that disturbed flow results in a less stable endothelial barrier.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-28607 and HL-44485.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.H.A., R.K.S., A.A., J.F.C., S.W., and F.-R.E.C. conception and design of research; R.H.A., R.K.S., A.A., and F.-R.E.C. analyzed data; R.H.A., R.K.S., A.A., J.F.C., and F.-R.E.C. interpreted results of experiments; R.H.A. and F.-R.E.C. prepared figures; R.H.A. and F.-R.E.C. drafted manuscript; R.H.A., J.F.C., and F.-R.E.C. edited and revised manuscript; R.H.A., R.K.S., A.A., J.F.C., S.W., and F.-R.E.C. approved final version of manuscript; R.K.S., A.A., and J.F.C. performed experiments.
REFERENCES
- 1. Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol 557: 889–907, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res 88: 344–351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adamson RH, Sarai RK, Clark JF, Altangerel A, Thirkill TL, Curry FE. Attenuation by sphingosine-1-phosphate of rat microvessel acute permeability response to bradykinin is rapidly reversible. Am J Physiol Heart Circ Physiol 302: H1929–H1935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406–H417, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 100: 174–190, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol Heart Circ Physiol 271: H2520–H2528, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca2+-dependent pathway. Am J Physiol Heart Circ Physiol 273: H687–H694, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Bode C, Sensken SC, Peest U, Beutel G, Thol F, Levkau B, Li Z, Bittman R, Huang T, Tolle M, van der Giet M, Graler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem 109: 1232–1243, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119: 1871–1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284: C1346–C1354, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85: 9–23, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Curry FE, Clark JF, Adamson RH. Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels. Am J Physiol Heart Circ Physiol 303: H825–H834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curry FE, Clough GF. Flow-dependent changes in microvascular permeability—an important adaptive phenomenon. J Physiol 543: 729, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton 40: 317–330, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J 21: 1202–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 18. He P, Zhang X, Curry FE. Ca2+ entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am J Physiol Heart Circ Physiol 271: H2377–H2387, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol Heart Circ Physiol 277: H508–H514, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Jo H, Dull RO, Hollis TM, Tarbell JM. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am J Physiol Heart Circ Physiol 260: H1992–H1996, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Kajimura M, Michel CC. Flow modulates the transport of K+ through the walls of single perfused mesenteric venules in anaesthetised rats. J Physiol 521: 665–677, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol 80: 359–372, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Kestin J, Sokolov M, Wakeham WA. Viscosity of liquid water in range −8°C to 150°C. J Phys Chem Ref Data 7: 941–948, 1978 [Google Scholar]
- 24. Kim MH, Harris NR, Tarbell JM. Regulation of capillary hydraulic conductivity in response to an acute change in shear. Am J Physiol Heart Circ Physiol 289: H2126–H2135, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Melchior B, Frangos JA. Galphaq/11-mediated intracellular calcium responses to retrograde flow in endothelial cells. Am J Physiol Cell Physiol 303: C467–C473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melchior B, Frangos JA. Shear-induced endothelial cell-cell junction inclination. Am J Physiol Cell Physiol 299: C621–C629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Michel CC, Mason JC, Curry FE, Tooke JE, Hunter PJ. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci 59: 283–309, 1974 [DOI] [PubMed] [Google Scholar]
- 29. Minnear FL, Zhu L, He P. Sphingosine 1-phosphate prevents platelet-activating factor-induced increase in hydraulic conductivity in rat mesenteric venules: pertussis toxin sensitive. Am J Physiol Heart Circ Physiol 289: H840–H844, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Montermini D, Winlove CP, Michel C. Effects of perfusion rate on permeability of frog and rat mesenteric microvessels to sodium fluorescein. J Physiol 543: 959–975, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neal CR, Bates DO. Measurement of hydraulic conductivity of single perfused Rana mesenteric microvessels between periods of controlled shear stress. J Physiol 543: 947–957, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res 102: 770–776, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ, Schnittler HJ. Endothelial barrier function under laminar fluid shear stress. Lab Invest 80: 1819–1831, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Sill HW, Chang YS, Artman JR, Frangos JA, Hollis TM, Tarbell JM. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am J Physiol Heart Circ Physiol 268: H535–H543, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Tanford C. Physical Chemistry of Macromolecules. New York: Wiley, 1961 [Google Scholar]
- 36. Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 87: 320–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res 103: 1164–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Williams DA. Intact capillaries sensitive to rate, magnitude, and pattern of shear stress stimuli as assessed by hydraulic conductivity (Lp). Microvasc Res 66: 147–158, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Wu NZ, Baldwin AL. Transient venular permeability increase and endothelial gap formation induced by histamine. Am J Physiol Heart Circ Physiol 262: H1238–H1247, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol Heart Circ Physiol 263: H641–H646, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Zhang G, Xu S, Qian Y, He P. Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules. Am J Physiol Heart Circ Physiol 299: H1494–H1504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zilberberg J, Harris NR. Role of shear and leukocyte adherence on venular permeability in the rat mesentery. Microvasc Res 62: 215–225, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Zilberberg J, Harris NR. Synergism between leukocyte adherence and shear determines venular permeability in the presence of nitric oxide. Microvasc Res 62: 410–420, 2001 [DOI] [PubMed] [Google Scholar]