Abstract
Dysregulation of the fear system is at the core of many psychiatric disorders. Much progress has been made in uncovering the neural basis of fear learning through studies in which associative emotional memories are formed by pairing an initially neutral stimulus (conditioned stimulus, CS; e.g., a tone) to an unconditioned stimulus (US; e.g., a shock). Despite significant recent advances, the question of how to persistently weaken aversive CS-US associations, or dampen traumatic memories in pathological cases, remains a major dilemma. Two paradigms (blockade of reconsolidation and extinction) have been used in the laboratory to reduce acquired fear. Unfortunately, their clinical efficacy is limited: reconsolidation blockade typically requires potentially toxic drugs and extinction is not permanent. Here we describe a novel behavioral design, in rats, in which a fear memory is destabilized and reinterpretated as safe by presenting an isolated retrieval trial prior to an extinction session. This procedure permanently attenuates the fear memory without the use of drugs.
When fearful memories are formed, they are initially labile but become progressively consolidated into persistent traces via the synthesis of new proteins (1, 2). Later retrieval of a consolidated fear memory engages two seemingly opposing mechanisms: reconsolidation and extinction (3-6). In the process of reconsolidation, a retrieved memory transiently returns to a labile state and requires new protein synthesis to persist further. During this labile state, the memory is amenable to enhancement or disruption (4, 8). The period of instability or lability, the reconsolidation window, persists for several hours following retrieval (9). Reconsolidation occurs in a broad range of learning paradigms (aversive and appetitive conditioning, explicit and implicit memory (5, 10) and species (from snails to humans) (11, 12). Its adaptive purpose might be to enable the integration of new information present at the time of retrieval into an updated memory representation (4, 13, 14). The possibility that reactivated memories may be modifiable was proposed many years ago (7,15), and since then, numerous studies have demonstrated that blockade of the updating process engaged during retrieval, usually via pharmacological intervention within the reconsolidation window, prevents memory re-storage and produces amnesia (loss of the specific memory that was reactivated in the presence of the drug or access to it) (4, 9, 13, 14). Thus, in the case of aversive memories, blocking reconsolidation weakens the emotional impact of a once fear-inducing stimulus by altering the molecular composition of the memory trace. This process generally requires the use of drugs that often cannot be readily administered to humans.
In contrast, fear extinction, a paradigm in which the CS is repeatedly presented in the absence of the US, leads to the progressive reduction in the expression of fear, but is not permanent because extinction does not directly modify the existing memory but instead leads to the formation of a new memory that suppresses activation of the initial trace (16-22). The efficacy of this inhibition, however, is strongly contingent upon spatial, sensory, and temporal variables. Specifically, the reemergence of a previously extinguished fear is known to occur, in rodents and humans alike, under three general conditions: (1) renewal, when the conditioned stimulus (CS) is presented outside of the extinction context (17, 18); (2) reinstatement, when the original unconditioned stimulus (US) is given unexpectedly (19, 20, 21, 22, 23), or (3) spontaneous recovery, when a substantial amount of time has passed (16, 17, 23). In clinical settings, where extinction-based exposure therapy is widely used as treatment for a number of anxiety-related disorders, including phobias and post-traumatic stress, exposure treatments are effective in some cases (e.g., 24, 25); however, they do not benefit everyone, and of those who do benefit, many show a return of fear due to spontaneous recovery, reinstatement or renewal (18, 23, 26, 27).
In the current study we devised an effective, drug-free paradigm for the persistent reduction o f learned fear, capitalizing on differences between reconsolidation and extinction. Given that extinction training reduces the threatening value of the CS, we reasoned that when applied within the reconsolidation window (after the memory is rendered unstable by presenting an isolated retrieval trial) extinction training would result in the storage of the new non-threatening meaning of the CS and prevent renewal, reinstatement and spontaneous recovery, thus resulting in a more enduring reduction in fear than extinction training conducted outside the reconsolidation window. Specifically, we predicted that an extinction session presented after an isolated retrieval trial would lead to a persistent revaluation of the CS as less threatening, and/or a weakening of the stored trace or access to it, and thus would prevent the return of fear in the three aforementioned tests.
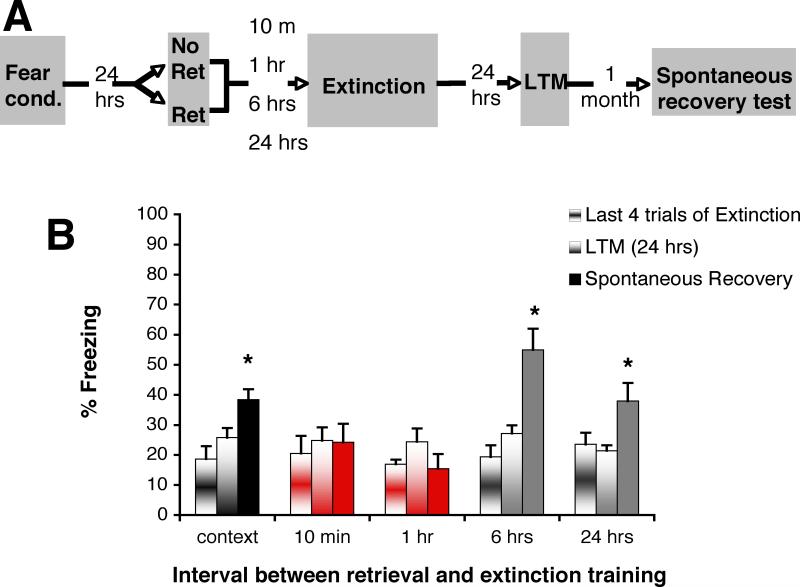
Six experiments were conducted. We first examined whether our behavioral paradigm could prevent the return of fear on a spontaneous recovery test, and if so, whether the observed effect was the result of an update during reconsolidation. We specifically designed this experiment based on the premise that the lability window engaged at the time of retrieval is temporary—in rat fear conditioning, it closes within 6 hours (4)— at which time the memory is thought to be reconsolidated (4). We posited that if the interval between the isolated retrieval cue and extinction training was brief enough to enable the repeated un-reinforced CSs to be presented within the lability window, that the new interpretation of the CS as no-longer threatening should be incorporated during reconsolidation. If, however, the interval between the isolated retrieval trial and the beginning of extinction was outside the lability boundary, standard extinction should take place (meaning that rather than targeting the initial fear memory during its reconsolidation, a new memory would be formed in parallel with it, and act to temporarily suppress it), and fear should re-emerge. Rats were fear conditioned as described above, and were then divided into 5 experimental groups. Two groups had a retrieval-extinction interval within (10 minutes, n=8; and 1 hour, n=8) and 2 groups outside the reconsolidation window (6, n=8; and 24 hrs, n=8). The fifth group was exposed to context, but did not receive a CS retrieval (n=12). All procedures were conducted in context A. All groups showed equivalent freezing for the last 4 trials of extinction (between-subjects ANOVA, P>.1; figure 1). Twenty-four hours later, all groups received an LTM test to assess consolidation of extinction; the groups did not differ from one another (repeated measures ANOVA, P>.1). All groups were tested one month after extinction, and their freezing to the CS was compared to their respective freezing at the 24 hr timepoint. A repeated measures ANOVA revealed a Group by Time interaction, suggesting that there was a differential effect between the groups between the 24 hr LTM test, and the one month test, F(1,39)=22.47, P<.0001. Simple main effects were then conducted to look at each group individually. The Ret groups with a retrieval-extinction interval outside the reconsolidation window, as well as the No Ret group, showed increased freezing (spontaneous recovery) relative to the 24 hr LTM test (within-subjects, two-tailed t-tests, no retrieval- t(11)=5.225, P<.0001; 6 hr – t(7)=5.671, P=.001; 24 hr –t(7)=2.681, P=.031), however, the groups with an interval within the lability window did not (within-subjects, two-tailed t-tests- 10 min-- t(7)=.146, P=.888; 1 hr—t(7)=1.59, P=.156) (see SOM for further details). These data are consistent with an update during reconsolidation.
Figure 1. Finite lability window to prevent return of fear via post retrieval extinction.
(A) Rats were fear-conditioned (Fear Cond) with three tone-shock pairings. Twenty-four hours later they were exposed either to an isolated cue retrieval trial (Ret) or context only (No Ret) followed by extinction training (Ext). The time interval between the retrieval trial (or context exposure, n=12) and the extinction was either within (10 min, n=8; 1 hr, n=8), or outside (6 hrs, n=8; 24 hrs, n=8) the reconsolidation window. Twenty-four hours after extinction, all groups were tested for long-term memory (LTM), and one month later for spontaneous recovery. The grey shading represents context A. (B) All groups were equivalent for the last 4 trials of extinction and at the 24 hr LTM test. One month after later, the Ret groups with an interval outside the reconsolidation window (grey), as well as the No Ret group (black), showed increased freezing (spontaneous recovery) relative to the 24 hr LTM test (no retrieval- P<.0001; 6 hr ITI-P=.001; 24 hr ITI- P=.031), however, the groups with an interval within the lability window (red) did not (10 min- P=.888; 1 hr- P=.156) (see SOM for further details). All data points show mean ± SEM.
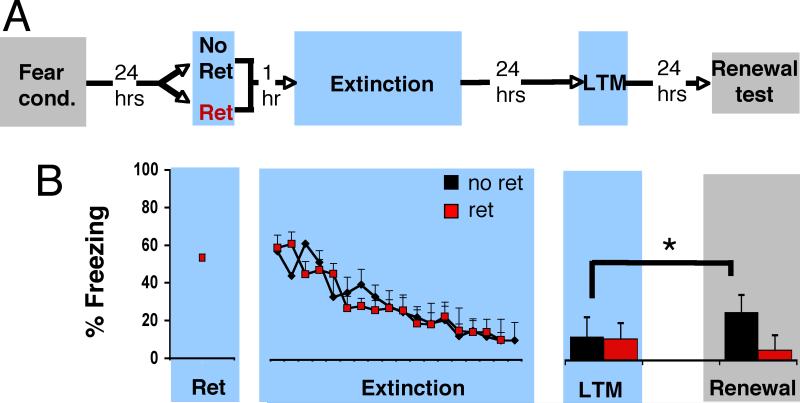
In order to more fully address whether our procedure could prevent the return of fear, we further examined its effect on two additional assays: renewal and reinstatement. Two groups of rats were fear conditioned with three tone-shock pairings (see Supplementary Online Material (SOM) for details). Twenty-four hours later, reconsolidation was initiated in one group by exposing the rats to an isolated retrieval trial (one tone presentation; Ret group, n=8), while the control group was placed in the same context but was not presented with a cue retrieval (no tone presentation; No Ret group, n=8). One hour later, extinction training occurred (the No Ret group was presented with 19, and the Ret group 18 CSs, in the absence of the US, that is, tones were repeatedly presented in the absence of shocks). In the Renewal experiment (Figure 2), rats were fear conditioned in context A, and then received the retrieval, or context-only exposure, and the extinction session in context B (see SOM for detailed descriptions of context A and B). Twenty-four hours later, they were tested for long-term memory in context B, and the next day were tested back in context A (renewal test). We found that the No Ret and Ret rats exhibited similar levels of freezing (a measure of fear expression) during fear conditioning (Repeated Measures ANOVA, P>.05), across the last 4 trials of extinction (Repeated Measures ANOVA, P>.1), and at the test of LTM (Repeated Measures ANOVA, P>.1). When placed back in context A (the original context in which fear to the CS was acquired), to assess whether they would show increased freezing relative to the extinction context (which would be indicative of fear renewal), there was a significant Group X Time of Test interaction, which suggested that the retrieval procedure induced a differential effect on behavior (F(1,14)= 13.522, p=.002). Follow up t-tests revealed that while the No Ret group showed an increase in freezing in context A relative to context B (P=.012), the Ret group did not (P>.1).
Figure 2. Attenuation of fear memory by presenting a single isolated retrieval trial followed by an extinction session prevents renewal.
(A) Rats were fear-conditioned (Fear Cond) in context A. Twenty-four hours later they were exposed either to an isolated cue retrieval trial (Ret, n=8) or context only (No Ret, n=8) in context B, followed, one hour later, by extinction training (Ext) in context B. Twenty-four hours after extinction, they were tested for long-term memory (LTM) in context B. The grey shading represents context A, and the blue shading represents context B (see SOM for details). (B) Rats from both experimental groups froze equivalently during the LTM test (all ANOVAs, P>.1). When they were placed back in the acquisition context, the No Ret group (black) showed fear renewal (P=.012), but the Ret group (red) did not (p>.1), relative to their respective LTM tests. All data points show mean ± SEM.
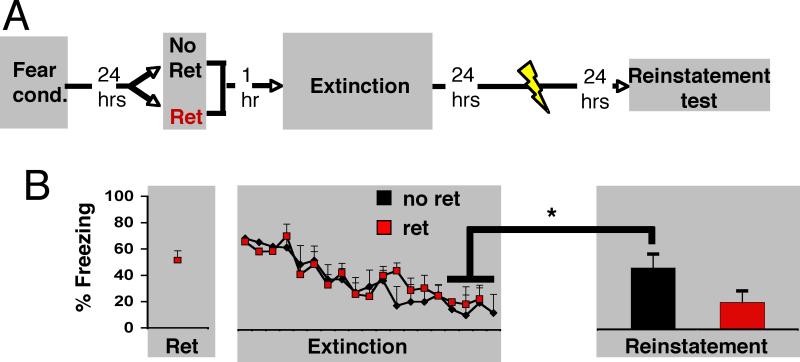
For the Reinstatement experiment, all procedures (described above) were conducted in Context A (Figure 3). Twenty-four hours after extinction, rats received five un-signaled footshocks and were tested for reinstatement the next day (Figure 3). The No Ret (n=8) and Ret (n=8) groups froze equivalently during conditioning, extinguished at the same rate, and did not differ during the last 4 trials of extinction (Repeated-measures ANOVAs, all tests, P>.1). There was a significant Group X Time of test interaction, which suggested that the retrieval procedure induced a differential effect on freezing behavior (F(1,14)= 5.456, p= .035). In agreement with previous research, follow-up comparisons revealed that the No Ret rats showed increased freezing 24 hours after the unsignaled footshocks (reinstatement) compared to the last 4 trials of extinction (P=.017), but rats in the Ret group did not (P>.1). There was no difference between the groups in pre-CS freezing (Figure S1)
Figure 3. Presenting a single isolated retrieval trial prior to an extinction session prevents reinstatement.
(A) Rats were fear-conditioned (Fear Cond). The next day, they were exposed either to an isolated cue retrieval trial (Ret, n=8) or context only (No Ret, n=8) followed, one hour later, by extinction training (Ext). Twenty-four hours after extinction, they received 5 unsignaled footshocks, and the next day were tested for reinstatement. The grey shading represents context A. (B) The No Ret and Ret groups froze equivalently to the last 4 CSs of extinction; however, 24 hours after the unsignaled footshocks the No Ret group (black) showed increased freezing (reinstatement) (p<.05), but the Ret group (red) did not (p>.05). All data points show mean ± SEM.
We next proceeded to determine what molecular mechanism might account for the clear behavioral effect of presenting a single isolated retrieval trial prior to extinction training. We wanted to use a design that would allow us to examine acute retrieval-induced biochemical changes that would be taking place on a brief time scale, but that would also be predictive of long-term synaptic plasticity, since there is an overlapping locus of plasticity for extinction and fear conditioning in the lateral amygdala (28). At initial retrieval (first CS presentation after conditioning), both reconsolidation and extinction mechanisms are engaged (5). Generally, as more CSs are presented, learning becomes biased towards extinction. In the current study, the only difference between our 2 experimental groups for the behavioral experiments was the interval between the first and second CSs. For these reasons, our hypothesis was that a different mechanism must be engaged early on (at the time of our differential manipulation), and that this would lead to a different long-term outcome. It was previously shown, in rats, that increasing cAMP-dependent protein kinase (PKA) signaling facilitates, and its blockade hinders, reconsolidation of fear memories (8). In addition, recent rat studies show that reconsolidation following retrieval of a fear memory requires phosphorylation of GluR1 receptors at the PKA site (ser845) (29). Phosphorylation at the S845 site is usually followed by GluR1 receptor insertion (29). GluR1 receptors insertion is indicative of synaptic plasticity, and takes place during consolidation of fear memories (30). In addition, Hu and colleagues (31) recently have shown that norepinephrine (which is known to be important for reconsolidation of fear memories in both rats (32) and humans (12) can trigger GluR1 phosphorylation via PKA (31). In the final experiment, we therefore examined the effect of an isolated retrieval on the phosphorylation of GluR1 at ser845, and then tested what the effect of a subsequent CS presentation would be.
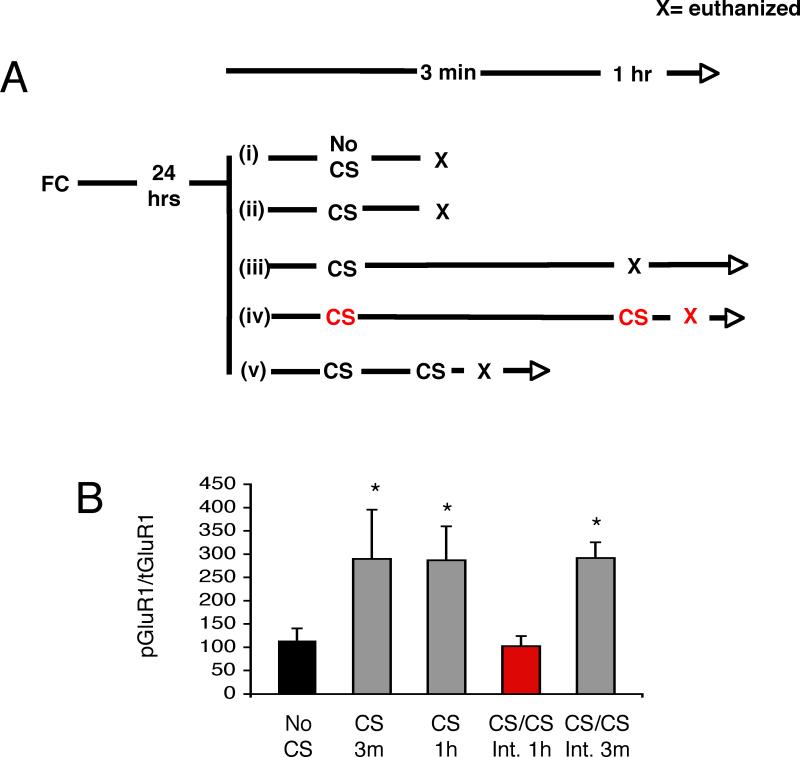
We examined the effect of a single CS presentation on Glur1 phosphorylation 3 min and 1 hr after the retrieval cue, and then asked what would happen if another CS was played 3 minutes vs. 1 hour after (Figure 4). These time points were chosen because our 2 experimental groups (No Ret vs. Ret) show a drastically different behavioral outcome, and their only distinguishing characteristic is a different interval between the first and second CS. We hypothesized that a certain time period might be necessary for the memory trace to be de-stabilized. Rats were fear conditioned, then 24 hrs later received (i) context exposure only (No CS) and euthanized 3 minutes later (n=6); (ii) a single CS retrieval and euthanized 3 min later (n=4); (iii) a single CS and euthanized 1 hr later (n=6); (iv) 2 CSs with a 3 min interval and euthanized 3 min later (n=6); or (v) 2 CSs with a 1 hr interval and euthanized 3 min later (n=6). At the time of euthanasia, the lateral amygdala was extracted, frozen, homogenized, and probed on Western blots for phospho-GluR1. We found that memory retrieval resulted in an increase in GluR1 phosphorylation at ser845 (omnibus ANOVA across all groups, P<.05; with significant posthoc comparisons (Tukey) between the CS-3 min and no CS groups, P<.05 and CS-1 hr group and no CS group, P<.05). A second CS presented one hour after initial retrieval resulted in de-phosphorylation of GluR1 within 3 min, possibly suggesting destabilization of the memory trace, and may underlie the lack of fear re-emergence observed in our behavioral experiments. This dephosphorylation of GluR1 was not simply due to the presentation of 2 CSs instead of one, because the presentation of 2 CSs with the 3 min interval used in standard extinction did not result in de-phosphorylation of GluR1 (Figure 4, Figure S2). These results were also confirmed by Enzyme-linked ImmunoSorbent Assay (ELISA) (Figure S3). These findings suggest that the 2 different treatments (Ret+Ext, vs. No Ret+ Ext) engage different molecular mechanisms in the lateral amygdala, and lead to a drastically different behavioral outcome.
Figure 4. De-phosphorylation of GluR1s845 underlies destabilization, and allows behavioral updating during reconsolidation.
(A) Rats were fear conditioned, then 24 hrs later received (i) context exposure only (No CS) and euthanized 3 minutes later (n=6); (ii) a single CS retrieval and euthanized 3 min later (n=4); (iii) a single CS and euthanized 1 hr later (n=6); (iv) 2 CSs with a 3 min interval and euthanized 3 min later (n=6); or (v) 2 CSs with a 1 hr interval and euthanized 3 min later (n=6). (B) Quantification showing an increase in GluR1 phosphorylation at S845 both 3 min and 1 hr after CS presentation (grey). A second CS presented one hour after initial retrieval leads to de-phosphorylation of the GluR1 receptors (red), while the presentation of 2 CSs with a 3 min interval (grey) does not result in de-phosphorylation. * signifies significantly different from the no CS group (black). Four Western blots were run, and all the data are included in the quantification graph. A representative western blot is shown in S1. All data points show mean ± SEM.
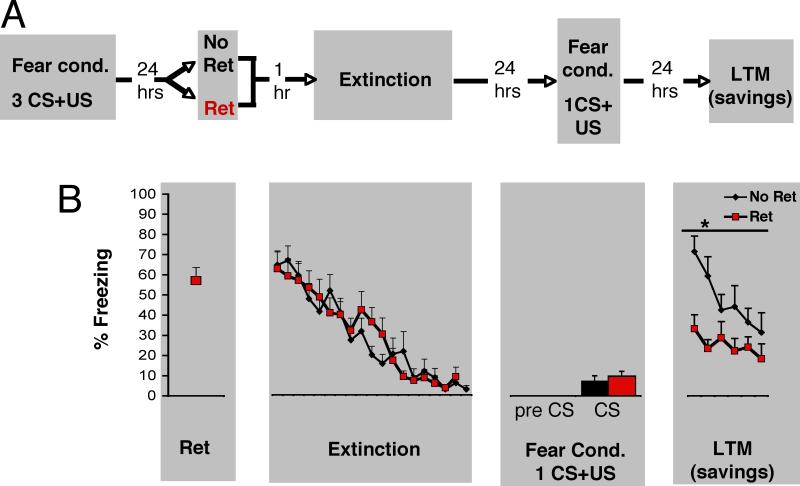
In order to better address whether our Ret+Ext paradigm led to a permanent re-valuing of the CS, we next sought to examine subsequent susceptibility to re-conditioning. We performed a savings experiment, in which the initial phases were identical to the ones presented in our original manuscript (day one: conditioning with 3 CS-US pairings; day two: No Ret+ Ext, or Ret+Ext, with a 1 hour interval between the retrieval and extinction phases). Then, on the third experimental day, we reconditioned rats using a single CS-US pairing. One additional group received the single CS-US pairing only. The fourth day, we tested the groups and compared them for savings (6 CS presentations). The results show that the No Ret group froze significantly more than the Ret group during the long-term memory (LTM) test presented 24 hours after the single CS-US training session, F(1,18)=11.679, P=.003 (Figure 5). The No Ret and Ret groups did not differ during extinction, or during the single CS-US pairing session (P>.1). These results could suggest either that the initial memory has been reversed (deconsolidated), and/or, that the valence associated to the CS has been permanently re-valued, and re-encoded as safe.
Figure 5. Presenting a single isolated retrieval trial prior to an extinction session leads to less fear memory savings than extinction alone.
(A) On day 1, rats were fear conditioned (Fear Cond). The next day, they received either No Ret+Ext (n=10), or Ret+Ext (n=10), with a 1 hour interval between the retrieval and extinction phases. Then, on the third experimental day, rats were reconditioned using a single CS-US pairing. The fourth day, we tested the groups and compared them for savings. (B) The No Ret group (black) froze significantly more than the Ret group (red) during the long-term memory (LTM-savings) test presented 24 hours after the single CS-US training session, F(1,18)=11.679, p=.003. The No Ret and Ret groups did not differ during extinction (p>.1), or during the single CS-US pairing session (p>.1), and no significant pre CS freezing was observed. All data points show mean ± SEM.
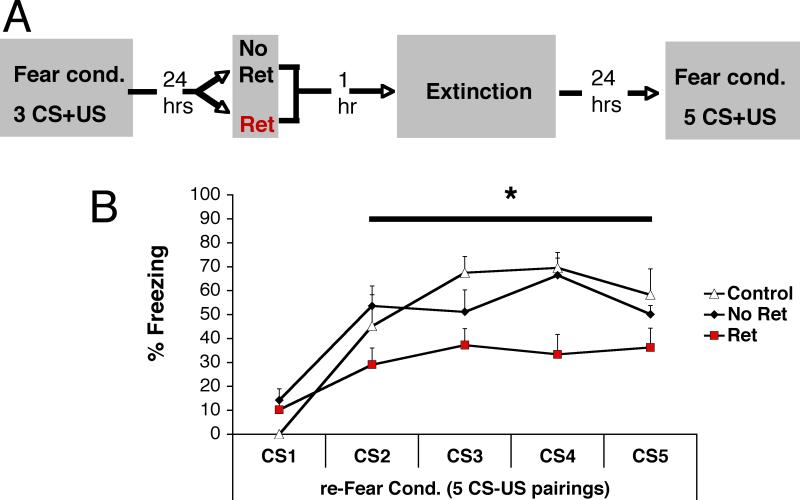
To address this further, we ran one additional experiment examining the effect of our Ret+Ext manipulation on the rate of fear re-acquisition. On day 1, rats were fear conditioned using 3 CS-US pairings. On day 2, they received either a retrieval (Ret+Ext, n=9) or not (No Ret+Ext, n=14), followed one hour later by an extinction session (18 CSs for the retrieval group, 19 CSs for the no retrieval group). On day three, we reconditioned these groups, as well as conditioned a naïve group of rats (control, n=7), using 5 CS-US pairings, to look at the effect of our treatment on re-acquisition. Our results suggest that not only does the Ret+Ext treatment does not lead to savings, it actually retards re-acquisition, relative to a group being conditioned for the first time (control) or the No Ret+ Ext group undergoing conditioning (Figure 6).
Figure 6. An isolated retrieval trial followed by an extinction leads to a re-valuation of the stimulus as safe, and retards subsequent acquisition of fear conditioning.
(A) On day 1, rats were fear conditioned (Fear Cond). On day 2, they received either a retrieval (Ret+Ext, n=9) or not (No Ret, n=14), followed one hour later by an extinction session (18 CSs for the Ret group, 19 CSs for the No Ret group). On day 3, we reconditioned these groups, as well as conditioned a naïve group of rats (control, n=7), using 5 CS-US pairings, to look at the effect of our treatment on re-acquisition. (B) The isolated retrieval presented before extinction (Ret+Ext, red) retards re-acquisition, relative to a naïve group (white) or the No Ret+ Ext group (black). Repeated measures ANOVA revealed a Main effect of Group, F(1,27)=85.85, p<.0001, and a Group by Trial interaction, F(2,27)=55.687, p=.016. Simple main effect follow-up showed that the Ret+Ext group was significantly lower than the Control (.019) and No Ret (.009) groups. The Control and No Ret groups were not significantly different from one another. All data points show mean ± SEM.
Taken together, our renewal, spontaneous recovery, reinstatement, and savings experiments point to a rather resilient decrease in fear induced by our Ret+ext paradigm. Our GluR1 results suggest that a process taking place in the lateral amygdala may underlie this effect. Furthermore, the re-acquisition experiment suggests not only that the CS no longer induces a fear response, but that it may now act as an inhibitor (similarly to what we might expect from a latent inhibition paradigm). This could mean that interference during reconsolidation either led to a progressive deconsolidation of the memory, followed by the learning of a new interpretation of the CS; or, that during reconsolidation, the new valence associated with the CS is incorporated in the updating. In either case, the initial valence conferred by the first conditioning session no longer seems to exist in its original fear-inducing form. In considering clinical implications, it will be important to pursue further what might underlie the retardation of re-acquisition induced by our behavioral procedure because it could, in principle, result in maladaptive behaviors in some cases. Future experiments will aim to determine whether we can successfully tailor our procedure to render a once-fear inducing stimulus simply neutral, without necessarily turning it into a safety signal. That is, we will establish whether the process described here involves destabilization, deconsolidation, and safe-updating, or simply destabilization and safe-updating during reconsolidation. Future studies will also disambiguate “fear expression” from “fear memory” in response to our procedure, and determine the effects on other fear-related assays. Extending these findings to humans would be particularly useful in addressing questions pertaining to the subjective experience resulting from the updated stimulus.
We have shown that presenting extinction training, within a reconsolidation window opened by an isolated CS, prevents renewal, reinstatement, and spontaneous recovery of fear memory. This suggests that a post-consolidation behavioral manipulation can render a memory labile and re-write and/or update it. In rodents, manipulating the intertrial interval of CS presentations during extinction (e.g., using massed vs. spaced training) has been previously reported to yield differential effects on extinction (33), but while massed training is better in the short-term, it worsens the long-term outcome (34). Thus, an important aspect of the current procedure in preventing the return of fear is that the initial CS be isolated from subsequent ones. It was also recently shown that extinction training applied shortly after fear conditioning can prevent memory consolidation, is resistant to the return of fear (35). However, subsequent experiments, in both rats and humans, that used variations of this protocol have met with limited success (36-37). This indicates that the contingencies that function to prevent fear re-emergence, either in the context of consolidation or reconsolidation, may be sensitive to subtle manipulations. Our results are consistent with the idea that an adaptive purpose of reconsolidation is to incorporate new information at the time of retrieval, and to update a memory (4, 8, 14)-- in the present case leading to destabilization of the initial trace in the lateral amygdala, and the re-encoding of the once fear-inducing CS as safe.
Supplementary Material
References
- 1.Squire LR, Davis HP. Annu. Rev. Pharmacol. Toxicol. 1981;21:323. doi: 10.1146/annurev.pa.21.040181.001543. [DOI] [PubMed] [Google Scholar]
- 2.McGaugh JL. Science. 2000;287:248. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 3.Lee JL, Milton AL, Everitt BJ. J. Neurosci. 2006;26:10051. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nader K, Schafe GE, LeDoux JE. Nature. 2000;406:722. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg MT, Kobilo, Dudai Y. Science. 2003;301:1102. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 6.Wolpe J. The practice of behavior therapy. Pergamon; New York: 1969. [Google Scholar]
- 7.Misanin JR, Miller RR, Lewis DJ. Science. 1968;160:554. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 8.Tronson NC, Wiseman SL, Olausson P, Taylor JR. Nat Neurosci. 2006;9:167. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- 9.Duvarci S, Nader K. J. Neurosci. 2004;24:9269. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida SJ. Neurosci. 2004;24:4787. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangha S, Scheibenstock A, Lukowiak K. J. Neurosci. 2003;23:8034. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindt M, Soeter M, Vervliet B. Nat. Neurosci. 2009 doi: 10.1038/nn.2271. In press. [DOI] [PubMed] [Google Scholar]
- 13.Alberini CM. Trends Neurosci. 2005;28:51. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Hupbach A, Gomez R, Hardt O, Nadel L. Learn Mem. 2007 Jan-Feb;14:47. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson R, Riccio DC, Jamis M, Cabosky J, Skoczen T. Am J Psychol. 1982;95:67. [PubMed] [Google Scholar]
- 16.Pavlov IV. In: Conditioned reflexes. Anrep GV, editor. Liveright publishing; New York: 1927. [Google Scholar]
- 17.Robbins SJ. J. Exp. Psychol. Anim. Behav. Process. 1990;16:235. [Google Scholar]
- 18.Effting M, Kindt M. Behav. Res. Ther. 2007;45:2002. doi: 10.1016/j.brat.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Rescorla RA, Heth CD. J. Exp. Psychol. Anim. Behav. Process. 1975;1:88. [PubMed] [Google Scholar]
- 20.Bouton ME, Bolles RC. J. Exp. Psychol. Anim. Behav. Process. 1979;5:368. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- 21.Bouton ME, Bolles RC. Learn. Motiv. 1979;10:455. [Google Scholar]
- 22.Westbrook RF, Iordanova M, McNally G, Richardson R, Harris JA. J. Exp. Psychol. Anim. Behav. Process. 2002;28:97. [PubMed] [Google Scholar]
- 23.Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, Phelps EA. Learn. Mem. 2008;15:394. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellstrom K, Ost LG. Behaviour Research and Therapy. 1995;33:959. doi: 10.1016/0005-7967(95)00028-v. [DOI] [PubMed] [Google Scholar]
- 25.Powers MB, Smits JA, Telch MJ. Journal of Consulting and Clinical Psychology. 2004;72:448. doi: 10.1037/0022-006X.72.3.448. [DOI] [PubMed] [Google Scholar]
- 26.Rowe MK, Craske MG. Behaviour Research and Therapy. 1998;36:719. doi: 10.1016/s0005-7967(97)10017-1. [DOI] [PubMed] [Google Scholar]
- 27.Rowe MK, Craske MG. Behaviour Research and Therapy. 1998;36:701. doi: 10.1016/s0005-7967(97)10016-x. [DOI] [PubMed] [Google Scholar]
- 28.Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. J. Neurosci. 2009;29:402. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. J. Neurosci. 1994;14:7585. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumpel S, LeDoux JE, Zador A, Malinow R. Science. 2005;308:83. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, Real E, Takamiya K, Kang MG, Ledoux JE, Huganir RL, Malinow R. Cell. 2007;131:160. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Debiec J, Ledoux JE. Neuroscience. 2004;129:267. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Cain CK, Blouin AM, Barad M. J Exp Psychol Anim Behav Process. 2003;29:323. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- 34.Li SH, Westbrook RF. J Exp Psychol Anim Behav Process. 2008;34:336. doi: 10.1037/0097-7403.34.3.336. [DOI] [PubMed] [Google Scholar]
- 35.Myers KM, Ressler KJ, Davis M. Learn Mem. 2006;13:216. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, Phelps EA. Learn Mem. 2008;15:394. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S, Chang CH. Proc Natl Acad Sci USA. 2006;103:18020. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JL. Nat. Neurosci. 2008;11:1264. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.