Abstract
Major depressive disorder (MDD) is characterized by impaired processing of negative information, possibly due to dysfunction in both, the bottom-up emotional network and top-down modulatory network. By acquiring functional magnetic resonance imaging (fMRI) on a pain-anticipation task, we tested the hypothesis that individuals with MDD would show increased negative biasing that may be associated with reduced frontal connectivity. Thirty-one (15 females) unmedicated young adults with current MDD and 22 (11 females) healthy subjects with no history of MDD were recruited. Groups did not differ significantly in age, race, level of education, marital status or gender distribution. fMRI data were collected during an event-related pain-anticipation paradigm, during which subjects were cued to anticipate painful heat stimuli of high or low intensity. All temperature stimuli were applied to each subject's left forearm. We found that relative to healthy comparison subjects, participants with MDD showed significantly stronger responses to high versus low pain anticipation within right ventral anterior insula (AI), but overlapping response within right dorsal AI, which correlated positively with the depression symptoms severity in the MDD group. Functional connectivity analyses showed increased functional connectivity between dorsal insula and posterior thalamus and decreased functional connectivity between dorsal insula and the right inferior frontal gyrus in the MDD compared with the non-MDD group. Our results demonstrate that unmedicated individuals with current MDD compared with healthy never-depressed subjects show both differential and overlapping response within AI during anticipation of pain. Furthermore, the overlapping insular response is less regulated by frontal brain systems and is more subservient to affective processing regions in the posterior thalamus in MDD. These results support and provide functional validation of the co-occurring enhanced ‘bottom-up' and attenuated ‘top-down' processing of salient, unpleasant emotional information in MDD.
Keywords: depression, emotion, fMRI, imaging, insula, modulation
Introduction
Individuals with major depressive disorder (MDD) focus more on negative stimuli,1, 2 and are less easily distracted from negative emotion processing.3, 4, 5, 6 Recent evidence suggests that MDD also interferes with anticipatory processing,7, 8 and increased negative biasing has been observed following anticipation of aversive images9, 10 or pain11 in MDD.
This negative biasing in MDD is thought to be influenced by: (1) increased ‘bottom-up' response to emotional stimuli and (2) decreased ‘top-down' modulation of emotion.12, 13, 14, 15 Using experimental pain, we have previously found that unmedicated individuals with MDD, in comparison with healthy volunteers, showed enhanced activation within amygdala, anterior insula (AI) and anterior cingulate cortex during anticipation of painful heat versus non-painful warmth and decreased activation within dorsolateral prefrontal cortex during actual pain experience.11 This is consistent with increased bottom-up processing during pain anticipation and decreased top-down control during pain experience. Influential models of MDD suggest that negative biasing in this disorder is probably due to a combination of both of these processes,13, 16 yet limited experimental data mechanistically links these notions. In addition, one of the main findings in our prior work was demonstration of ‘emotional allodynia' in the MDD subjects, whereby stimuli that were not perceived as painful were rated as highly unpleasant by the depressed participants. Therefore, it is possible that the differences in the perceived affect of temperature stimuli in MDD and non-MDD subjects could have potentially influenced the observed group differences in brain activation during anticipation and processing of pain in our prior work. Deciphering affective biasing from group effects is needed in order to understand the mechanisms underlying negative anticipation in MDD.
Recent evidence suggests that the AI region has an important role in emotional anticipation by acting as an integrator of physiological, cognitive and emotional experiences.17, 18, 19 Individuals with MDD consistently show increased activation within insula during processing and anticipation of negative emotional events.11, 20, 21, 22, 23 Recent imaging data suggest functional subdivisions within the AI, whereby dorsal anterior portion is more involved in cognitive processing and show strong functional connections with the dorsal attentional stream, whereas ventromedial aspects of the insula relate more to emotional processing and show strong functional connections with amygdala.24, 25 We have also recently shown the evidence of functional reorganization within AI in MDD, whereby emotion-related region of depressed subjects overlapped with pain-processing insular region in healthy controls.26
The aim of this study was to use functional magnetic resonance imaging (fMRI) together with a validated cued pain-anticipation paradigm27 in order to build on our prior evidence of dysfunctional anticipatory processes8, 11 and functional reorganization within insula cortex in MDD.26 Specifically, we directly tested the hypothesis that unmedicated individuals with MDD would show negative anticipatory biasing towards pain, which will be associated with maladaptive preparatory response within the insula, that would be related to both increased bottom-up (that is, thalamic) and decreased top-down (that is, prefrontal) response in the MDD. We aimed to directly test the current hypothesis by: (1) controlling for subjective negative biasing between the groups and (2) examining both contrast and connectivity effects.
Materials and methods
Subjects
Thirty-one unmedicated subjects with current MDD (15 females and 16 males) and twenty-two healthy subjects who never had MDD (non-MDD) (11 females and 11 males) gave written informed consent to participate in this study, which was approved by the University of California San Diego Human Research Protection Program and Veterans Affairs San Diego Healthcare System Research and Development Committee. Healthy control subjects were comparable to MDD participants on age (t (51)=0.35, P=0.72), race (χ2=0.091, P=0.993), education (t (51)=1.0, P=0.30), and gender (Yates corrected χ2=0.03, P=0.87) (Table 1). Subjects were recruited by using fliers at the University of California San Diego clinics, internet sites (for example, Craigslist), local papers and the word of mouth, and there was no overlap with our previously published sample.11
Table 1. Demographics, clinical and psychological variables.
| 22 Non-MDD | 31 MDD | Stats | ||||
|---|---|---|---|---|---|---|
| |
Mean |
s.d. |
Mean |
s.d. |
t/χ2 |
P |
| Demographic variables | ||||||
| Gender | 11 F | 11 M | 15 F | 16 M | 0.03 | 0.86 |
| Age (years) | 26.8 | 8.7 | 27.6 | 7.8 | 0.35 | 0.72 |
| Education (years) | 15.2 | 1.3 | 14.7 | 1.8 | 1.0 | 0.30 |
| Marital status | ||||||
| Married/living with partner | N=4 | N=4 | 2.4 | 0.30 | ||
| Single | N=18 | N=24 | ||||
| Separated/divorced | N=0 | N=3 | ||||
| Race | ||||||
| African American | N=2 | N=3 | 0.84 | 0.36 | ||
| Asian | N=5 | N=7 | ||||
| Caucasian | N=10 | N=13 | ||||
| Other | N=5 | N=8 | ||||
| Clinical variables | ||||||
| Age of MDD onset | 22 | 7 | ||||
| Number of previous episodes | 2 | 1 | ||||
| Comorbid diagnosis | ||||||
| Posttraumatic stress disorder | N=3 | |||||
| Generalized anxiety disorder | N=2 | |||||
| Panic disorder | N=4 | |||||
| Social phobia | N=2 | |||||
| Psychological variables | ||||||
| Beck Depression Inventory-2 | 1.1 | 1.9 | 25.5 | 8.4 | 13.0 | <0.01 |
| Post-scanner ratingsa | ||||||
| Low pain anticipationb | 1.5 | 0.4 | 1.6 | 0.3 | 0.19 | 0.8 |
| High pain anticipation | 4.3 | 0.7 | 4.4 | 0.6 | 0.14 | 0.9 |
| Low pain intensityc | 1.5 | 0.4 | 2.0 | 0.3 | 0.92 | 0.4 |
| Low pain unpleasantnessc | 1.3 | 0.4 | 1.6 | 0.3 | 0.55 | 0.6 |
| High pain intensity | 4.8 | 0.6 | 5.4 | 0.5 | 0.91 | 0.4 |
| High pain unpleasantness | 4.6 | 0.6 | 4.6 | 0.6 | 0.02 | 1.0 |
Abbreviations: F, females; M, males; MDD, major depressive disorder; Non-MDD, never-depressed controls.
Missing data in two MDD subjects.
Scale range from 0 to 5.
Scale range from 0 to 10 (see text for details).
To establish current and past psychiatric diagnoses, each subject underwent a Structured Clinical Interview for Diagnostic and Statistical Manual for Mental Disorders (DSM)-IV,28 which was administered by trained interviewers. Diagnosis was verified by consensus with a board certified Psychiatrist (SCM). The Beck Depression Inventory-229 was administered to quantify current depressive symptom severity. Subjects were excluded from the study if they: (1) used psychotropic medication within the last 30 days; (2) fulfilled DSM-IV criteria for alcohol/substance abuse or dependence within 30 days of study participation; (3) fulfilled DSM-IV criteria for lifetime bipolar or psychotic disorder; (4) had ever experienced a head injury; (5) had clinically significant comorbid medical conditions, such as cardiovascular and/or neurological abnormality, or any active serious medical problems requiring interventions or treatment; (6) had a history or current chronic pain disorder; (7) had irremovable ferromagnetic material; (8) were pregnant or claustrophobic; and (9) were left-handed. All female subjects were scanned during the first 10 days of their menstrual cycle.
Experimental pain paradigm
A validated cued pain-anticipation paradigm was used30 (Supplementary Figure 1s). Briefly, the paradigm had two temporal conditions (anticipation and stimulus), with the former having three stimulus conditions (anticipation of either high pain, low pain or uninformed pain) and the latter having two stimulus conditions (high pain stimulation or low pain stimulation). Thermal stimuli, experienced as moderately (6 s; 47.5 °C; rise/fall rate 10 °C s−1) and mildly (6 s; 45.5 °C; rise/fall rate 10 °C s−1) painful to the subject, were delivered in a pseudo-random and counterbalanced order through a 9-cm2 thermode (Medoc TSA-II, Ramat-Yishai, Israel) securely fastened to the subject's left volar forearm. Before scanning, subjects were pre-tested with several non-painful and painful temperature stimuli to ensure that temperatures were well tolerated. In the scanner, subjects were presented with a BLUE cross and were cued to anticipate ‘high pain' if the color of the cross changed to RED, to anticipate ‘low pain' if the color of the cross changed to GREEN and to anticipate ‘uninformed pain' (either high or low pain) if the color of the cross changed to YELLOW (50% probability). Subjects were instructed that during the task they would receive several thermal heat stimulations that produce high and low pain sensations. A total of 28 (14 high pain and 14 low pain) temperatures were delivered. High temperatures were preceded by the high anticipatory cue (that is, cross changed from BLUE to RED) seven times and by the uninformed cue (that is, cross changed from BLUE to YELLOW) seven times. Likewise, low temperatures were preceded by low anticipatory cue (that is, cross changed from BLUE to GREEN) seven times and by the uninformed cue seven times.
Post-task questionnaire
To measure the subjective experience of the task, subjects completed a post-task questionnaire. The following variables were measured: (1) anticipatory anxiety (from 0—‘not at all' to 5—‘extremely anxious'); (2) perceived pain intensity (0—‘no pain sensation' to 10—‘extreme pain sensation'); and (3) perceived pain unpleasantness (from 0—‘no unpleasantness' to 10—‘extreme unpleasantness'.
fMRI protocol
Two fMRI runs (412 brain volumes per run) sensitive to blood oxygenation level-dependent contrast were collected for each subject using 3.0 Tesla GE Signa EXCITE scanner (GE Healthcare, Milwaukee, WI, USA) (T2*-weighted echo planar imaging, TR=1500 ms, TE=30 ms, flip angle=90, FOV=23 cm, 64 × 64 matrix, 30 2.6-mm 1.4-mm gap axial slices) while they performed the paradigm described above (Supplementary Figure 1s). The fMRI acquisitions were time-locked to the onset of the task. During the same experimental session, a high-resolution T1-weighted image (FSPGR, TR=8 ms, TE=3 ms, TI=450 ms, flip angle=12, FOV=25 cm, 172 sagittal slices, 256 × 256 matrix, 1 × 0.97 × 0.97 mm3 voxels) was obtained for anatomical reference.
fMRI statistical analysis
All imaging data were analyzed with the Analysis of Functional NeuroImages software package.31 Preprocessed time-series data for each individual were analyzed using a multiple regression model corrected for autocorrelation consisting of three anticipation-related and two stimulus-related regressors, convolved with empirically derived HRF (http://afni.nimh.nih.gov/pub/dist/doc/program_help/waver.html), labeled as the Cox special. Anticipation-related regressors modeling the entire anticipation period consisted of: (1) anticipation of moderately painful heat stimulation, that is, high pain anticipation and (2) anticipation of mildly painful heat stimulation, that is, low pain anticipation. Because the uninformed cue did not contribute to our understanding of the specific mechanism of interest, this condition was modeled as a regressor of no interest in the current report, and a separate manuscript is being prepared that will explicitly examine the uninformed pain cue in a bigger sample. Stimulus-related regressors consisted of: (1) application of moderately painful heat, that is, high pain stimulation and (2) application of mildly painful heat, that is, low pain stimulation. Six additional regressors were included in the model as nuisance regressors: one outlier regressor to account for physiological and scanner noise (that is, the ratio of brain voxels outside of 2 s.d. of the mean at each acquisition), three movement regressors to account for residual motion (in the roll, pitch and yaw directions) and regressors for baseline and linear trends to account for signal drifts. To reduce the false positives induced by cross correlations of the time-series data were fit using the Analysis of Functional NeuroImages program 3dREMLfit. A Gaussian filter with a full-width half maximum of 4 mm was applied to the voxel-wise percent signal-change data to account for individual variation in the anatomical landmarks. Data from each subject were normalized to Talairach coordinates.32 The primary contrast between regression coefficients for the high versus low pain anticipation was entered into one-sample t-test to examine within-group brain response to pain anticipation, as well as a two-sample t-test to examine between-group differences in brain response to pain anticipation. A threshold adjustment method based on Monte Carlo simulations was used to guard against identifying false-positive areas of activation.33 Based on the whole-brain analysis using a 4-mm Gaussian filter, an a priori voxel-wise probability of P<0.05 in a cluster of 768 mm3 resulted in an a posteriori cluster-wise probability of P<0.05 (see Supplementary Information for further detail). The average percent signal changes only from clusters that survived this threshold/cluster method were extracted and used for exploratory post-hoc correlations. To verify task effects, the whole-brain activations within and between groups were also examined for high pain to low pain and are reported in the Supplementary Information. All post-hoc statistical analyses were performed with PASWStatistics18.0 (IBM, Chicago, IL, USA).
Functional connectivity analysis
We used functional connectivity method introduced by Fox et al.34 and a form of psychophysiological interaction method introduced by Friston et al.35 and adapted for the Analysis of Functional NeuroImages (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html). Data were preprocessed as above with minor modifications aimed at removing nonspecific physiological signals (see Supplementary Information for details). Individual time courses in these processed raw signal data sets were extracted for the seed region of interest within right AI (rAI) (from the high versus low pain-anticipation conjunction map) and the psychophysiological interaction regressors were created for each subject (see Supplementary Information for details). Dorsal rAI was chosen as a seed because of our aim to show that despite overlapping activation within this region during the anticipation of pain in both groups, dorsal rAI was still differentially utilized within a larger network in the MDD group. A multiple linear regression model as above was run thereafter to examine connectivity of the seed region during high versus low pain anticipation. Interaction time course was used as regressor of interest. The resulting correlation coefficient for the time course of interest was calculated for each voxel. This provided correlation maps for the time course in the seed region within rAI and the time course from all other brain voxels as a function of pain anticipation. The Fisher's z transforms of these correlation maps were then warped to conform to the Talairach atlas36 to allow for group comparisons of the Fisher's z transforms in rAI during high–low pain anticipation using independent two-sample t-tests. This assessed differences in functional connectivity in rAI during high versus low pain anticipation between MDD and non-MDD groups.
Results
Clinical measures
As indicated in Table 1, MDD subjects had significantly higher Beck Depression Inventory-2 scores consistent with moderate depressive symptoms. MDD subjects reported an average of two lifetime major depressive episodes. Non-MDD subjects had no history of MDD or other current or lifetime Axis-I psychiatric disorders. Among the MDD subjects, three male subjects met criteria for posttraumatic stress disorder, four males for panic disorder, two males for generalized anxiety disorder and two males for social phobia.
Post-task questionnaires
Subjects' ratings of their experience during the task are shown in Table 1. Repeated-measures analysis of variance with temperature (low and high) as a within-subject factor and group (MDD and non-MDD) as a between-subject factor showed no significant effect of group for either anticipatory anxiety (F (1, 29)=0.035; P=0.892), pain intensity (F (1, 29)=1.12; P=0.30) or pain unpleasantness rating (F (1, 28)=0.078; P=0.781). As expected, there was highly significant effect of temperature (or temperature cue), whereby high temperature resulted in higher rating of anticipatory anxiety (F (1, 49)=57.9; P<0.000), pain intensity (F (1, 49)=100.1; P<0.000) and pain unpleasantness (F (1, 48)=73.783; P<0.000). No significant group by temperature interactions were observed (anticipatory anxiety: F (1, 49)=0.02; P=0.965; pain intensity: F (1, 49)=0.12; P=0.73; and pain unpleasantness: F (1, 48)=0.227; P=0.636).
fMRI results
High versus low pain anticipation
Task effects (Figure 1a): Tables 2 and 3 show significant activation during high versus low pain anticipation in non-MDD and MDD subjects, respectively. Both groups showed significantly increased activation within bilateral insula, several areas within prefrontal and temporal cortices, as well as the cerebellum and decreased activation within parahippocampal gyrus.
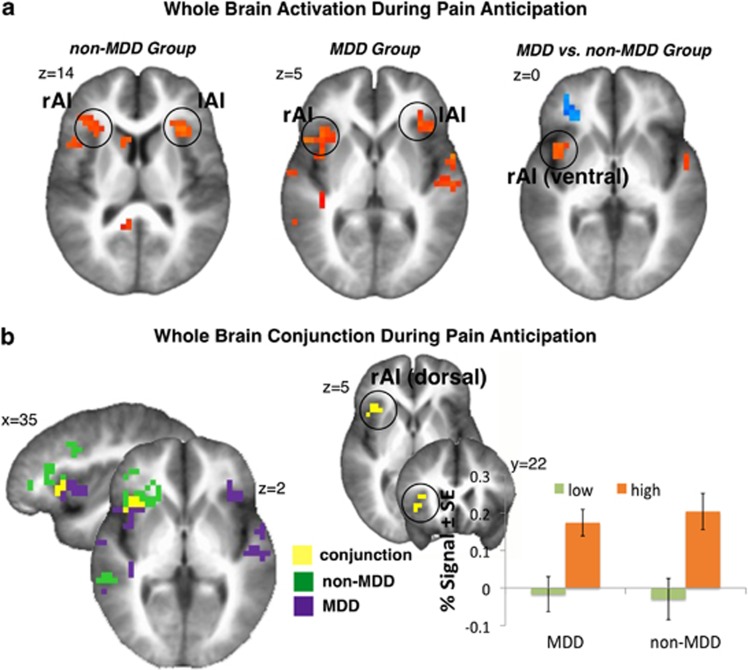
Figure 1.
Whole-brain significant task effects. (a) Significant whole-brain analysis of high versus low pain anticipation in the non-MDD (left), MDD (middle) and between-group contrast (right) showed increased bilateral dorsal AI activation in both groups and increased activation within ventral portion of the rAI in MDD (see Tables 2, 3, 4 for further details). (b) Whole-brain conjunction of high versus low pain anticipation in MDD (purple) and non-MDD (green) groups showed significant overlap (yellow) in right dorsal AI (x/y/z: 37/21/5, 512 mm3). Bar graphs indicate percent signal change within right dorsal AI during anticipation of low and high pain in MDD and non-MDD group. Left=Right. Non-MDD, healthy, never-depressed controls.
Table 2. High–low pain anticipation: non-MDD group whole brain.
| Brain region | Volume | x | y | z | Stat |
|---|---|---|---|---|---|
| High>low pain anticipation | |||||
| R. insula/IFG (BA 45) | 5184 | 32 | 28 | 6 | 2.66 |
| L. insula (BA 13) | 1216 | −35 | 21 | 14 | 2.53 |
| R. IFG (BA 9) | 1792 | 48 | 15 | 24 | 2.68 |
| R. MFG (BA 9) | 1408 | 41 | 12 | 34 | 2.72 |
| R. IPL (BA 40) | 2752 | 53 | −46 | 32 | 2.61 |
| L. cingulate (BA 23) | 1472 | −1 | −38 | 24 | 2.63 |
| R. MTG (BA 22) | 1216 | 54 | −38 | 3 | 2.68 |
| R. STG (BA 22) | 1024 | 48 | −19 | −5 | 2.52 |
| R. caudate | 768 | 11 | 9 | 11 | 2.83 |
| Cerebellar vermis | 768 | −3 | −29 | −12 | 2.84 |
| L. cerebellar tonsil | 896 | −38 | −46 | −37 | 2.45 |
| Low>high pain anticipation | |||||
| R. parahippocampal gyrus | 832 | 26 | −30 | −13 | 2.73 |
| L. precentral gyrus | 768 | −49 | −13 | 31 | 2.46 |
Abbreviations: BA, Brodmann area; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L., left; MFG, medial frontal gyrus; MTG, medial temporal gyrus; non-MDD, never-depressed controls; R., right; STG, superior temporal gyrus.
Table 3. High–low pain anticipation: MDD group whole brain.
| Brain region | Volume | x | y | z | Stat |
|---|---|---|---|---|---|
| High>low pain anticipation | |||||
| R. insula (BA 13) | 2688 | 37 | 16 | 5 | 2.62 |
| L. insula (BA 13) | 1920 | −38 | 18 | 10 | 2.66 |
| L. IFG (BA 47) | 960 | −37 | 26 | 2 | 2.95 |
| R. precentral gyrus | 768 | 55 | −12 | 10 | 2.39 |
| L. precentral gyrus | 832 | −57 | −5 | 6 | 2.45 |
| R. thalamus | 960 | 3 | −11 | 18 | 2.48 |
| R. STG (BA 22) | 2688 | 45 | −29 | −1 | 2.64 |
| L. STG (BA 21) | 1472 | −54 | −17 | 0 | 2.64 |
| L. culmen (BA 30) | 1216 | −9 | −32 | −7 | 2.61 |
| Low>high pain anticipation | |||||
| R. parahippocampal gyrus | 960 | 39 | −25 | −22 | 2.41 |
Abbreviations: BA, Brodmann area; IFG, inferior frontal gyrus; L., left; MDD, major depressive disorder; R., right; STG, superior temporal gyrus.
Group effects (Figure 1a): Table 4 shows significant between-group differences in brain activation during high versus low pain-anticipation contrast. MDD relative to non-MDD subjects showed increased activation within right ventral AI, left middle frontal gyrus, left cingulate and left superior temporal gyrus, whereas they showed decreased activation within right dorsolateral prefrontal and right orbitofrontal cortices.
Table 4. High–low pain anticipation: between-group differences whole brain.
| Brain region | Volume | x | y | z | Stat |
|---|---|---|---|---|---|
| MDD>non-MDD | |||||
| R. ventral anterior insula | 832 | 40 | 6 | 3 | 2.45 |
| L. MFG (BA 8) | 832 | −18 | 31 | 38 | 2.45 |
| L. cingulate gyrus (BA 31) | 1216 | −20 | −36 | 23 | 2.56 |
| L. STG (BA 22) | 896 | −52 | −1 | −3 | 2.59 |
| Non-MDD>MDD | |||||
| R. MFG (BA 47) (dlPFC, OFC) | 1472 | 32 | 37 | −2 | 2.46 |
| R. MFG (BA 9, dlPFC) | 896 | 43 | 12 | 34 | 2.61 |
Abbreviations: BA, Brodmann area; dlPFC, dorsolateral prefrontal cortex; L., left; MDD, major depressive disorder; MFG, medial frontal gyrus; non-MDD, never-depressed controls; OFC, orbitofrontal cortex; R., right; STG, superior temporal gyrus.
Conjunction: In order to examine in more detail pain anticipation-related insula activation, we performed conjunction analyses (Figure 1b). Both groups showed significant activation within dorsal rAI during high versus low pain anticipation, yet activation within the MDD group was more ventral and posterior. The area within dorsal AI of significant overlap between the two groups showed positive correlation with the Beck Depression Inventory-2 scores in the MDD group (ρ=0.40; P<0.05). This area was used as a seed for functional connectivity analyses in order to examine differences in recruited networks between the two groups.
Functional connectivity results
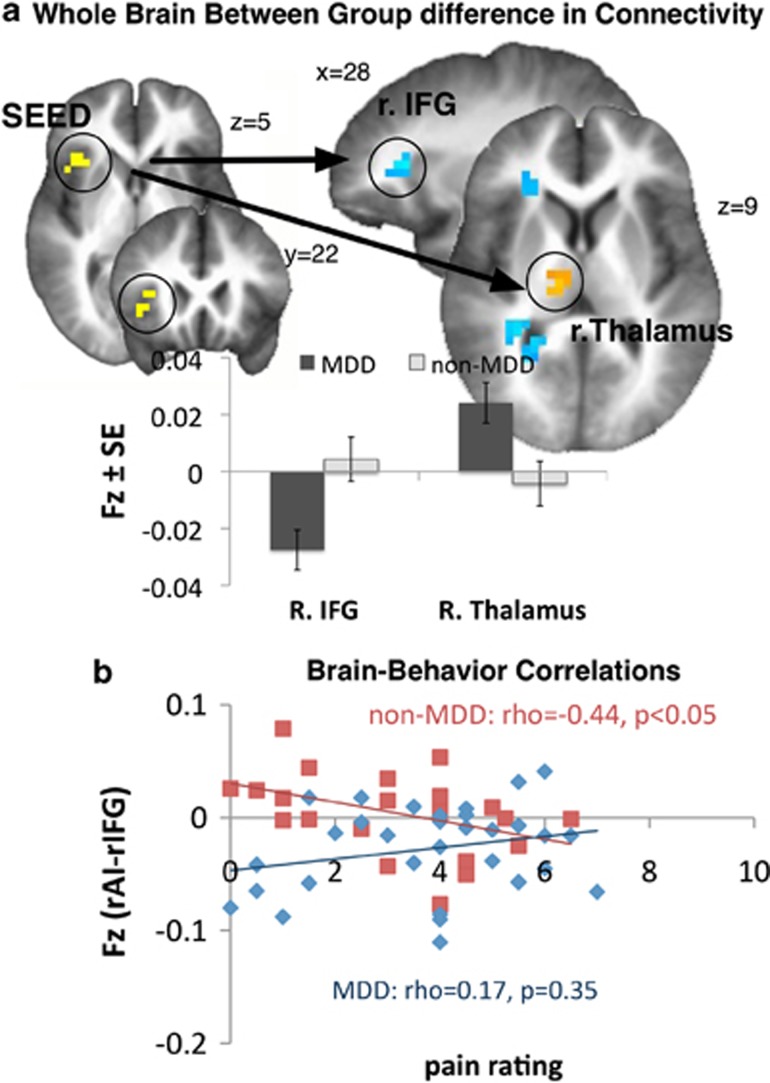
Using the dorsal rAI time series during high versus low pain anticipation from the conjunction map (see above) as a seed, four regions showed significant between-group differences in functional connectivity (Figure 2a, Table 5). Compared with healthy controls, right posterior thalamus and right middle prefrontal cortex (MPFC) showed increased connectivity, whereas right inferior frontal gyrus (rIFG) and cerebellum showed decreased connectivity during anticipation of pain in the MDD group.
Figure 2.
Whole-brain functional connectivity with dorsal insula. (a) Whole-brain between-group difference in functional connectivity using right dorsal AI (from the conjunction map) as a seed region (left) showed stronger rAI–rIFG connectivity in the non-MDD group and stronger rAI–thalamus connectivity in the MDD group during high versus low pain anticipation (see Table 5 for details). Bar graphs indicate Fisher's z transformations of the r values (Fz). (b) Significant negative correlation was found between the strength of functional connectivity between right dorsal AI and rIFG and subjective pain experience in the non-MDD group (red), whereby those subjects with strongest connections between rAI and rIFG during anticipation of pain provided lowest pain intensity (ρ=−0.43; P<0.05) and pain unpleasantness (ρ=−0.41; P=0.05) (not shown) rating. This relationship was not evident in the MDD group (blue) who showed nonsignificant relationship between rAI–rIFG connectivity and pain intensity (ρ=0.17; P=0.35), as well as pain unpleasantness (ρ=0.07; P=0.65) (not shown) rating. Furthermore, the between-group difference in the strengths of these correlations was significant for pain intensity (z=−2.16; P<0.05) and approached significance for pain unpleasantness (z=−1.72; P=0.08) rating.
Table 5. Functional connectivity with rAI: between-group differences whole brain.
| Brain region | Volume | x | y | z | Stat |
|---|---|---|---|---|---|
| MDD>non-MDD | |||||
| R. posterior thalamus | 576 | 10 | −16 | 7 | 2.2 |
| R. medial prefrontal cortex | 576 | 0 | 45 | 38 | 2.3 |
| MDD<non-MDD | |||||
| R. IFG | 768 | 25 | 29 | 10 | 2.4 |
| R. cerebellum | 1152 | 29 | −56 | −41 | 2.4 |
| 576 | 15 | −79 | −36 | 2.3 | |
Abbreviations: IFG, inferior frontal gyrus; L., left; MDD, major depressive disorder; non-MDD, never-depressed controls; rAI, right anterior insula; R., right.
Exploratory brain-behavior correlations
Prior literature by our group and others has shown that pain-related dorsolateral prefrontal cortex activation shapes pain experience.11, 37, 38 We therefore performed exploratory correlations between the strength of connectivity between dorsal AI and rIFG with the post-scan subjective pain rating (averaged across low and high pain) in both groups. Because both of the stimulations used in this study were painful and unpleasant (see Table 1), averaging across low and high pain was an optimal available proxy to the subjective experience in our subjects. A significant negative correlation was observed in the non-MDD group, whereby those subjects with the strongest connections between dorsal insula and rIFG during anticipation of pain provided the lowest pain intensity (ρ=−0.43; P<0.05) and pain unpleasantness (ρ=−0.41; P=0.05) rating (Figure 2b). Interestingly, this relationship was not evident in the MDD group who showed nonsignificant relationship between dorsal insula and rIFG connectivity and pain intensity (ρ=0.17; P=0.35), as well as pain unpleasantness (ρ=0.07; P=0.65) rating. Furthermore, the between-group difference in the strengths of these correlations was significant for pain intensity (z=−2.16; P<0.05) and approached significance for pain unpleasantness (z=−1.72; P=0.08) rating. We also explored whether anticipatory insula activation, and the connections between dorsal insula and thalamus, as well as dorsal insula and MPFC, related to subjective pain experience (both average and the difference in the perceived pain were examined). None of these correlations were significant (−0.25<ρ<0.25; P>0.05).
Discussion
One of the main findings of our study was that during anticipation of high pain compared with anticipation of low pain, the rAI activation differed in MDD compared with healthy subjects who never had MDD. We found that ventral insula was more strongly activated by the MDD group during anticipation of pain, whereas both groups similarly activated the dorsal insula. Furthermore, we found that despite similar recruitment of the dorsal insula by both groups, this region showed positive correlation with depressive symptom severity and stronger connectivity with posterior thalamus in our MDD subjects. Conversely, in the non-MDD group, dorsal insula was more strongly connected to the IFG, and the strength of this connection was inversely related to subjective pain experience in these subjects. Taken together, these results provide functional validation for the co-occurring enhanced ‘bottom-up' and attenuated ‘top-down' processing of salient, unpleasant emotional information in MDD.13
Besides segregated anatomical regions within the insular cortex,39, 40 several lines of evidence also clearly show that the insula is subdivided into several functional domains.17 In addition, meta-analyses based on published fMRI and positron emission tomography studies suggest that even within AI, that is, a region just anterior to the central sulcus of the insula, the processing of emotionally laden stimuli is represented in several regions.26, 41 Studies that examine cognitive aspects of emotional processing (for example, appraisal) find the peak coordinates in more dorsal aspects,26, 41 whereas studies that examine more visceral response associated with emotional experience (for example, heart rate and galvanic skin response) find the peak coordinates in more ventral aspects of the AI.42 This ventral insula is thought to integrate emotionally salient information from all sensory modalities with subcortical homeostatic control centers (amygdala and hypothalamus)17, 19, 43, 44 and is also the site of insular co-activation with the amygdala and pregenual anterior cingulate.25, 42, 45, 46 Therefore, increased activation within ventral insula in MDD during anticipation of pain found here is consistent with increased visceral response to salient cues in this disorder,13 and more intense affective experience.45
We also found that dorsal AI was similarly active between the two groups during anticipation of heat pain in our study. This dorsal insula is more involved in cognitive processing.18, 26, 41, 45, 47, 48, 49 Although both groups activated this region similarly, several results in the current study point to differential utilization of this region by the two groups. First, this warning signal was related to greater depressive symptomology in the MDD group, potentially, underlying increased hopelessness that can follow ineffective coping with salient cues in depressed individual.50 Second, this region showed differential connectivity. Specifically, we found that dorsal insula was more strongly connected to the rIFG in healthy volunteers than in our MDD subjects. This pattern of connectivity in healthy subjects is consistent with several recent functional24, 25, 45, 47, 51 and anatomical46 studies. For example, Peltz et al.51 found that during pain, right dorsal insula is connected with the prefrontal cortex, whereas Deen et al.25 found that during resting state, dorsal aspects of the rAI are connected with the rIFG. This is also consistent with Cauda et al.24 who found that dorsal insula is working together with the attentional network, which is critical for cognitive control of emotion, and the rIFG is a key factor in this network,52 and with Cerliani46 who found that dorsal insula has strong anatomic connections with the IFG. Taken together, these findings suggest that healthy, never-depressed subjects showed adaptive emotional response during pain anticipation, whereby they engaged frontal control network as evidenced by increase in connectivity between dorsal insula and rIFG in order to modulate the unpleasant experience of the upcoming pain. This adaptive and effective mechanism or ‘healthy' pain anticipation is further supported by our exploratory correlations, whereby significant negative relationship between the strength of this connection and subjective pain experience was observed only in the non-MDD subjects. Although biases in retrospective recalls cannot be ruled out, these findings are in direct agreement with prior literature37, 53, 54 and suggest that through greater frontal regulation healthy volunteers can modulate their subjective pain experience. This potential top-down control mechanism was diminished in our MDD group who employed different resources to regulate their subjective experience.
In the MDD group, the right posterior thalamus showed increased connectivity with dorsal insula. Specifically, this region lied within the medial dorsal nucleus and spanned from ventromedial to the pulvinar nuclei in the anterior-posterior direction. MDD has been associated with abnormalities within thalamus,55, 56, 57 particularly, within mediodorsal thalamus58 and pulvinar.13 The region of the thalamus observed in our study is known to project to the anterior agranular insula40, 59 and to the anterior cingulate44 and is highly interconnected with the amygdala.60 The pulvinar shows similar anatomical connections to amygdala61, 62 and the anterior cingulate.63 These connections are believed to relay salient emotional information about the environment to the limbic system.64 Strong functional connections between the AI and the thalamus65 are consistent with the cytoarchitecture and paralimbic emotional salience processing.66 Therefore, the heightened connectivity between dorsal insula and the thalamus during anticipation of pain in MDD points to increased bottom-up emotional response to aversive stimulation in this disorder. This fits recent findings showing that increased activation within rAI and this region of the thalamus was modulated by anxiety sensitivity during threat anticipation.67 Increased connectivity between dorsal insula and dorsal MPFC observed in the MDD group further strengthens this interpretation. Specifically, recent reviews of functional neuroimaging studies on fear conditioning attributed activation within MPFC to conscious appraisal of threat68 or expression of fear and anxiety.69 Therefore, increased connectivity between dorsal insula and MPFC in the MDD subjects during pain anticipation observed here was likely related to the initial response formation to the pain cue. Alternatively, increased bottom-up (that is, thalamic) response in MDD could, in part, be counterbalanced by greater top-down response within MPFC; this could potentially explain the lack of significant between-group difference in dorsal insula in our study. Taken together, the co-occurrence of the increased bottom-up and reduced top-down regulation during anticipation of pain that we observed here may underlie heightened somatic complaints70 and increased negative biasing71 in MDD.
This study had several limitations. Although the sample size is moderately large for an imaging study, a larger sample may help with the differentiation of functional subregions within the insula. Furthermore, we used brief experimental heat pain as a model for inducing acute pain and distress. Even though insula has an integral role in both acute and chronic stress and pain conditions,20, 72, 73 further studies are needed to determine generalizability of these findings to more chronic situations. Importantly, the experimental paradigm was designed to focus on the neural process during cued anticipation of pain and to compare this neural process on both contrast and connectivity levels between the groups. Although using explicit cuing and contrasting two painful stimuli minimized possible group differences in expectation biases, fear conditioning74 and/or learning, their contribution cannot be completely ruled out. Related to these concerns is also the fact that a cue influences the subsequent stimulus appraisal. Therefore, because of the known temporal biasing effects in depression, the interpretation of the pain findings in the current paradigm must not be overgeneralized to cases of uncued pain. Furthermore, we opted not to use in scanner ratings of subjective pain experience. Although implementation of a behavioral measure within the task could have potentially strengthened our interpretations, preparing to and making decisions about pain may also interfere with neural process of emotional anticipation.75 In addition, although prior research has shown that average within- and post-scan pain ratings do not differ significantly,76 retrospective ratings are subject to several types of response bias (for example, averaging and forgetting), which very well may differ between MDD and non-MDD subjects. The use of retrospective ratings could also have contributed to the lack of significant correlations between AI activation and subjective pain experience in our study. However, considering that the neural process, and also the subjective emotional experience, may not necessarily be reflected by observable behavior, we focused on the neural process of pain anticipation in the current design. Finally, we selected a group of non-medicated younger adults in this study to allow for as few confounds in understanding the underlying neural substrates in relation to depression. However, this may limit the generalizability of this sample to complicated, aging or medicated samples.
In summary, our data show increased ventral AI activation during pain anticipation in unmedicated subjects with MDD. This result is consistent with prior work describing maladaptive anticipatory response to salient emotional cues in this disorder. Furthermore, our functional connectivity data demonstrate the co-occurrence of the increased ‘bottom-up' and decreased ‘top-down' neural response during pain anticipation in MDD. These connectivity results provide mechanistic validation for several influential models of MDD.13, 16, 77 Taken together, our findings improve understanding of how MDD may affect ‘mind–body' connections and increase vulnerability for developing pain-related conditions.78
Acknowledgments
We thank the National Institute of Mental Health (MH8003 to IAS) and the Veterans Administration (Merit Grant to ANS, Clinical Science R&D Career Development Award to SCM, and the Center of Excellence in Stress and Mental Health to ANS and IAS) for supporting this work and to Lindsay E. Reinhardt for assisting with data collection. We are indebted to Dr Bud Craig for mentorship and conceptual input.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34 (Pt 1:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker CD. Stress and selective attention: the interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39:723–732. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. J Pers Soc Psychol. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Bates DE. Unmasking a cognitive vulnerability to depression: how lapses in mental control reveal depressive thinking. J Pers Soc Psychol. 1998;75:1559–1571. doi: 10.1037//0022-3514.75.6.1559. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN. Right anterior insula hypoactivity during anticipation of homeostatic shifts in major depressive disorder. Psychosom Med. 2010;72:316–323. doi: 10.1097/PSY.0b013e3181d07873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2006;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Herwig U, Brühl AB, Kaffenberger T, Baumgartner T, Boeker H, Jäncke L. Neural correlates of ‘pessimistic' attitude in depression. Psychol Med. 2010;40:789–800. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: multivariate granger causality analysis of resting-state fmri time-series data. Mol Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD.Interoception and emotion: a neuroanatomical perspectiveIn: Lewis M, Haviland-Jones JM, Barrett LF, (eds).Handbook of Emotions3rd edn.Guilford Publications: New York; 272–288.2008 [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- Herwig U, Bruhl AB, Kaffenberger T, Baumgartner T, Boeker H, Jancke L. Neural correlates of 'pessimistic' attitude in depression. Psychol Med. 2009;7:1–12. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Wagner G, Koschke M, Boettger S, Boettger MK, Schlosser R, et al. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. 2007;62:1281–1287. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2010;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN, Oberndorfer T, Klabunde M, Reinhardt LE, et al. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. Int J Eat Disord. 2012;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Clinician Version (SCID-1) American Psychiatric Press Inc.: Washington, DC; 1997. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Strigo I, Matthews S, AN S, Oberndorfer T, Klabunde M, LE R, et al. Altered insula activation during pain anticipaton in individuals recovered from anorexia nervosa: evidence of interoceptive dysregulation. Int J Eat Disord. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme: New York; 1988. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van EDC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126 (Pt 5:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil DCH, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex. 2010;20:1448–1461. doi: 10.1093/cercor/bhp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, et al. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, et al. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum Brain Mapp. 2011;33:2005–2034. doi: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2012;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Rumination, negative cognition, and their interactive effects on depressed mood. Emotion. 2007;7:555. doi: 10.1037/1528-3542.7.3.555. [DOI] [PubMed] [Google Scholar]
- Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien-Baumann B, Zundorf G, Triemer A, Ludecke S, Winiecki P, et al. Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand. 2004;110:184–194. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Young KA, Holcomb LA, Yazdani U, Hicks PB, German DC. Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry. 2004;161:1270–1277. doi: 10.1176/appi.ajp.161.7.1270. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Burton H, Jones EG. The posterior thalamic region and its cortical projection in new world and old world monkeys. J Comp Neurol. 1976;168:249–301. doi: 10.1002/cne.901680204. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain Res. 1976;104:142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Le Doux J. The Emotional Brain: the Misterious Underpinnings of Emotional Life. Simon & Schuster: New York, NY; 1996. [Google Scholar]
- Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, et al. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Ramel W, Edge MD, Hyde LW, Kuo JR, Goldin PR, et al. Neural mechanisms underlying 5-HTTLPR-related sensitivity to acute stress. Am J Psychiatry. 2012;169:397–405. doi: 10.1176/appi.ajp.2011.10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M-L, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2010;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameroff MJ, Olfson M. Major depressive disorder, somatic pain, and health care costs in an urban primary care practice. J Clin Psychiatry. 2006;67:1232–1239. doi: 10.4088/jcp.v67n0809. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, Experimental and Theorietical aspects. Hoeber Medical Division, Harper & Row: New York; 1967. [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32:1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Grimes EM, Allard CB, Reinhardt LE, et al. Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biological Psychiatry. 2010;68:442–450. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. A re-examination of pain-cognition interactions: implications for neuroimaging. Pain. 2007;130:8–13. doi: 10.1016/j.pain.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Christenfeld N. Memory for pain and the delayed effects of distraction. Health Psychol. 1997;16:327–330. doi: 10.1037//0278-6133.16.4.327. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.