Abstract
The antiphospholipid syndrome (APS) is characterized by recurrent vascular thrombosis, thrombocytopenia and fetal loss occurring in the presence of antiphospholipid antibodies (aPL). Along with arterial and venous thrombosis and pregnancy complications, patients with APS have an increased risk of myocardial infarction, stroke and coronary artery disease, resulting from vascular cell dysfunction induced by aPL. Accumulating evidence to date indicates that interactions between circulating aPL and cell surface molecules of target cells, primarily endothelial cells and platelets, underlie the vascular disease phenotypes of APS. However, the molecular basis of APS is poorly understood. Nitric oxide produced by endothelial cells is a key determinant of vascular health that regulates several physiologic processes including thrombosis, endothelial-leukocyte interaction, vascular cell migration, and the modulation of vascular tone. This review will discuss recent findings that indicate a novel mechanism by which aPL antagonize endothelial cell production of nitric oxide and thereby promote thrombosis.
Keywords: Antiphospholipid syndrome, Apolipoprotein E receptor 2, β2-glycoprotein I, Endothelium, Endothelial nitric oxide synthase, Nitric oxide, Platelets, Thrombosis, Nitric oxide, Inhibition
Introduction
The antiphospholipid syndrome (APS) is a systemic autoimmune disorder marked by the presence of antiphospholipid antibodies (aPL) in the circulation that contribute to enhanced risk for vascular thrombosis and pregnancy complications[1-4]. APS afflicts a significant number of patients with systemic lupus erythematosus (SLE), with as many as 34% of SLE patients having circulating aPL, as well as individuals without another underlying disorder[5]. Patients with APS also have an increased risk of cardiovascular diseases, such as coronary artery disease, myocardial infarction, and stroke stemming from vascular cell dysfunction[6]. Nitric oxide (NO) is a key determinant of vascular health that regulates several physiologic processes including thrombosis, endothelial-leukocyte interaction, vascular cell migration and proliferation, and the modulation of vascular tone and permeability[7]. Impaired NO bioavailability represents a central feature of endothelial and platelet dysfunction that contributes to intravascular thrombosis and a number of vascular diseases. The primary source of NO in the vascular wall under normal conditions is the endothelial isoform of NO synthase (eNOS).
This review will provide a brief overview of the role of NO and eNOS in regulation of platelet activation and thrombosis. It will then highlight the recent findings both in cultured cells and in mouse models that demonstrate the antagonism of eNOS by aPL. The molecular mechanisms by which aPL cause eNOS inhibition and thrombosis will be discussed in details. Novel interventions directly based on the pathogenetic mechanisms will be further considered that may be rapidly translated into new prophylactic or therapeutic strategies to combat the devastating impact of APS.
1. Anti-thrombotic Actions of Nitric Oxide
NO is a critical signal transduction molecule in the vascular system. NO is produced by three subtypes of NOS; nNOS (neuronal NOS, or NOSI), iNOS (inducible NOS or NOSII) and eNOS (endothelial NOS or NOSIII). The primary source of NO in the vascular wall under normal conditions is the endothelial isoform of NO synthase (eNOS). In addition to the endothelium, platelets and megakaryocytes express eNOS and they synthesize NO upon stimulation by a variety of agonists including thrombin and insulin [8-11].
The role of NO in the development of thrombosis has been investigated in animal models using inhibitors of NOS and its substrate L-arginine. In a rat model of thromboembolic stroke, infusion of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) caused an increase in platelet deposition and a reduction in global flow[12], indicating that both thrombotic and hemodynamic determinants contribute to the enhanced cerebral stroke. The role of endogenous NO production in the development of glomerular thrombosis associated with septic shock was studied an endotoxin-induced model of renal thrombosis[13]. Administration of endotoxin increased NO production, and this effect was inhibited by infusion of L-NAME. Kidneys from rats given endotoxin and L-NAME showed enhanced thrombosis in glomeruli as compared to those from rats given either endotoxin or L-NAME alone. In a rat model of nephrotoxic nephritis, enhanced NO production was observed, and the animals depleted of plasma L-arginine developed systemic hypertension and glomerular thrombosis, suggesting that the enhanced production of NO in this condition prevents acute glomerular injury[14]. In a canine model of coronary occlusion, thrombus formation in the coronary artery was delayed by administration of L-arginine[15]. Infusion of L-arginine also enhanced lysis of thrombus in the coronary arteries and inhibited platelet aggregations ex vivo. In a study of rabbit mesenteric venules, inhibition of NOS by Nω-nitro-L-arginine increased the duration of embolization and the number of emboli[16]. In contrast, infusion of L-arginine prevented the increase in venous embolization. Importance of eNOS in prevention of platelet aggregation and thrombosis has been also demonstrated in eNOS knockout mice[17]. These mice show increased propensity to thrombosis, stroke and atherosclerosis[18-21], and platelets isolated from eNOS deficient mice display enhanced aggregation[8]. The effect of exogenous NO on thrombosis has also been evaluated. Incubation with NO donor, S-nitroso-N-acetylcysteine, inhibits the upregulation of platelet surface glycoproteins including P-selectin and the integrin glycoprotein IIb/IIIa complex [22]. In a canine model of coronary artery stenosis associated with thrombus-dependent reductions in coronary blood flow, NO donor S-nitroso-bovine serum albumin reduced the frequency of flow cycles[23]. These cumulative studies in animal models highlight the anti-thrombotic actions of eNOS and NO.
The molecular basis of NO actions on platelets has been mainly attributed to its stimulation of soluble guanylate cyclase to produce cyclic GMP (cGMP)[24-26]. The increased production of cGMP leads to the stimulation of cGMP-dependent protein kinase that results in a reduction in fibrinogen binding to glycoprotein IIb/IIIa and modulation of phospholipase A2- and C-mediated responses[25;26]. NO also suppresses the agonist-dependent increase in cytosolic free calcium in a cGMP-dependent manner in isolated platelets[27]. More recently, cGMP-independent regulation of platelet function has also been reported[28;29]. NO suppresses exocytosis of Weibel-Palade bodies, endothelial granules that mediate vascular inflammation and thrombosis, by regulating the activity of N-ethylmaleimide-sensitive factor (NSF). NSF is a key component of the exocytic machinery, and NO inhibits NSF-mediated disassembly of soluble NSF attachment protein receptor complexes by nitrosylating critical cysteine residues of the factor.
Insufficient production of endogenous NO is also associated with thrombosis in clinical disorders in humans[30;31]. It is well established that thrombosis is a common cause of myocardial infarction and unstable angina[32;33], and platelets from patients with these conditions are activated with increased surface expression of P-selectin and active glycoprotein IIb/IIIa[34]. These platelets isolated from these patients produced less NO compared to patients with stable coronary artery disease, and the elevation of surface expression of P-selectin and glycoprotein GPIIb/IIIa was reduced by treatment with NO donors, such as nitroglycerin or S-nitrosoglutathione[35]. These results suggest that impaired NO production may contribute to the development of acute coronary syndromes by altering platelet function and consequent thrombus formation. Patients with atrial fibrillation, a condition associated with increased intracardiac thrombosis and cerebral embolism, have decreased plasma levels of nitrite and nitrate as well as lower levels of platelet cGMP[36]. Thrombosis has also been associated with NO deficiency in non-cardiac clinical disorders. Women with preeclampsia, a disorder with hypertension and intrarenal thrombosis during pregnancy, had lower levels of urinary cyclic GMP compared to noneclamptic women[37]. Recently, the role of human eNOS polymorphisms in thrombosis has been examined. Several polymorphisms in eNOS gene have been reported, including the ecNOS4a/4b in intron 4, the E298D mutation in exon 7, and the T786C in the promoter region. The E298D polymorphism of the eNOS gene was associated with an increased risk of hypertension, myocardial infarction and stroke in patients homozygous for this variant[38-41]. Another eNOS polymorphism ecNOS4a has been associated with severe stenosis in arteries and a history of myocardial infarction in smokers[42]. The polymorphism in the promoter region was associated with lower levels of platelet-derived NO and increased release of superoxide[43]. Taken together, these data suggest that select eNOS variants may influence thrombotic propensity in humans.
In summary, NO produced by eNOS in endothelial cells and platelets modulates a number of vascular processes including thrombosis that are known to be altered in APS patients.
2. NO Antagonism in Thrombosis in Antiphospholipid Syndrome
(a) eNOS Antagonism in Antiphospholipid Syndrome
As discussed above, eNOS and NO modulate critical vascular processes that are known to be adversely affected in APS. A potential link between APS and changes in bioavailable NO has been reported in both mouse models and humans. In mouse models, the administration of polyclonal aPL isolated from human patients or monoclonal β2GPI antibodies reduce plasma concentrations of NO metabolites and they also attenuate Ach-induced relaxation in isolated aortic rings, which is an NO-dependent process[60;61]. In humans, plasma aPL levels are inversely correlated with urinary NO metabolite excretion, and APS patients have lower levels of plasma nitrites compared to control subjects[62;63]. As such, the evidence both in mouse models and in humans supports a direct role for impaired NO production in the pathogenesis of APS.
This possibility has been directly investigated recently in studies by Ramesh et al., in which impact of aPL was tested in cultured endothelial cells and mouse models[64;65]. Cultured aortic endothelial cells were treated with aPL or normal human IgG (NHIgG) isolated from APS patients or healthy individuals, respectively, and their effects on eNOS activation induced by vascular endothelial growth factor (VEGF) was evaluated. Whereas NHIgG did not show any effect on VEGF-induced eNOS activation, aPL completely inhibited the activation. They further demonstrated that aPL induced increase in monocytes adhesion to the cultured endothelial cells and that this effect of aPL was prevented by addition of exogenous NO donor, S-Nitroso-N-acetylpenicillamine. These results demonstrate that aPL antagonize eNOS, leading to diminished NO production that induces monocytes adhesion in cultured endothelial cells. The antagonism of eNOS was further evaluated in vivo using carotid vascular conductance responses to acetylcholine (Ach) in mice, which are the indicator of endothelium-derived NO-dependent vascular relaxation[66]. Ach-mediated increases in carotid artery vascular conductance were assessed before and following the intravenous injection of either NHIgG or aPL. The mice injected with NHIgG showed normal response to Ach; in contrast, the mice injected with aPL showed marked attenuation of the vasodilatory response. These findings both in cell culture and in mice reveal for the first time that aPL have an inhibitory effect on eNOS.
Considering that NO plays a critical role in preventing thrombus formation and leukocyte adhesion to endothelium[67], Ramesh et al. further tested whether the eNOS antagonism by aPL is responsible for thrombus formation and leukocyte-endothelial cell adhesion in the mesenteric microcirculation using intravital microscopy[64]. Wild-type or eNOS deficient (eNOS−/−) mice were treated with either NHIgG or aPL, and 24 hours later endothelial cell-leukocyte adhesion and thrombosis induced by ferric chloride were studied. A marked increase in leukocyte adhesion and thrombus formation was observed in aPL-treated wild-type mice. In contrast, in eNOS−/− mice aPL did not induce increase in either leukocyte adhesion or thrombosis. These results provide the causal link between eNOS antagonism and the increase in leukocyte-endothelial cell adhesion and thrombus formation induced by aPL.
(b) Molecular Basis of eNOS Antagonism by Antiphospholipid Syndrome
Involvement of β2-glycoprotein I
Antibodies against phospholipid-binding proteins, particularly β2-glycoprotein I (β2GPI) are the major pathogenic antibodies in APS, and elevated levels of circulating anti-β2GPI antibodies are directly associated with both vascular and obstetric complications in APS patients[68;69]. β2GPI is a plasma protein composed of five distinctive domains (domains I-V), and it has been demonstrated that domain V of the protein binds to phospholipids on the surface of target cells such as platelets[69]. Studies using in vitro system have found that binding of antibody to phospholipid-bound β2GPI induces its dimerization and conformational change, which further increase its affinity for negatively-charged phospholipids on the cell surface[70;71]. In cultured endothelial cells, anti-β2GPI monoclonal antibodies have been shown to enhance adhesion molecule expression and the synthesis of cytokines, endothelin-1, and tissue factor, and in isolated platelets they increase the production of thromboxane B2, adhesion to collagen and aggregation[44;71-73]. In an animal model of photochemically induced arterial thrombosis, monoclonal β2GPI antibodies promote thrombus formation[47]. In APS patients, a strong relationship has been reported between the circulating levels of anti-β2GPI domain I antibodies and clinical symptoms[74-76].
Role of β2GPI in aPL antagonism of eNOS was first assessed by loss-of-function experiments comparing the actions of aPL in the presence or absence of β2GPI on the endothelial cell surface[64;77;78]. When cells were deprived of β2GPI, aPL did not cause eNOS inhibition, indicating that β2GPI is required for the aPL action. The role of β2GPI was further confirmed by evaluating the actions of anti-β2GPI monoclonal antibodies[79;80]. Monoclonal anti-β2GPI antibodies that specifically recognize domain I caused attenuation of VEGF-induced eNOS activation, mimicking the effect of the aPL that are isolated from APS patients. In contrast, another monoclonal antibody that recognizes domain II of β2GPI had no inhibitory effect on eNOS activation. The requirement for the β2GPI dimerization was further tested using purified β2GPI dimers on eNOS activation[64]. Whereas monomeric β2GPI had no effect on eNOS activation, dimerized β2GPI completely inhibited eNOS. These cumulative findings in cultured endothelial cells reveal that aPL-induced dimerization of β2GPI is required for aPL antagonism of eNOS, and that aPL binding to domain I of β2GPI likely mediates the process, providing a potential basis for the human studies[74].
Requirement for apoER2
Apolipoprotein E receptor 2 (apoER2), also known as LRP8, is a transmembrane protein that plays an important role in neuronal development as a signal transduction receptor for the glycoprotein Reelin[81]. Previous works using purified proteins in vitro have demonstrated that dimerized β2GPI binds to multiple members of the LDL receptor family including apoER2[82;83]. In addition to its abundant expression in brain, splice variants of apoER2 (designated apoER2’) have been detected in platelets and megakaryocytic cell lines[82-84]. Recent studies in isolated platelets have shown that platelet activation induced by dimerized β2GPI requires apoER2’[83].
Since aPL antagonism of eNOS is mediated by dimeric β2GPI and that apoER2 is expressed in endothelial cells, Ramesh et al. investigated the requirement for LDL receptor family protein, particularly apoER2, in aPL-induced eNOS antagonism[64]. In cultured endothelial cells, the LDL receptor family inhibitor, RAP (receptor-associated protein) [85] prevented aPL inhibition of eNOS, indicating that LDL receptor family protein is required for the aPL action. The involvement of apoER2 in particular has been evaluated using the specific knockdown of apoER2 protein by small interference RNA (siRNA). Knockdown of the receptor markedly attenuated the inhibitory effect of aPL on eNOS activation. It also prevented the enhancement of monocytes adhesion to the endothelium caused by anti-β2GPI monoclonal antibody. The role of β2GPI-apoER2 interaction in aPL antagonism of eNOS has been further investigated using a small peptide inhibitor. In previous studies in isolated platelets, a soluble peptide based on the sequence of the first LDL-binding domain of apoER2, designated sBD1, prevented the interaction of β2GPI with the receptor[83;84]. In cultured endothelial cells, sBD1 fully prevented the inhibition of eNOS by aPL, indicating that interaction between BD1 of the apoER2 and domain V of β2GPI is required for aPL antagonism of eNOS. These findings in cultured endothelial cells have revealed that apoER2 plays a critical link that connects the aPL recognition of β2GPI on the endothelial cell surface with the intracellular events leading to eNOS antagonism.
Ramesh and the colleagues further evaluated the role of apoER2 in aPL-induced thrombus formation and leukocyte adhesion in vivo using apoER2+/+ and apoER2−/− mice[64]. First to determine whether apoER2 is the transmembrane protein coupling aPL to eNOS, the effect of aPL on Ach-mediated increases in carotid vascular conductance was assessed in apoER2+/+ and apoER2−/− mice. Whereas the administration of aPL caused marked reduction of the vasodilatory response to Ach in apoER2+/+ mice, Ach-induced vasodilation was identical before and after aPL treatment in apoER2−/− mice. The involvement of apoER2 in aPL-induced increases in leukocyte adhesion to endothelium and in thrombus formation was also determined. ApoER2+/+ or apoER2−/− mice were treated with NHIgG or aPL, and 24 hours later endothelial cell-leukocyte adhesion or thrombus formation was evaluated using intravital microscopy. In wild-type apoER2+/+mice, aPL increased leukocyte adhesion to endothelium and it also enhanced thrombus formation. In contrast, aPL had no effect on leukocyte-endothelial cell adhesion or thrombosis in apoER2−/− mice. Importantly, the involvement of apoER2 in aPL-induced thrombosis has been independently demonstrated by other investigators[86]. They found that in wild-type mice human polyclonal aPL or murine anti-β2GPI monoclonal antibody increased tissue factor production and thrombus formation. In contrast, these pathologic effects of the antibodies were reduced in apoER2−/− mice. Furthermore, they showed that the blocking peptide sBD1 that interferes with β2GPI-apoER2 interaction attenuates these pathologic events invoked by aPL or anti-β2GPI antibody. These recent cumulative findings indicate that apoER2 mediates aPL-induced eNOS antagonism and tissue factor upregulation, contributing to the development of the vascular disease phenotypes of APS.
Intracellular Signaling Pathway Involved in aPL antagonism of eNOS
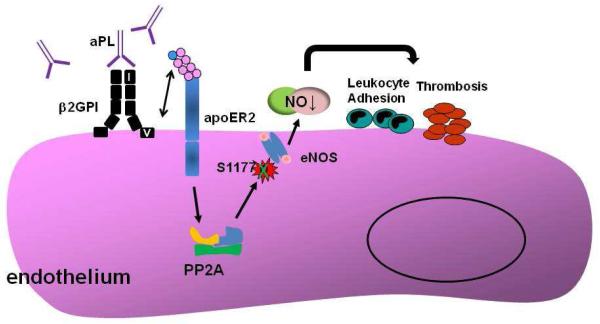
The molecular basis for aPL antagonism of eNOS was further investigated using cultured endothelial cells focusing on the regulation of the critical phosphorylation of the enzyme[64]. eNOS stimulation by multiple agonists including VEGF entails phosphorylation at the critical serine residue of the enzyme (S1177 in human) by the upstream kinase Akt [87;88]. Pre-incubation with aPL, but not with NHIgG, caused inhibition of VEGF-induced S1177 phosphorylation. To determine the basis for impaired S1177 phosphorylation by aPL, potential changes in Akt activation were then assessed. Whereas aPL treatment blunted eNOS S1177 phosphorylation induced by VEGF, activation of upstream kinase Akt was unaffected. This result suggests that aPL attenuate eNOS phosphorylation of S1177 via a mechanism distal to Akt. The phosphorylation of the enzymatic is known to be negatively regulated by the protein phosphatase 2A (PP2A)[89;90]. The role of PP2A in aPL-induced changes in eNOS activity was evaluated by siRNA knockdown. Decreased expression of PP2A by RNAi prevented aPL inhibition of eNOS. These findings in cultured endothelial cells indicate that aPL attenuate eNOS phosphorylation and activation through activation of PP2A. The pathway in which aPL leads to eNOS antagonism is depicted in Figure 1.
Figure 1.
The molecular mechanism of aPL antagonism of eNOS. Circulating aPL binding to domain I of β2GPI induces β2GPI dimerization, and interaction between domain V of β2GPI and the first LDL binding domain of apoER2 (shown in blue circle). Through yet-to-be-determined mechanism(s), the interaction of β2GPI with apoER2 causes increased activation of the phosphatase PP2A. This promotes the dephosphorylation of Ser1177 of eNOS yielding decreased enzyme activity and a decline in bioavailable NO, which contributes to increased leukocyte adhesion and enhanced thrombosis.
Unanswered Questions
In addition to recurrent arterial and venous thrombosis, patients with APS suffer from increased risk of cardiovascular diseases. Recent studies shed new lights on the underlying molecular mechanisms of vascular phenotypes of the disorder, revealing that aPL antagonize eNOS through binding to β2GPI on the cell surface and its interaction with the plasma membrane receptor apoER2. Experiments in mouse models indicate that the inhibition of eNOS and resulting attenuation in bioavailable NO contributes to thrombus formation and leukocyte adhesion to vascular endothelial cells. However, there are many aspects of the molecular mechanisms of aPL action that warrant further investigation. First, we do not know how the membrane receptor apoER2 transduces the signal initiated by binding to aPL-βGPI. Possible roles for known adaptor molecules of apoER2, such as Disabled-1, need to be considered [81;83]. Second, it is entirely unknown how apoER2 activates the phosphatase PP2A. PP2A is a serine/threonine phosphatase which regulates many cellular functions[91]. PP2A is composed of structural scaffolding (A), catalytic (C) and regulatory (B) subunits. The activity and substrate specificity of PP2A can be regulated through post-translational modification of the catalytic C subunit and through regulation of the B subunits, which are responsible for targeting different phosphoprotein substrates to PP2A. Each B-subunit has a distinct tissue and/or subcellular localization. The ability of aPL to promote dephosphorylation of eNOS via PP2A is not unique to aPL; prior studies have shown that endostatin, endothelial differentiation-related factor-1, peroxynitrite, and C-reactive protein all inhibit eNOS activity through PP2A[66;92-94]. How any of these factors regulates PP2A activity on eNOS in endothelial cells is yet to be determined. Third, it is not known whether aPL exert similar inhibitory effects on eNOS in platelets, which express the splice variant of apoER2. Platelets also express eNOS, which has been shown to attenuate platelet activation in an autocrine manner [8;9]. Lastly, we do not know how the mechanism leading to eNOS antagonism by aPL would explain the episodic characteristics of the thrombotic diathesis in APS. Despite the persistent presence of aPL in circulation, APS patients most often do not show clinical symptoms, suggesting that the presence of aPL is necessary but not sufficient for thrombosis or pregnancy complications. A hypothesis called “two-hit hypothesis” has been suggested, in which additional stimuli are required for manifestation of the clinical symptoms[95]. It has been proposed that aPL may decrease the threshold for activation in endothelial cells and platelets (first hit), and clinical events occur after another triggering event (second hit). Does impaired NO production and subsequent endothelial dysfunction induced by aPL represent “the first hit”? If so, what is the “second hit” that triggers the thrombotic events in APS? It may involve a proinflammatory stimulus or infection because rats administered aPL have spontaneous thromboses if they also receive lipopolysaccharide[96]. We also do not know whether or how the “first hit” and the “second hit” are mechanistically linked. As the molecular basis that underlies the “first hit” is further investigated, the nature of the processes that constitute “second hit” directly leading to thrombosis will become clear.
Conclusions
Patients with APS are currently treated with chronic anticoagulation medications such as heparin and warfarin or with medications that modulate the immune response. Despite long-term use of the anticoagulants, recurrent clinical events are reported in APS patients. Furthermore, use of oral anticoagulants is associated with a high risk for multiple complications including bleeding episodes that require frequent monitoring[97-100]. Building upon the novel findings discussed in this review, we anticipate that novel interventions can be developed that directly target the pathogenetic mechanisms, thereby affording greater efficacy and fewer complications in the management of this potentially life-threatening disorder APS.
Acknowledgments
Chieko Mineo has received grant support from the Alliance for Lupus Research and the National Institutes of Health.
Footnotes
Disclosure Chieko Mineo declares that she has no conflict of interest.
Reference List
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb.Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies) BMJ. 1997;314:253–257. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet.Gynecol. 1996;174:1584–1589. doi: 10.1016/s0002-9378(96)70610-5. [DOI] [PubMed] [Google Scholar]
- 4.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N.Engl.J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 6.Soltesz P, Szekanecz Z, Kiss E, Shoenfeld Y. Cardiac manifestations in antiphospholipid syndrome. Autoimmun.Rev. 2007;6:379–386. doi: 10.1016/j.autrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Loscalzo J. Nitric oxide and vascular disease. N.Engl.J.Med. 1995;333:251–253. doi: 10.1056/NEJM199507273330410. [DOI] [PubMed] [Google Scholar]
- 8.Freedman JE, Sauter R, Battinelli EM, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 9.Freedman JE, Loscalzo J, Barnard MR, et al. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin.Invest. 1997;100:350–356. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta JL, Chen LY, Kone BC, et al. Identification of constitutive and inducible forms of nitric oxide synthase in human platelets. J.Lab Clin.Med. 1995;125:370–377. [PubMed] [Google Scholar]
- 11.Sase K, Michel T. Expression of constitutive endothelial nitric oxide synthase in human blood platelets. Life Sci. 1995;57:2049–2055. doi: 10.1016/0024-3205(95)02191-k. [DOI] [PubMed] [Google Scholar]
- 12.Stagliano NE, Zhao W, Prado R, et al. The effect of nitric oxide synthase inhibition on acute platelet accumulation and hemodynamic depression in a rat model of thromboembolic stroke. J.Cereb.Blood Flow Metab. 1997;17:1182–1190. doi: 10.1097/00004647-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Shultz PJ, Raij L. Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J.Clin.Invest. 1992;90:1718–1725. doi: 10.1172/JCI116045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddington S, Cook HT, Reaveley D, et al. L-arginine depletion inhibits glomerular nitric oxide synthesis and exacerbates rat nephrotoxic nephritis. Kidney Int. 1996;49:1090–1096. doi: 10.1038/ki.1996.158. [DOI] [PubMed] [Google Scholar]
- 15.Yao SK, Ober JC, Krishnaswami A, et al. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation. 1992;86:1302–1309. doi: 10.1161/01.cir.86.4.1302. [DOI] [PubMed] [Google Scholar]
- 16.Broeders MA, Tangelder GJ, Slaaf DW, et al. Endogenous nitric oxide protects against thromboembolism in venules but not in arterioles. Arterioscler.Thromb.Vasc.Biol. 1998;18:139–145. doi: 10.1161/01.atv.18.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch. 2010;460:965–974. doi: 10.1007/s00424-010-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefer DJ, Jones SP, Girod WG, et al. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am.J.Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Huang PL, Ma J, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J.Cereb.Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Atochin DN, Wang A, Liu VW, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J.Clin.Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhlencordt PJ, Rosel E, Gerszten RE, et al. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am.J.Physiol Cell Physiol. 2004;286:C1195–C1202. doi: 10.1152/ajpcell.00546.2002. [DOI] [PubMed] [Google Scholar]
- 22.Michelson AD, Benoit SE, Furman MI, et al. Effects of nitric oxide/EDRF on platelet surface glycoproteins. Am.J.Physiol. 1996;270:H1640–H1648. doi: 10.1152/ajpheart.1996.270.5.H1640. [DOI] [PubMed] [Google Scholar]
- 23.Folts JD, Stamler J, Loscalzo J. Intravenous nitroglycerin infusion inhibits cyclic blood flow responses caused by periodic platelet thrombus formation in stenosed canine coronary arteries. Circulation. 1991;83:2122–2127. doi: 10.1161/01.cir.83.6.2122. [DOI] [PubMed] [Google Scholar]
- 24.Mergia E, Friebe A, Dangel O, et al. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J.Clin.Invest. 2006;116:1731–1737. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thromb.Haemost. 1993;70:36–41. [PubMed] [Google Scholar]
- 26.Radomski MW, Moncada S. The biological and pharmacological role of nitric oxide in platelet function. Adv.Exp.Med.Biol. 1993;344:251–264. doi: 10.1007/978-1-4615-2994-1_20. [DOI] [PubMed] [Google Scholar]
- 27.Negrescu EV, Sazonova LN, Baldenkov GN, et al. Relationship between the inhibition of receptor-induced increase in cytosolic free calcium concentration and the vasodilator effects of nitrates in patients with congestive heart failure. Int.J.Cardiol. 1990;26:175–184. doi: 10.1016/0167-5273(90)90031-y. [DOI] [PubMed] [Google Scholar]
- 28.Lowenstein CJ. Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc.Res. 2007;75:240–246. doi: 10.1016/j.cardiores.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita K, Morrell CN, Cambien B, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman JE, Loscalzo J. Nitric oxide and its relationship to thrombotic disorders. J.Thromb.Haemost. 2003;1:1183–1188. doi: 10.1046/j.1538-7836.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 31.Freedman JE. Oxidative stress and platelets. Arterioscler.Thromb.Vasc.Biol. 2008;28:s11–s16. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 32.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N.Engl.J.Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 33.Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985;71:699–708. doi: 10.1161/01.cir.71.4.699. [DOI] [PubMed] [Google Scholar]
- 34.Langford EJ, Wainwright RJ, Martin JF. Platelet activation in acute myocardial infarction and unstable angina is inhibited by nitric oxide donors. Arterioscler.Thromb.Vasc.Biol. 1996;16:51–55. doi: 10.1161/01.atv.16.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Freedman JE, Ting B, Hankin B, et al. Impaired platelet production of nitric oxide predicts presence of acute coronary syndromes. Circulation. 1998;98:1481–1486. doi: 10.1161/01.cir.98.15.1481. [DOI] [PubMed] [Google Scholar]
- 36.Minamino T, Kitakaze M, Sato H, et al. Plasma levels of nitrite/nitrate and platelet cGMP levels are decreased in patients with atrial fibrillation. Arterioscler.Thromb.Vasc.Biol. 1997;17:3191–3195. doi: 10.1161/01.atv.17.11.3191. [DOI] [PubMed] [Google Scholar]
- 37.Clark BA, Ludmir J, Epstein FH, et al. Urinary cyclic GMP, endothelin, and prostaglandin E2 in normal pregnancy and preeclampsia. Am.J.Perinatol. 1997;14:559–562. doi: 10.1055/s-2007-994334. [DOI] [PubMed] [Google Scholar]
- 38.Lacolley P, Gautier S, Poirier O, et al. Nitric oxide synthase gene polymorphisms, blood pressure and aortic stiffness in normotensive and hypertensive subjects. J.Hypertens. 1998;16:31–35. doi: 10.1097/00004872-199816010-00006. [DOI] [PubMed] [Google Scholar]
- 39.Elbaz A, Poirier O, Moulin T, et al. Association between the Glu298Asp polymorphism in the endothelial constitutive nitric oxide synthase gene and brain infarction. The GENIC Investigators. Stroke. 2000;31:1634–1639. doi: 10.1161/01.str.31.7.1634. [DOI] [PubMed] [Google Scholar]
- 40.Hingorani AD, Liang CF, Fatibene J, et al. A common variant of the endothelial nitric oxide synthase (Glu298-->Asp) is a major risk factor for coronary artery disease in the UK. Circulation. 1999;100:1515–1520. doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- 41.Shimasaki Y, Yasue H, Yoshimura M, et al. Association of the missense Glu298Asp variant of the endothelial nitric oxide synthase gene with myocardial infarction. J.Am.Coll.Cardiol. 1998;31:1506–1510. doi: 10.1016/s0735-1097(98)00167-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang XL, Sim AS, Badenhop RF, et al. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat.Med. 1996;2:41–45. doi: 10.1038/nm0196-41. [DOI] [PubMed] [Google Scholar]
- 43.Tanus-Santos JE, Desai M, Deak LR, et al. Effects of endothelial nitric oxide synthase gene polymorphisms on platelet function, nitric oxide release, and interactions with estradiol. Pharmacogenetics. 2002;12:407–413. doi: 10.1097/00008571-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Pierangeli SS, Chen PP, Raschi E, et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin.Thromb.Hemost. 2008;34:236–250. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 45.Pierangeli SS, Gharavi AE, Harris EN. Experimental thrombosis and antiphospholipid antibodies: new insights. J.Autoimmun. 2000;15:241–247. doi: 10.1006/jaut.2000.0420. [DOI] [PubMed] [Google Scholar]
- 46.Oku K, Amengual O, Atsumi T. Pathophysiology of thrombosis and pregnancy morbidity in the antiphospholipid syndrome. Eur.J.Clin.Invest. 2012;42:1126–1135. doi: 10.1111/j.1365-2362.2012.02697.x. [DOI] [PubMed] [Google Scholar]
- 47.Jankowski M, Vreys I, Wittevrongel C, et al. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101:157–162. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 48.Pierangeli SS, Colden-Stanfield M, Liu X, et al. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99:1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 49.Espinola RG, Liu X, Colden-Stanfield M, et al. E-Selectin mediates pathogenic effects of antiphospholipid antibodies. J Thromb.Haemost. 2003;1:843–848. doi: 10.1046/j.1538-7836.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 50.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–3083. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vega-Ostertag ME, Ferrara DE, Romay-Penabad Z, et al. Role of p38 mitogen-activated protein kinase in antiphospholipid antibody-mediated thrombosis and endothelial cell activation. J.Thromb.Haemost. 2007;5:1828–1834. doi: 10.1111/j.1538-7836.2007.02680.x. [DOI] [PubMed] [Google Scholar]
- 52.Vega-Ostertag M, Liu X, Kwan-Ki H, et al. A human monoclonal antiprothrombin antibody is thrombogenic in vivo and upregulates expression of tissue factor and E-selectin on endothelial cells. Br.J.Haematol. 2006;135:214–219. doi: 10.1111/j.1365-2141.2006.06283.x. [DOI] [PubMed] [Google Scholar]
- 53.Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88:245–250. doi: 10.1161/01.res.88.2.245. [DOI] [PubMed] [Google Scholar]
- 54.Branch DW, Rodgers GM. Induction of endothelial cell tissue factor activity by sera from patients with antiphospholipid syndrome: a possible mechanism of thrombosis. Am J Obstet.Gynecol. 1993;168:206–210. doi: 10.1016/s0002-9378(12)90915-1. [DOI] [PubMed] [Google Scholar]
- 55.Atsumi T, Khamashta MA, Haworth RS, et al. Arterial disease and thrombosis in the antiphospholipid syndrome: a pathogenic role for endothelin 1. Arthritis Rheum. 1998;41:800–807. doi: 10.1002/1529-0131(199805)41:5<800::AID-ART5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 56.Dunoyer-Geindre S, de MP, Galve-de RB, et al. NFkappaB is an essential intermediate in the activation of endothelial cells by anti-beta(2)-glycoprotein 1 antibodies. Thromb.Haemost. 2002;88:851–857. [PubMed] [Google Scholar]
- 57.Lopez-Pedrera C, Buendia P, Cuadrado MJ, et al. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-kappaB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Rheum. 2006;54:301–311. doi: 10.1002/art.21549. [DOI] [PubMed] [Google Scholar]
- 58.Simoncini S, Sapet C, Camoin-Jau L, et al. Role of reactive oxygen species and p38 MAPK in the induction of the pro-adhesive endothelial state mediated by IgG from patients with anti-phospholipid syndrome. Int.Immunol. 2005;17:489–500. doi: 10.1093/intimm/dxh229. [DOI] [PubMed] [Google Scholar]
- 59.Vega-Ostertag M, Casper K, Swerlick R, et al. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52:1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 60.Belizna C, Lartigue A, Favre J, et al. Antiphospholipid antibodies induce vascular functional changes in mice: a mechanism of vascular lesions in antiphospholipid syndrome? Lupus. 2008;17:185–194. doi: 10.1177/0961203307086931. [DOI] [PubMed] [Google Scholar]
- 61.Delgado AJ, Mason LJ, Ames PR, et al. Antiphospholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model. Rheumatology.(Oxford) 2005;44:1238–1244. doi: 10.1093/rheumatology/keh722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ames PR, Tommasino C, Alves J, et al. Antioxidant susceptibility of pathogenic pathways in subjects with antiphospholipid antibodies: a pilot study. Lupus. 2000;9:688–695. doi: 10.1191/096120300677692516. [DOI] [PubMed] [Google Scholar]
- *63.Ames PR, Batuca JR, Ciampa A, et al. Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome. J.Rheumatol. 2010;37:2523–2530. doi: 10.3899/jrheum.100494. *This is one of the series of studies published by the group that provide the link between nitric oxide and APS in humans.
- **64.Ramesh S, Morrell CN, Tarango C, et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J.Clin.Invest. 2011;121:120–131. doi: 10.1172/JCI39828. **This work demonstrates for the first time that aPL antagonism of eNOS plays an important role in the abnormal vascular phenotypes in APS.
- 65.Mineo C, Shaul PW. New Insights into the Molecular Basis of the Antiphospholipid Syndrome. Drug Discov.Today Dis.Mech. 2011;8:e47–e52. doi: 10.1016/j.ddmec.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mineo C, Gormley AK, Yuhanna IS, et al. FcgammaRIIB mediates C-reactive protein inhibition of endothelial NO synthase. Circ Res. 2005;97:1124–1131. doi: 10.1161/01.RES.0000194323.77203.fe. [DOI] [PubMed] [Google Scholar]
- 67.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 68.Galli M, Luciani D, Bertolini G, Barbui T. Anti-beta 2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003;102:2717–2723. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- 69.Miyakis S, Giannakopoulos B, Krilis SA. Beta 2 glycoprotein I--function in health and disease. Thromb.Res. 2004;114:335–346. doi: 10.1016/j.thromres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Ninivaggi M, Kelchtermans H, Lindhout T, de Laat B. Conformation of beta2glycoprotein I and its effect on coagulation. Thromb.Res. 2012;130 Suppl 1:S33–S36. doi: 10.1016/j.thromres.2012.08.269. [DOI] [PubMed] [Google Scholar]
- 71.de Groot PG, van Lummel M, Pennings M, et al. Beta2-glycoprotein I and LDL-receptor family members. Thromb.Res. 2004;114:455–459. doi: 10.1016/j.thromres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Urbanus RT, Derksen RH, de Groot PG. Platelets and the antiphospholipid syndrome. Lupus. 2008;17:888–894. doi: 10.1177/0961203308096344. [DOI] [PubMed] [Google Scholar]
- 73.Pierangeli SS, Vega-Ostertag M, Harris EN. Intracellular signaling triggered by antiphospholipid antibodies in platelets and endothelial cells: a pathway to targeted therapies. Thromb.Res. 2004;114:467–476. doi: 10.1016/j.thromres.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 74.de Laat B, de Groot PG. Autoantibodies directed against domain I of beta2-glycoprotein I. Curr.Rheumatol.Rep. 2011;13:70–76. doi: 10.1007/s11926-010-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–1545. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 76.de Laat B, Pengo V, Pabinger I, et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J.Thromb.Haemost. 2009;7:1767–1773. doi: 10.1111/j.1538-7836.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 77.Del Papa N, Guidali L, Sala A, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40:551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 78.Del Papa N, Guidali L, Spatola L, et al. Relationship between anti-phospholipid and anti-endothelial cell antibodies III: beta 2 glycoprotein I mediates the antibody binding to endothelial membranes and induces the expression of adhesion molecules. Clin.Exp.Rheumatol. 1995;13:179–185. [PubMed] [Google Scholar]
- 79.He J, Luster TA, Thorpe PE. Radiation-enhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin.Cancer Res. 2007;13:5211–5218. doi: 10.1158/1078-0432.CCR-07-0793. [DOI] [PubMed] [Google Scholar]
- 80.Ran S, He J, Huang X. Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin.Cancer Res. 2005;11:1551–1562. doi: 10.1158/1078-0432.CCR-04-1645. Set al. [DOI] [PubMed] [Google Scholar]
- 81.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat.Rev.Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 82.Pennings MT, van Lummel M, Derksen RH, et al. Interaction of beta2-glycoprotein I with members of the low density lipoprotein receptor family. J Thromb.Haemost. 2006;4:1680–1690. doi: 10.1111/j.1538-7836.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- 83.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2′. J.Thromb.Haemost. 2008;6:1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 84.Pennings MT, Derksen RH, Urbanus RT, et al. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J Thromb.Haemost. 2007;5:1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 85.Herz J, Goldstein JL, Strickland DK, et al. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol.Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- **86.Romay-Penabad Z, guilar-Valenzuela R, Urbanus RT, et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117:1408–1414. doi: 10.1182/blood-2010-07-299099. **The paper provides concrete evidence that apoER2 is required for aPL-induced thrombus formation in vivo.
- 87.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol.Exp.Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 88.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu.Rev.Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 89.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry (Mosc) 2002;41:15845–15853. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 90.Michell BJ, Chen Z, Tiganis T, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J.Biol.Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 91.Mumby MC, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 92.Leidi M, Mariotti M, Maier JA. EDF-1 contributes to the regulation of nitric oxide release in VEGF-treated human endothelial cells. Eur.J.Cell Biol. 2010;89:654–660. doi: 10.1016/j.ejcb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Urbich C, Reissner A, Chavakis E, et al. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002;16:706–708. doi: 10.1096/fj.01-0637fje. [DOI] [PubMed] [Google Scholar]
- 94.Wu F, Wilson JX. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc.Res. 2009;81:38–45. doi: 10.1093/cvr/cvn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meroni PL, Raschi E, Testoni C, et al. Innate immunity in the antiphospholipid syndrome: role of toll-like receptors in endothelial cell activation by antiphospholipid antibodies. Autoimmun.Rev. 2004;3:510–515. doi: 10.1016/j.autrev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 96.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 97.Pierangeli SS, Erkan D. Antiphospholipid syndrome treatment beyond anticoagulation: are we there yet? Lupus. 2010;19:475–485. doi: 10.1177/0961203310361489. [DOI] [PubMed] [Google Scholar]
- 98.Erkan D, Harrison MJ, Levy R, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum. 2007;56:2382–2391. doi: 10.1002/art.22663. [DOI] [PubMed] [Google Scholar]
- 99.Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS) J.Thromb.Haemost. 2005;3:848–853. doi: 10.1111/j.1538-7836.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 100.Garcia DA, Khamashta MA, Crowther MA. How we diagnose and treat thrombotic manifestations of the antiphospholipid syndrome: a case-based review. Blood. 2007;110:3122–3127. doi: 10.1182/blood-2006-10-041814. [DOI] [PubMed] [Google Scholar]