Abstract
Objective
Several studies have demonstrated differences in omega-3 fatty acid composition in plasma and in erythrocyte membranes in patients with ADHD compared to unaffected controls. Omega-3 fatty acids have anti-inflammatory properties and can alter central nervous system cell membrane fluidity and phospholipid composition. Cell membrane fluidity can alter serotonin and dopamine neurotransmission. The goal of this meta-analysis is to examine the efficacy of omega-3 fatty acid supplementation in children with ADHD.
Method
We searched PubMED for randomized, placebo-controlled trials examining omega-3 fatty acid supplementation in children with ADHD symptomatology. Our primary outcome measure was standardized mean difference in rating scales of ADHD severity. We conducted secondary analyses to determine the effects of dosing of different omega-3 fatty acids in supplements.
Results
Ten trials involving 699 children were included in this meta-analysis. Omega-3 fatty acid supplementation demonstrated a small, but significant effect in improving ADHD symptoms. Eicosapentaenoic acid (EPA) dose within supplements was significantly correlated with supplement efficacy. We found no evidence of publication bias or heterogeneity between trials.
Conclusion
Omega-3 fatty acid supplementation, particularly with higher doses of EPA, was modestly effective in the treatment of ADHD. The relative efficacy of omega-3 fatty acid supplementation was modest compared to currently available pharmacotherapies for ADHD such as psychostimulants, atomoxetine or alpha-2 agonists. However, given its relatively benign side-effect profile and evidence of modest efficacy, it may be reasonable to use omega-3 fatty supplementation to augment traditional pharmacological interventions or for families who decline other psychopharmacological options.
Keywords: Attention-Deficit Disorder with Hyperactivity, polyunsaturated fatty acids, omega-3 fatty acids, Eicosapentaenoic acid (EPA), meta-analysis
Introduction
Attention-Deficit/ Hyperactivity Disorder (ADHD) is characterized by developmentally inappropriate and impairing inattention, impulsivity and hyperactivity (DSM).1 ADHD is one the most common and impairing health conditions affecting school-aged children.2, 3 Several pharmacotherapies have demonstrated significant short-term efficacy for the treatment of ADHD. More than 70% of children with ADHD respond to psychostimulant medications (i.e. methylphenidate and dextroamphetamine derivatives).4 Other medications such as atomoxetine, alpha-2 agonists and desipramine have also demonstrated efficacy in treating ADHD.5–7 However, many families elect not to use traditional pharmacotherapies to treat ADHD. This decision is often related to concerns over possible short-term side effects or doubts regarding long-term efficacy or effects on development of these medications.8–11 Instead, alternative and complementary treatments such as natural supplements are often used by families to treat ADHD.12
Omega-3 fatty acid supplementation is one of the most studied alternative treatments for ADHD.13 Omega-3 fatty acids cannot be synthesized de novo by humans and instead are required in our diet. In the Western diet omega-6 fatty acids or their precursors (e.g. linoleic acid) are much more abundant than omega-3 fatty acids or their precursors (e.g. alpha-linolenic acid).14 A high omega-6 to omega-3 ratio can alter cell membrane properties and increase production of inflammatory mediators because arachidonic acid, an omega 6 fatty acid found in cell membranes, is the precursor of inflammatory eicosanoids, such as prostaglandins and thromboxanes.15 By contrast, omega-3 fatty acids are anti-inflammatory.15 Therefore, a high dietary omega-6 to omega-3 fatty ratio could promote neuroinflammation Increased omega-3 fatty acid concentration in the diet may also act by altering central nervous system cell membrane fluidity and phospholipid composition which may alter the structure and function of the proteins embedded in it.16 By this mechanism, increased omega-3 fatty acid concentrations in cell membranes have been shown to affect serotonin and dopamine neurotransmission especially in the frontal cortex.17
Through these mechanisms omega-3 fatty acid consumption has been hypothesized to alter risk for a variety of psychiatric conditions including psychosis, depression, dementia and ADHD.18, 19 Several studies have demonstrated differences in omega-3 fatty acid composition in plasma and in erythrocyte membranes in ADHD patients compared to unaffected controls.20–26 Furthermore, omega-3 fatty acid supplementation has been consistently demonstrated to alter cell membrane composition in vivo.25, 27
Several double-blind, placebo-controlled trials have been conducted to assess the efficacy of omega-3 fatty acid supplementation in the treatment of children with ADHD. The results of these trials have been mixed leading to considerable confusion and controversy in the field. For instance, one recent systematic review published in the last year described the results of current trials in the area “very disappointing” with “most randomized trials have clearly demonstrated lack of superiority or arbitrary findings (which may be a result of multiple analyses without appropriate statistical correction) compared with placebo.”13 Another recent review, evaluating the same literature stated “that the administration of specific combinations of long chain- polyunsaturated fatty acids (LC-PUFAs) can have a positive effect in children with ADHD” but that the “optimum LC-PUFA composition and dose needs to be established.”28
To our knowledge, no meta-analysis has been conducted in order to determine the efficacy of omega-3 fatty acid supplementation for children with ADHD. Our goal was to conduct a meta-analysis to determine the efficacy of omega-3 fatty acid supplementation in ADHD. Given the considerable heterogeneity expected in the literature, we also wanted to use meta-regression to examine how the use of different omega-3 fatty acid compositions in supplementation affected treatment efficacy.
Method
Search Strategy
All meta-analytic methods and sensitivity analyses were specified prior to conducting the meta-analysis but were not registered online. PubMED (1965-December 2010) was searched by two reviewers (MHB and AQ) for relevant trials using the search strategy (Attention Deficit Disorder with Hyperactivity“[Mesh] AND “Fatty Acids, Unsaturated“[Mesh]). The results of the search were further limited to randomized control trials and meta-analyses. The references of eligible trials for this meta-analysis as well as any appropriate review articles in this area were additionally searched for citations of further relevant published and unpublished research. We additionally searched for unpublished or ongoing trials on the cinicaltrials.gov website using search terms fatty acid, omega-3 OR omega-6 and ADHD. There were no language limitations.
Criteria for Inclusion of Studies in this Review
Studies were included in this meta-analysis if they were (1) randomized, placebo-controlled trials examining the efficacy of omega-3 fatty acid supplementation in children with ADHD or targeting ADHD symptoms in other children (undiagnosed or with comorbid conditions) and (2) used a validated rating scale to measure ADHD severity during the trial. Trials were considered randomized when investigators explicitly represented them as such in the methods section of their published manuscript. Trials in which other psychoactive substances were started at the same time as omega-3 fatty acid supplementation were also excluded.
Meta-Analytic Methods
Data extraction was performed on specially designed Microsoft Excel spreadsheets. Data were also collected on methods, participants, intervention and outcome measurements, and other relevant attributes and results of the studies. Any missing information was requested from the study investigators when possible. The outcome measure selected from each included trial was the difference in mean improvement between omega-3 fatty acid supplementation and placebo group in a clinical rating scale measuring ADHD severity over the course of the trial. Preferred rating scales for rating of ADHD severity (in order of preference) were the ADHD Rating Scale, Conner's Rating Scales for Teachers or Parents, the Disruptive Behavior Disorder Rating Scale. When both parents and teachers completed the same rating scale, we used the responding group which had the greatest number of completed rating scales. A hierarchy of preferred ADHD rating scale for our primary outcome was established a priori (as opposed to utilizing the ADHD rating scale indentified as primary by the trial investigator) in order to avoid any possible inflation of treatment effects caused by possible reporting bias towards measures that showed the greatest efficacy. As many rating scales have excellent psychometric properties in evaluating ADHD, this hierarchy of preferred rating scales for ADHD is not meant to reflect the relative merit of these measures. When the standard deviation of the mean improvement on placebo or omega-3 fatty acid supplementation was not reported in individual studies this was imputed based on the standard deviation of reported baseline and endpoint ADHD severity using Cochrane methodology.29
Standard mean difference (SMD) was chosen as the summary statistic for meta-analysis and calculated by pooling the standardized mean improvement of each study using RevMan 5. SMD was favored over weighted mean difference because rating scales differed across studies. A fixed effects model was chosen for meta-analysis because this method is favored when testing for subgroup differences in stratified meta-analysis. Publication bias was assessed by plotting the effect size against sample size for each trial (funnel plot).30 Publication bias was also statistically tested by testing the association between sample size and effect size in meta-regression. Heterogeneity of treatment response was assessed visually from the forest plot of weighted mean differences and relative risk of individual studies. Statistical estimates of heterogeneity were also assessed using the I-square heterogeneity statistic in RevMan. We conducted a sensitivity analysis to examine our decision to use a random-effects rather than fixed effects model for meta-analysis.
For secondary analysis, we used the same methodology as above to examine the effect of omega-3 supplementation for the symptoms of inattention and hyperactivity/impulsivity separately. We also examined the effect of omega-3 supplementation on parent ratings of ADHD symptoms. We were unable to examine the effects of supplementation on teacher ratings of ADHD because a minority of trials reported on this outcome. We also performed several subgroup analyses and meta-regression. For subgroup analyses trials were stratified based on (1) whether the omega-3 supplement was given as monotherapy or given as an augmentation agent to pharmacological treatment, (2) methodological quality of trials, (3) diagnosis (undiagnosed population, confirmed ADHD and ADHD symptoms in comorbid condition), (4) analysis method (intention-to-treat or completers analysis) and (5) type of placebo. Overall methodological quality of trials was assessed using the JADAD Scale.31, 32 We used the test for subgroup differences in RevMan to determine whether subgroups reduced overall heterogeneity.33
Meta-regression was performed in SPSS 19.0 using linear regression. Trials were weighted using the generic inverse variance method. Effect size (SMD) of trials was entered as the dependent variable with the variables of interest being the independent variable. We used a meta-regression techniques to examine the association between omega-3 and naturally continuous variables such as (1) trial duration, (2) proportion of dropouts in trials using completers analysis and (3) doses of omega-3 fatty acids in supplementation preparations. We examined doses of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and α-linolenic acid (ALA) in omega-3 fatty acid preparations. For our primary analysis examining the efficacy of omega-3 fatty acids for ADHD symptoms we used a significance threshold of p<0.05. For all subgroup analyses and meta-regression we used the same threshold for statistical significance. Any significant findings should be regarded as exploratory because we did not adjust for inflation of false-positive error from our 13 secondary analyses.
Results
Selection of Studies
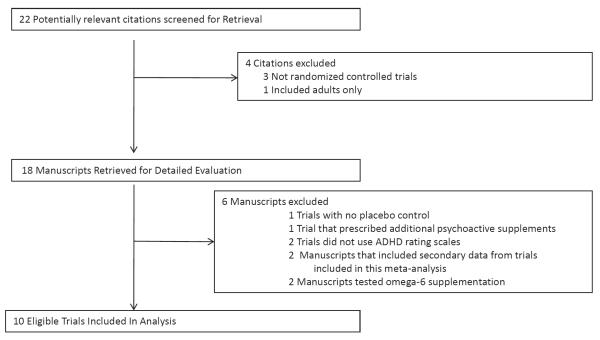
Our PubMED search identified 18 manuscripts that were potentially eligible for inclusion in this meta-analysis. We identified 4 potentially eligible trials from the references of relevant reviews. Figure 1 demonstrates a flow diagram depicting our selection procedure for this meta-analysis. One randomized controlled trial was excluded because it included supplementation with additional psychoactive substances (including Gingko Biloba, l-glutamate, Grapine, Melissa officinalis and dimethylaminoethanol).34 Two randomized controlled trials were excluded because they used number of DSM ADHD symptoms present rather than a rating scale to assess ADHD severity.35, 36 Two trials were additionally excluded because they studied omega-6 rather than omega-3 fatty acid supplementation.37, 38
Figure 1. Flow Chart Depicting Selection of Studies.
Note: ADHD = attention-deficit/hyperactivity disorder.
We identified 10 eligible trials with 11 appropriate treatment arms for inclusion in this review. Table 1 depicts the characteristics of included trials.25, 39–47,38Only 2 of these 10 trials reported a statistically significant benefit of omega-3 fatty acid supplementation.41, 45 Six trials showed no benefit of omega-3 fatty acid supplementation compared to placebo.25, 37–40, 43, 44, 46 Two trials demonstrated a benefit on some but not most ADHD rating scales when no measure was specified a priori.42, 47
Table 1.
Characteristics of Included Trials
| Study | Year | Design | Study Duration | Monotherapy vs. Augmentation | N | Mean Age | Gender (%Male) | Rating | JADAD | Analysis | EPA Dose (in mg) | DHA Dose) (in mg) | ALA Dose (in mg) | Placebo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voigt44 | 2001 | Parallel | 4 mo. | augmentation | 63 | 9.3 | 78 | CBCL Attention Subscale | 5 | Completer | 0 | 345 | 0 | -- |
| Richardson42 | 2002 | Parallel | 12 wks. | monotherapy | 41 | 10.3 | 85 | CPRS-L | 4 | Completer | 186 | 480 | 0 | Olive oil |
| Stevens25 | 2003 | Parallel | 4 mos. | augmentation | 47 | 9.8 | 87 | ASQ (DBD-teacher)b | 3 | Completera | 80 | 480 | 0 | Olive oil |
| Richardson41 | 2005 | Parallel | 3 mos. | monotherapy | 117 | 8.8 | 67 | CTRS-L | 5 | ITT | 558 | 174 | 0 | Olive oil |
| Sinn45 | 2007 | Parallel | 15 wks. | monotherapy | 104 | 9.4 | 74 | CPRS-L | 2 | Completer | 558 | 174 | 0 | Palm oil |
| Johnson39 | 2008 | Parallel | 3 mos. | monotherapy | 75 | 12.0 | 85 | ADHD-RS | 5 | Modified ITT | 558 | 174 | 0 | Olive oil |
| Vaisman43 | 2008 | Parallel | 3 mos. | monotherapy | 55 | 9.2 | 77 | CPRS-R-S | 4 | Completer | 156 | 95 | 1 | Canola oil |
| Vaiseman43 | 2008 | Parallel | 3 mos. | monotherapy | 54 | 9.4 | 71 | CPRS-R-S | 4 | Completer | 153 | 96 | 2 | Canola oil |
| Belanger47 | 2009 | Crossover | 16 wks. | monotherapy | 26 | 9.2 | 69 | CPRS-L | 3 | Completer | 750 | 300 | 0 | Sunflower oil |
| Gustafsson46 | 2010 | Parallel | 15 wks. | monotherapy | 92 | 7–12 | -- | CTRS | 5 | Modified ITT | 500 | 2.7 | 0 | Canola oil |
| Rax40 | 2010 | Parallel | 7 wks. | monotherapy | 78 | 10.5 | 60 | ADHD-RS | 3 | Completer | 0 | 0 | 120 | Vitamin C |
Note: Twelve trials involving 735 children with attention-deficit/hyperactivity disorder (ADHD) were included in this meta-analysis. ADHD-RS = ADHD rating scale; ALA = α-linolenic acid; ASQ = Connors' parental abbreviated symptom questioner; CBCL= Connors' Behavioral Checklist; CPRS-L = Connors' Parents Rating Scale-Long Version; CPRS-R-S = Connors' Parents Rating Scale-Revised Short Version; CTRS-L = Connors' Teachers Rating Scale-Long Version; DBD = Disruptive Behavior Disorders Scale; DHA = docosahexaenoic acid; EPA= eicosapentaenoic acid; ITT = intention-to-treat; N = number of individuals contributing data to meta-analysis from trial.
presented data for completers analysis only but gave p-values for ITT sample.
for inattention and hyperactivity measures DBD scale was used
Efficacy of Omega-3 Fatty Acid Supplementation for ADHD
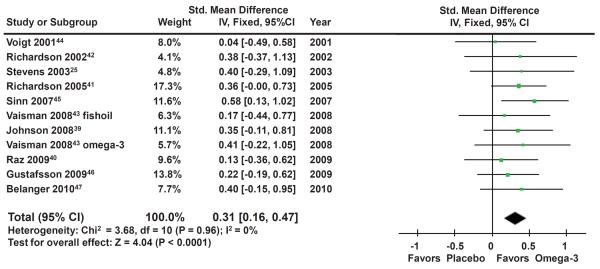
Overall meta-analysis of 10 trials involving 699 participants demonstrated a small but significant effect of omega-3 fatty acid supplementation for ADHD (standardized mean difference (SMD): 0.31 (95% Confidence Interval (CI): 0.16–0.47), z=4.04, p~0.0001). Figure 2 provides a forest plot depicting the significant benefit of omega-3 fatty acid supplementation in the treatment of ADHD. There was no evidence of significant heterogeneity (Heterogeneity: Chi2 = 3.68, df = 10 (P = 0.96); I2 = 0%). A funnel plot indicated no evidence of publication bias in the literature. A regression of sample size versus trial effect size also showed no evidence of publication bias (β=0 (95% CI: −0.004–0.005), t=0.20, p=0.84). Sensitivity analysis demonstrated that our findings were identical if we used a random effects model. Additionally, when parental ratings of ADHD were used from each trial omega-3 supplementation showed similar benefits when compared to placebo (SMD: 0.29 (95% CI: 0.14–0.44), z=3.72, p=0.0002). Teacher ratings of ADHD were not analyzed as an outcome because a minority of trials reported on this outcome.
Figure 2. Omega-3 Fatty Acid Supplementation for Attention-Deficit Hyperactivity Disorder (ADHD).
Note: Forest plot depicting efficacy of omega-3 fatty acid supplementation compared to placebo in the treatment of children with ADHD symptoms. Ten studies involving 699 participants contributed to this analysis. There was a significant benefit of omega-3 supplementation compared to placebo and no evidence of heterogeneity or publication bias. CI = confidence interval.
Meta-analysis also demonstrated similar effect sizes of omega-3 fatty acid supplementation in the treatment of both inattentive (SMD=0.29 (95%CI: 0.07–0.50), z=2.63, p=0.009) and hyperactivity SMD=0.23 (95%CI: 0.07–0.40), z=2.78, p=0.005) symptoms separately. Data were available for only 8 of 10 eligible trials for these analyses and involved 602 participants. There was no significant heterogeneity or publication bias evident for these measures.
Dosing of Omega-3 Fatty Acid Supplementation
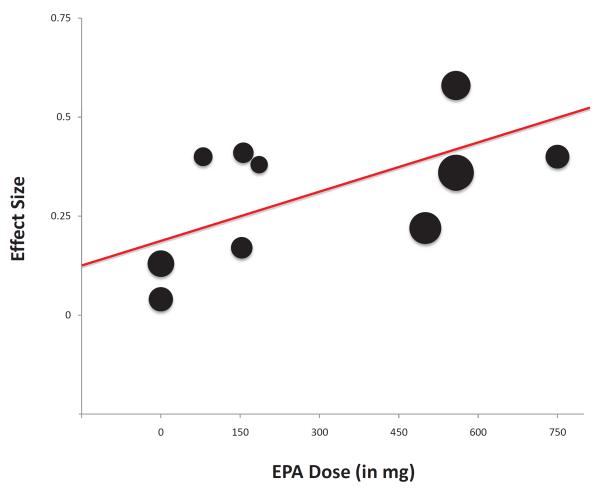
Higher doses of EPA within omega-3 fatty acids supplements were significantly associated with increased efficacy in treating ADHD symptoms (β=0.36 (95% CI: 0.01–0.72), t=2.30, p=0.04, R2=0.37). Figure 3 is a scatterplot that depicts the relationship between EPA dose and effect size of supplementation for individual trials.
Figure 3. Eicosapentaenoic acid dose and Efficacy of Omega-3 Fatty Acid Supplementation for Attention-Deficit Hyperactivity Disorder (ADHD).
Note: Scatterplot of measured efficacy of omega-3 fatty acid supplementation in trials as a function of Eicosapentaenoic acid (EPA) dose utilized. Trials were weighted (size of circles) using the generic inverse variance method. Higher doses of EPA within essential fatty acids supplements was significantly associated with increased efficacy in treating ADHD symptoms (β=0.36 (95% CI: 0.01–0.72), t=2.34, p=0.04, R2=0.38). Two trials, Johnson 200839 (effect size=0.35, EPA dose=558mg weight=11.1%) and Richardson 200541 (effect size=0.36, EPA dose=558mg weight=17.3%) had overlapping point estimates on this figure. CI = confidence interval.
Doses of other omega-3 fatty acids within supplements such as DHA (β=0.24 (95% CI: −0.54–1.02), t=0.70, p=0.50) and ALA (β=−1.71 (95% CI: −4.62–1.19), t=−1.33, p=0.22) were not significantly associated with the measured efficacy of supplements.
Augmentation versus Monotherapy
We found no significant difference in the efficacy of omega-3 fatty acid supplementation based on whether they were given as monotherapy versus as augmentation to other traditional ADHD medications (Test for subgroup differences: Chi2 = 0.45, df = 1 (P = 0.50, I2 = 0%). There was no difference in efficacy when omega-3 fatty acid supplementation was given as monotherapy (SMD=0.33 (95%CI: 0.17–0.50, z=4.01, p<0.0001) compared to augmentation (SMD=0.18 (95%CI: −0.25–0.60, z=0.82, p=0.41).
Primary Psychiatric Diagnosis of Subjects
The efficacy of omega-3 fatty acid supplementation did not significantly (Test for subgroup differences: Chi2 = 0.12, df = 1 (P = 0.73), I2 = 0%) differ whether ADHD was the subjects' primary diagnoses (SMD=0.30 (95% CI: 0.13–0.47, z=3.42, p=0.0006) or whether ADHD symptoms were being targeted in another psychiatric condition (SMD=0.36 (95% CI: 0.04–0.69, z=2.18, p=0.03).
Trial Duration
Trial duration ranged from 4 weeks to 4 months in included trials. Meta-regression demonstrated no significant relationship between trial duration and measured efficacy of supplementation (β=0.002 (95% CI: −0.004–0.007), t=0.63, p=0.55).
Type of Placebo
We found no significant effect of type of placebo on the measured effect of omega-3 supplementation in trials (Test for subgroup differences: Chi2 = 2.26, df = 4 (P = 0.69), I2 = 0%). Four trials that used olive oil as the placebo demonstrated a modest effect size (SMD=0.36 (95% CI: 0.12–0.61), z=2.87, p=0.004)25, 41, 42 similar to that seen in trials using canola oil as a placebo (SMD=0.25 (95% CI: −0.05–0.55), z=1.62, p=0.11)43, 46 and individual trials that utilized vitamin C40, sunflower oil47 and palm oil45 as placebo,
Study Quality
We found no significant effect of study quality on the measured efficacy of omega-3 fatty acid supplementation in the treatment of ADHD (Test for subgroup differences: Chi2 = 0.41, df = 1 (P = 0.52), I2 = 0%). Lower quality studies (JADAD =2 or 3: SMD=0.38 (95% CI: 0.12–0.64), z=2.87, p=0.004) did not show a significantly greater efficacy of omega-3 fatty acid supplementation compared than higher quality studies (JADAD =4 or 5: SMD=0.28 (95% CI: 0.09–0.46), z=2.92, p=0.004).
Method of Analysis
Trials that relied on completers' analysis (SMD=0.32 (95%CI: 0.12–0.52) z=3.09, p=0.002) did not demonstrate a significantly greater efficacy (Test for subgroup differences: Chi2 = 0, df = 1 (P = 0.98), I2 = 0%) of omega-3 fatty acid supplementation than trials that utilized ITT or modified ITT analysis methods (SMD=0.31 (95%CI: 0.08–0.55), z=2.6, p=0.009).
Effects of Subject Dropouts
The proportion of dropouts within trials employing completers' analysis was not significantly associated with measured efficacy of supplementation (β=0.51 (95% CI: − −0.28–1.29), t=1.46, p=0.18).
Discussion
In this meta-analysis we report a small but significant benefit of omega-3 fatty acid supplementation. Meta-analysis found no evidence of publication bias or of significant heterogeneity between trials. In secondary analysis, we found no evidence that poor study quality or that inappropriate treatment of study dropouts in some studies affected overall findings.
These results reporting a significant benefit of omega-3 supplementation stand in contrast to the conclusions of most of the individual trials included in meta-analysis. The effect size of 0.31 reported for omega-3 fatty acid supplementation, although significant is quite modest. In order to have sufficient power (β=80%, 2-sided α=0.05) to detect a significant benefit of omega-3 fatty acid supplementation compared to placebo assuming the effect size observed in this meta-analysis, clinical trials would require a sample size of approximately 330 children. By contrast, the omega-3 fatty acid supplementation trials examining childhood ADHD have employed 26–117 participants. Thus insufficient power in the original trials likely account for the different conclusion reached in this meta-analysis.
The statistically significant benefits of omega-3 fatty acid supplementation are modest compared to the efficacy of currently available pharmacological treatments for ADHD. For example, recent meta-analyses have estimated the effect sizes of commonly prescribed pharmacological treatments for ADHD such as methylphenidate (ES=0.78 (95% CI 0.64–0.91), clonidine (ES=0.58 (95% CI 0.27–0.89) and atomoxetine (ES= 0.64 (95% CI 0.51–0.76) to be higher.5–7 Based on the currently available evidence, we would not recommend using omega-3 fatty acid supplementation in lieu of traditional pharmacological treatments in children with significant ADHD symptoms. However, given evidence of modest efficacy of omega-3 fatty acid supplementation and given its relatively benign side-effect profile, omega-3 fatty acid supplementation, particularly with higher doses of EPA, is a reasonable treatment strategy as augmentation to traditional pharmacotherapy or for those families reticent to use psychopharmacological agents.
Meta-regression also demonstrated a significant association between efficacy and dose of EPA given in supplements. EPA and DHA are omega-3 fatty acids. Omega-3 fatty acids have anti-inflammatory properties.15 Omega-3 fatty acids are also known to alter cell membrane fluidity in the CNS which affects dopamine and serotonin neurotransmission.17 It remains unclear why supplementation with EPA may improve ADHD symptoms while supplementation with DHA may not to the same degree. Of note, EPA and not DHA supplementation has also been demonstrated to effective in omega-3 supplementation to treat depression.48 Oxidized derivatives of DHA are known to have pro-inflammatory effects, while oxidized derivatives of EPA have anti-inflammatory effects.49 Thus, in a situation of excess supplementation with omega-3 fatty acids EPA would still produce anti-inflammatory effects while DHA would not.
Although we reported a significant effect of omega-3 fatty acid supplementation in treating ADHD symptomatology, there are several weaknesses and limitations to the current meta-analysis. In general, the clinical trials conducted in this area have been of rather poor quality, many with JADAD scores of 2 or 3 indicating potential issues with randomization, blinding and/or tracking dropouts. Additionally, many trials included in this analysis did not account for dropouts in their analysis method, which could introduce further bias into our results. Lastly, questions have been raised regarding the adequacy of blinding in early trials using fish oil because of a fishy aftertaste present when the active formulations are refluxed.50 Based on the data presented in manuscripts it is not possible to evaluate the effectiveness of blinding in these trials. Inadequate blinding has the potential to introduce bias and can inflate estimates of efficacy. Despite these limitations of individual trials, overall meta-analysis demonstrates no evidence of publication bias, heterogeneity between trials and effect of subject dropout or poor study quality. Likely some evidence of each of these phenomena would be present if any of these types of bias were driving our results. Statistical measures of heterogeneity, publication bias, and the effects of subject dropout and study quality were not only statistically insignificant, they were negligible.
Overall this meta-analysis demonstrates a small but statistically significant benefit of omega-3 fatty acid supplementation in the treatment of ADHD. Furthermore, we demonstrate a significant association between EPA dose in supplements and their measured efficacy. Because of poor quality and potential issues of blinding in many of the included trials, further clinical trials are needed to replicate the results of this meta-analysis. In particular, clinical trials involving at least 330 children with ADHD are needed to demonstrate efficacy of these supplements. We would also recommend these trials utilize supplements with high concentrations of EPA, an omega-3 fatty acid, given the evidence of a dose-response relationship in meta-regression. Given omega-3 fatty acid supplementation's modest efficacy compared to other available pharmacological treatments for ADHD, we would not recommend its use in lieu of traditional psychopharmacological agents for ADHD. However, given its relatively benign side-effect profile and evidence of modest efficacy, it may be reasonable to use omega-3 fatty supplementation to augment traditional pharmacological interventions or for families who decline all other psychopharmacological options.
Acknowledgments
The authors acknowledge the National Institute of Mental Health support of the Yale Child Study Center Research Training Program (MHB), the National Institutes of Health (NIH) grant 1K23MH091240-01 (MHB), the American Psychiatric Institute for Research and Education (APIRE) / Eli Lilly and Co. Psychiatric Research Fellowship (MHB), the American Academy of Child and Adolescent Psychiatry / Eli Lilly and Co. Pilot Research Award (MHB), the Trichotillomania Learning Center (MHB), National Alliance for Research on Schizophrenia and Depression (MHB), and UL1 RR024139 from the National Center for Research Resources, a component of NIH, and NIH Roadmap for Medical Research (MHB).
Footnotes
Disclosure: Drs. Bloch and Qawasmi report no biomedical financial interests or potential conflicts of interest to disclose
References
- 1.Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; 1994. [Google Scholar]
- 2.Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. American Academy of Pediatrics. Pediatrics. 2000;105(5):1158–70. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 3.Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100–7. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 4.Spencer T, Biederman J, Wilens T, Harding M, O'Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409–32. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165(11):1475–88. [PMC free article] [PubMed] [Google Scholar]
- 6.Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1551–9. doi: 10.1097/00004583-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JY, Chen RY, Ko JS, Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology (Berl) 2007;194(2):197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT. Psychotropic drug use in very young children. JAMA. 2000;283(8):1059–60. doi: 10.1001/jama.283.8.1059. [DOI] [PubMed] [Google Scholar]
- 9.Dunnick JK, Hailey JR. Experimental studies on the long-term effects of methylphenidate hydrochloride. Toxicology. 1995;103(2):77–84. doi: 10.1016/0300-483x(95)03109-s. [DOI] [PubMed] [Google Scholar]
- 10.Klein RG, Landa B, Mattes JA, Klein DF. Methylphenidate and growth in hyperactive children. A controlled withdrawal study. Arch Gen Psychiatry. 1988;45(12):1127–30. doi: 10.1001/archpsyc.1988.01800360075011. [DOI] [PubMed] [Google Scholar]
- 11.Nasrallah HA, Loney J, Olson SC, McCalley-Whitters M, Kramer J, Jacoby CG. Cortical atrophy in young adults with a history of hyperactivity in childhood. Psychiatry Res. 1986;17(3):241–6. doi: 10.1016/0165-1781(86)90052-1. [DOI] [PubMed] [Google Scholar]
- 12.Chan E, Gardiner P, Kemper KJ. At least its natural: herbs and dietary supplements in ADHD. Contemporary Pediatrics. 2000;17:116–30. [Google Scholar]
- 13.Raz R, Gabis L. Essential fatty acids and attention-deficit-hyperactivity disorder: a systematic review. Dev Med Child Neurol. 2009;51(8):580–92. doi: 10.1111/j.1469-8749.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- 14.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54(3):438–63. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 15.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 16.Freeman MP, Rapaport MH. Omega-3 fatty acids and depression: from cellular mechanisms to clinical care. J Clin Psychiatry. 2011;72(2):258–9. doi: 10.4088/JCP.11ac06830. [DOI] [PubMed] [Google Scholar]
- 17.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):259–69. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Peet M, Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65(8):1051–9. doi: 10.2165/00003495-200565080-00002. [DOI] [PubMed] [Google Scholar]
- 19.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–67. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62(4):761–8. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 21.Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem. 2004;15(8):467–72. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case-control study. Nutr J. 2008;7:8. doi: 10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laasonen M, Hokkanen L, Leppamaki S, Tani P, Erkkila AT. Project DyAdd: Fatty acids in adult dyslexia, ADHD, and their comorbid combination. Prostaglandins Leukot Essent Fatty Acids. 2009;81(1):89–96. doi: 10.1016/j.plefa.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids. 2003;38(10):1007–21. doi: 10.1007/s11745-006-1155-0. [DOI] [PubMed] [Google Scholar]
- 26.Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids. 2004;39(2):117–23. doi: 10.1007/s11745-004-1209-3. [DOI] [PubMed] [Google Scholar]
- 27.Joshi K, Lad S, Kale M, Patwardhan B, Mahadik SP, Patni B, et al. Supplementation with flax oil and vitamin C improves the outcome of Attention Deficit Hyperactivity Disorder (ADHD) Prostaglandins Leukot Essent Fatty Acids. 2006;74(1):17–21. doi: 10.1016/j.plefa.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr. 2010;169(2):149–64. doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. John Wiley & Sons, Ltd.; Chishester, UK: 2005. [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73. doi: 10.1016/0197-2456(94)00031-w. [DOI] [PubMed] [Google Scholar]
- 32.Moncrieff J, Churchill R, Drummond C, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Methods in Psychiatric Research. 2001;10:126–33. [Google Scholar]
- 33.Deeks J, Higgins J, Altman D. Cochrane Reviewers' Handbook 4.2.1. John Wiley & Sons, Ltd; 2003. [Google Scholar]
- 34.Brue AW, Oakland TD, Evans RA. The Use of Dietary Supplement Combination and an Essential Fatty Acid as an Alternative and Complementary Treatment for Children with Attention-Deficit/Hyperactivity Disorder. Scientific Review of Alternative Medicine. 2001;5:187–94. [Google Scholar]
- 35.Itomura M, Hamazaki K, Sawazaki S, Kobayashi M, Terasawa K, Watanabe S, et al. The effect of fish oil on physical aggression in schoolchildren--a randomized, double-blind, placebo-controlled trial. J Nutr Biochem. 2005;16(3):163–71. doi: 10.1016/j.jnutbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder - a placebo-controlled double-blind study. Eur J Clin Nutr. 2004;58(3):467–73. doi: 10.1038/sj.ejcn.1601830. [DOI] [PubMed] [Google Scholar]
- 37.Aman MG, Mitchell EA, Turbott SH. The effects of essential fatty acid supplementation by Efamol in hyperactive children. J Abnorm Child Psychol. 1987;15(1):75–90. doi: 10.1007/BF00916467. [DOI] [PubMed] [Google Scholar]
- 38.Arnold LE, Kleykamp D, Votolato NA, Taylor WA, Kontras SB, Tobin K. Gamma-linolenic acid for attention-deficit hyperactivity disorder: placebo-controlled comparison to D-amphetamine. Biol Psychiatry. 1989;25(2):222–8. doi: 10.1016/0006-3223(89)90167-4. [DOI] [PubMed] [Google Scholar]
- 39.Johnson M, Ostlund S, Fransson G, Kadesjo B, Gillberg C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Atten Disord. 2009;12(5):394–401. doi: 10.1177/1087054708316261. [DOI] [PubMed] [Google Scholar]
- 40.Raz R, Carasso RL, Yehuda S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: a double-blind placebo-controlled study. J Child Adolesc Psychopharmacol. 2009;19(2):167–77. doi: 10.1089/cap.2008.070. [DOI] [PubMed] [Google Scholar]
- 41.Richardson AJ, Montgomery P. The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115(5):1360–6. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- 42.Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(2):233–9. doi: 10.1016/s0278-5846(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 43.Vaisman N, Kaysar N, Zaruk-Adasha Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr. 2008;87(5):1170–80. doi: 10.1093/ajcn/87.5.1170. [DOI] [PubMed] [Google Scholar]
- 44.Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139(2):189–96. doi: 10.1067/mpd.2001.116050. [DOI] [PubMed] [Google Scholar]
- 45.Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. 2007;28(2):82–91. doi: 10.1097/01.DBP.0000267558.88457.a5. [DOI] [PubMed] [Google Scholar]
- 46.Gustafsson PA, Birberg-Thornberg U, Duchen K, Landgren M, Malmberg K, Pelling H, et al. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010;99(10):1540–9. doi: 10.1111/j.1651-2227.2010.01871.x. [DOI] [PubMed] [Google Scholar]
- 47.Belanger SA, Vanasse M, Spahis S, Sylvestre MP, Lippe S, L'Heureux F, et al. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr Child Health. 2009;14(2):89–98. doi: 10.1093/pch/14.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28(5):525–42. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 49.Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008;283(18):12043–55. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sontrop J, Campbell MK. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev Med. 2006;42(1):4–13. doi: 10.1016/j.ypmed.2005.11.005. [DOI] [PubMed] [Google Scholar]