Abstract
Purpose
To investigate the effects of surface modification of extrasynovial tendon with a carbodiimide derivatized synovial fluid (SF) on the gliding ability of extrasynovial tendon for a possible tendon graft application.
Methods
We used 63 peroneus longus tendons from canine hind legs. We immediately assessed 3 tendons morphologically using a scanning electron microscope (SEM); these served as the normal tendon group. The other 60 tendons were randomly assigned to each of 6 experimental groups treated with (1) control (saline); (2) 1% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) plus 1% N-hydroxysuccinimide (NHS) (cd only); (3) 1% EDC/NHS plus 10% gelatin (cd-G); (4) SF plus 1% EDC/NHS plus 10% gelatin (cd-SF-G); (5) SF only; or (6) SF plus 1% EDC/NHS (cd-SF). We measured the gliding resistance for 1,000 cycles of simulated flexion-extension motion. We also observed the tendon surface smoothness by SEM.
Results
Compared with the first cycle in each group, the gliding resistance after 1,000 cycles of tendon motion was significantly increased in the control, cd only, cd-gelatin, SF only, and cd-SF groups (p<.05). In contrast, we found no significant difference in gliding resistance between the first cycle and 1,000 cycles for the cd-SF-G–treated group. In addition, the gliding resistance in the cd-SF, cd-G, and cd-SF-G groups was significantly lower than the control group after 1,000 cycles of tendon motion (p<.05) and the gliding resistance of the cd-SF-G group was significantly lower than both the cd-G and cd-SF groups (p<.05). On SEM, the surface treated with cd-SF-G was smooth after 1,000 cycles, whereas the other surfaces were rough.
Conclusions
Surface modification of extrasynovial tendon with cd-SF-G improves tendon gliding ability. This treatment may be useful clinically in improving the outcomes of tendon autografts.
Keywords: Extrasynovial tendon, flexor tendon, gliding resistance, graft, surface modification
Injuries to the flexor tendons in the digits are common and usually result in considerable disability. Although immediate primary repair after injury has been generally accepted and outcomes have been improved with postoperative controlled mobilization programs,1–4 functional outcomes are occasionally poor. In the most complex cases, tendon grafts have an important role in salvage.5 However, clinical outcomes after tendon autograft are generally poor owing to complications such as adhesion formation, which impairs hand function. Although the most complex injuries occur to intrasynovial tendons, usually tendon grafts are obtained from extrasynovial tendons because extrasynovial tendons are easily harvested without major ramification to the donor site6–8 and intrasynovial sources are not readily available. However, experimental studies have shown that extrasynovial tendon grafts are associated with more adhesions to the surrounding tissues than are intrasynovial tendon grafts9,10 and grafts harvested from intrasynovial tendons have better outcomes than extrasynovial grafts.11–13 In addition, the gliding resistance of extrasynovial tendon is greater than that of intrasynovial tendon14,15 and the surface of the extrasynovial tendon is rougher than that of intrasynovial tendon.14,16
Previous studies have also shown that higher gliding resistance results in greater adhesion formation17 and extrasynovial tendon has a higher gliding resistance than intrasynovial tendon.14 Tendon gliding resistance is an important factor influencing the outcome of tendon repair. To improve its gliding ability, surface modification of the tendon has been studied using surface lubricants such as hyaluronic acid, lubricin, and phospholipids.15,18–20 Treating the extrasynovial tendon surface with a carbodiimide derivatized hyaluronic acid and gelatin mixture has been shown to improve tendon gliding ability in vitro15,19,21 and improve digit function in vivo.20,22 Phospholipid surface modification can also decrease tendon gliding resistance and improve outcome after flexor tendon repair.18 Recently, the addition of lubricin has demonstrated dramatic improvement in extrasynovial tendon gliding ability in a canine in vitro model.19 All 3 lubricating molecules exist in native synovial fluid (SF) and are found on the intrasynovial tendon surface; each has an important role in joint and tendon lubrication.23–30
In this study, we used native SF as a lubricating material and used a relatively simple tissue engineering technique, carbodiimide derivatization, to fix the SF onto an extrasynovial tendon surface in a canine model in vitro, to test the hypothesis that fixation of SF decreases gliding resistance and smooths the tendon surface.
MATERIALS AND METHODS
Specimen preparation
We harvested 63 peroneus longus (PL) tendons from the hind legs of 32 adult mongrel dogs, weighing approximately 20 kg each, which were killed for other studies approved by our Institutional Animal Care and Use Committee. The superficial aspect of the paratenon around the PL tendon was carefully removed, as recommended clinically when extrasynovial tendons are used for tendon grafting.16 The second digit was dissected from each hind paw. The proximal pulley, proximal and middle phalanx, and flexor digitorum superficialis tendon insertion was carefully preserved and the flexor digitorum profundus tendon was removed. Then the proximal interphalangeal joint was fixed in full extension with a longitudinal 2-mm K-wire (Fig. 1). We immediately assessed 3 PL tendons for morphological evaluation using scanning electron microscopy (SEM); these tendons served as the normal tendon group. We randomly assigned the other 60 PL tendons to 6 groups of 10 tendons each. Each group was then treated by one of the 6 solutions described below and then evaluated mechanically and morphologically.
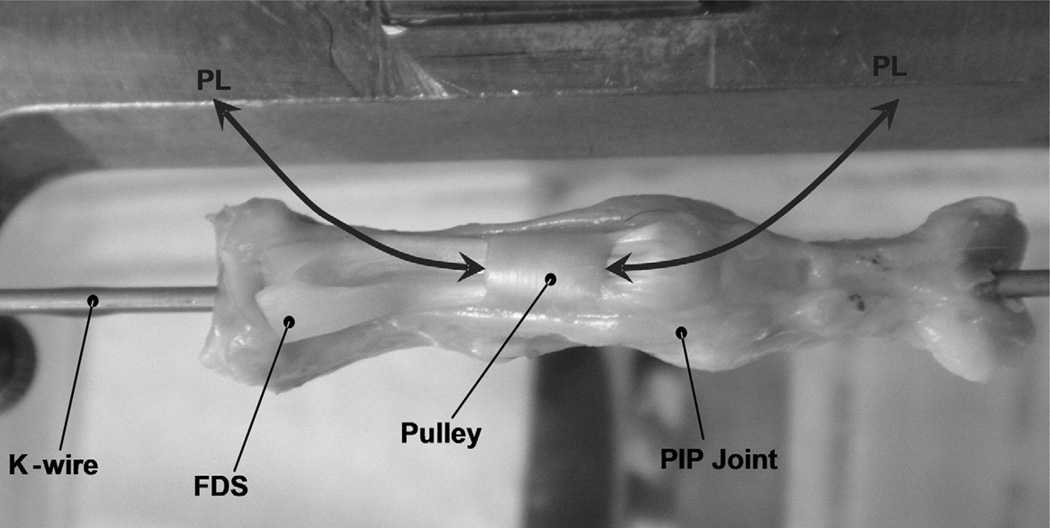
FIGURE 1.
Pulley unit: The proximal phalanx and intact A2 pulley were mounted on the device and fixed in full extension of the proximal interphalangeal joint by a longitudinal K-wire.
Control (saline)
We treated the tendons with 0.9% NaCl, 0.1 mol/L Mes (Sigma Chemical, St. Louis, MO), pH 6.0.
Carbodiimide derivatization only (cd only)
We treated the tendons with 1% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma Chemical)31 and 1% N-hydroxysuccinimide (NHS; Pierce, Rockford, IL), 0.1 mol/L Mes, pH 6.0. In the carbodiimide derivatization reaction, EDC couples carboxylic acid groups to primary animo groups, resulting in the formation of amide bond (Fig. 2). This crosslinking reaction is usually performed between pH 4.5 and 7.5 and requires only a few minutes for completion.32
FIGURE 2.
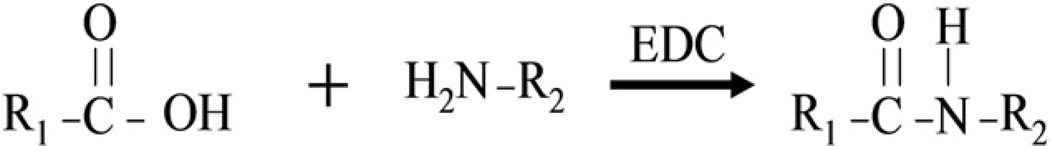
Carbodiimide derivitization reaction.
Carbodiimide derivatization plus gelatin (cd-G)
We treated the tendons with 10% gelatin (Sigma Chemical), 1% EDC, 1% NHS, 0.1 mol/L Mes, pH 6.0.
Carbodiimide derivatized synovial fluid plus gelatin (cd-SF-G)
We treated the tendons with 88% SF, 10% gelatin, 1% EDC, 1% NHS, 0.1 mol/L Mes, pH 6.0.
SF Only
We treated the tendons with 100% SF, 0.1 mol/L Mes, pH 6.0.
Carbodiimide derivatized SF (cd-SF)
We treated the tendons with 98% SF, 1% EDC, 1% NHS, 0.1 mol/L Mes, pH 6.0.
Treatment of tendon surface
We collected 1 to 2 mL SF from knee joints immediately after the dogs were killed and stored it at −80°C for future use. After we collected a total of 60 mL SF, we defrosted and pooled it and divided it into 2-mL aliquots, each stored in a separate vial and frozen again. Just before each experiment, a synovial bottle was randomly chosen, defrosted, and then used for tendon surface modification. The PL tendons were immersed for 30 seconds in one of the 6 solutions. After immersion, each tendon was placed in an incubator at 100% humidity at 37°C for 15 minutes, wrapped with wet towels, kept at 37°C for 1 hour, and then tested.
Measurement of friction force
We evaluated the gliding resistance between the PL tendon and proximal pulley using a testing device developed previously.33–35 A normal canine proximal phalanx with the intact A2 pulley was secured on the custom-made device with the volar side upward (Fig. 1) in a saline bath (ISOTEMP 202; Fisher Scientific, Houston, TX) at 37°C. Each tendon was tested against its own phalanx and pulley. The tendon was then pulled at a rate of 2 mm/s by the actuator against the weight with a fixed excursion of 16 mm, an estimate of normal canine tendon excursion distance based on previous studies.36 The experimental setup consists of one mechanical actuator, a linear potentiometer, and a tensile load transducer, which were connected to the proximal tendon end. The resolution of the recorded output of the force transducers in this setup is less than 1 g. Another tensile load transducer, a pulley unit, and a 4.9-N weight were connected to the distal tendon end. The motion between tendon and pulley was repeated for 1,000 cycles. The number of simulated repetitive cycles, 1,000, was determined based on the estimated total number of postoperative passive motions in a 6-week in vivo canine tendon rehabilitation model.22,36,37 The data of the gliding resistance were recorded after every 50 cycles up to 500 cycles, and then every 100 cycles up to 1,000 cycles. The first 2 cycles of frictional testing were used as preconditioning and to remove any loose superficial reagent; we collected data from the third cycle motion as the first data collection cycle. The force difference between the proximal and distal tendon ends represents the gliding resistance of the flexor digitorum profundus tendon against the A2 pulley. The gliding resistance was obtained from the equation (F2flexion−F2extension)/2.38
Evaluating by SEM
After 1,000 cycles of testing, we prepared 3 tendons in each group for SEM. The selected tendons were washed in phosphate-buffered saline solution and fixed in a solution of buffered glutaraldehyde and osmium tetroxide. After dehydration in graded acetone, the specimens were coated with gold/palladium alloy and examined by SEM. The specimens were mounted on a Hitachi 4700 scanning electron microscope at 3 kV. The surface of the tendon was qualitatively assessed, paying particular attention to the smoothness of the tendon. As noted above, 3 PL tendons were evaluated as a normal extrasynovial tendon just after death.
Statistical analysis
We determined the mean ± SD of the gliding resistance for each treatment group. We used one-way analysis of variance to compare friction data among the 6 experimental groups. For effects of interest, least square means, differences in means, and 95% confidence intervals were determined. We used Tukey-Kramer post-hoc test for each pairwise comparison when there was a significant difference. A p value less than .05 was considered to indicate statistical significance in all cases.
RESULTS
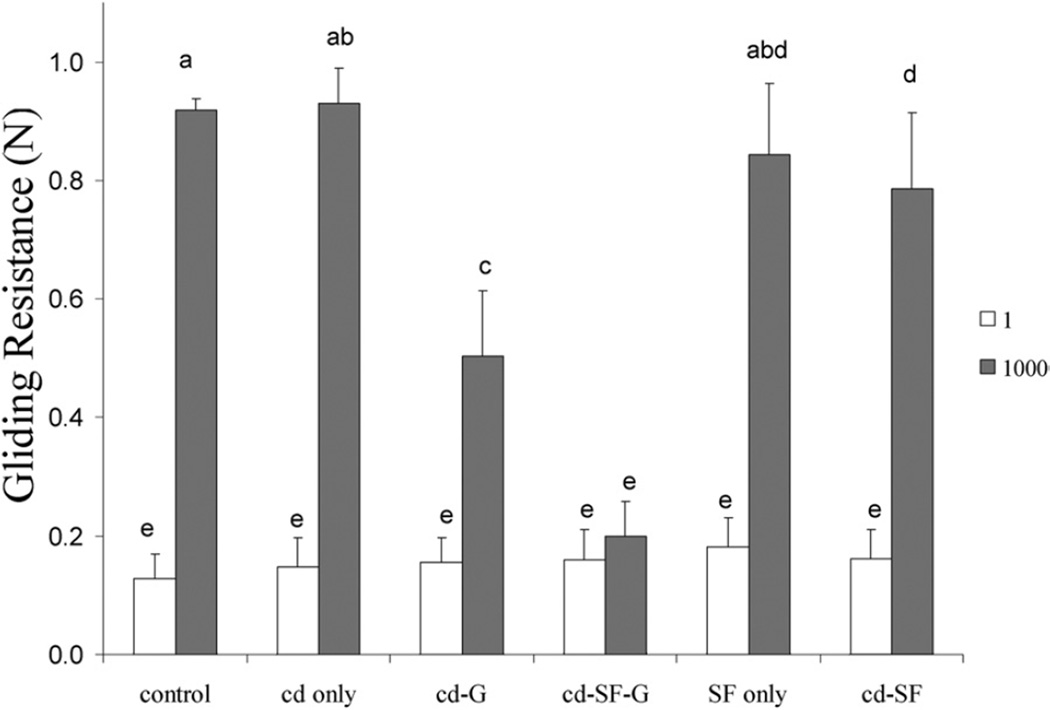
There was no significant difference in gliding resistance of the PL tendon before treatment among the 6 experimental groups (Fig. 3). After 1,000 cycles of tendon gliding motion, the gliding resistance in saline, cd only, cd-G, cd-SF-G, SF only, and cd-SF groups was 0.92 ± 0.02, 0.93 ± 0.06, 0.50 ± 0.11, 0.20 ± 0.06, 0.84 ± 0.12, and 0.79 ± 0.13 N, respectively (Fig. 3). We found a significant difference in gliding resistance between the first cycle and 1,000 cycles in all groups (p<.05) except the cd-SF-G group (Fig. 3). There was no significant difference in gliding resistance after 1,000 cycles between the saline control group and those with tendon treated with cd only and SF only, but gliding resistance in the cd-G, cd-SF-G, and cd-SF groups was significant lower than in the saline control group after 1,000 cycles of tendon motion (p<.05) (Fig. 3). In addition, gliding resistance in the cd-SF-G group was significantly lower than in the cd-G and cd-SF groups (p<.05). Tendons treated with cd-SF-G showed the lowest gliding resistance after 1,000 cycles of tendon motion, increasing by 25% compared with the first cycle; whereas that of the saline control group increased 608%.
FIGURE 3.
Frictional force at first cycle and 1,000 cycles in each group. Different letters indicate significant differences (p<.05).
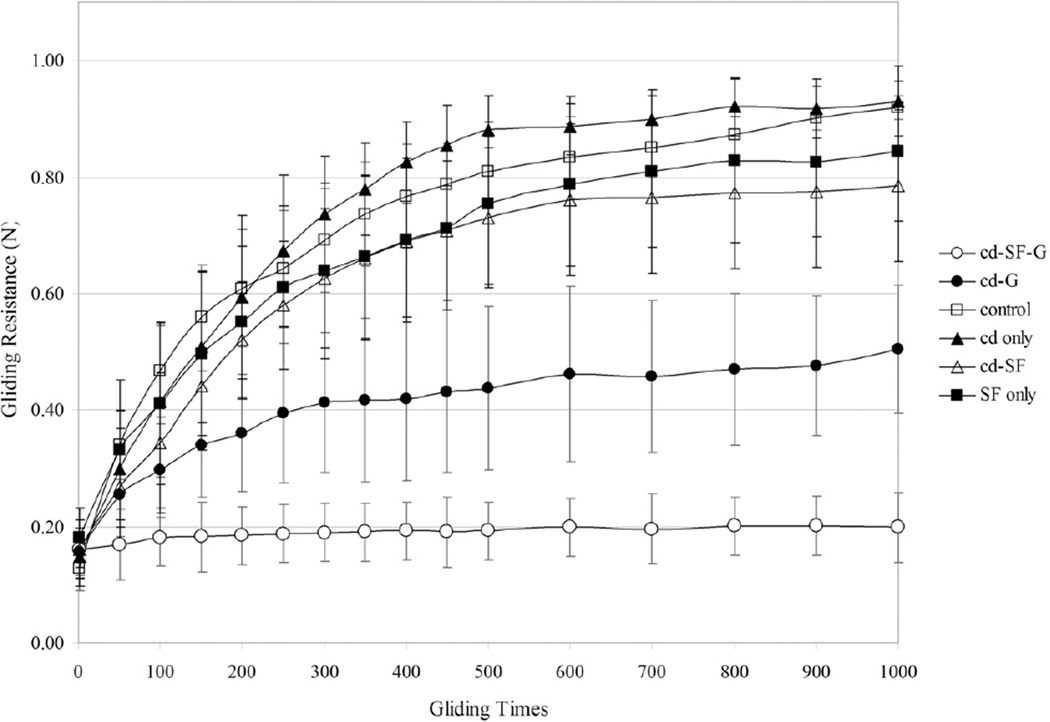
Figure 4 shows the trend of gliding resistance for each group. Gliding resistance of the PL tendons treated with saline, cd only, SF only, and cd-SF groups increased more over the first 500 cycles than in the next 500. Gliding resistance of the PL tendon in the cd-G group increased at a more gradual rate over the 1,000 cycles. Gliding resistance of the PL tendon treated with cd-SF-G was the most stable over the 1,000 cycles.
FIGURE 4.
The trend of gliding resistance from the first cycle to 1,000 cycles.
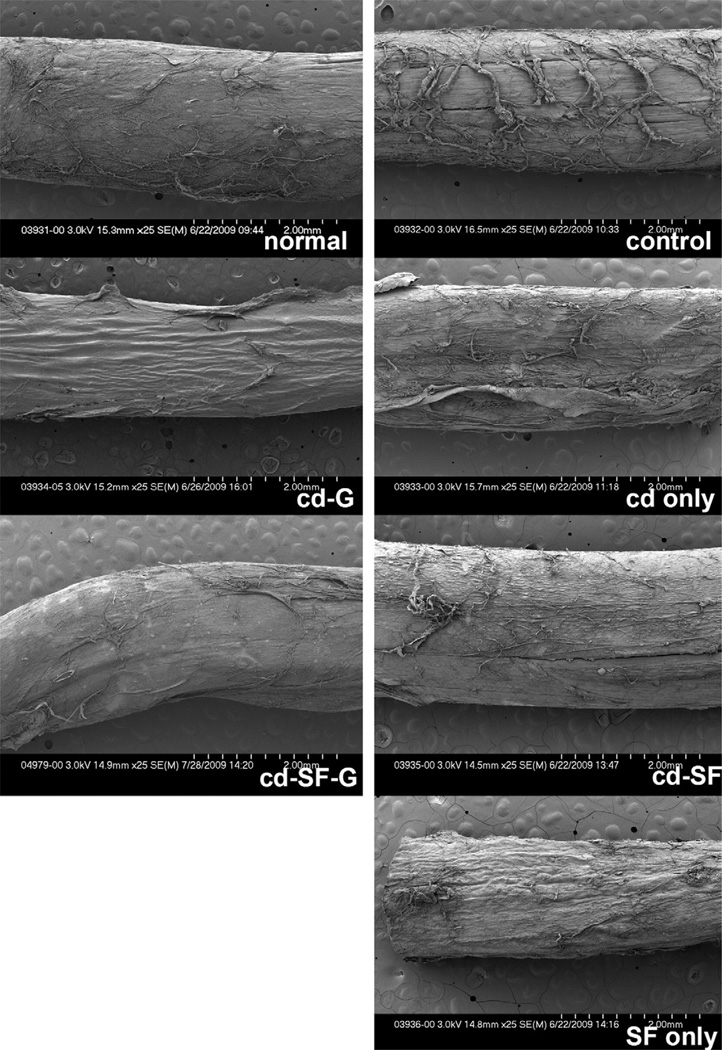
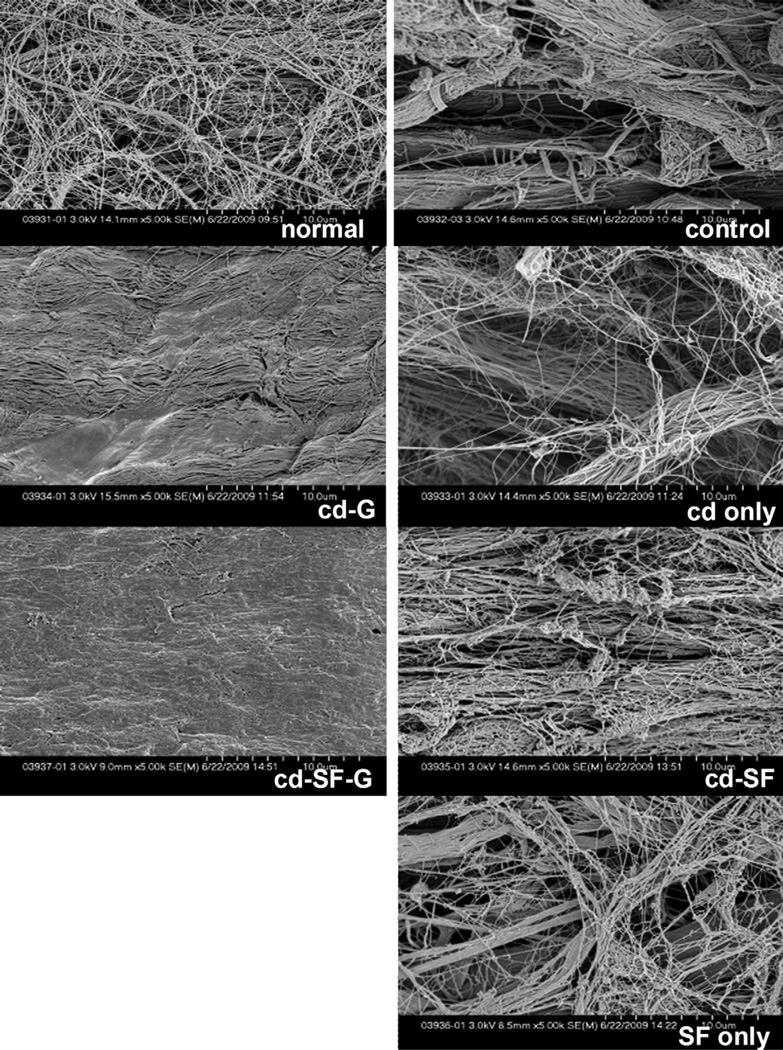
On SEM evaluation at low magnification (Fig. 5), the tendon surface after 1,000 cycles of motion in the saline control, cd only, SF only, and cd-SF groups appeared to be rough, with irregularly arranged collagen bundles around the tendon surface, whereas that of the tendons treated with cd-G and cd-SF-G were still smooth. At high SEM magnification, the collagen was fully exposed on the surface of normal PL, saline control, cd only, SF only, and cd-SF groups after 1,000 cycles of tendon motion, but in the cd-G and cd-SF-G groups the collagen was not exposed (Fig. 6).
FIGURE 5.
Scanning electron microscope images after 1,000 cycles of motion. (Low magnification, ×25.) A smoother surface was noted in the groups treated with cd-SF-gelatin and cd-gelatin.
FIGURE 6.
Scanning electron microscope images after 1,000 cycles of motion. (High magnification, ×5,000.) A smoother surface was noted in the groups treated with cd-SF-gelatin and cd-gelatin.
DISCUSSION
Because of its smooth surface, low friction, and durability, an intrasynovial tendon is the ideal graft tendon for reconstruction of finger function.14,37,39 The 3 principal biological lubricants are hyaluronic acid, lubricin, and phospholipids.23,30,40 Lubricin is the principal lubricant in joints.41 Phospholipids not only function as a joint lubricant but also lubricate other gliding surfaces42,43 including tendon.29,30 Improvement in tendon gliding ability by applying each of these biological lubricants has been reported. Momose et al44 reported that surface modification of extrasynovial tendon with carbodiimide derivatized hyaluronic acid improved tendon gliding ability, and that SF simply placed on the PL tendon surface did not bind strongly enough to the PL tendon surface, so that it was easily rubbed away with repeated tendon motion. Other studies have confirmed these results in extrasynovial and intrasynovial tendon and after tendon repair and graft, both in vitro and in vivo.15,19–22,45,46 Recently Taguchi et al19 combined 2 lubricating molecules, hyaluronic acid and lubricin, and found better results compared with either lubricant used alone.
Our results demonstrated that surface modification using cd-SF-G significantly improved extrasynovial tendon gliding ability during 1,000 cycles of tendon motion. In addition to the natural combination of biological lubricants in SF, the other advantages of using native SF are that it is easily obtained and cost-effective. The same principle could be further investigated in other applications, such as treating arthritic joints. This study also emphasizes the role of gelatin in improving gliding resistance over and above the effect of the lubricant, especially when looking at the effect over 1,000 cycles. We believe that this effect is a result of gelatin working as a grout, filling the interstices of the surface collagen network. In addition, the gelatin may provide more binding sites for the carbodiimide-activated lubricant.
There are some limitations to this study. First, it is an in vitro investigation, which may not apply to an in vivo study. The biological effect of this intervention on tendon graft function in vivo is not known. Second, although we used SEM to identify smoothness on the tendon surface, we did not perform a quantitative evaluation. Third, other tendon mechanical properties such as tensile or compressive moduli were not assessed at this time. Finally, other than surface imaging, we performed no surface assay to confirm that crosslinked SF remained on the tendon surface.
Surface modification of extrasynovial tendons with carbodiimide derivatized SF plus gelatin improves tendon gliding ability in vitro. If confirmed in vivo, this finding may have implications for clinical tendon graft surgery.
Acknowledgments
Supported by a grant from the Mayo Foundation.
Footnotes
No benefits in any form have been received or will be receive related directly or indirectly to the subject of this article.
REFERENCES
- 1.Kleinert HE, Schepel S, Gill T. Flexor tendon injuries. Surg Clin North Am. 1981;61:267–286. doi: 10.1016/s0039-6109(16)42381-9. [DOI] [PubMed] [Google Scholar]
- 2.Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg. 1977;2:441–451. doi: 10.1016/s0363-5023(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 3.Chow JA, Thomes LJ, Dovelle S, Milnor WH, Seyfer AE, Smith AC. A combined regimen of controlled motion following flexor tendon repair in “no man’s land”. Plast Reconstr Surg. 1987;79:447–455. doi: 10.1097/00006534-198703000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg. 1989;14B:383–391. doi: 10.1016/0266-7681_89_90152-6. [DOI] [PubMed] [Google Scholar]
- 5.Amadio PC, Wood MB, Cooney WP, 3rd, Bogard SD. Staged flexor tendon reconstruction in the fingers and hand. J Hand Surg. 1988;13A:559–562. doi: 10.1016/s0363-5023(88)80095-9. [DOI] [PubMed] [Google Scholar]
- 6.White WL. Tendon grafts: a consideration of their source, procurement and suitability. Surg Clin North Am. 1960;40:403–413. doi: 10.1016/s0039-6109(16)36047-9. [DOI] [PubMed] [Google Scholar]
- 7.Wehbe MA. Tendon graft donor sites. J Hand Surg. 1992;17A:1130–1132. doi: 10.1016/s0363-5023(09)91079-6. [DOI] [PubMed] [Google Scholar]
- 8.Carlson GD, Botte MJ, Josephs MS, Newton PO, Davis JL, Woo SL. Morphologic and biomechanical comparison of tendons used as free grafts. J Hand Surg. 1993;18A:76–82. doi: 10.1016/0363-5023(93)90249-3. [DOI] [PubMed] [Google Scholar]
- 9.Gelberman RH, Seiler JG, 3rd, Rosenberg AE, Heyman P, Amiel D. Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg. 1992;26:257–264. doi: 10.3109/02844319209015268. [DOI] [PubMed] [Google Scholar]
- 10.Seiler JG, 3rd, Chu CR, Amiel D, Woo SL, Gelberman RH. The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997;345:239–247. [PubMed] [Google Scholar]
- 11.Abrahamsson SO, Gelberman RH, Amiel D, Winterton P, Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58–66. doi: 10.1002/jor.1100130110. [DOI] [PubMed] [Google Scholar]
- 12.Duffy FJ, Seiler JG, Hergrueter CA, Kandel J, Gelberman RH. Intrinsic mitogenic potential of canine flexor tendons. J Hand Surg. 1992;17B:275–277. doi: 10.1016/0266-7681(92)90114-h. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi M, Seiler JG, 3rd, Boardman ND, 3rd, Tramaglini DM, Gelberman RH, Woo SL. Tensile properties of canine intrasynovial and extrasynovial flexor tendon autografts. J Hand Surg. 1997;22A:457–463. doi: 10.1016/S0363-5023(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 14.Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg. 1997;79A:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Momose T, Amadio PC, Zobitz ME, Zhao C, An KN. Effect of paratenon and repetitive motion on the gliding resistance of tendon of extrasynovial origin. Clin Anat. 2002;15:199–205. doi: 10.1002/ca.10009. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Moro-oka T, Miura H, Mawatari T, Kawano T, Nakanishi Y, Higaki H, et al. Mixture of hyaluronic acid and phospholipid prevents adhesion formation on the injured flexor tendon in rabbits. J Orthop Res. 2000;18:835–840. doi: 10.1002/jor.1100180523. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg. 2008;90A:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Zhao C, Sun YL, Zobitz ME, An KN, Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg. 2007;32A:876–881. doi: 10.1016/j.jhsa.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Sun YL, Zhao C, Zobitz ME, An KN, Amadio PC. Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. J Orthop Res. 2006;24:1555–1561. doi: 10.1002/jor.20205. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg. 2006;88A:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 24.Mazzucco D, Scott R, Spector M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: correlation with flow properties. Biomaterials. 2004;25:4433–4445. doi: 10.1016/j.biomaterials.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Haubeck HD, Kock R, Fischer DC, Van de Leur E, Hoffmeister K, Greiling H. Transforming growth factor beta 1, a major stimulator of hyaluronan synthesis in human synovial lining cells. Arthritis Rheum. 1995;38:669–677. doi: 10.1002/art.1780380515. [DOI] [PubMed] [Google Scholar]
- 26.Radin EL, Paul IL, Swann DA, Schottstaedt ES. Lubrication of synovial membrane. Ann Rheum Dis. 1971;30:322–325. doi: 10.1136/ard.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchiyama S, Amadio PC, Ishikawa J, An KN. Boundary lubrication between the tendon and the pulley in the finger. J Bone Joint Surg. 1997;79A:213–218. [PubMed] [Google Scholar]
- 28.Rees SG, Davies JR, Tudor D, Flannery CR, Hughes CE, Dent CM, et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593–602. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Chen MY, Zhao C, An KN, Amadio PC. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J Orthop Res. 2008;26:1225–1229. doi: 10.1002/jor.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills PC, Hills Y, Hills BA. Surface-active phospholipid (surfactant) in equine tendon and tendon sheath fluid. N Z Vet J. 2005;53:154–156. doi: 10.1080/00480169.2005.36494. [DOI] [PubMed] [Google Scholar]
- 31.Ferrier BM, Branda LA. Synthesis and some biological properties of 1-deamino-4-glu-oxytocin (1-beta-mercaptopropionic acid-4-glutamic acid-oxytocin) and its use in preparing a hormone-agarose complex. Can J Biochem. 1975;53:21–27. doi: 10.1139/o75-004. [DOI] [PubMed] [Google Scholar]
- 32.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 33.An KN, Berglund L, Uchiyama S, Coert JH. Measurement of friction between pulley and flexor tendon. Biomed Sci Instrum. 1993;29:1–7. [PubMed] [Google Scholar]
- 34.Uchiyama S, Coert JH, Berglund L, Amadio PC, An KN. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13:83–89. doi: 10.1002/jor.1100130113. [DOI] [PubMed] [Google Scholar]
- 35.Coert JH, Uchiyama S, Amadio PC, Berglund LJ, An KN. Flexor tendon-pulley interaction after tendon repair. A biomechanical study. J Hand Surg. 1995;20B:573–577. doi: 10.1016/s0266-7681(05)80113-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop Relat Res. 2002;396:223–230. doi: 10.1097/00003086-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 37.Zhao C, Amadio PC, Momose T, Zobitz ME, Couvreur P, An KN. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20:857–862. doi: 10.1016/S0736-0266(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 39.Gelberman RH, Chu CR, Williams CS, Seiler JG, 3rd, Amiel D. Angiogenesis in healing autogenous flexor-tendon grafts. J Bone Joint Surg. 1992;74A:1207–1216. [PubMed] [Google Scholar]
- 40.Blewis ME, Nugent-Derfus GE, Schmidt TA, Schumacher BL, Sah RL. A model of synovial fluid lubricant composition in normal and injured joints. Eur Cell Mater. 2007;13:26–39. doi: 10.22203/ecm.v013a03. [DOI] [PubMed] [Google Scholar]
- 41.Jay GD. Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 42.Hills BA. Surface-active phospholipid: a Pandora’s box of clinical applications. Part II. Barrier and lubricating properties. Intern Med J. 2002;32:242–251. doi: 10.1046/j.1445-5994.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 43.Hills BA. Oligolamellar lubrication of joints by surface active phospholipid. J Rheumatol. 1989;16:82–91. [PubMed] [Google Scholar]
- 44.Momose T, Amadio PC, Sun YL, Zhao C, Zobitz ME, Harrington JR, et al. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res. 2002;59:219–224. doi: 10.1002/jbm.1235. [DOI] [PubMed] [Google Scholar]
- 45.Yang C, Amadio PC, Sun YL, Zhao C, Zobitz ME, An KN. Tendon surface modification by chemically modified HA coating after flexor digitorum profundus tendon repair. J Biomed Mater Res B Appl Biomater. 2004;68:15–20. doi: 10.1002/jbm.b.10074. [DOI] [PubMed] [Google Scholar]
- 46.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27:257–263. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]