Abstract
We conducted a meta-analysis of randomized, placebo-controlled trials of omega-3 fatty acid treatment of major depressive disorder in order to determine efficacy and to examine sources of heterogeneity between trials. PubMED (1965-May 2010) was searched for randomized, placebo-controlled trials of omega-3 fatty acids for major depressive disorder. Our primary outcome measure was standardized mean difference in a clinical measure of depression severity. In stratified meta-analysis we examined the effects of trial duration, trial methodological quality, baseline depression severity, diagnostic indication, dose of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in omega-3 preparations, and whether omega-3 fatty acid was given as monotherapy or augmentation.
In 13 randomized, placebo-controlled trials examining the efficacy of omega-3 fatty acids involving 731 participants, meta-analysis demonstrated no significant benefit of omega-3 fatty acid treatment compared to placebo (SMD=0.11, 95% CI: -0.04, 0.26). Meta-analysis demonstrated significant heterogeneity and publication bias. Nearly all evidence of omega-3 benefit was removed after adjusting for publication bias using the trim-and-fill method (SMD=0.01, 95% CI: -0.13, 0.15). Secondary analyses suggested a trend towards increased efficacy of omega-3 fatty acids in trials of lower methodological quality, trials of shorter duration, trials, which utilized completers rather than intention-to-treat analysis, and trials in which study participants had greater baseline depression severity,
Current published trials suggest a small, non-significant benefit of omega-3 fatty acids for major depression. Nearly all of the treatment efficacy observed in the published literature may be attributable to publication bias.
Background
Pharmacological and behavioral treatments for Major Depressive Disorder (MDD) show only modest efficacy. For instance, serotonin reuptake inhibitors (SRIs) produce response in fewer than 60% who complete a full course of SRI pharmacotherapy.1 These medications are not always well tolerated; in randomized controlled trials (RCT), ∼30% of subjects who begin treatment with SSRIs or tricyclic antidepressants (TCA) drop-out.2 Thus there is a continued need for the development of both better and better-tolerated pharmacological interventions for MDD
Fatty acids are essential components of cell membranes. Unsaturated fatty acids have one or more double-bonds between carbon atoms; when the double bond is in position 6 the unsaturated fatty acid is called an “omega-6 fatty acids” while those with a double bond in position 3 are “omega-3 fatty acids”, examples of which are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). In the Western diet omega-6 fatty acids or their precursors (e.g. linoleic acid) are much more abundant than omega-3 fatty acids or their precursors (e.g. alpha-linolenic acid). A high omega-6 to omega-3 ratio can alter cell membrane properties and increase production of inflammatory mediators because arachidonic acid, an omega 6 fatty acid found in cell membranes, is the precursor of inflammatory eicosanoids, such as prostaglandins and thromboxanes. By contrast, omega-3 fatty acids are anti-inflammatory. Therefore, a high dietary omega-6 to omega-3 fatty ratio could promote neuroinflammation. Increased omega-3 fatty acids concentration in the diet may also act by altering CNS cell membrane fluidity and phospholipid composition which may alter the structure and function of the proteins embedded in it. By this mechanism, increased omega-3 fatty acid concentrations in cell membranes have been shown to affect serotonin and dopamine neurotransmission.3
Evidence from both ecologic, cross-sectional and case-control studies suggest that fish consumption and omega-3 fatty acid intake may affect the prevalence of MDD. There is a strong negative correlation between fish consumption and national rates of MDD.4. Cross-sectional studies have demonstrated higher rates of MDD in individuals who rarely consume fish.5 In case-control studies, individuals with MDD have higher ratio of arachidonic acid to eicosapentaenoic acid (EPA) in both cell membrane cholesteryl esters and serum phospholipids, and significantly higher omega-6/omega-3 ratio than non-depressed controls.6 Similarly, case-control studies have demonstrated significantly lower omega-3 levels in erythrocyte membranes of depressed patients.7
Several double-blind, placebo-controlled trials have studied the efficacy of omega-3 supplementation in adults with MDD. In 2006-2007, three meta-analyses reported a significant benefit of omega-3 fatty acids for the treatment of depressed mood.8-10 These meta-analyses suggested a modest effect size of treatment (effect size: 0.13-0.61), a large degree of heterogeneity between studies, and evidence of publication bias in the literature.8-10 These meta-analysis also included trials that examined depressed mood as an outcome in patients with other primary psychiatric disorders such as bipolar disorder, schizophrenia, obsessive-compulsive disorder, personality disorders or chronic fatigue syndrome. Since these meta-analyses were published, there have been at least 9 additional randomized, placebo-controlled trials examining the efficacy of omega-3 fatty acids for MDD. The addition of these trials more than doubles the sample size of previous meta-analyses and gives us the power to examine the efficacy of omega-3 FAs in MDD specifically. Two recent meta-analyses reported significant treatment benefits of omega-3 fatty acids in depressed mood consistent with the earlier studies (effect size: 0.10-0.47), but still included patients with other primary psychiatric disorders and failed to adjust for publication bias.11, 12 Trials that report on depressive symptoms as a secondary outcome in other psychiatric illnesses may be particularly prone to publication bias as authors are less likely to analyze and report secondary measures that are non-significant and therefore non-informative. Furthermore, adding trials in which exclusively included individuals with other primary psychiatric disorders will likely increase heterogeneity between trials.
The goals of this meta-analysis are two-fold: (1) to analyze the efficacy of omega-3 fatty acids in the treatment of MDD and (2) to examine possible sources of heterogeneity between trials. We specifically hypothesize that possible sources of heterogeneity include (1) omega-3 fatty acid dose, (2) trial duration, (3) diagnostic heterogeneity, (4) the use of omega-3 treatment as monotherapy vs. augmentation, and (5) baseline depression severity. These sources of heterogeneity represent potentially important differences affecting the efficacy omega-3 fatty acid pharmacotherapy in clinical practice.
Methods
Search Strategy
All meta-analytic methods and sensitivity analyses were specified prior to conducting the meta-analysis but were not registered online. PubMED (1965-May 2010) was searched by two reviewers (JH and MHB) for relevant trials using the search strategy (omega-3 OR polyunsaturated FAs OR fish oil OR eicosapentaenoic acid OR EPA OR docosahexaenoic acid OR DHA OR alpha-linolenic acid OR cod liver oil) AND (depression OR depressive disorder OR depressed mood OR dysthymic OR postpartum depression). The results of the search were further limited to randomized control trials and meta-analyses. The references of eligible trials for this meta-analysis as well as any appropriate review articles in this area were additionally searched for citations of further relevant published and unpublished research. No additional efforts were made to search for unpublished works. There were no language limitations.
Criteria for Inclusion of Studies in this Review
Studies were included in this meta-analysis if they were (1) randomized, placebo-controlled trials examining the efficacy of omega-3 fatty acids in adults with MDD. Trials were considered randomized when investigators explicitly represented them as such in the methods section of their published manuscript. Trials examining the efficacy of omega-3 fatty acids in treating MDD in subjects with medical comorbidity (i.e. cardiac disease, Parkinson's disease) or pregnancy were included in this meta-analysis. Trials were included if they examined the efficacy of omega-3 fatty acids to target depressive symptoms (as a primary outcome) in patients who may not have received a formal psychiatric diagnosis (i.e. in a primary care setting). Trials examining the efficacy of omega-3 FAs in treating depressive symptoms in the context of another primary psychiatric disorder, such as bipolar disorder or schizophrenia, were not included. Trials in which pharmacological agents of known efficacy were started at the same time as omega-3 fatty acids were also excluded.
Meta-Analytic Methods
Data extraction was performed by two independently working reviewers (MHB and JH) on specially designed Microsoft Excel spreadsheets. Reviewers collected data on methods, participants, intervention and outcome measurements, and other relevant attributes and results of the studies. Any disagreement between reviewers was resolved through discussion and obtaining more information from the study investigators when possible.
The outcome measure selected from each included trial was the difference in mean improvement between the omega-3 fatty acid and placebo group in a clinical rating scale measuring depression severity over the course of the trial. Preferred rating scales for measuring depression severity were the Hamilton Depression Rating Scale (HDRS), either the 9-item short form, 17-item, 21-item or 25-items scales, and the Montgomery Åsberg Depression Rating Scale (MADRS).13-15 When available, HDRS score from each study was used. If the HDRS was not available we used the MADRS. If neither HDRS nor MADRS data were available, we used the clinician rated measure of depression that the investigators identified as their primary outcome. When no clinician-rated measures of depression severity were available from a trial then self-report measures were utilized. When the standard deviation of the mean improvement on placebo or omega-3 fatty acids was not reported in individual studies this was imputed based on the standard deviation of reported baseline and endpoint depression severity using Cochrane methodology.16 The formula SD(improvement) = √(SD(baseline)2+SD(endpoint)2-2*r*SD(baseline)*SD(endpoint) where r=correlation coefficient was used to calculate the standard deviation of mean improvement. A correlation coefficient of 0.4 was used for this calculation based on the average of computed correlation coefficients in included studies where all information on standard deviations was available.
Standard mean difference (SMD) was chosen as the summary statistic for meta-analysis and calculated by pooling the standardized mean improvement of each study using RevMan 5. SMD was favored over weighted mean difference because rating scales differed across studies. A fixed effects model was chosen for meta-analysis because this method of analysis is favored when there is evidence of publication bias in the literature as this method gives less weight to small studies. Previous meta-analyses in this area have suggested significant publication bias in this literature.8, 9
Publication bias was assessed by plotting the effect size against sample size for each trial (funnel plot).17 We used the rank correlation test in Mix 1.7 to statistically test for evidence of publication bias.18 and the trim-and-fill method to adjust our analysis for the effects of publication bias on the available data.18
Heterogeneity of treatment response was assessed visually from the forest plot of weighted mean differences and relative risk of individual studies. Statistical estimates of heterogeneity were also assessed using the I-square heterogeneity statistic in RevMan.
For secondary analyses we performed several subgroup analyses and meta-regression. For subgroup analyses trials were stratified based on (1) baseline depression severity (mild, moderate or severe) (2) diagnosis (depression with or without co-morbid medical problems, postpartum depression), (3) whether the omega-3 FA preparation was given as monotherapy for depression or given as an augmentation agent to pharmacological treatment, (4) methodological quality of trials and (5) analysis method (intention-to-treat, modified intention-to-treat or completers). For stratification of trials by baseline depression severity we used traditional cutoffs for the HDRS-17 (mild: <18, moderate: 18-28, severe: >28).19 Trials that measured depression severity on scales other than the HDRS-17 were converted to this scale based on previously defined algorithms.20 Overall methodological quality of trials was assessed using the JADAD Scale.21, 22 Because the subgroup analysis based on study quality was not specified a-priori we defined subgroups based on a median split of trial scores on this scale (JADAD=5 vs. JADAD<5). We used the test for subgroup differences in RevMan to determine whether subgroups reduced overall heterogeneity.23
Meta-regression was performed in SPSS 19.0 using linear regression. Trials were weighted using the generic inverse variance method. Effect size (SMD) of trials was entered as the dependent variable with the variables of interest being the independent variable. We used a meta-regression techniques to examine the association between omega-3 and naturally continuous variables such as (1) trial duration, (2) EPA dose and (3) DHA dose in omega-3 preparations. For our primary analysis examining the efficacy of omega-3 FAs for MDD we used a significance threshold of p<0.05. For secondary analyses we used a Bonferroni correction and set the threshold at p<0.006 in order to adjust for inflation of false-positive error from our 8 secondary analyses.
Sensitivity analyses were conducted to determine the robustness of reviewers' conclusions to methodological assumptions made in conducting this systematic review. We conducted a sensitivity analysis to examine our decision to use a fixed-effects rather than random effects model for meta-analysis. We also conducted a sensitivity analysis excluding studies that did not require a formal psychiatric diagnosis of MDD to determine if including studies that targeted depressive symptoms without a formal psychiatric diagnosis influenced our findings.
Results
Selection of Studies
Our search strategy in PubMED yielded 97 articles for consideration in this review. Studies were excluded because the intervention studied was not omega-3 fatty acids (n=46), research was not performed in humans (n=7), the design was not a randomized controlled trial (n=6) or subjects were psychiatrically healthy (n=12). Additional randomized controlled trials examining the efficacy of omega-3 fatty acids for psychiatric conditions were excluded because subjects had bipolar disorder24-26, obsessive-compulsive disorder27, recurrent self-harm28, borderline personality disorder29, Alzheimer's disease30, or because the trial did not include a placebo control group31, examined pediatric depression32, or involved starting concomitant antidepressant medications at the same time as starting omega-3 supplementation.33, 34
Characteristics of Included Studies
Table 1 depicts characteristics of trials included in this meta-analysis. We found 13 separate randomized, double-blind, placebo-controlled trials involving 731 participants that compared omega-3 FAs to placebo for the treatment of depressive disorders. Four studies35-38 reported a significant benefit of omega-3 fatty acids in the treatment of depression and 9 studies reported no significant difference of omega-3 fatty acids compared to placebo.39-47 No studies reported a worsening of depressive symptoms with omega-3 fatty acid therapy compared to placebo.
Table 1. Characteristics of Trials Included in the Meta-Analysis of the Efficacy of Omega-3 Fatty Acids for Depression.
All 13 trials included in this meta-analysis were randomized, double-blind and placebo-controlled. All trials were additionally conducted in an outpatient setting relying on referred volunteers. For classification of trials by baseline depression severity we used traditional cutoffs for the HDRS-17 (mild: <18, moderate: 18-28, severe: >28) 19. Trials that measured depression severity on scales other than the HDRS-17 were converted to this scale based on previously defined algorithms 20. Abbreviations: EPA= eicosapentaenoic acid, DHA=docosahexaenoic acid,, EPA/DHA=Ratio of EPA to DHA, QRS=Quality Rating Scale, PD=Parkinson Disease, HAM-D=Hamilton Rating Scale for Depression, BDI=Beck Depression Inventory, MADRS= Montgomery Åsberg Depression Rating Scale, ITT=intention-to-treat analysis, modified ITT=ITT analysis where subjects were required to either take one medication dose or make one follow-up visit to be included in the analysis, + = positive study which showed a benefit of omega-3 fatty acids compared to placebo, and - =negative study which failed to show benefit of omega-3 FA.
| Study | Year | Indicaton | Formulation | EPA Dose | DHA Dose | EPA:DHA Ratio | Study Duration | Mono. V. Aug. | N | Mean Age | Gender (% Male) | Location | Setting | Rating | Depression Severity | Dropouts | Analysis | QRS | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peet (34) | 2002 | Depression | EPA | 1,2 or 4g | 0 | ∞ | 12 weeks | Augmentation | 70 | 45 | 16% | UK | Outpatient, Primary Care | HAM-D-17 | Moderate | 10 (14%) | ITT | 31 | + for 1g only |
| Nemets (35) | 2002 | Depression | EPA | 2g | 0 | ∞ | 4 weeks | Augmentation | 20 | 53 | 15% | Israel | Outpatient | HAM-D-24 | Moderate | 1 (5%) | ITT | 25 | + |
| Su (37) | 2003 | Depression | Omega-3 | 4.4g | 2.2g | 2 | 8 weeks | Augmentation | 28 | 39 | 18% | Taiwan | Outpatient | HAM-D-SF | Moderate | 6 (21%) | Completers | 21 | + |
| Marangell (38) | 2003 | Depression | DHA | 0 | 2g | 0 | 6 weeks | Monotherapy | 36 | 47 | 22% | USA | Outpatient | HAM-D-28 | Moderate | 1 (3%) | Modified ITT | 27 | - |
| Silvers (39) | 2005 | Depression | Omega-3 | 0.6g | 2.4g | 0.25 | 12 weeks | Augmentation | 77 | 39 | 47% | New Zealand | Outpatient, Primary Care | HAM-D-SF | Moderate | 18 (23%) | ITT | 38 | - |
| Grenyer (40) | 2007 | Depression | Omega-3 | 0.6g | 2.2g | 0.27 | 16 weeks | Augmentation | 83 | 45 | 32% | Australia | Outpatient | BDI | Mild | 23 (28%) | ITT | 35 | - |
| Su (36) | 2008 | Perinatal Depression | Omega-3 | 2.2g | 1.2g | 1.83 | 8 weeks | Monotherapy | 36 | 31 | 0 | Taiwan | Outpatient, OB Clinic | HAM-D-21 | Moderate | 12 (33%) | Modified ITT | 33 | + |
| Rogers (45) | 2008 | Mild Depression | Omega-3 | 0.6g | 0.85g | 0.71 | 12 weeks | Monotherapy | 218 | 38 | 23% | UK | Outpatient, Surgery Clinics | DASS | Mild | 28 (13%) | ITT | 42 | - |
| Rees (42) | 2008 | Perinatal Depression | Omega-3 | 0.4g | 1.6g | 0.25 | 6 weeks | Monotherapy | 26 | 33 | 0 | Australia | Outpatient, OB and Psychiatric | HAM-D-17 | Moderate | 5 (19%) | ITT | 35 | - |
| Freeman (41) | 2008 | Perinatal Depression | Omega-3 | 1.1g | 0.8g | 1.38 | 8 weeks | Monotherapy | 51 | 30 | 0 | USA | Outpatient | HAM-D-21 | Mild | 20 (34%) | Modified ITT | 33 | - |
| da Silva (46) | 2008 | Depression + PD | Omega-3 | 0.7g | 0.5g | 1.4 | 12 weeks | Augmentation | 31 | 64 | 42% | Brazil | Outpatient, Neurology Clinic | MADRS | Mild | 2 (7%) | Completers | 27 | + |
| Mischoulon (44) | 2009 | Depression | EPA | 1g | 0 | ∞ | 8 weeks | Monotherapy | 35 | 45 | 37% | USA | Outpatient | HAM-D-17 | Moderate | 11 (31%) | Modified ITT | 28 | - |
| Lucas (43) | 2009 | Depression | Omega-3 | 1.05g | 0.15g | 7 | 8 weeks | Monotherapy | 29 | 48 | 0 | Canada | General Population | HAM-D-21 | Mild | 3 (10%) | ITT | 44 | - |
Efficacy of Omega-3 for Depression
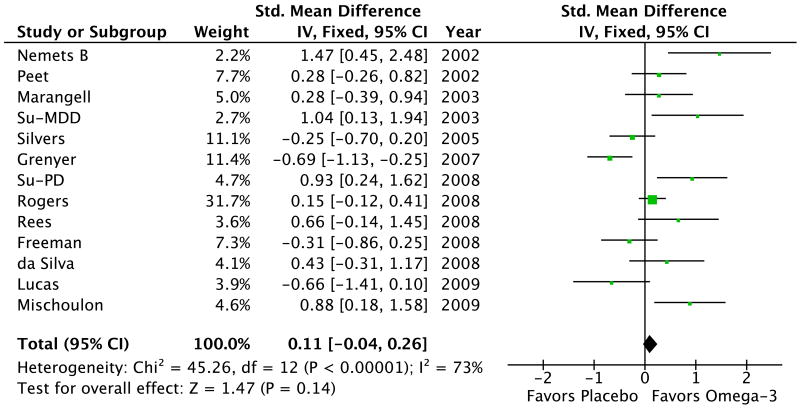
Meta-analysis showed no significant effect of omega-3 fatty acids for the treatment of depression (SMD=0.11, 95% Confidence Interval (CI): -0.04, 0.26, p=0.14). Figure 1 depicts a forest plot of the effect of omega-3 fatty acids for the treatment of depression compared to placebo. There was evidence of significant heterogeneity among studies (χ2 = 45.3, df = 12 (P < 0.00001); I2 = 73%). Sensitivity analysis using a random-effects model (SMD=0.25, 95% CI: -0.07, 0.56, p= 0.13) did not affect the overall findings. Similarly excluding the 2 trials that did not require a formal psychiatric diagnosis did not affect our overall findings (SMD=0.07, 95%CI: -0.12, 0.27, p=0.45).
Figure 1. Forest Plot of Omega-3 Fatty Acids for Depression.
There was no significant effect of omega-3 fatty acids for major depression compared to placebo. There was significant evidence of heterogeneity between trials. Significant publication bias was also evident using the rank correlation test (τb=0.47, z=2.3, p=0.02).
The funnel plot was asymmetric and demonstrated an excess of small positive studies suggesting publication bias in the literature (Figure 2). The rank correlation test for publication bias was statistically significant (τb=0.47, z=2.3, p=0.02). When the trim-and-fill method was used to adjust our results for the potential impact of publication bias nearly all benefits of omega-3 FAs were eliminated (SMD=0.01, 95% CI: -0.13, 0.15).
Figure 2. Funnel Plot Examining Publication Bias in Omega-3 Fatty Acid Trials to Treat Major Depression.
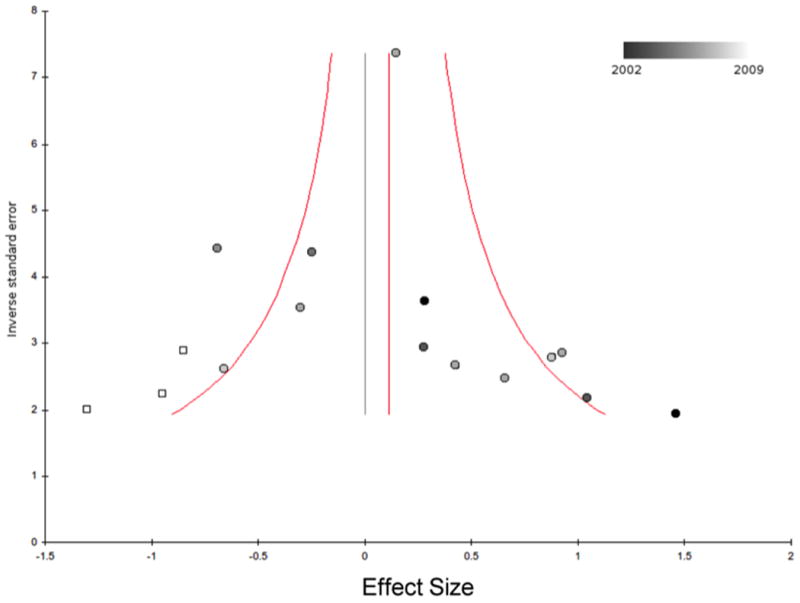
This funnel plot depicts the effect size of trials versus their inverse standard error. Published trials are depicted as circles and are shaded from darkest to lightest based on their publication year. White squares represent potentially missing trials that were imputed based on the trim-and-fill method. The red line represents the point estimate for omega-3 treatment effects based on published trials (SMD=0.11, 95% CI: -0.04, 0.26), p=0.14). The red curves bracket the 95% CI for the expect results of trials given this estimated underlying effect size. The black vertical line represents the new point estimate for the effect size of omega-3 fatty acids when publication bias is adjusted for using the trim-and-fill method. (SMD=0.01, 95% CI: -0.13, 0.15). Abbreviations: SMD=standardized mean difference, CI=confidence interval.
EPA Dose
Meta-regression demonstrated no significant difference in efficacy of omega-3 fatty acid preparations based on dose of EPA utilized (β=0.17 ± standard error (SE)=0.14, 95%CI: (-0.14, 0.47), p=0.26).
DHA Dose
Meta-regression demonstrated no significant difference in the efficacy of omega-3 fatty acids in trials based on dose of DHA utilized ((β=-0.25 ± 0.18, 95%CI: (-0.64, 0.14), p=0.19).
Omega-3 Monotherapy vs. Augmentation Therapy
There was no significant difference in estimates of omega-3 fatty acid efficacy based on whether omega-3 fatty acids were given as monotherapy or augmentation (test for subgroup differences χ2 = 1.8, df = 1 (p= 0.17); I2 = 46%). The estimate of effect in trials which omega-3 FAs were given as monotherapy was 0.20 (95%CI: 0,0.39), whereas the effect estimate was -0.02 (95%CI: -0.26,0.22) in trials in which concomitant pharmacotherapy was allowed.
Diagnostic Indication
There was no significant difference in estimates of omega-3 fatty acid efficacy based on whether trials examined their efficacy in MDD, peripartum MDD or MDD with medical comorbidities (Test for subgroup differences: χ2 = 1.9, df = 2 (p= 0.39), I2 = 0%), and the efficacy was similar for those three subgroups (SMD=0.06, 95% CI: -0.10, 0.23), (SMD=0.29, 95% CI: -0.09, 0.67) and (SMD=0.43, 95% CI: -0.31, 1.17), respectively.
Baseline Depression Severity
Trials in which participants had moderate depressive symptoms at baseline reported a greater efficacy of omega-3 fatty acids when compared to trials in which participants were only mildly depressed (test for subgroup differences: χ2 = 11.7, df = 1 (P < 0.0006), I2 = 91%). The effect size for omega-3 in trials in which participants were at least moderately depressed on average at baseline was 0.42 (95% CI: 0.19, 0.65) compared to trials in which participants had only mild depression on average at baseline (SMD=-0.11, (95% CI: -0.30, 0.09) (see Figure 3).
Figure 3. Forest Plot of Omega-3 Fatty Acids for Depression Stratified by Baseline Depression Severity.
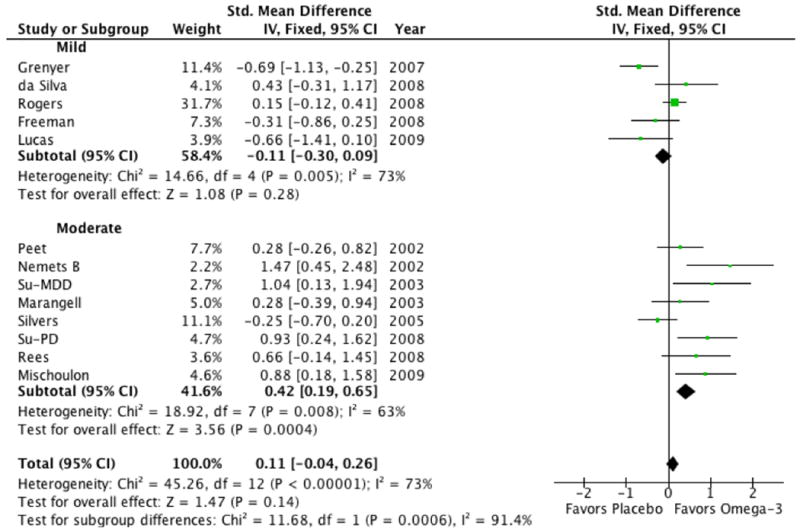
Trials in which participants were at least moderately depressed on average before starting treatment reported a greater efficacy of omega-3 fatty acids when compared to trials in which participants were only mildly depressed (test for subgroup differences: χ2 = 11.7, df = 1 (P < 0.0006), I2 = 91%). For stratification of trials by baseline depression severity we used traditional cutoffs for the HAM-D-17 (mild: <18, moderate: 18-28, severe: >28) 19. Trials that measured initial depression severity on scales other than the HAM-D-17 were converted to this scale based on previously defined algorithms 20. Abbreviation: CI= confidence interval.
Trial Duration
Meta-regression revealed a strong trend towards shorter duration trials demonstrating a greater efficacy of omega-3 fatty acids in the treatment of depression (β=-0.11 ± 0.04, 95%CI: (-0.20, -0.01), p=0.028, R2=0.37) (see Figure 4). However, this association did not reach the strict threshold of statistical significance set forth for secondary analyses in this meta-analysis.
Figure 4. Meta-Regression of Effect Size of Omega-3 Fatty Acids versus Trial Duration.
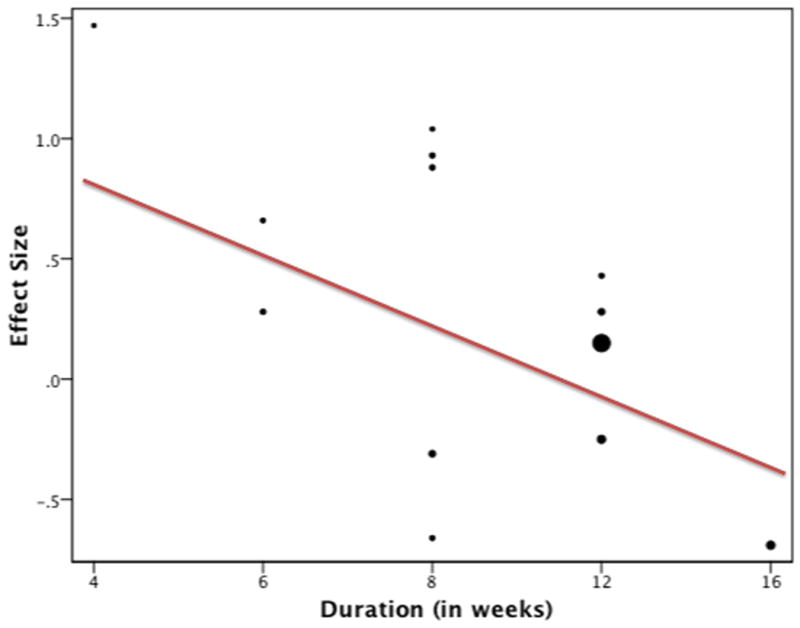
Trials of shorter duration tended to show a greater efficacy of omega-3 fatty acid. The size of the circle representing each trial is proportional to its weighting on the overall analysis (β=-0.11 ± 0.04, 95%CI: (-0.20, -0.01), p=0.028, R2=0.37). Trials were weighted using the generic inverse variance method.
Trial Quality
Omega-3 fatty acids showed greater efficacy in trials judged of lower quality (JADAD=3 or 4) than in trials judged of higher quality (JADAD=5; Test for subgroup differences: Chi2 = 7.2, df = 1 (P = 0.007), I2 = 86%). The effect size for omega-3 fatty acids in lower quality trials was 0.47 (95% CI: 0.17 0.76) compared to 0 (95% CI: -0.17, 0.17) in higher quality trials (see Figure 5). Lower quality trials as rated by the JADAD scale generally provided insufficient evidence about the appropriateness of randomization or blinding techniques (or both).
Figure 5. Forest Plot of Omega-3 Fatty Acids for Depression Stratified by Methodological Quality of Trials.
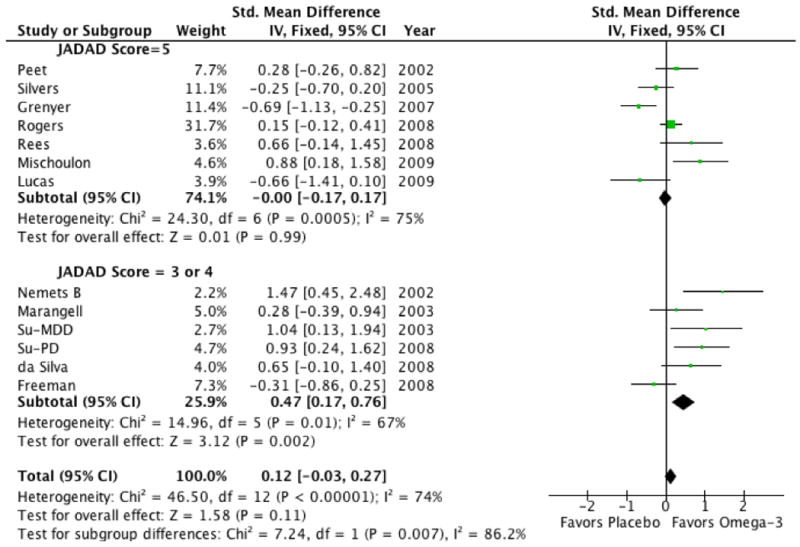
Omega-3 fatty acids tended to show greater efficacy in trials judged of lower quality (JADAD=3 or 4) than in trials judged of higher quality (JADAD=5 Test for subgroup differences: Chi2 = 7.2, df = 1 (P = 0.007), I2 = 86%). Clinical trials were stratified based on a median split of scores on the JADAD Scale The JADAD scale rates trials on presence and appropriateness of randomization and blinding as well as whether the number and reasons for dropouts was described in each trial.
Trial Analysis Method
There was no significant effect of analysis method on the measured efficacy of omega-3 FAs for depression (Test for subgroup differences: Chi2 = 9.5, df = 1 (P = 0.009), I2 = 79%). However, there was a strong trend towards trials relying on completers' analysis (SMD=0.81, 95% CI: 0.23, 1.38) showing greater efficacy than trials relying on modified ITT (SMD=0.35, 95% CI: 0.03, 0.67) and ITT (SMD=-0.01, 95% CI: 0.19,0.16) analysis (see Figure 6).
Figure 6. Forest Plot of Omega-3 Fatty Acids for Depression Stratified by Method of Accounting for Dropouts.
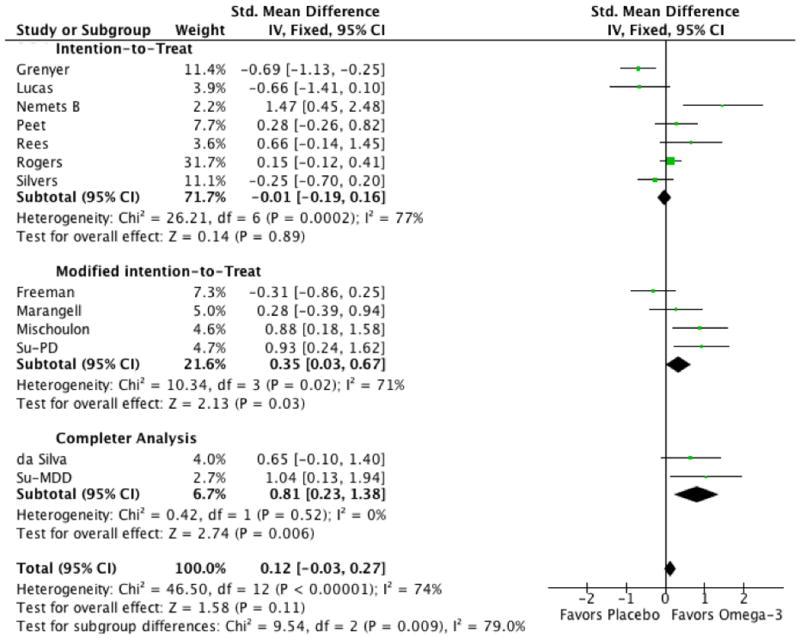
Omega-3 fatty acids tended to shower greater efficacy in trials that utilized completers analysis compared to trials that employed a modified intention-to-treat or an intention-to-treat analysis (Test for subgroup differences: Chi2 = 9.5, df = 1 (P = 0.009), I2 = 79%).
Discussion
Our meta-analysis demonstrates a small, non-significant benefit of omega-3 fatty acids for the treatment of MDD. There was evidence of publication bias, and statistical adjustment or this bias eliminated nearly all of the observed benefit of omega-3 fatty acids. This result stands in contrast to many previous meta-analyses that have reported significant benefits of omega-3 fatty acids in MDD. 8-12
Several factors are likely responsible for the difference in our results from previous meta-analyses. Since the publication of many previous meta-analyses, 8 additional trials examining the efficacy of omega-3 fatty acids for depression have been published.8-10 These trials have cumulatively more than tripled the number of MDD participants compared to earlier meta-analyses. The publication of a couple larger trials as well as the relatively recent requirement for public registration of trials and reporting of their primary outcome may be responsible for the decreased evidence of publication bias and treatment effects in later trials (see figure 2). A greater awareness of the methodological limitations of early omega-3 trials such as blinding issues caused by a fishy aftertaste present when the active formulations are refluxed may have inflated estimates of efficacy in earlier trials.48 The adoption of using flavoring such as orange or peppermint oil or adding a small amount of fish oil to the placebo preparations has increased in recent years.48 Also, the use of less fishy tasting formulations may have also improved blinding in later trials. To further support this view, trials of lower quality estimated a greater effect size of omega-3 fatty acids for depression than higher quality studies (see figure 5). Additionally, trials that did not account for dropouts in their analysis showed a greater effect of omega-3 fatty acids than trials that employed intention-to-treat analysis.
Another methodological difference compared to other reviews is that this systematic review excluded omega-3 trials of primary psychiatric disorder other than MDD (e.g. bipolar disorder, schizophrenia and OCD).49 These patients have distinct psychiatric conditions as recognized in DSM-IV or ICD-10 and are often known to be treated with and respond to different pharmacological interventions. In these trials depression was typically a secondary outcome and reporting of secondary outcomes may be particularly prone to publication bias (especially when specific, detailed data is required for meta-analysis). The inclusion of different psychiatric conditions is also likely a source for increased heterogeneity in previous meta-analyses. This increased heterogeneity may occur because improvement in depression symptoms in these trials may be particularly prone to confounding by other factors. For instance, previous meta-analyses have included trials of subjects with schizophrenia.50, 51 A rated improvement in depressive symptoms in a population of schizophrenics could be attributable to (1) improvement in the underlying psychosis that secondarily affect depression measures (e.g. a subject's depression rating improves because he is happy because his hallucinations are less), (2) improvement in some psychotic symptoms may directly lower depression rating scores (e.g. improvement in negative symptoms of schizophrenia would lead to an improvement in depression ratings). Or (3) improvement could occur in other symptoms commonly experienced by schizophrenics that could affect depression ratings (e.g. if omega-3 fatty acid supplementation decreased the side-effects of antipsychotic medications (such as fatigue, emotional blunting) depression ratings would improve). Despite these differences, the updated results of a systematic review which examined improvement in depressive symptoms among this broad population reported fairly similar findings.49 This systematic review reported a small (ES=0.10) but significant effect of omega-3 fatty acids with considerable evidence of publication bias and heterogeneity.49 A subsequent correspondence suggested that nearly all the treatment effect observed in the literature might be due to publication bias and that claims regarding efficacy of omega-3 given the publication bias were inappropriate.52 Our meta-analysis serves to reinforce the findings of this previous systematic review and reinforce the interpretation of later critiques with data. We further demonstrate no evidence of a beneficial treatment effect when the data is specific to those with MDD, which is in contrast to this previous meta-analysis.49
Our meta-analysis also demonstrated significant heterogeneity between trials. We conducted several secondary analyses to examine possible sources of this heterogeneity. Although, we did not demonstrate a significant overall effect of omega-3 fatty acids in MDD, our results suggest that they may be effective in patients with more severe depression. Trials where participants were at least moderately depressed on average showed a significant and medium sized effect of omega-3 treatment. This finding is quite similar to a recent meta-analysis using patient-level data that demonstrated a significantly greater effect of the antidepressants paroxetine and imipramine in severe depression.53 On the other hand, trials that included subjects with more severe depression severity also tended to be of lower methodological quality, employ completer rather than intention-to-treat and have smaller numbers of subjects (and thus be more prone to publication bias). We are unsure of whether this significant finding in secondary analysis is emblematic of a significant treatment effect or due to confounding by these other factors.
There were also several other potential sources of heterogeneity between trials that we were not able to investigate in this meta-analysis. Research has suggested the possibility that genetic variation may influence the extent to which individuals require to omega-3 fatty acids.54 A hypothesis exists that the relationship between omega-3 fatty acids and depression may be the result of an interaction between low intake of these polyunsaturated fatty acids in combination with a genetically determined predisposition towards abnormal phospholipid synthesis that results in a reduced cellular uptake of omega-3 fatty acids.55 Reduced cellular uptake of omega-3 fatty acids has been linked to low activity of FA CoA lipase 4 and/or Type IV phospholipase A2 (PLA2) and low functioning variants for each of these genes has been associated with MDD.56-58 Also, the ability of omega-3 fatty acids to suppress production of the inflammatory cytokine, TNF-α may be related to a polymorphism within the lymphotoxin gene.59 Thus differences between trials regarding omega-3 fatty acid diet of the samples and genetic heterogeneity could plausibly influence trial results. Few trials measure the omega-3 deficiency status of their subjects and even fewer trials assess potentially important sources of genetic heterogeneity. These measures thus represent potentially important sources of heterogeneity that could serve moderators or mediators of treatment effects and deserves further research.60
Our meta-analysis has several limitations. The use of patient-level rather than study-level data would have allowed us more powerfully and accurately to examine several potential moderators of treatment effect such as the influence of baseline depression severity, the use of omega-3 fatty acid supplementation as monotherapy versus as augmentation therapy. The use of patient-level data would have allowed us to also look at other potential moderators of treatment effect such as daily dietary fish consumption or other sources of omega-3 fatty acids, or mediators such as plasma omega-3 to omega-6 ratio in cell plasma membranes. We suspect that the use of study level data rather than patient-level decreased our power to detect differences in moderators of treatment effect when we were able to examine them. Furthermore, our examination of several secondary factors (in subgroup or meta-regression analysis) leaves open the strong possibility of confounding. For example, a few early trials that were poorly conducted, of short duration, used highly depressed patients, and high doses of EPA, generally reported the largest effects of omega-3 fatty acids. Without patient-level data or a vastly increased population of studies it is impossible to determine which of these factors are most influential.
Our meta-analysis also suggested possible publication bias in the literature. Because publication bias may have inflated our and others estimates of omega-3 efficacy, we decided to adjust for publication bias using the trim-and-fill method. The detection and adjustment for publication bias is difficult and somewhat controversial when there is a small number of trials.61 We therefore decided to present our results before and after adjusting for publication bias and made the primary data for this decision available in figure 2 so the reader can make their own decision about validity. There are clearly other reasons that the results of smaller trials may differ systematically from larger trials such as the use of more severely ill patients, the use of higher doses of omega-3 fatty acids or greater treatment fidelity or adherence in smaller studies. However, there are no clear reasons to believe this is the case. Although we examined sources of heterogeneity by conducting secondary analyses, this only explained part of the heterogeneity. We believe that other differences between trials that we were unable to measure such as differences in (1) participant characteristics – i.e. baseline dietary fish consumption, and (2) trial methodology – i.e. depression scale utilized, adequacy of blinding, or tolerability of intervention may be additional sources of heterogeneity.
Despite these limitations our meta-analysis has several important findings. After the inclusion of several recent studies examining the efficacy of omega-3 fatty acids in MDD, there is no longer significant evidence of efficacy. Meta-analysis also suggests that omega-3 fatty acids have at most minimal efficacy in treating depression. Further statistical adjustment for publication bias eliminates nearly all of the treatment benefits observed in the published literature. These findings are particularly sobering in light of the 7 additional double-blind placebo-controlled trials of omega-3 fatty acids in the treatment of MDD, (including 4 federally-funded trials), being currently conducted according to clinicaltrials.gov (accessed December 9, 2010). These ongoing trials plan to enroll over 1000 additional subjects with depression. Although there is still strong evidence based on the epidemiologic and cellular literature that omega-3/omega-6 FA balance may play an important role in the pathogenesis of depression, there is limited evidence for omega-3 fatty acid supplementation being an effective acute treatment for it.
Acknowledgments
The authors acknowledge the National Institute of Mental Health support of the Yale Child Study Center Research Training Program (MHB), the National Institutes of Health 1K23MH091240-01 (MHB), the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), the AACAP/Eli Lilly Pilot Research Award (MHB), the Trichotillomania Learning Center (MHB), NARSAD (MHB and JH), and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB and JH).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose
References
- 1.Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J. SSRIs versus other antidepressants for depressive disorder. Cochrane Database Syst Rev. 2000;(2):CD001851. doi: 10.1002/14651858.CD001851. [DOI] [PubMed] [Google Scholar]
- 2.Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58(1):19–36. doi: 10.1016/s0165-0327(99)00092-0. [DOI] [PubMed] [Google Scholar]
- 3.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4-5):259–69. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 5.Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamaki H, et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52(4):529–31. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38(1):35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 7.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43(5):315–9. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 8.Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr. 2006;84(6):1308–16. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 9.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–61. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 10.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–67. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 11.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 91(3):757–70. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 12.Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS. Efficacy of omega-3 Fatty acids in mood disorders - a systematic review and metaanalysis. Psychopharmacol Bull. 2009;42(3):39–54. [PubMed] [Google Scholar]
- 13.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. Chishester, UK: John Wiley & Sons, Ltd.; 2005. [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angst J, Amrein R, Stabl M. Moclobemide and tricyclic antidepressants in severe depression: meta-analysis and prospective studies. J Clin Psychopharmacol. 1985;15(S2):16S–26S. doi: 10.1097/00004714-199508001-00004. [DOI] [PubMed] [Google Scholar]
- 20.Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D, et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol. 2006;16(8):601–11. doi: 10.1016/j.euroneuro.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73. doi: 10.1016/0197-2456(94)00031-w. [DOI] [PubMed] [Google Scholar]
- 22.Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al. A systematic review of controlled trials of the effectiveness and cost effectiveness of brief psychological treatments for depression. Health Technology Assessment. 2001;5 doi: 10.3310/hta5350. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Altman DG, Bradburn MJ, editors. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. London: BMJ Publication Group; 2001. [Google Scholar]
- 24.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):407–12. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 25.Keck PE, Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60(9):1020–2. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 26.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 27.Fux M, Benjamin J, Nemets B. A placebo-controlled cross-over trial of adjunctive EPA in OCD. J Psychiatr Res. 2004;38(3):323–5. doi: 10.1016/S0022-3956(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 28.Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry. 2007;190:118–22. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- 29.Zanarini MC, Frankenburg FR. omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry. 2003;160(1):167–9. doi: 10.1176/appi.ajp.160.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–8. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 31.Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18(9):639–45. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163(6):1098–100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 33.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192–8. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 34.Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. Jama. 2009;302(15):1651–7. doi: 10.1001/jama.2009.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59(10):913–9. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 36.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159(3):477–9. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 37.Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644–51. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 38.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(4):267–71. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 39.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160(5):996–8. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 40.Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72(3):211–8. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393–6. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110(1-2):142–8. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- 44.Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Effects of ethyl-eicosapentaenoic acid omega-3 fatty acid supplementation on hot flashes and quality of life among middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Menopause. 2009;16(2):357–66. doi: 10.1097/gme.0b013e3181865386. [DOI] [PubMed] [Google Scholar]
- 45.Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009;70(12):1636–44. doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–31. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 47.da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, et al. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351–9. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Sontrop J, Campbell MK. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev Med. 2006;42(1):4–13. doi: 10.1016/j.ypmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91(3):757–70. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 50.Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158(12):2071–4. doi: 10.1176/appi.ajp.158.12.2071. [DOI] [PubMed] [Google Scholar]
- 51.Peet M, Horrobin DF. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36(1):7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- 52.Laurin D, Carmichael PH. Combining or not combining published results in the presence of heterogeneity? Am J Clin Nutr. 2010;92(3):669–70. doi: 10.3945/ajcn.2010.29767. author reply 70-1. [DOI] [PubMed] [Google Scholar]
- 53.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. Jama. 303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 55.Ross BM. Omega-3 fatty acid deficiency in major depressive disorder is caused by the interaction between diet and a genetically determined abnormality in phospholipid metabolism. Med Hypotheses. 2007;68(3):515–24. doi: 10.1016/j.mehy.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 56.Covault J, Pettinati H, Moak D, Mueller T, Kranzler HR. Association of a long-chain fatty acid-CoA ligase 4 gene polymorphism with depression and with enhanced niacin-induced dermal erythema. Am J Med Genet B Neuropsychiatr Genet. 2004;127B(1):42–7. doi: 10.1002/ajmg.b.20156. [DOI] [PubMed] [Google Scholar]
- 57.Papadimitriou GN, Dikeos DG, Souery D, Del-Favero J, Massat I, Avramopoulos D, et al. Genetic association between the phospholipase A2 gene and unipolar affective disorder: a multicentre case-control study. Psychiatr Genet. 2003;13(4):211–20. doi: 10.1097/00041444-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Pae CU, Yu HS, Kim JJ, Lee CU, Lee SJ, Lee KU, et al. BanI polymorphism of the cytosolic phospholipase A2 gene and mood disorders in the Korean population. Neuropsychobiology. 2004;49(4):185–8. doi: 10.1159/000077364. [DOI] [PubMed] [Google Scholar]
- 59.Grimble RF, Howell WM, O'Reilly G, Turner SJ, Markovic O, Hirrell S, et al. The ability of fish oil to suppress tumor necrosis factor alpha production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor alpha production. Am J Clin Nutr. 2002;76(2):454–9. doi: 10.1093/ajcn/76.2.454. [DOI] [PubMed] [Google Scholar]
- 60.Sontrop JM, Campbell MK, Evers SE, Speechley KN, Avison WR. Fish consumption among pregnant women in London, Ontario: associations with socio-demographic and health and lifestyle factors. Can J Public Health. 2007;98(5):389–94. doi: 10.1007/BF03405425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]