Abstract
Static muscle contraction activates the exercise pressor reflex, which in turn increases sympathetic nerve activity (SNA) and blood pressure (BP). Bradykinin (BK) is considered as a muscle metabolite responsible for modulation of the sympathetic and cardiovascular responses to muscle contraction. Prior studies have suggested that kinin B2 receptor mediates the effects of BK on the reflex SNA and BP responses during stimulation of skeletal muscle afferents. In patients with peripheral artery disease and a rat model with femoral artery ligation, amplified SNA and BP responses to static exercise were observed. This dysfunction of the exercise pressor reflex has previously been shown to be mediated, in part, by muscle mechanoreflex overactivity. Thus, in this report, we determined whether kinin B2 receptor contributes to the augmented mechanoreflex activity in rats with 24 h of femoral artery occlusion. First, Western blot analysis was used to examine protein expression of B2 receptors in dorsal root ganglion tissues of control limbs and ligated limbs. Our data show that B2 receptor displays significant overexpression in ligated limbs as compared with control limbs (optical density: 0.94 ± 0.02 in control and 1.87 ± 0.08 after ligation, P < 0.05 vs. control; n = 6 in each group). Second, mechanoreflex was evoked by muscle stretch and the reflex renal SNA (RSNA) and mean arterial pressure (MAP) responses to muscle stretch were examined after HOE-140, a B2 receptors blocker, was injected into the arterial blood supply of the hindlimb muscles. The results demonstrate that the stretch-evoked reflex responses were attenuated by administration of HOE-140 in control rats and ligated rats; however, the attenuating effects of HOE-140 were significantly greater in ligated rats, i.e., after 5 μg/kg of HOE-140 RSNA and MAP responses evoked by 0.5 kg of muscle tension were attenuated by 43% and 25% in control vs. 54% and 34% in ligation (P < 0.05 vs. control group; n = 11 in each group). In contrast, there was no significant difference in B1 receptor expression in both experimental groups, and arterial injection of R-715, a B1 receptors blocker, had no significant effects on RSNA and MAP responses evoked by muscle stretch. Accordingly, results obtained from this study support our hypothesis that heightened kinin B2 receptor expression in the sensory nerves contributes to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion.
Keywords: peripheral artery disease, blood pressure, sympathetic nerve activity, muscle afferent nerves, static exercise
two neural mechanisms are suggested to evoke sympathetic nerve and cardiovascular responses during exercise. The first is referred to as the exercise pressor reflex, which is evoked by mechanical and metabolic stimuli that activate thin-fiber muscle afferents in the working muscle (15, 34, 35). Thus the exercise pressor reflex has two functional components, namely the muscle mechanoreflex and metaboreflex. Specifically, mechanical deformation mostly stimulates thinly myelinated group III and muscle metabolites mostly stimulate unmyelinated group IV afferent fibers (15, 17–19). Consequently, the brainstem nuclei that regulate cardiovascular activities are stimulated and sympathetic nerve activity (SNA) and arterial blood pressure (BP) increase (15, 35). The second mechanism is termed central command, which originates in the higher brain responsible for recruiting motor units and is engaged in cardiovascular and respiratory regulation during exercise (12, 57).
Peripheral artery disease (PAD), caused by a restriction of the blood flow in the lower limbs, is typically common in older adults (5, 36, 38). Prior studies have shown that SNA and BP responses during activation of the exercise pressor reflex are augmented in human and rat models with PAD (1, 2, 53). This reflex dysfunction has previously been shown to be mediated, in part, by muscle mechanoreflex overactivity (31). With the use of a rat model of PAD, prior studies have further demonstrated that a number of receptors on muscle afferent nerves contribute to the amplified BP response during the exercise pressor reflex (24, 54, 55). For example, blocking acid sensing ion channels and thromboxane TP receptors, and stimulating μ-opioid receptors can attenuate the augmented pressor response to muscle contraction (24, 54, 55). Nevertheless, the underlying mechanisms by which femoral artery occlusion augments responsiveness of SNA and BP to activation of muscle mechano- and metabosensitive afferents remain to be determined.
A number of metabolites produced in contracting muscles, such as ATP, bradykinin (BK), cyclooxygenase products, lactic acid, and potassium, etc., have been considered as potential stimulants and/or sensitizers of muscle afferents responding to mechanical and metabolic stimuli during the exercise pressor reflex (15, 45). Among those metabolites, BK is an autacoid produced within the interstitium of most tissues and is synthesized from its precursor kininogen after activation of the enzyme, kallikrein. A previous study has shown that kinin B2 receptor, but not B1 receptor, mediates the effects of BK on cardiovascular responses to stimulation of skeletal muscle afferents in anesthetized cats (40). In addition, it is found that muscle BK is increased during exercise and there is a close relationship between the levels of BK and ventilatory response during postexercise ischemia (42). Our recent study further suggests that blocking kinin B2 receptor significantly attenuates SNA response to activation of the muscle mechanoreceptors in rats with heart failure (21). Thus the purpose of this study was to determine the role for BK and its receptors (kinin B1 and B2) in modulating the muscle mechanoreflex in rats with PAD induced by 24 h of femoral artery ligation. Our general hypothesis was that heightened kinin B2 receptor expression in the sensory nerves contributes to the exaggerated muscle mechanoreflex in PAD rats. Specifically, we hypothesized that protein expression of B2 receptors in dorsal root ganglion (DRG) tissues of ligated limbs of rats is upregulated compared with control limbs. In addition, we hypothesized that SNA and BP responses to stimulation of mechanically sensitive muscle afferent nerves evoked by muscle stretch are inhibited to a greater degree in ligated rats than that in control rats with B2 receptor antagonist injected into the arterial blood supply of the hindlimb muscles.
Our previous study demonstrated that 24 h of femoral artery ligation significantly enhanced the SNA and BP responses to muscle stretch, and there were no differences in those responses between rats with 24 h and those with 72 h of femoral artery ligation (31). Also, nerve growth factor (NGF) played a role in regulating expression of kinin B2 receptor (41) and the levels of NGF were significantly increased in the DRG tissues 24 h after femoral artery ligation. However, there were no significant differences in increased NGF between 24 and 48 h after ligation (61). Thus 24 h of ligation was selected for the experiments in this report.
METHODS
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State College of Medicine and complied with the National Institutes of Health (NIH) guidelines.
Ligation of Femoral Artery
Under inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen), the surgical procedures were performed in 54 male Sprague-Dawley rats (6 to 8 wk old) as previously described (28, 58, 61). For the Western blotting experiments, femoral arteries of the right hindlimb of 12 rats were surgically exposed, dissected, and ligated ∼3 mm distal to the inguinal ligament; this served as ligated limb. The same procedures were performed on the left hindlimb except that a suture was placed below the femoral artery but was not tied; this served as control limb. For the experiment of SNA and BP recording, 42 rats were equally divided between those that had the right femoral artery ligated and those that had sham surgery on the right hindlimb and served as controls. The ligated rats and control rat were defined in this experiment. For both experiments, 24 h were then allowed for recovery before the experiments began.
Western Blot Analysis
Twelve rats were used to examine expression of kinin B2 and B1 receptors protein in lumbar (L4–6) DRGs of control and ligated limbs. Western blot methods were performed as previously described (28, 30). In brief, DRGs of the rats were removed. All DRG tissues from individual rats were sampled for Western blot analysis. Total protein was then extracted by homogenizing DRG sample in ice-cold radioimmunoprecipitation assay buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% SDS with protease inhibitor cocktail kit (Sigma-Aldrich, St. Louis, MO). The lysates were centrifuged at 15,000 g for 15 min at 4°C; the supernatants were collected for measurements of protein concentrations using a bicinchoninic acid assay reagent kit (Pierce Biotech, Rockford, IL) and then stored in −80°C for later use.
After being denatured by heating at 95°C for 5 min in an SDS sample buffer (Cell Signaling Technology, Danvers, MA), the supernatant samples containing 20 μg of protein were loaded onto 4–20% Mini-PROTEAN TGX gels (Bio-Rad Laboratories, Hercules, CA) and then electrically transferred to a polyvinylidene fluoride membrane (GE Water & Process Tech, Trevose, PA). The membrane was blocked in 5% nonfat milk in 0.1% Tween-TBS buffer for 1 h and then incubated overnight with primary antibodies (mouse anti-kinin B2 receptor at 1:200; rabbit anti-kinin B1 receptor at 1:200; Santa Cruz Biotechnology).
After being fully washed, the membrane was incubated with horseradish peroxidase-linked anti-mouse secondary antibody (1:1,000) and anti-rabbit secondary antibody (1:1,000). The immunoreactive proteins were detected by enhanced chemiluminescence system (Cell Signaling Technology). The bands recognized by the primary antibody were visualized by exposure of the membrane onto an X-ray film. The membrane was stripped and incubated with mouse anti-β-actin (Sigma-Aldrich) to show equal loading of the protein in the Western blot analysis. The film was then scanned, and the optical density of kinin B2, B1 receptors, and β-actin bands was analyzed using the NIH Scion Image Software.
Examination of Muscle Mechanoreflex
Surgical preparations.
Forty-two rats were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). Note that this was a different group of rats than those used in the Western blot analysis. An endotracheal tube was inserted and attached to a ventilator (Model AWS; Hallowell EMC, Pittsfield, MA). Polyethylene (PE-50) catheters were inserted into an external jugular vein and the common carotid arteries for the purposes of drug administration and measurement of arterial BP, respectively. The femoral artery was isolated in the previously ligated limb/control limb. An incision was made in the artery between the previous suture and the popliteal artery. A catheter (PE-10) was then inserted into the femoral artery, and the tip of the catheter was placed in the popliteal artery for injection of drugs into the arterial blood supply of the hindlimb muscles. As there is collateral flow to maintain limb perfusion, a catheter inserted into the popliteal artery is unlikely to occlude the circulation of the limb being tested. The skin covering the triceps surae muscle and femoral region was surgically separated from the muscle below to eliminate inputs from cutaneous afferents in the hindlimb.
During the experiments, the animals were artificially ventilated, and tidal CO2 was monitored by a respiratory gas monitor (Model 5250; Datex-Ohmeda, Madison, WI) and maintained within normal ranges, as previously described (11, 14, 22, 59). Body temperature was carefully maintained at 37.5–38.5°C by a heating pad and external heating lamps. A continuous infusion of physiological saline (0.1 ml/h) into the venous line was established by using a syringe pump (Medical Industries, Subiaco, Australia). This maintained fluid balance and basal BP.
The rats were placed in a spinal unit (Kopf Instruments). A bundle of the renal nerves on the left side was carefully dissected from other connective tissues. A piece of laboratory film was placed under the isolated nerves, and two tips of a bipolar electrode used to record neural activity were placed between the nerves and the film; these were embedded in a silicone gel. Once the gel hardened, the silicone rubber was fixed to the surrounding tissue with a glue containing α-cyanoacrylate. The skin and muscle tissues in the region of incision were used to form a pool that was filled with warm (37°C) mineral oil. The renal SNA (RSNA) signal was amplified with an amplifier (P511; Grass Instruments) with a band-pass filter of 300 Hz in low-cut frequency and of 3 kHz in high-cut frequency and recorded as previously described (28, 58). Note that signal-to-noise ratio for the baseline RSNA was examined to validate data obtained.
Decerebration was performed as previously described (11, 28, 58) to avoid the confounding effects of anesthesia on the reflex pressor response. A transverse section was made anterior to the superior colliculus and extending ventrally to the mammillary bodies. All brain tissues rostral to the section were removed. After this procedure, the anesthesia was withdrawn from the rats. The calcaneal bone of right hindlimb was cut and its tendon was attached to a force transducer (Grass FT10), and the knee joints were secured by clamping the patellar tendon to a spinal unit. A recovery period of 60 min was allowed before the experiment.
Experimental protocols.
The purpose of this experiment was to examine if femoral artery occlusion altered the effects of blocking kinin B2 and B1 receptors on the sympathetic and pressor responses evoked by stimulation of mechanically sensitive muscle afferent nerves. Thus muscle stretch was performed to activate the mechanoreceptor component of the exercise pressor reflex in control rats and rats whose femoral artery was ligated for 24 h. Note that each muscle stretch was performed 15 min after arterial injection of saline (control and recovery) and each antagonist. Then, 20 min was allowed after stretch and before the next injection. The injected volume was 0.1 to 0.15 ml, and the duration of injections was 1 min. Thus there was a ∼36-min resting period between bouts of muscle stretch. In the first group, saline was injected into the arterial line before muscle stretch was evoked to obtain a control. The RSNA, BP, and heart rate (HR) responses to muscle stretch were then examined. Next, to examine effects of B2 receptors, 5 μg/kg of HOE-140 (a specific B2 receptor antagonist) was administered into the arterial blood supply of hindlimb muscles of control rats and ligated rats (n = 11 in each group) before muscle stretch. After this, saline was arterially given and then the reflex RSNA, BP, and HR responses were examined to obtain a recovery. In the second group, in the same way, in control rats and ligated rats (n = 10 in each group) saline was injected to obtain a control. R-715 (20 μg/kg; a specific B1 receptor antagonist) was given to examine effects of B1 receptors, and a recovery was examined after saline injection. The effective doses of B2 and B1 receptor antagonists were determined based on the previous work from our laboratory and others (7, 9, 21). In this experiment, we attempted to stimulate muscle mechanoreceptors and minimize engagement of other receptors (i.e., pain), although it is unlikely to rule out their effects. Muscle stretch (0.5 kg tension) was produced manually over ∼5 s by using a rack and pinion attached to the Achilles′ tendon. Each bout of muscle stretch was maintained for 30 s after 0.5 kg of tension was achieved. The levels of tension and intervention have been reported to generate sufficient RSNA and BP responses in rats (10).
Data acquisition.
All measured data of RSNA, BP, and HR were continuously recorded and stored on a computer with PowerLab system (AD instruments, Castle Hill, Australia). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. HR was calculated on a basis of beat to beat from the arterial pressure pulse. The peak responses of MAP and HR were determined by the peak change from the control value (average over 30 s). RSNA signals were transformed into absolute values, integrated over 1-s interval, and subtracted by 1 s of integrated background noise. To quantify RSNA response to muscle stretch and arterial injection, baseline values were obtained by taking the mean value for the 30 s immediately before each injection and by ascribing the mean value of 100%, and relative change from baseline during the injection was then evaluated.
Data Statistical Analysis
All statistical analyses were performed using SPSS for Windows version 19.0 (SPSS Sci, Chicago, IL). One-way repeated-measures ANOVA was performed to compare variables for RSNA, MAP, and HR, as well as kinin B1 and B2 receptors optical density, followed by Tukey's post hoc test as appropriate. All values were presented as means ± SE. For all analyses, differences were considered significant at P < 0.05.
RESULTS
Effects of Femoral Occlusion on Protein Levels of B2 and B1 Receptors
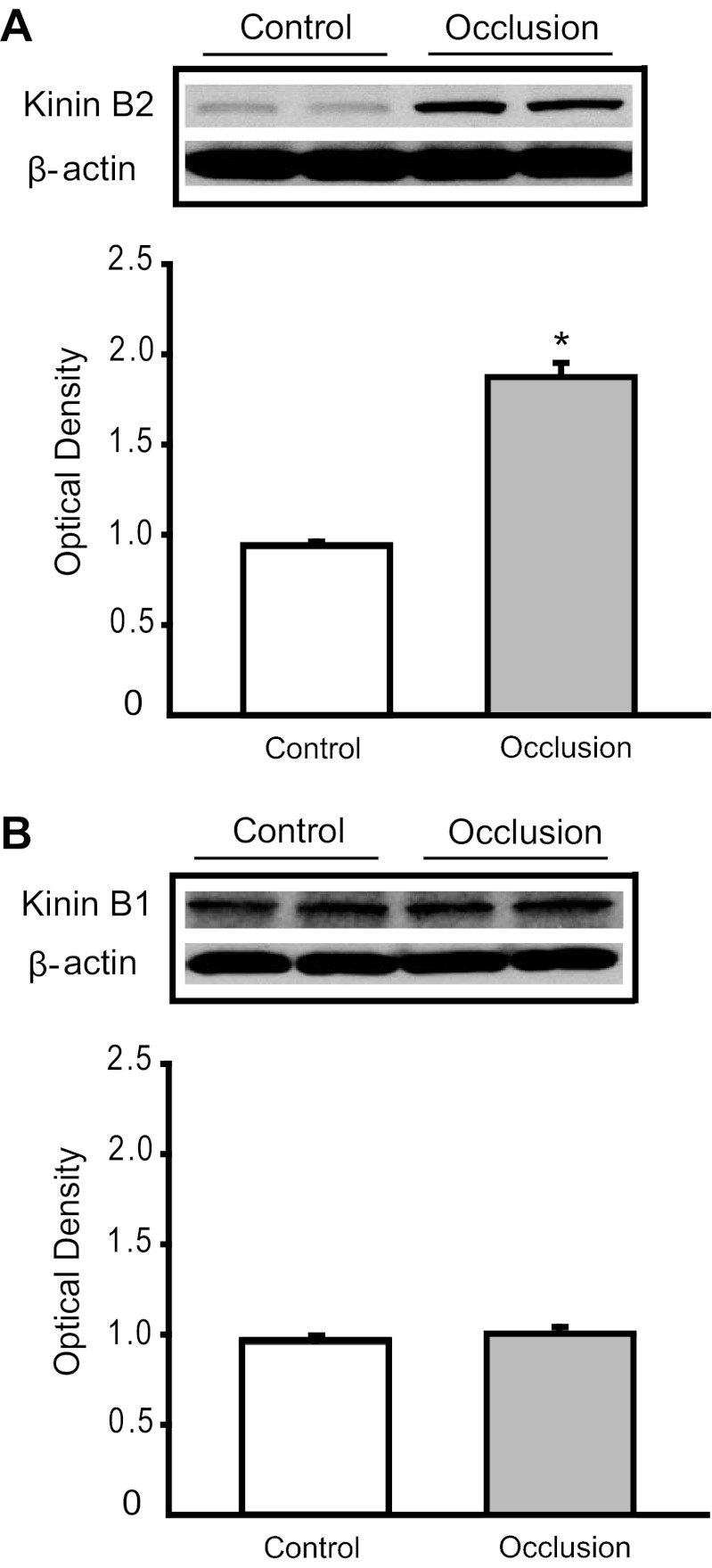
Figure 1A shows that femoral artery occlusion significantly increased expression of B2 receptor in DRG. The intensity of the signal in lumbar DRG tissues of ligated limbs was ∼1.99-fold greater than that in control limbs (optical density, 1.87 ± 0.08 in occlusion vs. 0.94 ± 0.02 in control, P < 0.05; n = 6 in each group). Figure 1B further shows that no differences were seen in the expression of B1 receptor in DRG of control limbs and ligated limbs (optical density, 1.01 ± 0.03 in occlusion vs. 0.97 ± 0.01 in control, P > 0.05; n = 6 in each group).
Fig. 1.
Analysis of kinin B2 and B1 receptors protein expression in control limbs and limbs with 24 h of femoral artery ligation. Western blot assays were performed on dorsal root ganglion tissues from control limbs and ligated limbs. A, top: typical bands. The results of Western blot assays illustrate that optical density of B2 receptor protein is higher in a ligated limb than that in a control limb. Bands of β-actin are used as control for an equal protein loading. A, bottom: average data. Significant B2 receptor overexpression was seen in ligated limbs over control limbs. Results represent means ± SE of n = 6. *P < 0.05 compared with control. B, top: representative bands of B1 receptor expression. B, bottom: average data. Results represent means ± SE of n = 6. No significant difference was observed between control and ligated groups.
Effects of Blocking B2 and B1 Receptors on Responses of RSNA, MAP, and HR during Muscle Stretch
Effects of HOE-140.
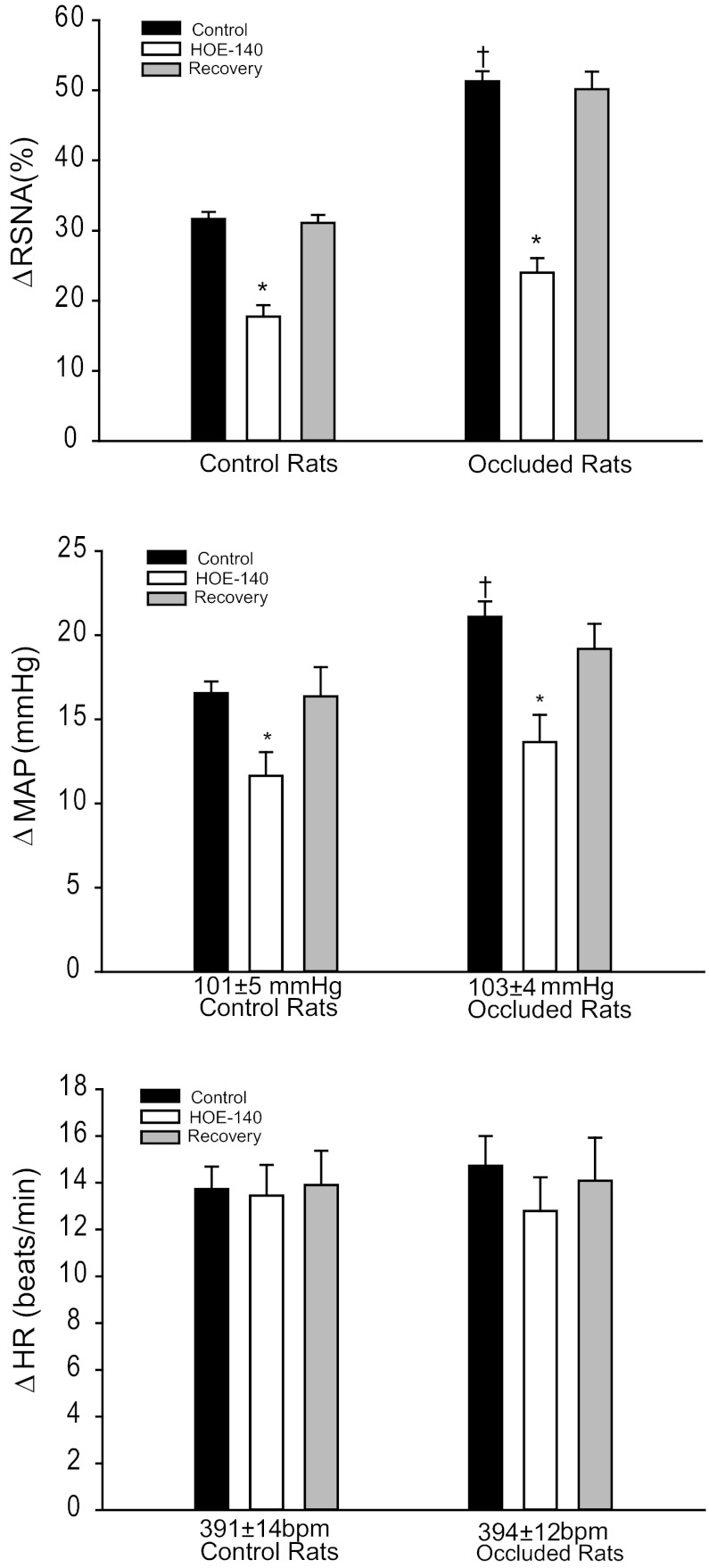
Baseline MAPs and HRs were 101 ± 5 mmHg; 391 ± 14 beats/min in control rats (n = 11) and 103 ± 4 mmHg; 394 ± 12 beats/min in rats whose femoral artery was ligated for 24 h (n = 11) (P > 0.05, vs. control). Muscle stretch was performed in both groups after each intervention. Typical traces and average data are shown in Figs. 2 and 3. Twenty-four hours of femoral occlusion significantly increased the responses of RSNA and MAP evoked by muscle stretch with 0.5 kg of muscle tension. No significant differences in the HR response were seen between the two groups. Moreover, Figs. 2 and 3 show RSNA, MAP, and HR responses to muscle stretch after inhibition of B2 receptors with arterial injection of 5 μg/kg of HOE-140 in control rats and ligated rats. HOE-140 significantly inhibited RSNA and MAP responses induced by muscle stretch in control and ligated groups. In control rats, the RSNA and MAP responses were 32 ± 1% and 16 ± 1 mmHg with saline injection and 18 ± 2% and 12 ± 1 mmHg with HOE-140 treatment (P < 0.05, HOE-140 treatment vs. saline control for both RSNA and MAP responses). In ligated rats, the RSNA and MAP responses were 51 ± 2% and 21 ± 1 mmHg with saline injection and 24 ± 2% and 14 ± 2 mmHg with HOE-140 treatment (P < 0.05, HOE-140 treatment vs. saline control for both RSNA and MAP responses). The attenuating effects of HOE-140 on RSNA and MAP responses were greater in the ligated group than in the control group (percentage reduction on RSNA and MAP: 43 ± 3% and 25 ± 5% in control vs. 54 ± 3% and 34 ± 5% in ligation, P < 0.05 vs. control). In addition, the attenuated RSNA and MAP responses during muscle stretch returned to their control levels after a recovery period. HOE-140 treatment had no effects on HR responses to muscle stretch in either group.
Fig. 2.
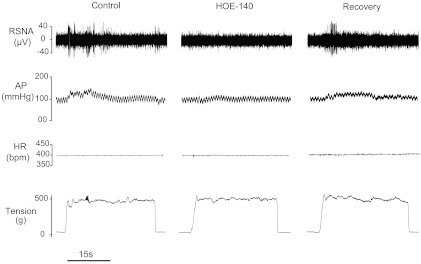
Original recordings show that renal sympathetic nerve activity (RSNA), arterial blood pressure (AP), and heart rate (HR) responses to muscle stretch were attenuated by the prior arterial administration of HOE-140 to inhibit kinin B2 receptors. The data were obtained from a rat with 24 h of femoral artery ligation. The reflex RSNA and BP responses were recovered to the levels of control ∼36 min after the end of HOE-140 intervention. Bpm, beats/min.
Fig. 3.
The changes of RSNA, mean arterial pressure (MAP), and HR were measured in response to muscle stretch after B2 receptor inhibition by HOE-140. Average data show that RSNA and MAP responses to muscle stretch were significantly attenuated in both control rats and ligated rats after blocking B2 receptors. Note that the attenuating effects of HOE-140 appeared to be a greater degree in ligated group. The responses of RSNA and MAP returned to their pre-inhibitory state after a recovery period. No significant difference was observed in baseline MAP and HR in control rats and ligated rats. Also, there were no significant differences in signal-to-noise (S/N) ratio for the basal RSNA in control rats (3.5 ± 0.6) and ligated rats (3.6 ± 0.5, P > 0.05 vs. control). The reflex responses to muscle stretch with saline control were higher in ligated rats versus control animals. *P < 0.05 vs. saline control; †P < 0.05, significant differences in changes in RSNA and MAP between control group and ligated group. The number of animals is 11 in each group. Note that the same level of tension was loaded in all interventions.
Effects of R-715.
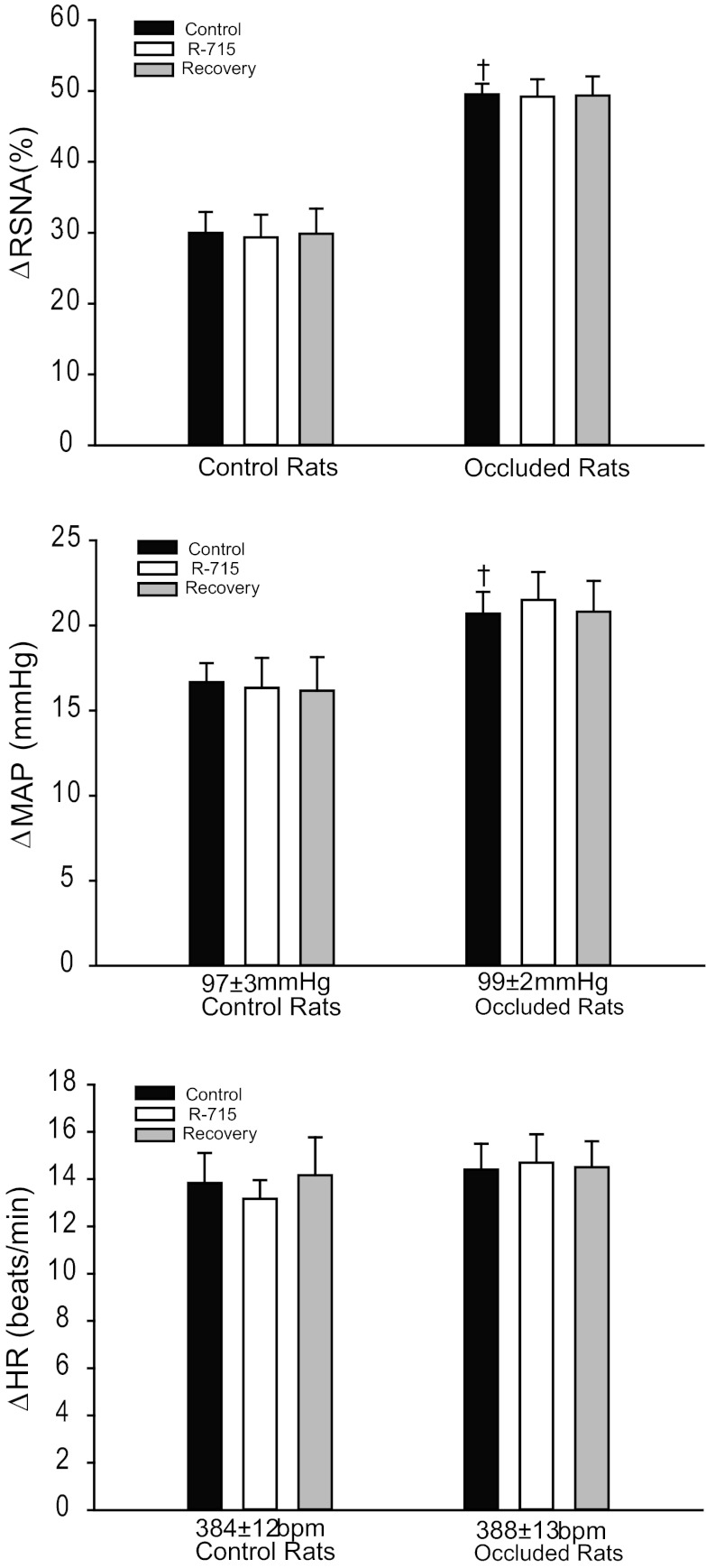
There were no significant differences in baseline values for MAP and HR in control rats and ligated rats (n = 10 in each group). Baseline MAPs and HRs were 97 ± 3 mmHg, 384 ± 12 beats/min, in control rats; and 99 ± 2 mmHg, 388 ± 13 beats/min, in ligated rats. Figures 4 and 5 are typical traces and average data demonstrating RSNA, MAP, and HR responses to muscle stretch during each intervention. Before B1 receptor inhibition, significantly higher RSNA and MAP responses were observed in the ligated group versus the control group. No significant HR responses were seen in the two groups during stretch. Blocking B1 receptors with arterial injection of 20 μg/kg of R-715 did not elicit significant changes in RSNA, MAP, and HR responses during muscle stretch in either the control rats or the ligated animals, i.e., in ligated rats, RSNA and MAP responses induced by 0.5 kg of muscle tension were 50 ± 2% and 21 ± 1 mmHg in control and 49 ± 3% and 22 ± 2 mmHg (P > 0.05 vs. control) after 20 μg/kg of R-715.
Fig. 4.
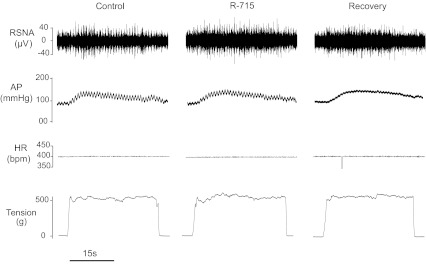
Original recordings show the changes in the RSNA, AP, HR, and muscle tension in saline control, after arterial administrations of R-715 (a B1 receptor inhibitor), and recovery in a rat with 24 h of femoral artery ligation. R-715 failed to attenuate the increases in RSNA, AP, and HR elicited by muscle stretch.
Fig. 5.
The changes of RSNA, MAP, and HR were measured in response to muscle stretch after B1 receptor inhibition by R-715. Average data show that 24 h of femoral artery occlusion significantly increased the responses of RSNA and MAP evoked by muscle stretch with 0.5 kg of muscle tension. Blocking B1 receptors did not significantly influence RSNA, MAP, and HR responses to muscle stretch in control and ligated groups. Both control and ligated groups displayed very little changes in overall RSNA, MAP, and HR responses after inhibition of B1 receptor. †P < 0.05, significant differences in changes in RSNA and MAP between control group and ligated group. The number of animals is 10 in each group. Note that the same level of tension was loaded in 3 interventions. Also, there were no differences in S/N ratio for the basal RSNA in control rats (3.6 ± 0.4) and ligated rats (3.4 ± 0.4, P > 0.05 vs. control).
DISCUSSION
The present study has demonstrated that a significant B2 receptor overexpression is observed in the DRG tissues of ligated limbs as compared with control limbs. This result suggests that femoral artery occlusion amplifies expression of B2 receptors in the sensory neurons. In contrast, no significant difference in levels of B1 receptor protein in the DRG was observed between the two conditions, suggesting that femoral artery occlusion is unlikely to change expression of B1 receptors in the sensory neurons. Given that DRG cells are the primary sensory projections to group III and IV fibers afferent nerves, expression and characteristics of sensory receptors in DRG neurons are generally examined to study receptor physiology (4, 25, 27, 56). The receptors in question are found in both the peripheral terminals and cell bodies of sensory DRG neurons. Receptor activity and characteristics of the DRG cell body have been used to reflect activity and characteristics of the receptors located at the nerve endings (4, 25, 27, 56). Accordingly, B2 receptors on the mechanosensitive nerve endings in the muscle interstitium are likely upregulated in this report.
In agreement with the findings of BK receptors expression, our data have further shown that intra-arterial injection of HOE-140, a specific kinin B2 receptor blocker, into the hindlimb circulation significantly attenuates RSNA and BP responses evoked by muscle stretch in both control rats and ligated rats. However, the effects of HOE-140 were greater in ligated rats than they were in control rats. On the other hand, arterial injection of R-715, a B1 receptors blocker, had no effect on RSNA and BP responses to muscle stretch in either experimental group.
During muscle contraction, an increase in the levels of BK (23, 42, 43, 49) stimulates and/or sensitizes muscle afferents responding to contraction, thereby contributing to autonomic function during exercise (8, 16, 40, 50, 51). Recent findings have suggested that femoral artery occlusion augments responsiveness of SNA and BP to stimulation of muscle mechano- and metabosensitive afferents (28, 29, 31, 53, 55, 58, 60, 61). Muscle stretch used in the present experiment is considered to mainly stimulate muscle mechanoreceptors. Thus, based on the data obtained from this study, it is well reasoned that BK sensitizes mechanosensitive muscle afferents via B2 receptors. Due to overexpression of B2 receptors, RSNA and BP responses induced by the mechanoreflex are attenuated to a larger degree in ligated rats when B2 receptors are blocked. Overall, the data suggest that expression of kinin B2 receptor in the sensory nerves is heightened in rats with the hindlimb ischemia, which thereby results in a greater stimulating and/or sensitizing effect on the sympathetic and pressor responses to stimulation of the muscle mechanoreceptor during muscle stretch.
Similarly, our previous study has demonstrated that blocking kinin B2 receptor with HOE-140 can significantly attenuate SNA responses to intermittent muscle contraction (an intervention considered to largely stimulate muscle mechanoreceptors) to a greater degree in rats with heart failure induced by myocardial infarction as compared with control rats (21). Given that the abnormalities in the muscle pressor reflex are mediated primarily by muscle mechanoreflex overactivity in heart failure (22, 26, 46), together with findings of our current study, this suggests kinin B2 receptor is generally engaged in the exaggerated sympathetic responses during stimulation of the muscle mechanoreceptor as the blood flow to active muscle is insufficient.
Both heart failure and PAD can cause skeletal muscle ischemia when muscle is active. Thus there is a possibility that any condition that causes muscle ischemia could evoke such B2 receptor-induced sensitizing effect on skeletal muscle mechanoreceptors. Nonetheless, it should be noted that heart failure is considered as a chronic low flow state compared with femoral artery occlusion-induced hindlimb ischemia employed in the present study. Left ventricular function is largely diminished in rats 6–8 wk after ligation of the coronary artery (11, 21, 26, 46, 47, 59). In contrast, the femoral artery occlusion is an insufficient low flow directed to the hindlimb leading to localized muscle ischemia. Thus the mechanisms responsible for the amplified mechanoreflex could be different.
Also, it should be noted that intermittent muscle contraction is believed to be due, primarily, to activation of mechanoreceptors and also it is logical to assume that this increase in muscle activity could have led to local production of BK as shown in response to static contraction (49). B2 receptors are then stimulated by BK (40). However, there is no evidence that muscle stretch, as was used in the present study, increases BK production (or metabolic activity in general). Thus a question is how activation of B2 receptors would have occurred under these circumstances. It seems that there are other mechanisms responsible for sensitization of mechanoreceptors in our current study using muscle stretch to stimulate muscle mechanoreceptors (48).
It is well-known that ischemia can generally induce inflammation, which can provoke the release of BK (41). Thus femoral artery ligation likely increased baseline levels of BK, and this is likely to have contributed to the concomitant upregulation of B2 receptors observed in the present study. In addition, substantial evidence has demonstrated that BK can activate and/or sensitize thin-fiber afferents to mechanical stimulation of numerous organs (6, 32, 33, 37, 39, 52, 62), suggesting that BK is engaged in enhancement of mechanoreceptor sensitivity. As a result, it is reasoned that elevated baseline levels of BK by femoral artery occlusion are likely to lead to sensitization of mechanoreceptors. Nevertheless, whether resting levels of BK in the muscle is greater in the ligated rats than that in the control rats needs to be studied to better clarify this issue.
With regard to amplified expression of B2 receptor in sensory nerves, NGF should be considered as an important player in regulating kinin B2 receptor (41). Our prior study demonstrates that 24 h of femoral ligation increases the levels of NGF in the DRG tissues (61), which is very likely to enhance expression of B2 receptors. Even though the consistent expression of B1 receptors in the DRG tissue was observed in control limbs and ligated limbs, there were no significant differences in B1 receptors in between groups in the present study. Consistently, B1 receptor did not appear to contribute to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion. It is assumed that femoral artery occlusion-evoked NGF specifically modulates B2 receptors in the DRG but not B1 receptors.
Exercise decreases muscle pH of the exercising limb and increases venous level of lactic acid (43, 44). Of note, a prior study (49) has reported that the extent of the release of BK from contracting skeletal muscle was correlated with the magnitude of the decrease in venous pH and the increase in venous lactate. Thus it is speculated that the release of BK is likely to be more in active muscle, thereby leading to upregulation of B2 receptor if femoral artery occlusion induces interstitial pH to a less degree compared with controls. However, a previous study has demonstrated that there are no significant differences in resting levels of intramuscular pH in control limbs and ligated limbs of rats 12 h and 4, 7, and 14 days after the surgery (3). This result is consistent with findings in PAD patients, suggesting that muscle pH is not altered in symptomatic legs (13, 20). These data do not support the idea that B2 receptors in sensory nerves are upregulated via the greater releases of BK by a lower muscle pH in ligated rats.
Limitations of the Study
In rats, femoral artery ligation (i.e., 24–72 h) can cause an insufficient low flow directed to the hindlimb and this likely leads to localized muscle ischemia when muscle is active. In contrast, PAD is mainly due to atherosclerotic vascular disease and atherosclerosis develops over time in patients (5, 36, 38), and it is unlikely that acute ligation creates the same conditions. Thus the potential problems in translating this relatively acute rat model of PAD, to the human chronic PAD situation should be noted.
In summary, the data of this report support the hypothesis that kinin B2 receptor, but not B1 receptor, is overexpressed in the DRG tissues of rats after 24 h of femoral artery occlusion. Furthermore, specific inhibition of B2 receptors significantly attenuates the augmented SNA and BP responses to muscle stretch in occluded rats to a greater degree compared with control animals. In addition, the data demonstrate that inhibiting B1 receptors does not significantly affect the muscle stretch-induced SNA and BP responses in either experimental group, suggesting that kinin B1 receptor is unlikely to directly modulate the muscle mechanoreflex. Notably, the role of B2 and B1 receptors in sensitizing muscle mechanoreceptive afferents in a rat model of PAD is congruent with their expression in the sensory nerves. Therefore, the current study has identified kinin B2 receptor as a primary contributor to the over-reactive muscle mechanoreflex observed in PAD.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants NIH-R01-HL090720 and NIH-P01-HL096570 and American Heart Association Established Investigator Award 0840130N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J. Lu, J.X., and J. Li conception and design of research; J. Lu performed experiments; J. Lu and J. Li analyzed data; J. Lu, J.X., and J. Li interpreted results of experiments; J. Lu and J. Li prepared figures; J. Lu and J. Li drafted manuscript; J. Lu, J.X., and J. Li edited and revised manuscript; J. Lu, J.X., and J. Li approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Chunying Yang for technical assistance in experiments of the sympathetic nerve activity recording.
REFERENCES
- 1. Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Challiss RA, Hayes DJ, Petty RFH, Radda GK. An investigation of arterial insufficiency in rat hindlimb: a combined 31P-nmr and bloodflow study. Biochem J 236: 461–467, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on painsensing and stretchsensing neurons. Nature 387: 505–508, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 290: 86–97, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species and inflammatory mediators enhance muscle spindles mechanosensitivity in rats. Pflügers Arch 457: 877–884, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Eric J, Gabra BH, Sirois P. Implication of the bradykinin receptors in antigen-induced pulmonary inflammation in mice. Br J Pharmacol 138: 1589–1597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franz M, Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res 92: 369–383, 1975 [DOI] [PubMed] [Google Scholar]
- 9. Gabra BH, Sirois P. Kinin B1 receptor antagonists inhibit diabetes-induced hyperalgesia in mice. Neuropeptides 37: 36–44, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gao Z, Koba S, Sinoway L, Li J. 20-HETE increases renal sympathetic nerve activity via activation of chemically and mechanically sensitive muscle afferents. J Physiol 586: 2581–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol 292: H939–H945, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol (London) 226: 173–190, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greiner A, Esterhammer R, Messner H, Biebl M, Mühlthaler H, Fraedrich G, Jaschke WR, Schocke MFH. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg 43: 978–986, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24: 4709–4717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. Chapter 10. In: Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. New York: Oxford University Press, 1996, p. 381–447 [Google Scholar]
- 16. Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982 [DOI] [PubMed] [Google Scholar]
- 17. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, Gilling-Smith GL, Brennan J, Rankin A, Frostick SP. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg 34: 1103–1110, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Koba S, Xing J, Sinoway LI, Li J. Bradykinin receptor blockade reduces sympathetic nerve response to muscle contraction in rats with ischemic heart failure. Am J Physiol Heart Circ Physiol 298: H1438–H1444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H311–H321, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Langberg H, Bjørn C, Boushel R, Hellsten Y, Kjær M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol 542: 977–983, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leal AK, Mccord JL, Tsuchimochi H, Kaufman MP. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H2140–H2146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis C, Neidhart S, Holy C, North RA, Buell G, Suprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATPgated currents in sensory neurons. Nature 377: 432–435, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu J, Li J, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomytes. Am J Physiol Heart Circ Physiol 295: H1956–H1965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu J, Xing J, Li J. Role for NGF in augmented sympathetic responses to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion. J Appl Physiol 113: 1311–1322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lynn PA, Chen BN, Zagorodnyuk VP, Costa M, Brookes SJH. TNBS-induced inflammation modulates the function of one class of low-threshold rectal mechanoreceptors in the guinea pig. Am J Physiol Gastrointest Liver Physiol 295: G862–G871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 36. Muir RL. Peripheral arterial disease: Pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs 27: 26–30, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H, Mizumura K. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness). J Neurosci 30: 3752–3761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ouriel K. Peripheral arterial disease. Lancet 358: 1257–1264, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol 281: G1449–G1459, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75: 2061–2068, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Scott AC, Wensel R, Davos CH, Georgiadou P, Ceri Davies L, Coats AJ, Francis DP, Piepoli MF. Putative contribution of prostaglandin and bradykinin to muscle reflex hyperactivity in patients on Ace-inhibitor therapy for chronic heart failure. Eur Heart J 25: 1806–1813, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ, Piepoli MF. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation 106: 214–220, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol 84: 1551–1559, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 69: 1225–1230, 1990 [DOI] [PubMed] [Google Scholar]
- 50. Stebbins CL, Longhurst JC. Bradykinin-induced chemoreflexes from skeletal muscle: implications for the exercise reflex. J Appl Physiol 59: 56–63, 1985 [DOI] [PubMed] [Google Scholar]
- 51. Stebbins CL, Longhurst JC. Bradykinin in reflex cardiovascular responses to static muscular contraction. J Appl Physiol 61: 271–279, 1986 [DOI] [PubMed] [Google Scholar]
- 52. Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 80: 2632–2644, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsuchimochi H, Mccord JL, Kaufman MP. Peripheral u-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion (Abstract). J Physiol 589: 6173–6189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. Chapter 9. In: Handbook of Physiology - Section 12, Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. New York: Oxford University Press, 1996, p. 333–380 [Google Scholar]
- 58. Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xing J, Koba S, Kehoe V, Gao Z, Rice K, King N, Sinoway L, Li J. Interstitial norepinephrine concentrations in skeletal muscle of ischemic heart failure. Am J Physiol Heart Circ Physiol 293: H1190–H1195, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Xing J, Lu J, Li J. ASIC3 Function and immunolabeling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol 590: 1261–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zagorodnyuk VP, Brookes SJH, Spencer NJ, Gregory S. Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J Physiol 587: 3523–3538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]