Abstract
Although we sometimes use the intrasynovial tendon allograft as a donor, the gliding ability of allograft prepared by lyophilization is significantly decreased. The gliding ability of the grafted tendon after tendon reconstruction is very important because the high gliding resistance causes more adhesion and leads to poor clinical results. We recently revealed that tendon surface treatment with a carbodiimide derivatized HA (cd-HA)-gelatin mixture for intrasynovial tendon allograft significantly improved its gliding ability. The purpose of this study was to investigate whether this cd-HA-gelatin treatment affects the tendon mechanical property or not. A total of 40 flexor digitorum profundus (FDP) tendons from canines were evaluated for compressive property by using indentation test. Indentation stiffness was measured for normal tendon, rehydrated tendon after lyophilization, rehydrated tendon after lyophilization that was implanted 6 weeks in vivo, and cd-HA treated rehydrated tendon after lyophilization that was implanted 6 weeks in vivo. The results for all groups showed no significant difference in the tendon compressive properties. The findings of these results demonstrate that cd-HA treatment for intrasynovial tendon allograft is an excellent method to improve the tendon gliding ability after lyophilization without changing the compressive property of donor tendon.
Keywords: Tendon graft, Allograft, Lyophilization, Mechanical property
1. Introduction
Although immediate primary repair after flexor tendon injury has been generally accepted and outcome has improved (Kleinert et al., 1973; Strickland, 1989), poor functional outcome continues to be a difficult problem in 5–10% of cases (Amadio, 2005; Jansen and Watson, 1993). In such cases, tendon grafts play an important role to restore hand function. However, clinical outcomes after tendon graft are also often poor, due to complications such as adhesion formation.
We have shown that tendon gliding resistance is an important factor influencing the outcome of tendon repair (Zhao et al., 2001a, 2001b). We have also demonstrated that extrasynovial tendon has a higher gliding resistance than the native intrasynovial tendon (Uchiyama et al., 1997). However, extrasynovial tendons are usually used as a donor because they are easily harvested (Wehbe, 1992) without any risk of important functional loss and the potential availability of intrasynovial autologous tendons are limited. Because of the limited availability of autologous intrasynovial tendon graft donor sites, a lyophilized intrasynovial tendon allograft is another option for flexor tendon reconstruction. However, the gliding ability of intrasynovial tendons prepared by lyophilization, a common method of preservation, is significantly decreased (Ikeda et al., 2010). To increase the tendon gliding ability, surface modification with carbodiimide derivatized hyaluronic acid (cd-HA) has been well studied (Ikeda et al., 2010; Momose et al., 2002; Sun et al., 2004; Taguchi et al., 2008; Tanaka et al., 2006; Yang et al., 2004). This surface modification indeed has been recently reported to mitigate this adverse effect, leading to decreased postoperative adhesions (Zhao et al., 2010).
The mechanism of this chemical modification involves cross-linking HA onto the tendon surface. If this chemical reaction occurs within the tendon tissue, it carries the risk of cross-linking within the tendon, which could alter tendon stiffness. The purpose of this study was therefore to measure the stiffness of intrasynovial tendon allografts that were treated with this novel method, using indentation testing as a measure of stiffness.
2. Materials and methods
2.1. Intrasynovial tendon preparation for allograft
A total of 40 flexor digitorum profundus (FDP) tendons from the second and fifth digits were harvested from adult mongrel dog hind paws that were approved for use by our Institutional Animal Care and Use Committee (IACUC). Immediately after sacrifice, the hind paws were frozen in a −80 °C freezer. Following thawing at room temperature, 10 FDP tendons were immediately tested for indentation stiffness and served as a normal tendon control group. The other 30 tendons were immediately submerged into liquid nitrogen for 1 min and then thawed in warmed saline solution (0.9%) at 37 °C for 5 min to create necrosis of the intrinsic fibroblasts in FDP tendons to reduce immunogenicity. This procedure was repeated five times and the tendons were then immediately lyophilized. Before the experiment, each tendon was sterilized with ethylene oxide gas and thawed in saline bath at 4 °C over night for rehydration. Ten randomly selected tendons were immediately assessed for mechanical evaluation, as the lyophilized tendon control group. The other twenty allografts were randomly divided into two groups, one group treated with cd-HA-gelatin (Sun et al., 2004; Tanaka et al., 2007; Zhao et al., 2006) and the other would serve as the allograft in vivo control group.
2.2. Allograft implantation and postoperative rehabilitations
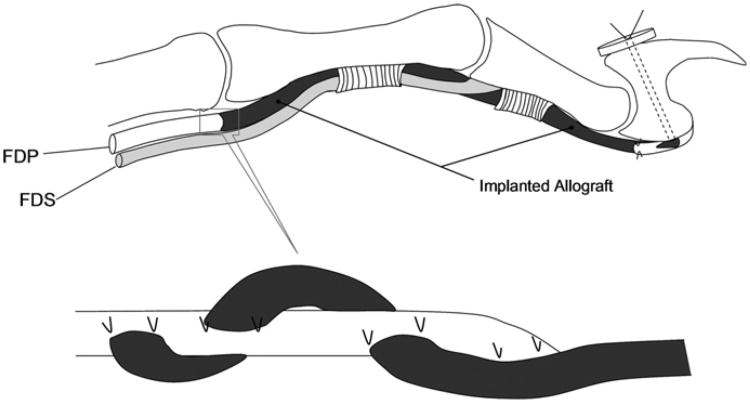
After the dogs were anesthetized with intravenous ketamine and diazepam, both forelimbs were shaved, scrubbed with povidone–iodine and sterilely draped. An elastic bandage was used to exsanguinate the forelimb and to act as a tourniquet for the procedure. The second and fifth digits were used as the flexor tendon allograft recipient sites. Both tendons were excised between a level 5 mm proximal to its attachment to the distal phalanx and the level of the middle of the metacarpal bone. One digit was assigned as the cd-HA-gelatin treated allograft and the other the untreated graft recipient site. In each grafted digit, the proximal ends of the grafts were sutured to the proximal FDP tendons using a Pulvertaft weave technique, secured with two sutures of 4/0 braided Dacron (Ethicon, Somerville, NJ, USA). The distal tendon graft was secured by a pull-out suture of 3/0 nylon (Ethicon, Somerville, NJ, USA), passed through drill holes in the distal phalanx and fixed to a tie-over button dorsally (Fig. 1). The distal end was fixed first, so that the tension of the tendon graft could be adjusted at the time of the proximal repair. Following the tendon graft, the incisions were closed in layers. A high radial neurectomy was performed to prevent the canine from bearing weight on the operated forelimb. A sterile dressing was applied and a sling was used to maintain the operated paw underneath the chest without weight bearing. The dogs were allowed immediate cage activity. On postoperative day 3, rehabilitation was started with a synergistic wrist digit motion protocol (Zhao et al., 2002a, 2002b), performed daily until sacrifice. The dogs were sacrificed with an intravenous overdose of pentobarbital 6 weeks after implantation. The surgical digits were excised after sacrifice for the mechanical evaluations. The second FDP from the contralateral side was also harvested to serve as a control.
Fig. 1.
Schema of allograft implantation.
2.3. Indentation testing
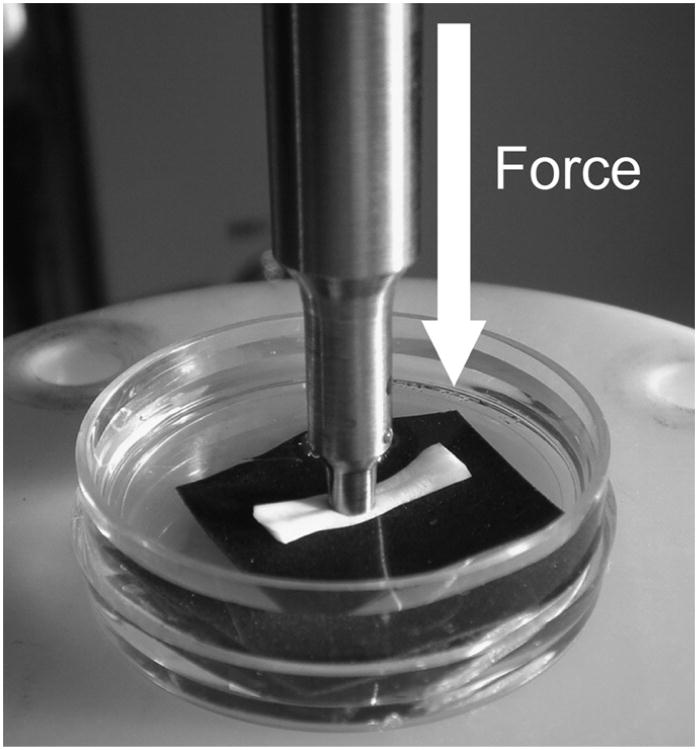
The longitudinal tendinous segments, approximately 10 mm long, were cut from each group. The samples were harvested from Okuda's zone D of the canine FDP (Okuda et al., 1987). The sample pieces were fixed to small tissue culture dishes (Falcon, 35 mm × 10 mm polystyrene Becton-Dickinson Ltd., Franklin Lakes, NJ) using sand paper to avoid tendon slipping. Tissue culture dishes were then filled with normal saline solution (0.9%) so that specimens were fully immersed (Fig. 2). Indentation testing was performed using a BOSE ElectroForce® 3200 test instruments (Bose Corporation, Eden Prairie, MN, USA). The indentation force was monitored with a load transducer (Transducer Techniques, MDB-10, Temecula, CA, USA). An indenter with a flat and non-porous 3 mm diameter was used. Indentation testing was performed at a loading rate of 10N/min to a maximum force of 5 N.
Fig. 2.
Overview of indentation testing. Transducer was placed under the experimental dish.
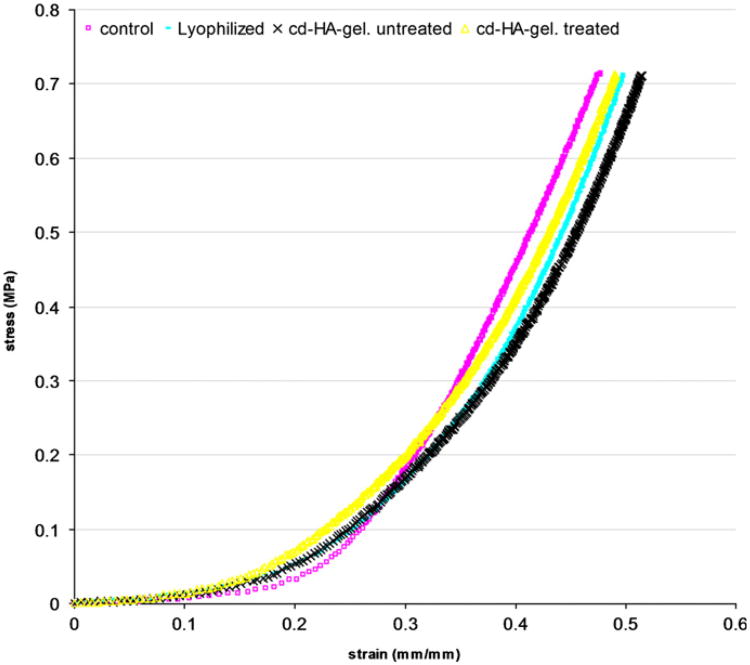
The indentation data was plotted on a stress–strain curve. The stress was calculated by dividing the force by the area of the indenter and the strain was calculated based on the indentation depth relative to the initial specimen height. The slope of the linear region that followed the initial toe region of the stress–strain curve was considered as the indentation stiffness.
2.4. Data analysis
A sample size of 10 was chosen based on a previous publication (Shin et al., 2008), in which the compressive moduli of the intrasynovial and extrasynovial tendons were 4.08 (SD±1.02) and 2.79 (SD±0.53), respectively. With a power of 80%, the sample size of 9 would be sufficient to detect a difference of this magnitude with a p value of less than 0.05.
A single factor analysis of variance (ANOVA) test was used to determine statistically significant differences in indentation stiffness among these groups. A Holm t-test was used to detect significant difference in the stiffness between the different tendons. A statistical significance level of p<0.05 was used in all cases.
3. Results
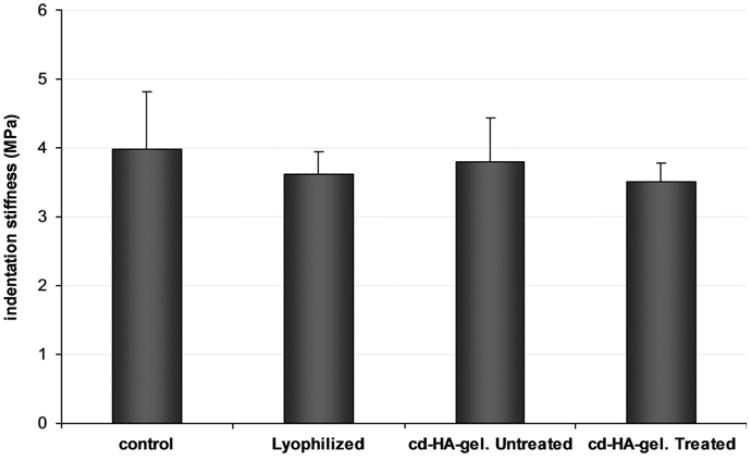
The data from the indentation test showed a non-linear relationship between stress and strain (Fig. 3). The mean stiffness was: of the control group 3.99±0.83 MPa, lyophilized intrasynovial tendon group 3.62±0.33 MPa, without cd-HA treated (saline) group 3.8070.633 MPa, and cd-HA treated group 3.51±0.27 MPa. None of the differences in indentation stiffness among the experimental groups were statistically significant (Fig. 4).
Fig. 3.
A typical sample of stress–strain curve for four different groups of tendon.
Fig. 4.
The indentation stiffness of each experimental group (mean, SD). There are no significant differences to compare with each group.
4. Discussion
The best donor for a tendon graft in the finger is probably an intrasynovial tendon autograft, because it has the greatest potential to improve finger function (Duffy et al., 1992; Gelberman et al., 1992; Noguchi et al., 1997). However, due to the limitations in the availability of suitable intrasynovial autografts, an extrasynovial allograft is often used for hand reconstruction. Lyophilized flexor tendons have been reported as a satisfactory substitute for injured tendon (Cameron et al., 1971; Peacock and Madden, 1967). Potenza (1964) and Potenza and Melone (1978) showed that lyophilized implanted flexor tendons in canine model can function very well and are histologically well tolerated. However, to eliminate allograft immunogenicity, the donor graft cells must be removed in some way. The multiple deep freeze-thaw technique is a common procedure to eliminate the cells during tendon allograft preparation. For the purpose of long term preservation, lyophilization is combined as well. However, lyophilization changes tendon surface morphology and results in higher gliding resistance (Ikeda et al., 2010), which may hinder tendon gliding when such tendons are subsequently rehydrated and used in vivo. Because high gliding resistance causes more adhesions (Zhao et al., 2001a, 2001b) we tried to decrease the gliding resistance for extrasynovial tendon autografts. We found that tendon surface modification with chemically modified hyaluronic acid could improve grafted extrasynovial tendon gliding ability in vitro and in vivo (Momose et al., 2002; Sun et al., 2004; Taguchi et al., 2008; Tanaka et al., 2006, 2007; Yang et al., 2004; Zhao et al., 2006). In addition, we recently reported that this tendon surface treatment, combined with gelatin, significantly improved gliding ability of lyophilized tendon as well (Ikeda et al., 2010). It is possible, though, that this surface modification may also affect tendon stiffness.
While there have been some studies describing the mechanical properties of tendons (Gibbons et al., 1991; Lee et al., 2000; Mae et al., 2003; Salehpour et al., 1995; Zobitz et al., 2001), especially in regards to anterior cruciate ligament reconstruction (Bechtold et al., 1994; Kaminski et al., 2009; Park et al., 2009; Smith et al., 1996), very little is known about the mechanical properties of intrasynovial flexor tendons that are prepared for use as allografts. Webster and Werner (1983) reported on the mechanical properties of grafted intrasynovial flexor tendon allograft and he concluded that lyophilized flexor tendon allograft was a satisfactory alternative to free tendon autografting. Shin et al. (2008) also described the mechanical properties of intrasynovial and extrasynovial tendons.
In the current study, we evaluated the indentation stiffness of the surface modified intrasynovial tendon allograft with and without cd-HA-gelatin in an in vivo canine model. We were unable to find any significant difference in the indentation stiffness of treated tendons, compared to normal ones. The advantages of the indentation test we used versus compression testing include (1) the contact area (indenter area) is more accurately known, which allows us to accurately calculate the tissue stiffness (or hardness), (2) the indenter diameter is small relative to the tendon thickness, and thus is less susceptible to non-uniform stress distribution, which is often an issue for compression testing of thin specimens, and (3) complications such as buckling, bulging, and material densification are not an issue with indentation testing. Additionally, tendon is a homogeneous tissue, therefore sampling is not a factor.
This study has several limitations. First, while tensile properties are important, we only evaluated tendon indentation stiffness. Second, we only evaluated 6 weeks as an in vivo time period. Indentation stiffness may differ with shorter or longer time points.
In conclusion, tendon surface modification with cd-HA-gelatin may improve the tendon gliding ability without compromising the normal indentation stiffness. This tendon modification has shown some promise in a canine model in vivo and may have clinical application.
Acknowledgments
This study was funded by a grant from the Musculoskeletal Transplant Foundation.
Footnotes
Conflict of interest: The authors confirm that there is no potential conflict of interest including employment, consultancies, stock ownership, honoraria, and paid expert testimony, and patent applications influencing this work.
References
- Amadio PC. What's new in hand surgery. Journal of Bone and Joint Surgery—American. 2005;87:468–474. doi: 10.2106/JBJS.D.02807. [DOI] [PubMed] [Google Scholar]
- Bechtold JE, Eastlund DT, Butts MK, Lagerborg DF, Kyle RF. The effects of freeze-drying and ethylene oxide sterilization on the mechanical properties of human patellar tendon. American Journal of Sports Medicine. 1994;22:562–566. doi: 10.1177/036354659402200421. [DOI] [PubMed] [Google Scholar]
- Cameron RR, Conrad RN, Sell KW, Latham WD. Freeze-dried composite tendon allografts: an experimental study. Plastic and Reconstructive Surgery. 1971;47:39–46. doi: 10.1097/00006534-197101000-00009. [DOI] [PubMed] [Google Scholar]
- Duffy FJ, Seiler JG, Hergrueter CA, Kandel J, Gelberman RH. Intrinsic mitogenic potential of canine flexor tendons. Journal of Hand Surgery—British. 1992;17:275–277. doi: 10.1016/0266-7681(92)90114-h. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Chu CR, Williams CS, Seiler JG, 3rd, Amiel D. Angiogenesis in healing autogenous flexor-tendon grafts. Journal of Bone and Joint Surgery—American. 1992;74:1207–1216. [PubMed] [Google Scholar]
- Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. Journal of Orthopaedic Research. 1991;9:209–218. doi: 10.1002/jor.1100090209. [DOI] [PubMed] [Google Scholar]
- Ikeda J, Zhao C, Sun YL, An KN, Amadio PC. Carbodiimide-derivatized hyaluronic acid surface modification of lyophilized flexor tendon: a biomechanical study in a canine in vitro model. Journal of Bone and Joint Surgery—American. 2010;92:388–395. doi: 10.2106/JBJS.H.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CW, Watson MG. Measurement of range of motion of the finger after flexor tendon repair in zone ii of the hand. Journal of Hand Surgery—American. 1993;18:411–417. doi: 10.1016/0363-5023(93)90083-f. [DOI] [PubMed] [Google Scholar]
- Kaminski A, Gut G, Marowska J, Lada-Kozlowska M, Biwejnis W, Zasacka M. Mechanical properties of radiation-sterilised human bone–tendon–bone grafts preserved by different methods. Cell Tissue Bank. 2009;10:215–219. doi: 10.1007/s10561-008-9112-1. [DOI] [PubMed] [Google Scholar]
- Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthopedic Clinics of North America. 1973;4:865–876. [PubMed] [Google Scholar]
- Lee DH, Robbin ML, Galliott R, Graveman VA. Ultrasound evaluation of flexor tendon lacerations. Journal of Hand Surgery—American. 2000;25:236–241. doi: 10.1053/jhsu.2000.jhsu25a0236. [DOI] [PubMed] [Google Scholar]
- Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clinical Orthopaedics and Related Research. 2003:305–314. doi: 10.1097/01.blo.0000079440.64912.c3. [DOI] [PubMed] [Google Scholar]
- Momose T, Amadio PC, Sun YL, Zhao C, Zobitz ME, Harrington JR, An KN. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. Journal of Biomedical Materials Research. 2002;59:219–224. doi: 10.1002/jbm.1235. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Seiler JG, 3rd, Boardman ND, 3rd, Tramaglini DM, Gelberman RH, Woo SL. Tensile properties of canine intrasynovial and extrasynovial flexor tendon autografts. Journal of Hand Surgery—American. 1997;22:457–463. doi: 10.1016/S0363-5023(97)80013-5. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Gorski JP, An KN, Amadio PC. Biochemical, histological, and biomechanical analyses of canine tendon. Journal of Orthopaedic Research. 1987;5:60–68. doi: 10.1002/jor.1100050109. [DOI] [PubMed] [Google Scholar]
- Park HJ, Urabe K, Naruse K, Onuma K, Nemoto N, Itoman M. The effect of cryopreservation or heating on the mechanical properties and histomorphology of rat bone–patellar tendon–bone. Cell Tissue Bank. 2009;10:11–18. doi: 10.1007/s10561-008-9109-9. [DOI] [PubMed] [Google Scholar]
- Peacock EE, Jr, Madden JW. Human composite flexor tendon allografts. Annals of Surgery. 1967;166:624–629. doi: 10.1097/00000658-196710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza AD. The healing of autogenous tendon grafts within the flexor digital sheath in dogs. Journal of Bone and Joint Surgery—American. 1964;46:1462–1484. [PubMed] [Google Scholar]
- Potenza AD, Melone C. Evaluation of freeze-dried flexor tendon grafts in the dog. Journal of Hand Surgery—American. 1978;3:157–162. doi: 10.1016/s0363-5023(78)80065-3. [DOI] [PubMed] [Google Scholar]
- Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. Journal of Orthopaedic Research. 1995;13:898–906. doi: 10.1002/jor.1100130614. [DOI] [PubMed] [Google Scholar]
- Shin RH, Zhao C, Zobitz ME, Amadio PC, An KN. Mechanical properties of intrasynovial and extrasynovial tendon fascicles. Clinical Biomechanics. 2008;23:236–241. doi: 10.1016/j.clinbiomech.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Smith CW, Young IS, Kearney JN. Mechanical properties of tendons: changes with sterilization and preservation. Journal of Biomechanical Engneering. 1996;118:56–61. doi: 10.1115/1.2795946. [DOI] [PubMed] [Google Scholar]
- Strickland JW. Flexor tendon surgery. Part 2: free tendon grafts and tenolysis. Journal of Hand Surgery—British. 1989;14:368–382. doi: 10.1016/0266-7681_89_90151-4. [DOI] [PubMed] [Google Scholar]
- Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. Journal of Orthopaedic Research. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, An KN, Amadio PC. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. Journal of Bone and Joint Surgery—American. 2008;90:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Sun YL, Zhao C, Zobitz ME, An KN, Amadio PC. Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. Journal of Orthopaedic Research. 2006;24:1555–1561. doi: 10.1002/jor.20205. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Zhao C, Sun YL, Zobitz ME, An KN, Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. Journal of Hand Surgery—American. 2007;32:876–881. doi: 10.1016/j.jhsa.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the a2 pulley. Journal of Bone and Joint Surgery—American. 1997;79:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- Webster DA, Werner FW. Freeze-dried flexor tendons in anterior cruciate ligament reconstruction. Clinical Orthopaedics and Related Research. 1983:238–243. [PubMed] [Google Scholar]
- Wehbe MA. Tendon graft donor sites. Journal of Hand Surgery—American. 1992;17:1130–1132. doi: 10.1016/s0363-5023(09)91079-6. [DOI] [PubMed] [Google Scholar]
- Yang C, Amadio PC, Sun YL, Zhao C, Zobitz ME, An KN. Tendon surface modification by chemically modified ha coating after flexor digitorum profundus tendon repair. Journal of Biomedical Materials Research—Part B, Applied Biomaterials. 2004;68:15–20. doi: 10.1002/jbm.b.10074. [DOI] [PubMed] [Google Scholar]
- Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. Journal of Trauma-Injury Infection and Critical Care. 2001a;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Zhao C, Amadio PC, Momose T, Zobitz ME, Couvreur P, An KN. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. Journal of Orthopaedic Research. 2002a;20:857–862. doi: 10.1016/S0736-0266(01)00168-1. [DOI] [PubMed] [Google Scholar]
- Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Gliding resistance after repair of partially lacerated human flexor digitorum profundus tendon in vitro. Clinical Biomechanics. 2001b;16:696–701. doi: 10.1016/s0268-0033(01)00056-0. [DOI] [PubMed] [Google Scholar]
- Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clinical Orthopaedics and Related Research. 2002b:223–230. doi: 10.1097/00003086-200203000-00033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. Journal of Bone and Joint Surgery—American. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun YL, Ikeda Y, Kirk RL, Thoreson AR, Moran SL, An K, Amadio PC. Improvement of flexor tendon reconstruction using carbodiimide derivatized hyaluronic acid and gelatin modified intrasynovial allografts with a primary repair failure model. Journal of Bone and Joint Surgery—American. 2010;92:2817–28. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobitz ME, Zhao C, Erhard L, Amadio PC, An KN. Tensile properties of suture methods for repair of partially lacerated human flexor tendon in vitro. Journal of Hand Surgery—American. 2001;26:821–827. doi: 10.1053/jhsu.2001.26031. [DOI] [PubMed] [Google Scholar]