Abstract
Objective
The dialysis dietary regimen is complicated, and computer-based dietary self-monitoring may be useful for helping dialysis patients manage their dietary regimen. In this report we describe dietary self-monitoring rates among study participants randomized to the intervention arms of two pilot studies.
Methods
Both studies tested similar interventions involving dietary counseling paired with personal digital assistant (PDA)-based self-monitoring. One study was performed in hemodialysis (HD) and one in peritoneal dialysis (PD) patients.
Results
HD intervention participants entered an average of 244.9 meals (median=288; IQR: 186–342) over the 16-week intervention, 2.2 meals per day (median=2.6; IQR; 1.7–3.1), and 73% of expected meals (median=86; IQR: 55–102), assuming intake of 3 meals per day. At least some meals were entered in 87% of the observed weeks (median=100%; IQR: 81–100). PD intervention participants entered an average of 212.1 meals (median=203; IQR: 110–312) over the 16-week intervention, 1.9 meals per day (median=1.8; IQR: 1–2.8), and 63% of expected meals (median=60; IQR: 33–93) assuming 3 meals per day. At least some meals were entered in 80% of the observed weeks (median=94; IQR: 50–100).
Conclusion
These HD and PD patients demonstrated excellent rates of self-monitoring. Additional research with a larger sample is required to confirm these findings.
Keywords: hemodialysis, self care, computers, handheld, personal digital assistant, randomized clinical trial, behavioral research, peritoneal dialysis, nutrition
INTRODUCTION
The incidence of end stage renal disease (ESRD) has grown exponentially reaching 354 per million population in 2007. (1) By the end of 2007, the Medicare ESRD Program was treating 527,282 patients by either dialysis or transplantation. (1) Of the patients treated with dialysis therapy, more than 341,000 were on hemodialysis (HD) and 26,340 were on peritoneal dialysis (PD). (1,2) According to recent projections, nearly 774,000 patients will be enrolled in the ESRD program by the year 2020, with the majority being on some type of dialysis therapy. (1)
Dialysis does not entirely replace kidney function, and these treatments are associated with a variety of complications and poor outcomes for patients. The overall life expectancy for ESRD patients is 5.9 years, compared to 25.2 years for age matched individuals in the general population who are not on dialysis. (3) The most common cause of death, cardiac events, accounts for over 40% of all deaths. (4, 5, 6) The most common cardiac alteration, left ventricular hypertrophy, is related to an increased cardiac work load due to expanded extracellular fluid volume and hypertension. (5, 6) A high sodium intake contributes to volume overload by stimulating the thirst center, resulting in excess fluid intake. Because of the importance of volume control in managing hypertension and improving cardiac outcomes, dialysis patients are encouraged to restrict their dietary intake of sodium. (5, 6, 7)
Dietary change is widely known to be difficult to achieve and sustain. Even the most effective dietary interventions may be less successful in dialysis patients than in other populations because of the complex nature of the treatment regimen and other dietary demands. In addition to limiting dietary sodium intake, dialysis patients are required to moderate their consumption of potassium and phosphorus. Additionally, they are predisposed to protein energy malnutrition and, so, are encouraged to maintain adequate intake of calories and high quality protein. Sodium intake is particularly difficult to control because many foods are naturally high in sodium, and most prepared/prepackaged foods have substantial amounts of sodium added to enhance taste and prolong shelf-life. Thus, it is not surprising that many patients have difficulty with the recommended dialysis diet in general and sodium restrictions in particular.
Several strategies to improve the efficacy of dietary interventions have been evaluated, mostly in the area of weight loss. Lasser and Wilbur discuss the importance of self-monitoring of both diet and exercise. (8, 9, 10) Diet and exercise diaries, in which participants are asked to record their food intake and/or track physical activity, can improve adherence and build rapport in group counseling sessions. (11, 8)
Computer-based dietary self-monitoring may help dialysis patients to manage their dietary regimen. This investigative team recently completed two pilot studies to evaluate a personal digital assistant (PDA)-based dietary intervention designed to moderate dietary sodium intake in HD (the BalanceWise-HD Study) and PD (the BalanceWise-PD Study). In this report we describe the self-monitoring patterns of those individuals randomized to the intervention groups in these 2 studies.
METHODS
Design
Both BalanceWise-HD and BalanceWise-PD were randomized controlled trials of 16 weeks duration testing a dietary education intervention to moderate sodium intake in HD and PD patients. The primary results of these studies will be reported elsewhere.. In this brief report, we describe the PDA self-monitoring behavior of participants randomized to the intervention arms of the 2 studies
Participants
Participants for both studies were recruited from three Dialysis Clinic, Inc. (DCI) dialysis clinics located in Southwestern Pennsylvania. In order to be eligible for the study, participants were literate, English-speaking, community-dwelling adults over 18 years of age. We excluded those who could not read or write, planned to move out of the area or change dialysis centers during the study period, were scheduled for a living donor transplant, had significant cognitive impairment, or were deemed by staff to have a life expectancy of less than 1 year. We excluded individuals who were living in an institutional setting (e.g. nursing home, personal care facility, or jail) because they would have little control over their dietary intake. We also excluded those who could not see the PDA screen or use the stylus to make selections from the PDA screen. Because the intervention required adherence to computer-based self-monitoring, in the BalanceWise-HD study, dialysis center staff who were responsible for prescreening patients were initially asked to eliminate from consideration those individuals who missed 25% or more of their scheduled dialysis treatments. This exclusion was later dropped from the study, and was never an exclusionary factor in the BalanceWise-PD study.
A HIPAA authorization was obtained by a DCI staff person prior to a patient being approached for recruitment. All participants gave full informed and signed consent. The study was approved by the Institutional Review Board of the University of Pittsburgh and the DCI Administrative Review Office.
Intervention
The intervention in both studies involved 16 weeks of dietary counseling based on Social Cognitive Theory (12, 13), paired with PDA-based dietary self-monitoring. Participants were provided with a PalmOne Tungsten/E2 PDA with BalanceLog® software by MicroLife. (14) BalanceLog® contained a food database of more than 4300 foods, developed using nutrient composition data from the United States Department of Agriculture. Prior to training, BalanceLog® was programmed for the individual calorie and nutrient requirements per the DCI renal dietitian. The software was programmed to permit 4 meals logs (breakfast, lunch, dinner, and snack). With one-on-one instruction, participants were taught how to search the database, enter foods they ate, and examine (meal-by-meal and day-by-day) the extent to which they achieved their dietary goals. The intervention focused primarily on moderating dietary sodium. However, if the participant’s electronic record suggested inadequate intake of calories or protein, the study dietitian would provide appropriate counseling. If routine monthly laboratory testing suggested hyperphosphatemia or hyperkalemia, the electronic record was searched to identify foods that may have contributed to these abnormal findings.
In the BalanceWise-HD study, counseling was performed during regularly scheduled in-center dialysis treatments. With each contact the study dietitian provided dietary counseling based on the electronic dietary record. Intervention contacts occurred twice a week during weeks 1–6, weekly during weeks 7–12, and every other week for weeks 13–16. In BalanceWise-PD, the intent was to provide counseling on the same schedule as the HD version of the study. However, because participants often lived at great distances from the dialysis unit, they visited the dialysis unit for routine care only 1–2 times/month. Consequently, the interventionist used a variety of means to provide counseling, including meeting participants at a convenient location or at regularly scheduled dialysis clinic appointments, or calling by telephone when face-to-face meetings were not possible.
Measures
Sociodemographic data were obtained from a baseline questionnaire. Duration and etiology of ESRD were abstracted from the participant’s medical record. With each face-to-face contact, electronic logs of the meals entered into the PDA were uploaded to a laptop computer. These files were later abstracted to capture the number of meal entries made each week (valid range 0–28).
Analysis
If the participant voluntarily withdrew from the study or did not return the PDA to the investigators for uploading, the number of meals entered per week following the last PDA upload was coded as “0” (i.e., a conservative assumption was made that the participant had discontinued dietary self-monitoring altogether). For those participants who died, were transplanted, changed dialysis treatment modality, or relocated outside of the study site, the number of meals entered per week following the last PDA upload was coded as missing. Self-monitoring patterns were described for each study in terms of number and percent of expected meals entered each week, assuming 3 meals a day. Means, medians, and interquartile ranges (IQRs) were reported for the numbers of meals because these numbers were quite skewed. We also summarized the number and percent of weeks for which any meals were entered into the PDA.
RESULTS
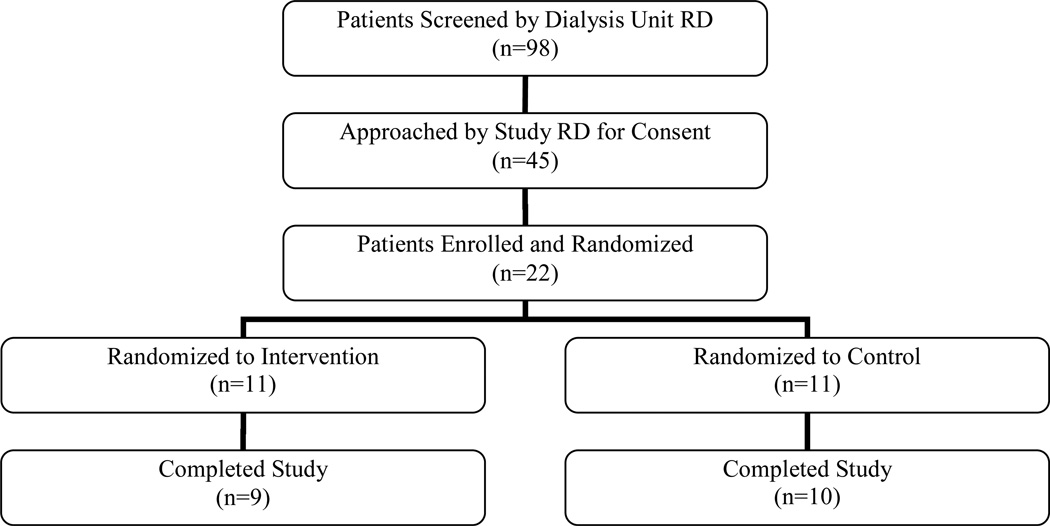
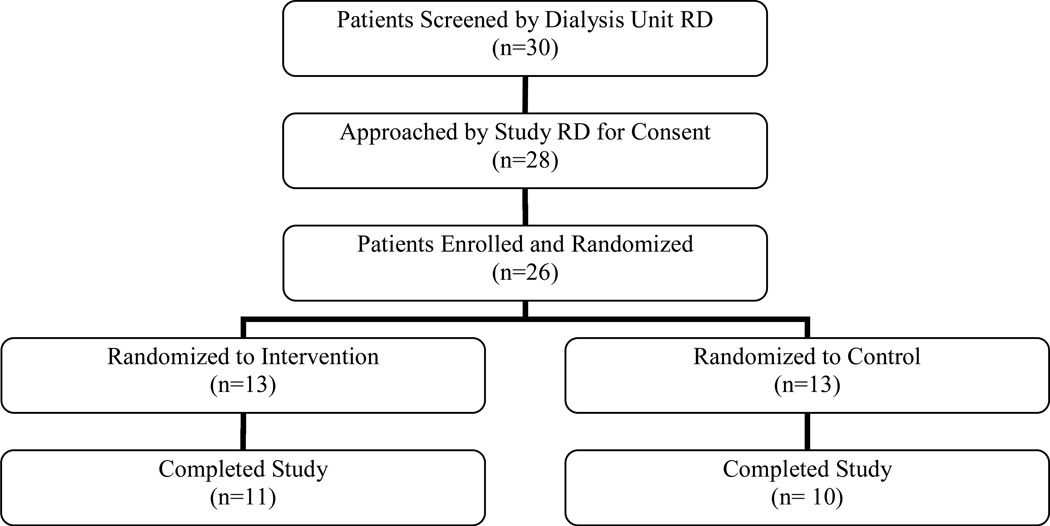
A total of 98 HD and 30 PD patients were screened by the dialysis center dietitian for eligibility to participate in these studies. Of the 22 HD and 26 PD patients enrolled in the study, 11 HD and 13 PD patients were randomized to the intervention. Figures 1 and 2 summarize the flow of participants from initial screening to completion of the HD and PD studies, respectively. The mean age of HD participants was 56.0 years, and 51.7 years in PD patients (Table 1). The mean duration of dialysis was 4.7 years for HD patients and 2.1 years for PD patients. .
Figure 1.
Recruitment and retention for BalanceWise-HD Study
Figure 2.
Recruitment and retention for BalanceWise-PD Study
Table 1.
Characteristics of intervention participants in the BalanceWise Hemodialysis (HD) and BalanceWise Peritoneal Dialysis (PD) studies
| HD (N=10) | PD (N=13) | |
|---|---|---|
| Mean (SD) | ||
| Age (years) | 56.0 (15.9) | 51.7 (19.8) |
| Duration of dialysis (years) | 4.7 (7.0) | 2.1 (2.1) |
| N (%) | ||
| Male | 6 (60) | 7 (54) |
| Minority race | 9 (90) | 8 (31) |
| >High School education | 8 (80) | 6 (46) |
| Etiology of ESRD | ||
| Hypertension | 5 (50) | 2 (15) |
| Diabetes | 3 (30) | 6 (46) |
| Glomerulonephritis | 0 (0) | 0 (0) |
| Other | 2 (20) | 5 (38) |
In the HD study, participants entered an average of 244.9 meals (median=288; IQR: 186–342) over the 16-week intervention, or 2.2 meals per day (median=2.6; IQR: 1.7–3.1). The average number of weeks that HD patients entered any meals into the PDA was 13.9 (median=16; IQR: 13–16) or 87% of observed weeks (median= 100%; IQR: 81–100). If we assume 3 meals per day were consumed, participants entered an average of 73% of expected meals (median=86%; IQR: 55–102).
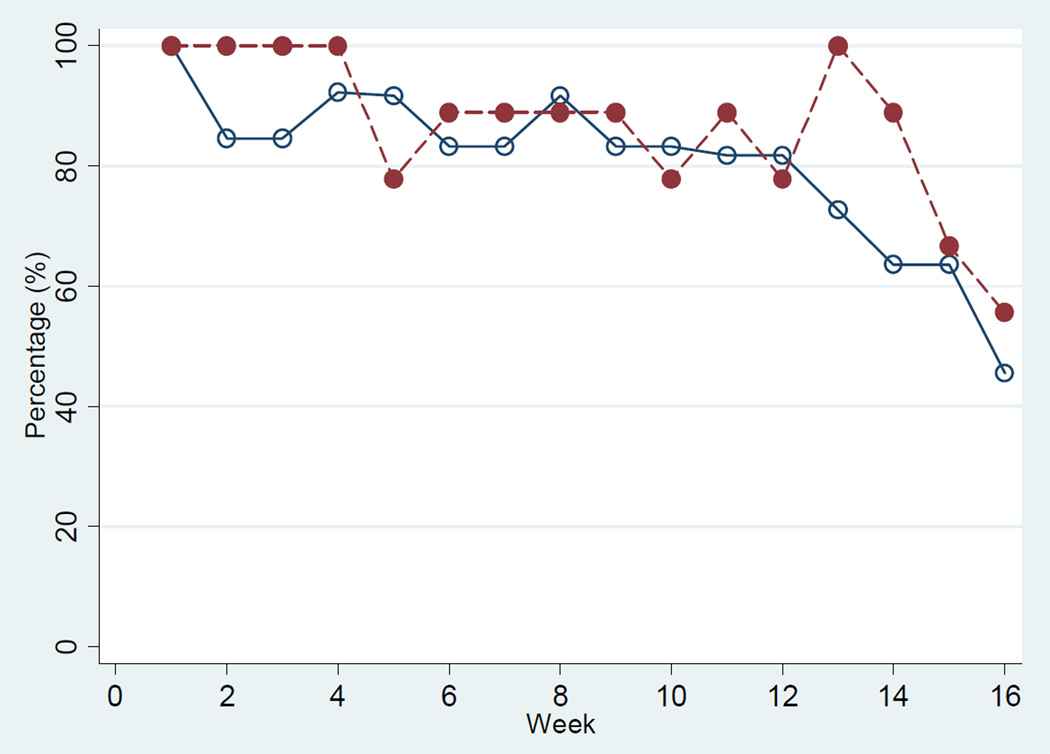
In the PD study, participants entered an average of 212.1 meals (median=203; IQR: 110–312) over the 16-week intervention, or 1.9 meals per day (median=1.8; IQR: 1–2.8). The average number of weeks that PD patients entered any meals into the PDA was 12.7 (median=15; IQR: 8–16), or 80% of observed weeks (median=94%; IQR: 50–100). If we assume 3 meals per day, participants entered an average of 63% of their expected meals (median=60; IQR: 33–93). Figure 3 shows the average percent of meals entered per week by intervention participants in each study by week of follow-up. Adherence was quite high initially in both groups (generally better than 80%), but declined in both groups during the last few weeks of follow-up. In each study, only 2 cases did not complete the intervention. Thus, loss to follow-up does not appear to play a dominant role in the reduction in self-monitoring behavior near the end of the study.
Figure 3.
Weekly percentage of participants who enter any meals in the BalanceWise-HD and BalanceWise-PD Studies
DISCUSSION
The HD and PD patients in these two pilot studies demonstrated excellent rates of self-monitoring. However, the small samples, which were drawn from three dialysis centers in Western Pennsylvania, limits the generalizability of our results. Because the mean ages of the HD and PD patients are somewhat lower than that of prevalent dialysis patients (60.9 years) (15), the generalizability of these results may be limited to younger and healthier dialysis patients.
Given the onerous and time consuming nature of dialysis treatments and dietary regimens, we were surprised at the high rates of self-monitoring among participants. HD and PD patients frequently experience loss of appetite. PD patients often complain of a sense of fullness due to the presence of dialysate fluid in the abdominal cavity and satiety from glucose that is absorbed from the dialysate. HD patients spend 4–5 hours at the dialysis center on treatment days and, typically, are not permitted to eat during treatments. After dialysis HD patients often feel “washed” out and have little energy or desire to prepare meals. Consuming 3 meals on a dialysis day is a challenge for many HD and PD patients. Consequently, our expectation that patients consumed three meals a day during the study may have resulted in underestimation of adherence to PDA-based dietary self-monitoring.
A possible factor contributing to the high rate of dietary self-monitoring observed was a sense of accountability to the interventionist who reviewed PDA entries with them during the scheduled counseling sessions. HD patients, who had a higher number of face-to-face contacts with the interventionist than the PD patients, also had a higher rate of self monitoring (87% of observed weeks and 73% of observed meals in HD patients compared to 80% of observed weeks and 63% of observed meals in PD patients). More research is needed to ascertain the amount of interventionist contact required to sustain high levels of self-monitoring and the degree of self-monitoring adherence required to elicit the desired dietary behavior change.
The high rate of self-monitoring may also be due to the theoretical approach driving the intervention in which self monitoring was used to increase awareness of food intake, monitor progress and promote self-efficacy. The immediate feedback from the PDA about dietary intake may have led to an improved sense of success in being able to manage their diet which, in turn, positively reinforced self-monitoring behavior.
It is possible that some patients consumed more than 4 meals per day. Because we limited the electronic log to 4 meals per day, it is possible that our analysis approach over-estimated adherence. Future researchers may wish to employ a self-monitoring approach that permits the entry of a greater number of meals. Regardless of the number of meals accommodated by the software, a more accurate evaluation of self-monitoring adherence would require an independent measure of the number of meals consumed (e.g., an additional self-report measure of meals consumed each day or direct observation).
We compared the self-monitoring rates obtained from BalanceWise-HD and BalanceWise-PD with those reported in a recent review (16). While several studies report on the effect of self monitoring on achieving a specific dietary goal (e.g. in weight loss interventions, those with greater rates of self-monitoring lose more weight) the actual rates of self-monitoring usually are not reported. Those studies that do report adherence to self monitoring do not measure it in a consistent manner.. The most frequent approach is to report a count of the number of diet diaries submitted. (16)
The use of technology in dietary self-monitoring is a relatively new development. In a 6-month weight loss study that employed a PDA for monitoring diet and physical activity, Yon et al. (17) reported that participants submitted electronic records on approximately 50% of study weeks; the number of meals entered per week and the exact percentage of weeks participants submitted records were not reported. Glanz et al. (18) conducted a 1-month pilot study of PDA use by participants in the Dietary Modification Arm of the Women’s Health Initiative. The dietary intervention was designed to reduce dietary fat intake to 20% of total calories and to increase intake of fruits and vegetables to at least 5 servings per day and grains to at least 6 servings per day. They reported that 50% of participants entered foods into the PDA on at least 6 days each week. We recently completed a clinical trial that used BalanceLog software in a diabetes self-management intervention (19). By the end of that study, only 20% of intervention participants were entering more than 50% of their expected meals into the PDA, assuming that study participants consumed 3 meals per day (20). Because a different metric was used to evaluate adherence to the self-monitoring protocols and the studies differed in duration, we cannot make direct comparisons to the rates observed in BalanceWise-HD and BalanceWise-PD.
Because of differences in the software programs used, the patient populations recruited, and duration of interventions, caution must be used in comparing PDA self-monitoring rates from these different studies. As new technologies and software become available (e.g. cellular-and web-based programs), additional research will be needed to determine the extent to which such technologies reduce the burden of self-monitoring and strengthen interventions.
CONCLUSION
HD and PD patients in our studies demonstrated excellent rates of PDA-based dietary self-monitoring. These pilot results suggest that electronic technology is feasible and may be useful for assisting dialysis patients in adhering to a complex dietary regimen. While technology based self monitoring has been found to improve outcomes in other populations, no studies other than our own have evaluated it in the dialysis population. A larger scale randomized controlled trial in underway to evaluate technology based self monitoring for control sodium intake in patients on hemodialysis.
ACKNOWLEDGEMENTS
The work of this paper was supported by the following grants: Paul Teschan Research Foundation, NIH/NIDDK/DK-R21DK067181, NIH/NCRR/CTSA-UL1-RR024153, and NIH/NCRR/GCRC-M01- RR000056. The authors take full responsibility for the contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government. The authors would like to acknowledge the individual contributions of: Rita Marsh, RN, MSN; Deborah Klinvex, BA; and Tienna Luster.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. [Accessed May 21, 2010];Volume Two: Atlas of End-Stage Renal Disease. Chapter 2 Incidence and Prevalence. http://www.usrds.org/2009/pdf/V2_02_INC_PREV_09.PDF. [Google Scholar]
- 2.U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. [Accessed May 21, 2010];Volume Two: Atlas of End-Stage Renal Disease. Chapter 4 Treatment Modalities. http://www.usrds.org/2009/pdf/V2_04_09.PDF. [Google Scholar]
- 3.U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. [Accesssed May 21, 2010];Volume Three: Reference Tables on End-Stage Renal Disease. Section H. Mortality http://www.usrds.org/2009/ref/H_Ref_09.pdf. [Google Scholar]
- 4.U.S. Renal Data System, USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. [Accessed December 28, 2009]; http://www.usrds.org/adr_2007.htm. [Google Scholar]
- 5.National Kidney Foundation. K/DOQIClinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am J Kidney Dis. 2005;45(suppl 3):S1–S154. [PubMed] [Google Scholar]
- 6.National Kidney Foundation. 2006. Clinical practice guidelines for hemodialysis adequacy, Update 2006. Am J Kidney Dis. 2006;48:S13–S97. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Peritoneal Dialysis Adequacy Work Group. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 2006;48:S98–S129. doi: 10.1053/j.ajkd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Lasser VI, Raczynski JM, Stevens VJ, Mattfeldt-Beman MK, Kukmanyika S, Evans M, Danielson E, Dalcin A, Batey DM, Belden LK, Brewer AA. Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Trials of hypertension prevention, phase ii structure and content of the weight loss and dietary sodium reduction interventions. Ann Epidemiol. 1995;5:156–164. doi: 10.1016/1047-2797(94)00060-7. [DOI] [PubMed] [Google Scholar]
- 9.Wilbur J, Michaels-Miller A, Chandler P, McDevitt J. Determinants of physical activity and adherence to a 24-week home-based walking program in African American and Caucasian women. Res Nurs Health. 1995;26:213–224. doi: 10.1002/nur.10083. [DOI] [PubMed] [Google Scholar]
- 10.Wilbur J, Vassalo A, Chandler P, McDevitt J, Miller AM. Midlife women’s adherence to home-based walking during maintenance. Nurs Res. 2005;54:33–40. doi: 10.1097/00006199-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Elmer PJ, Laing BM, Clearman DR. Food and fitness guide: What you need to know about calories, fat and sodium in foods. Minneapolis: University of Minnesota; 1991. [Google Scholar]
- 12.Bandura A. Social Foundations of Thought & Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 13.Bandura A. Self-Efficacy: The Exercise of Control. New York: Freeman and Co.; 1997. [Google Scholar]
- 14. [Accessed August 2, 2010]; http://metabolicratetest.com/microlife-balancelog-software/.
- 15.U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. [Accessed May 21, 2010];Volume Two: Atlas of End-Stage Renal Disease. Chapter 3 Patient Characteristics. http://www.usrds.org/2009/pdf/V2_03_09.PDF. [Google Scholar]
- 16.Burke LE, Wang J, Sevick MA. Self-Monitoring in Weight Loss: A Systematic Review of the Literature. Journal of the American Dietetic Association. doi: 10.1016/j.jada.2010.10.008. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yon BA, Johnson RK, Harvey-Berino J, Gold BC, Howard AB. Personal digital assistants are comparable to traditional diaries for dietary self-monitoring during a weight loss program. J Behav Med. 2007;30:165–175. doi: 10.1007/s10865-006-9092-1. [DOI] [PubMed] [Google Scholar]
- 18.Glanz K, Murphy S, Moylan J, Evensen D, Curb JD. Improving dietary self-monitoring and adherence with hand-held computers: a pilot study. Am J Health Promot. 2006;20:165–170. doi: 10.4278/0890-1171-20.3.165. [DOI] [PubMed] [Google Scholar]
- 19.Sevick MA, Zickmund S, Korytkowski M, Piraino B, Sereika S, Ren D, Mihalko S, Snetselaar L, Stumbo P, Marsh R, Sakraida T, Gibson J, Safaien M, Starrett TJ, Burke LE. The ENHANCE Study: a randomized clinical trial testing the efficacy of a behavioral intervention paired with personal digital assistant-based dietary self-monitoring in type 2 diabetes – a unique intervention approach. Contemp Clin Trials. 2008;29:396–409. doi: 10.1016/j.cct.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevick MA, Stone RA, Zickmund S, Wang Y, Korytkowski M, Burke LE. Factors Associated with probability of personal digital assistant-based dietary self-monitoring in those with type 2 diabetes. Journal of Behavioral Medicine. 2010 Aug;33(4):315–325. doi: 10.1007/s10865-010-9257-9. Epub 2010 Mar 16.PMID: 20232131. [DOI] [PubMed] [Google Scholar]