Abstract
Learning and memory processes may be influenced by fluctuations in steroid hormones, such as estrogens and progestins. In this study, we have used an animal model to investigate the effects of endogenous fluctuations in ovarian steroids in intact female rats and effects of administration of ovarian steroids to ovariectomized rats for non-spatial, working memory using the object recognition task. Performance in this task relies on cortical and hippocampal function. As such, serum, cortical, and hippocampal concentrations of estradiol (E2), progesterone (P4), and P4’s metabolite, 5α-pregnan-3α-ol-20-one (3α, 5α-THP), were measured by radioimmunoassay. Experiment 1: Rats in behavioral estrus, compared to those in diestrus or estrus, spent a greater percentage of time exploring a novel object concomitant with increases in serum E2, P4, and 3α, 5α-THP levels. Regression analyses revealed that there was a significant positive relationship between E2 levels in the hippocampus and 3α, 5α-THP levels in the hippocampus and cortex and performance in this task. Experiment 2: Administration of E2 and/or P4 immediately post-training increased the percentage of time spent exploring the novel object and produced levels of E2, P4, and 3α, 5α-THP akin to that of rats in behavioral estrus. Experiment 3: Post-training administration of selective estrogen receptor modulators, including 17β-E2, propyl pyrazole triol, and diarylpropionitrile increased the percentage of time spent exploring the novel object compared to vehicle-administration. Experiment 4: Post-training P4 or 3α, 5α-THP administration, compared to vehicle, increased the percentage of time spent exploring the novel object and produced P4 and/or 3α, 5α-THP levels within the physiological range typically observed for rats in behavioral estrus. Experiment 5: If post-training administration of E2 and/or P4 was delayed one hour, no enhancement in object recognition was observed. Together, these results suggest that E2 and progestins can have mnemonic effects through actions in the cortex and/or hippocampus.
Keywords: Estrogen, Progesterone, Allopregnanolone, Selective estrogen receptor modulators, Prefrontal cortex, Hippocampus, Learning, Memory
1. Introduction
Fluctuations in ovarian steroids, such as estrogens and progestins, across the estrous cycle alter several behavioral processes among rodents, including cognitive performance. Circulating estradiol (E2) levels are low early in diestrus, increase later in diestrus, peak during behavioral estrus, and decline during estrus. Progesterone (P4) and its metabolite, 5α-pregnan-3α-ol-20-one (3α, 5α-THP), are low during diestrus and increase following the peak in E2 levels during behavioral estrus (Frye & Bayon, 1999). The extent to which these endogenous fluctuations in estrogens and progestins influence cognitive performance is not entirely clear. Acute elevations to physiological levels of E2 and progestins in young, intact female rats are associated with enhanced cognitive performance in some tasks. For example, in the classical eye-blink conditioning task and the inhibitory avoidance task, performance is better during behavioral estrus compared to other phases of the cycle (Rhodes & Frye, 2004a; Shors, Lewczyk, Pacynski, Mathew, & Pickett, 1998; Wood, Beylin, & Shors, 2001). No differences, or poorer performance, are associated with estrous cycle variations in E2 that would not be expected to produce low physiological levels of E2 during memory consolidation in tasks that are dependent upon the hippocampus (i.e., water and radial arm maze) or hippocampus and amygdala (i.e., active avoidance; Frye, 1995; Galea, Kavaliers, Ossenkopp, & Hampson, 1995; Mora, Dussaubat, & Diaz-Veliz, 1996; Stackman, Blasberg, Langan, & Clark, 1997; Warren & Juraska, 1997). Thus, these data from tasks that involve hippocampal function suggest that the hippocampus is a target of E2 for its effects to enhance cognitive performance.
Although the data discussed above suggest that there may be concentration-dependent effects of endogenous E2 across the estrous cycle to alter cognitive performance, other steroids, such as progestins, also vary with the estrous cycle. One way to investigate E2’s effects is to administer E2 to ovariectomized (OVX) rats. Administration of E2 that likely produces low, physiological levels of E2 during training and/or testing (similar to naturally receptive rats) improves performance in tasks that are mediated by the striatum, hippocampus, amygdala, and/or neocortex (Bimonte & Denenberg, 1999; Daniel, Fader, Spencer, & Dohanich, 1997; Davis, Jacobson, Aliakbari, & Mizumori, 2005; Diaz-Veliz, Urresta, Dussaubat, & Mora, 1991; Gibbs, 1999, 2000; Gibbs, Gabor, Cox, & Johnson, 2004; as reviewed in Korol, 2004; Korol & Kolo, 2002; Luine, Richards, Wu, & Beck, 1998; Marriott & Korol, 2003; O’Neal, Means, Poole, & Hamm, 1996; Sandstrom & Williams, 2001, 2004; Vazquez-Pereyra, Rivas-Arancibia, Loaeza-Del Castillo, & Schneider-Rivas, 1995). However, beneficial effects of E2 are not observed in all studies using tasks that are dependent upon the hippocampus (Morris water maze, radial arm maze) or striatum (win-stay task, conditioned place preference) when E2 is administered pre-training and/or pre-testing (Chesler & Juraska, 2000; Galea et al., 2001, Galea, Lee, Kostaras, Sidhu, & Barr, 2002; Holmes, Wide, & Galea, 2002). It may be that clearer effects of E2 to enhance learning/memory are observed when E2 is present during the post-training/consolidation period (Packard, 1998). Together, these findings suggest that effects of E2 to enhance learning/memory may be dependent upon many factors, such as regimen utilized, task difficulty, type of task, strategy utilized, etc. Furthermore, it may be that some of these variable effects of E2 for cognitive function are due to actions at different putative receptor substrates of E2.
In considering the effects of E2 for cognitive performance, it is also of interest to examine putative mechanisms for these effects. For instance, studies utilizing mice with deletions of the intracellular estrogen receptor (ER) suggest that E2’s effects at ERs may be critical for its effects on cognitive behavior (Fugger, Foster, Gustafsson, & Rissman, 2000). Furthermore, there is some suggestion from studies with knockout mice and administration of selective estrogen receptor modulator (SERMs) that have greater affinity for the α or β isoform of the ER that E2 can have effects involving both of these isoforms (Fugger et al., 2000; Gibbs et al., 2004; Luine, Jacome, & Maclusky, 2003; Lund & Lephart, 2001; Rhodes & Frye, in press; Rissman, Heck, Leonard, Shupnik, & Gustafsson, 2002). Notably, both ERα and ERβ have been localized in brain regions considered important for learning and memory processes, such as the hippocampus and prefrontal cortex (PFC; Shughrue, Lane, & Merchenthaler, 1997, 1998).
The behavioral data discussed above regarding E2’s effects for cognitive performance suggest that a likely target of E2 for these effects is the hippocampus. However, the PFC may be another important target for steroid hormones’ effects on cognitive performance. Two tasks that are typically utilized to assess PFC function are the T-maze and object recognition tasks (Ennaceur, Neave, & Aggleton, 1997; Granon, Vidal, Thinus-Blanc, Changeux, & Poucet, 1994). E2 administration to young or aged mice and young, OVX rats enhances performance in both of these tasks (Fader, Hendricson, & Dohanich, 1998; Heikkinen, Puolivali, & Tanila, 2004; Luine et al., 2003; Miller et al., 1999; Vaucher et al., 2002). These data suggest that the PFC may also be a target for E2’s effects on cognitive performance.
Although the data discussed above provide support for E2’s effects to alter cognitive performance, the role of progestins for these effects has received less attention and is also of interest. First, in addition to circulating E2, progestin levels change over the estrous cycle, such that increased P4 and 3α, 5α-THP levels may improve cognitive performance. Second, there is also evidence from extirpation and replacement studies that progestins may enhance cognitive performance of young rats. Young, OVX rats show consistent decrements in water maze and inhibitory avoidance that can be ameliorated with administration of P4 or 3α, 5α-THP (Frye & Lacey, 2001; Frye & Sturgis, 1995). Co-administration of E2 and P4 enhances performance of young OVX rats, with or without neurodegeneration, in the hippocampally-mediated water or radial arm maze (Sandstrom & Williams, 2001; Sato et al., 2004; Vongher & Frye, 1999). Although there has been less investigation of the effects of progestins to modulate performance in cognitive tasks mediated by the PFC and hippocampus, in one study, administration of E2 alone, or with P4, to OVX rats attenuated scopolamine-induced deficits in T-maze alternations (Dohanich, Fader, & Javorsky, 1994). Third, it may be that E2’s effects to enhance cognitive performance are through modulating endogenous production and/or functional effects of progestins. For example, E2 enhances central 3α, 5α-THP synthesis (Cheng & Karavolas, 1973; Malendowicz, 1976; Resko, Stadelman, & Handa, 1986; Vongher & Frye, 1999) and can enhance anti-nociceptive and/or neuroprotective effects of neuroactive progestins (Frye & Duncan, 1996; Vongher & Frye, 1999). Thus, it is intriguing to consider how E2 and/or progestins may interact in the PFC and hippocampus to alter cognitive performance.
To investigate the role of ovarian hormones’ actions in the PFC and hippocampus for cognitive performance, we examined effects of estrogens and progestins for performance in the object recognition task, a memory task that relies on the hippocampus and PFC. We hypothesized that if estrogens and/or progestins have beneficial effects to enhance cognitive performance that involves the PFC and hippocampus, then higher endogenous levels of estrogens and/or progestins in the PFC and hippocampus should occur with enhanced performance in the object recognition task. Further, administration of estrogens and/or progestins post-training to OVX rats should produce increased levels of these steroids in the PFC and hippocampus concomitant with enhanced performance in this task.
2. Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at S.U.N.Y.-Albany.
Adult, female Long-Evans rats (N = 160), approximately 55 days old, were obtained from the breeding colony at S.U.N.Y.-Albany (stock originally purchased from Taconic Farms, Germantown, NY). Rats were housed four per cage in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility in The Life Sciences Research Building. Rats were maintained on a 12/12 h reversed-light cycle (lights off at 0800) with continuous access to Purina Rat Chow and tap water.
In Experiment 1, effects of endogenous variations in E2 and progestins for cognitive performance were examined. Intact rats were cycled to determine estrous cycle phase. As per previously published methods, estrous cycle phase was determined with examination of vaginal cytology (Frye & Bayon, 1999; Long & Evans, 1922). Upon examination of vaginal cytology, nucleated cells were used to identify rats in behavioral estrus, cornified cells were used to identify rats in estrus, and a heterogenous vaginal epithelia were used to identify rats in diestrus. Immediately after screening (~0800–900), diestrous (n = 6), estrous (n = 7), and behavioral estrous (n = 6) rats were trained in the object recognition task. Data from one diestrous rat was excluded because central levels of E2 and progestins could not be determined.
Rats in Experiments 2–5 were ovariectomized under Rompum (12 mg/kg; Bayer Corp., Shawnee Mission, KS) and Ketaset (80 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia at least 1 week before behavioral testing commenced. In Experiment 2, OVX rats received subcutaneous (SC) injections of sesame oil vehicle (0.2 cc; n = 15), 17β-estradiol (17β-E2; Sigma Chemical Co.; 0.9 mg/kg; n = 14), P4 (Sigma Chemical Co.; 4 mg/kg; n = 16), or 17β-E2 (0.9 mg/kg) and P4 (4 mg/kg; n = 16) immediately after training in the object recognition task. One week later, in Experiment 3, OVX rats (n = 15/condition) were administered SC injections of sesame oil vehicle, 17β-E2 (0.9 mg/kg), or SERMs that have greater affinity for estrogen receptor (ER) α (propyl pyrazole triol; PPT; 0.9 mg/kg; Stauffer et al., 2000) or ERβ (diarylpropionitrile; DPN; 0.9 mg/kg; Meyers et al., 2001) immediately after training. The next week, in Experiment 4, OVX rats were administered SC sesame oil vehicle (0.2 cc; n = 11), P4 (4 mg/kg; n = 14), or 3α, 5α-THP (4 mg/kg; n = 14) immediately after training. One week later in Experiment 5, OVX rats were administered sesame oil vehicle (0.2 cc; n = 7), 17β-E2 (0.9 mg/kg; n = 5), or P4 (4 mg/kg), or 17β-E2 and P4 (n = 5) 1 h following training to address whether steroid hormones would enhance performance if present outside the 1 h post-training consolidation period. The same OVX rats were retested once per week in different conditions that were randomized with no evidence of test-decay effects, as has been previously demonstrated (Luine et al., 2003).
The object recognition task is a working memory task that relies on cortical and hippocampal functioning (Ennaceur et al., 1997). This task was used as modified from previously published methods (Ennaceur & Delacour, 1988; Frye & Lacey, 2001; Luine et al., 2003). During training, rats are placed in a white open field (76 × 57 cm with 35 cm high walls) in the brightly lit testing room. During training, the open field has two identical objects in adjacent corners. The identical objects were colored spheres (“orange” or “lemon” plastic toys). During testing, one sphere was replaced by a cone-shaped object (blue or red “buoy” plastic toys) of a similar size. Four hours after training, rats are placed back in the open field with two objects, one that rats had previously been exposed to during training and one novel object. Rats were allowed to explore the objects for 3 min in both trials. Rats spent equal amounts of time exploring objects in this task (typically 4.7 ± 1.0 s) and only data of rats that spent time exploring both objects during training are considered in analyses. Although rats did not show a bias towards exploring objects on the right vs. left side of the open field, placement of the novel object was counterbalanced across treatment groups and testing sessions to eliminate any possible effects of side preference. An increased percentage of time spent exploring the novel object as a function of the total amount of time spent exploring both objects during testing (duration spent with novel object/(duration spent with novel object + duration spent with familiar object) × 100) is considered an index of enhanced cognitive performance in this task.
Immediately after testing, intact female rats (n = 18) and, a group of OVX rats that were trained, administered vehicle (n = 2), E2 (n = 3), E2 and P4 (n = 3), P4 (n = 3), or 3α, 5α-THP (n = 3) post-training, and were tested 1 week following completion of Experiment 5, were rapidly decapitated. Blood and whole brains were collected for later hormonal measurement by radioimmunoassay. After decapitation, blood was collected in empty glass test tubes on ice and then blood was spun in a centrifuge 3000g for 10 min. After spinning, serum was decanted into empty plastic eppendorf tubes and stored at −80 °C (~1 week). Whole brains were dissected out from the skull in under 30 s and were placed on weighboats on dry ice. Whole brains were stored at −80 °C (~1 week) until immediately before radioimmunoassay when bilateral sections of the hippocampus and cortex were dissected out on ice. These dissected out sections were then homogenized with a glass/glass homogenizer and steroids were extracted from serum and CNS tissue on the same day as radioimmunoassay was performed. Radioimmunoassay was utilized to determine E2, P4, and 3α, 5α-THP levels in these tissues, as per previously published methods (Frye & Bayon, 1999).
Data from rats were analyzed using analyses of variance (ANOVAs) to determine effects of hormone condition on performance in the object recognition task. In Experiment 1, simple regression analyses were carried out to determine the contribution of steroid hormone levels in intact, cycling rats to performance in the object recognition task. An α level of p < .05 was used to determine statistical significance. When appropriate, significant overall analyses were further analyzed with Fisher PLSD comparisons to determine group differences.
3. Results
3.1. Experiment 1—Endogenous variations in object recognition
Endogenous variations in E2 and progestin levels influence performance in the object recognition task. There was a main effect for endogenous hormone condition to alter percentage of time spent with the novel object F (2, 15) = 10.23, p < .01 (Fig. 1). Post hoc analyses revealed that rats in behavioral estrus (4.7 ± 0.6 s with novel object vs. 1.3 ± 0.4 s with familiar object) spent a greater percentage of time with the novel object compared to estrous (4.1 ± 0.6 s with novel object vs. 2.0 ± 0.3 s with familiar object) or diestrous (2.6 ± 0.7 s with novel object vs. 3.0 ± 0.8 s with familiar object) rats. Estrous rats spent a significantly greater percentage of time with the novel object than did diestrous rats.
Fig. 1.
Percentage of time spent exploring the novel object during testing of intact, cycling rats. Rats in behavioral estrus (n = 6) spent a significantly higher percentage of time exploring the novel object than did estrous (n = 7) or diestrous rats (n = 5; **). Estrous rats spent a significantly higher percentage of time exploring the novel object than did diestrous rats (*).
3.1.1. Experiment 1—Endogenous variations in E2 and progestin levels
There were endogenous variations in E2 and progestin levels in serum, cortex and hippocampus (Table 1). Serum E2 F (2, 16) = 30.77, p < 0.01, P4 F (2, 16) = 23.73, p < 0.01, and 3α, 5α-THP F (2, 16) = 4.48, p ≤ 0.03 concentrations were increased in rats in behavioral estrus compared to diestrus. Cortical P4 F (2, 16) = 4.21, p ≤ 0.03, and 3α, 5α-THP F (2, 16) = 5.49, p ≤ 0.02 concentrations were significantly increased in rats in behavioral estrus compared to diestrus. Hippocampal E2 F (2, 16) = 4.04, p < 0.03, and 3α, 5α-THP F (2, 16) = 50.04, p < 0.01 concentrations were increased in rats in behavioral estrus compared to diestrus.
Table 1.
Serum, cortical, and hippocampal (hippo) concentrations of E2, P4, and 3α, 5α-THP, when rats in different endogenous hormonal milieu are tested in the object recognition task
| Condition | Estrogen levels
|
Progesterone Levels
|
3α, 5α-THP levels
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum (pg/ml) | Cortex (ng/mg) | Hippo (ng/mg) | Serum (ng/ml) | Cortex (ng/g) | Hippo (ng/g) | Serum (ng/ml) | Cortex (ng/g) | Hippo (ng/g) | |
| Diestrus | 4.9 ± 1.2 | 6.0 ± 0.8 | 2.1 ± 0.5 | 5.3 ± 1.6 | 2.6 ± 1.2 | 9.1 ± 4.5 | 1.2 ± 2.00 | 4.7 ± 0.5 | 3.2 ± 0.3 |
| Behavioral Estrus | 19.1 ± 1.9a,b | 3.8 ± 0.8 | 4.9 ± 0.8a | 21.1 ± 1.9a,b | 12.9 ± 2.6a | 27.3 ± 7.2 | 11.5 ± 0.9a | 9.6 ± 1.4a | 13.7 ± 0.8a,b |
| Estrus | 16.6 ± 0.4 | 4.1 ± 0.5 | 3.2 ± 0.5 | 12.8 ± 1.2a | 9.0 ± 2.8 | 18.9 ± 3.4 | 8.7 ± 0.5 | 7.3 ± 0.7 | 5.3 ± 1.0 |
Different from diestrus (p ≤ 0.05).
Different from estrus (p ≤ 0.05).
3.1.2. Experiment 1—Regression analyses of endogenous variations in steroids and object recognition
Regression analyses revealed that physiological steroid hormone concentrations, as measured in serum, cortex, and hippocampus, contributed to performance in the object recognition task (Fig. 2). There were positive relationships between serum levels of E2 (r = 0.67; r2 = 0.45; p ≤ 0.01, P4 (r = 0.67; r2 = 0.44; p ≤ 0.01), and 3α, 5α-THP (r = 0.57; r2 = 0.32; p ≤ 0.01) and the percentage of time spent exploring the novel object among intact females. In analyzing the contribution of E2 or progestin concentrations in the cortex for object recognition, a positive relationship between cortical levels of 3α, 5α-THP (r = 0.48; r2 = 0.23; p ≤ 0.04), but not E2 or P4, and total percentage of time spent exploring the novel object was found. Positive relationships between hippocampal levels of E2 (r = 0.60; r2 = 0.36; p ≤ 0.01), and 3α, 5α-THP (r = 0.67; r2 = 0.46; p ≤ 0.01), and the percentage of time spent exploring the novel object among intact females were also found.
Fig. 2.
Scatter plots depicting regression analyses of cortical and hippocampal E2, P4, and 3α, 5α-THP concentrations and the percentage of time spent by cycling rats exploring the novel object. Black circles represent data of behavioral estrous rats. Gray circles represent data of estrous rats. White circles represent data of diestrous rats.
3.2. Experiment 2—Effects of 17β-E2 and/or P4 to OVX rats for object recognition
A similar pattern of results were observed in experiments investigating effects of administration of ovarian steroids to OVX rats. There was a main effect of 17β-E2 and/or P4 treatment F (3, 57) = 3.44, p ≤ 0.02. Rats administered 17β-E2 (2.7 ± 0.4 s with novel object vs. 1.2 ± 0.3 s with familiar object), P4 (3.8 ± 0.6 s with novel object vs. 1.8 ± 0.4 s with familiar object), or 17β-E2 and P4 (2.7 ± 0.3 s with novel object vs. 0.9 ± 0.2 s with familiar object) spent a significantly greater percentage of time exploring the novel object compared to rats administered vehicle (2.3 ± 0.7 s with novel object vs. 0.8 ± 0.2 s with familiar object; Fig. 3).
Fig. 3.
Percentage of time spent exploring the novel object during testing of OVX, hormone-primed rats. Rats administered 17β-E2 (n = 14), P4 (n = 16), or 17β-E2 + P4 (n = 16) immediately post-training had a significantly higher percentage of time exploring the novel object than did rats administered vehicle (n = 15; *).
3.2.1. E2 and progestin levels produced in OVX rats by E2 and/or P4 administration
Results of radioimmunoassay demonstrate that serum, cortex, and hippocampal levels of E2 and progestins were increased to a physiological range 4 h after training and post-training E2 and/or P4 injections, when rats were tested and tissues were collected (Table 2). E2 levels were significantly increased in serum F (4, 9) = 5.74, p ≤ 0.01, hippocampus F (4, 23) = 22.31, p ≤ 0.01, and cortex F (4, 23) = 2.65, p ≤ 0.05 of rats administered E2 or E2 + P4 compared to rats administered vehicle or progestins. Cortical P4 F (4, 23) = 3.28, p ≤ 0.03 and 3α, 5α-THP F (4, 23) = 28.12, p ≤ 0.01 and hippocampal P4 F (4, 23) = 4.33, p ≤ 0.01, and 3α, 5α-THP F (4, 23) = 16.12, p ≤ 0.01 levels were significantly increased by E2 + P4 or P4, compared to E2 or vehicle, administration to OVX rats. There was a tendency for serum levels of P4 to be different among groups F (4, 9) = 3.16, p ≤ 0.07, such that rats administered E2 + P4 or P4 had higher serum P4 levels compared to all other groups. Although not statistically different, rats administered E2, E2 + P4, or P4, had higher serum 3α, 5α-THP levels than did rats administered vehicle.
Table 2.
Serum, cortical, and hippocampal (hippo) concentrations of E2, P4, and 3α, 5α-THP at test-time of OVX rats administered vehicle, E2 and/or P4, or 3α, 5α-THP subcutaneously
| Condition | Estrogen levels
|
Progesterone Levels
|
3α, 5α-THP levels
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum (pg/ml) | Cortex (ng/mg) | Hippo (ng/mg) | Serum (ng/ml) | Cortex (ng/g) | Hippo (ng/g) | Serum (ng/ml) | Cortex (ng/g) | Hippo (ng/g) | |
| OVX Vehicle SC | 0 | 0.1 ± 0.1 | 0 | 0.6 ± 0.6 | 1.2 ± 0.1 | 0.9 ± 0.3 | 1.6 ± 1.6 | 0.5 ± 0.1 | 0.9 ± 0.3 |
| OVX E2 SC | 17.2 ± 2.9a,d,e | 3.0 ± 1.2a,e | 1.5 ± 0.2a,d,e | 4.2 ± 1.5 | 1.3 ± 0.4 | 2.7 ± 1.3 | 6.1 ± 4.4 | 1.3 ± 0.4 | 1.2 ± 0.1c,d,e |
| OVX E2 + P4 | 13.0 ± 6.7a,d,e | 1.3 ± 0.1 | 1.3 ± 0.2a,d,e | 50.9 ± 18.3 | 15.4 ± 5.0a,b,e | 6.7 ± 1.3 | 5.9 ± 0.5 | 7.1 ± 0.5a,b | 9.3 ± 1.0a,a,b |
| OVX P4 SC | 0 | 1.1 ± 0.9 | 0.1 ± 0.0 | 59.9 ± 28.6 | 9.3 ± 3.8 | 18.6 ± 4.3a,b,e | 10.2 ± 2.3 | 6.7 ± 0.9a,b | 9.6 ± 1.1a,b |
| OVX 3α, 5α-THP SC | 0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 2.2 ± 1.1 | 6.0 ± 2.5 | 14.9 ± 6.0a,b | 6.1 ± 0.6 | 9.0 ± 0.9a,b,c,d | 11.9 ± 2.1a,b |
Different from vehicle (p ≤ .05).
Different from E2 (p ≤ .05).
Different from E2 + P (p ≤ .05).
Different from P (p ≤ .05).
Different from THP (p ≤ .05).
3.3. Experiment 3—Effects of 17β-E2 or SERMs to OVX rats for object recognition
There was a main effect of estrogen treatment F (3, 56) = 4.01, p ≤ 0.01. Rats administered 17β-E2 (3.8 ± 0.9 s with novel object vs. 2.0 ± 0.5 s with familiar object), PPT (4.3 ± 0.8 s with novel object vs. 2.7 ± 0.7 s with familiar object), or DPN (5.5 ± 1.2 s with novel object vs. 2.7 ± 0.7 s with familiar object) spent a significantly longer percentage of time with the novel object compared to rats administered vehicle (2.7 ± 0.7 s with novel object vs. 3.1 ± 0.5 s with familiar object; Fig. 4).
Fig. 4.
Percentage of time spent exploring the novel object during testing of OVX rats administered ER ligands. Rats administered 17β-E2, the ERα ligand (PPT), the ERβ ligand (DPN) immediately post-training had a significantly higher percentage of time exploring the novel object than did rats administered vehicle (n = 15/group; *).
3.4. Experiment 4—Effects of Progestins to OVX rats for object recognition
There was a main effect of progestin treatment F (2, 36) = 4.77, p ≤ 0.02 for object recognition task performance. Administration of P4 (2.4 ± 0.5 s with novel object vs. 1.2 ± 0.2 s with familiar object) or 3α, 5α-THP (2.4 ± 0.5 s with novel object vs. 1.7 ± 0.6 s with familiar object) significantly increased the percentage of time spent with the novel object compared to vehicle-administration (1.5 ± 0.5 s with novel object vs. 1.5 ± 0.4 s with familiar object; Fig. 5).
Fig. 5.
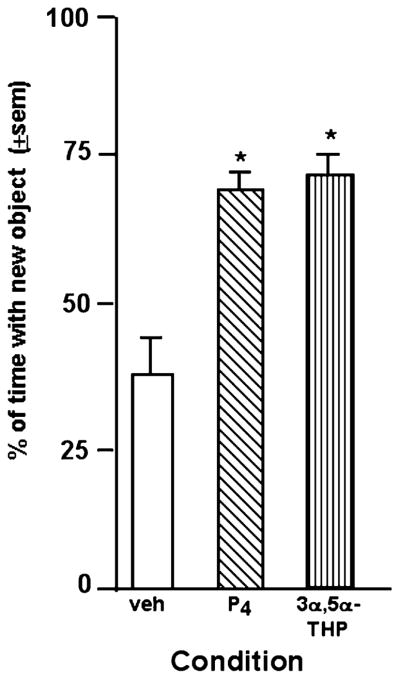
Percentage of time spent exploring the novel object during testing of OVX, progestin-administered rats. Rats administered P4 (n = 14) or 3α, 5α-THP (n = 14) immediately post-training had a significantly higher percentage of time exploring the novel object than did rats administered vehicle (n = 11; *).
3.4.1. Progestin levels in OVX rats administered P4 or 3α, 5α-THP
Results of radioimmunoassay demonstrate that serum, cortex, and hippocampal levels of progestins were increased to a physiological range 4 h after training and post-training P4 (as described above in Section 3.2.1) and 3α, 5α-THP injections, when rats were tested and tissues were collected (Table 2). Administration of 3α, 5α-THP increased cortical F (4, 23) = 28.12, p ≤ 0.01 and hippocampal F (4, 23) = 16.12, p ≤ 0.01, 3α, 5α-THP levels compared to vehicle or E2 administration. Rats administered 3α, 5α-THP had the highest cortical concentration of 3α, 5α-THP compared to all other groups. Although not statistically different, rats administered 3α, 5α-THP had higher serum 3α, 5α-THP levels than did rats administered vehicle.
3.5. Experiment 5—Effects of 1 h delay in post-training E2 or P4 administration to OVX rats for object recognition
There was no main effect of hormone treatment, when rats were administered the drug 1 h after training was completed (p = 0.90). The percentage of time spent with the novel object was similar in rats administered vehicle (3.0 ± 0.9 s with novel object vs. 1.7 ± 0.5 s with familiar object), E2 (4.0 ± 0.8 s with novel object vs. 2.8 ± 0.8 s with familiar object), P4 (3.5 ± 0.6 s with novel object vs. 1.5 ± 0.5 s with familiar object), or E2 + P4 (4.6 ± 0.6 s with novel object vs. 2.4 ± 0.7 s with familiar object) 1 h post-training (Fig. 6).
Fig. 6.
Percentage of time spent exploring the novel object during testing of OVX rats administered 17β-E2 (n = 5), P4 (n = 6), or 17β-E2 + P4 (n = 5), or vehicle (n = 7) 1 h after training. All rats in this experiment had similar percentages of time exploring the novel object that were not above chance levels.
4. Discussion
These results supported our hypothesis that estrogens and/or progestins from endogenous sources can have beneficial effects to enhance object recognition performance coincident with increasing endogenous steroid levels in serum, cortex, and hippocampus. Regression analyses suggest that E2 levels in the hippocampus and/or 3α, 5α-THP levels in the cortex or hippocampus may be particularly important for the observed effects. Administration of these steroids immediately post-training in concentrations that produced similar physiological levels as were observed in behavioral estrous rats enhanced object recognition performance. ER ligands with selective affinity for ERα, ERβ, or both increased performance in the object recognition task compared to vehicle-administration. Furthermore, administration of E2 and/or P4 1 h after training did not alter performance of OVX rats, suggesting that there is a critical time frame in which steroids must be present in order for observed beneficial effects to occur. Thus, these data support a role of E2 and progestins having effects in the cortex and hippocampus to enhance object recognition.
The present results that behavioral estrous rats and OVX rats administered E2 + P4, both of which had physiological E2 and P4 levels, had better performance in the object recognition task confirm previous reports of the beneficial effects of physiological E2 and/or P4 levels in the hippocampus for cognitive performance. Administration of SC E2 regimen that typically produces physiological, proestrous-like serum levels of E2 in OVX mice (Edwards, 1970; Morgan, Schulkin, & Pfaff, 2004) enhances performance in the water maze of aged mice and increases hippocampal E2, synaptophysin, and BDNF levels compared to aged mice administered vehicle, which had hippocampal E2 levels at nadir (Frick, Fernandez, & Bulinski, 2002; Frye, Rhodes, & Dudek, 2005). In the inhibitory avoidance task, a task that is considered to be mediated by the hippocampus and amygdala, naturally receptive rats perform better than do non-receptive rats when rats are tested 4 h later (Rhodes & Frye, 2004a). Notably, the present study, investigating effects of estrous cycle differences and effects of exogenous E2 or progestin administration (that produces similar circulating and central E2, P4, and 3α, 5α-THP levels), suggests that ovarian steroids may enhance performance in the object recognition task, a task that relies on the functioning of the cortex and the hippocampus. Furthermore, these data suggest that there may be a relationship between levels of E2 in the hippocampus, but not the cortex, and 3α, 5α-THP in the hippocampus and cortex and performance of rats in the object recognition task. Higher cortical levels of E2 appear to be disruptive to performance among cycling females in this task, which may be related to regimen-dependent effects of E2, as already discussed. Although it is not evident from these data how E2 and 3α,5α-THP may be interacting to produce these functional effects, E2 can increase production of 3α, 5α-THP by altering activity of enzymes necessary for the metabolism of P4 to 3α, 5α-THP and this may underlie the observed effects (Cheng & Karavolas, 1973; Malendowicz, 1976; Resko et al., 1986). Administration of a lower dose of E2 (2 μg) than was used in this study, increases 3α, 5α-THP levels in the hippocampus of OVX rats (Frye & Rhodes, 2005). Together, these data suggest a role of these steroids in the cortical-hippocampal circuitry underlying performance in this non-spatial, working memory task.
The present study confirms and extends previous reports on the effects of estrogens on object recognition memory and putative central mechanisms for these effects. Oral administration of E2 to middle-aged mice enhances object recognition (Fernandez & Frick, 2004). In the present study, similar effects were observed in rats administered estrogens with different affinity for ERα and ERβ. 17β-E2, which is a ligand for both ERα and ERβ, produced consistent effects across experiments to enhance performance in the object recognition task when it was administered post-training compared to that observed in OVX, vehicle-administered rats. Furthermore, rats administered the ERβ-selective ligand, PPT, or the ERα-selective ligand, DPN, had similar increases over that observed with vehicle-administration in the percentage of time spent exploring the novel object. We and others have demonstrated that cognitive enhancing effects may occur through actions at ERα and/or ERβ using specific ligands for these receptors or knockout mice (Fugger et al., 2000; Gibbs et al., 2004; Luine et al., 2003; Lund & Lephart, 2001; Rhodes & Frye, in press; Rissman et al., 2002). The present study utilized post-training administration of 17β-E2 in higher dosages than that observed to enhance object recognition in another study (Luine et al., 2003). It may be that higher dosages of these SERMs could eliminate the specificity of these ligands for ERα vs. ERβ and this would be an important distinction to determine in future studies. The present results that 17β-E2, DPN, or PPT enhance object recognition performance confirm that actions at ERα and/or ERβ may enhance cognitive performance.
The ability of progestins to modulate cognitive performance of female rodents was confirmed by the present study and others investigating the effects of progestins. In young rodents, evidence suggests that P4 administration can have beneficial effects on cognitive performance (Sandstrom & Williams, 2001; Sato et al., 2004; Vongher & Frye, 1999). Furthermore in situations associated with degeneration, P4 can enhance T-maze performance (Dohanich et al., 1994). In aged rats, with low endogenous progestin levels, the beneficial effects of P4 in various tasks are less clear owing to the fact that basal levels of P4 have not been addressed (as reviewed in Arnaiz et al., 2004; Bimonte-Nelson, Singleton, Williams, & Granholm, 2004; Blank, Nijholt, Kye, Radulovic, & Spiess, 2003; Kiray, Uysal, Sonmez, Acikgoz, & Gonenc, 2004; McEchron, Cheng, & Gilmartin, 2004; Warren & Juraska, 2000). It may be that beneficial effects of progestins to enhance cognitive performance depend on both ligand availability and/or activity of metabolism enzymes, both of which are different in the aging population (reviewed in, Chakraborty & Gore, 2004). Indeed, our results suggest that enhancing 3α, 5α-THP levels with SC administration of 3α, 5α-THP increases both P4 and 3α, 5α-THP levels in the hippocampus (Table 2). Although the P4 antibody used cross-reacts with 3α, 5α-THP and dihydroprogesterone (less than 4%) and has negligible binding to other pregnane and cortico-steroids, effects of other progestins as a source of increases in P4 cannot be entirely ruled out (Purdy et al., 1990). A possibility more likely than artifactual increases in P4 associated with radioimmunoassay is that P4 was increased in the hippocampus due to enhanced de novo synthesis. Indeed, it has been demonstrated that exposure to a novel open field for behavioral testing can increase de novo production of progestins (Frye & Bayon, 1999; Frye & Rhodes, 2006). Alternatively, increased P4 levels in the hippocampus following 3α, 5α-THP administration could be due to back-conversion of 3α, 5α-THP to its precursors. However, this is unlikely given that 3α, 5α-THP can be oxidized to dihydroprogesterone, but dihydroprogesterone does not typically get back-converted to P4 (as reviewed in Martini, Celotti, & Melcangi, 1996). Another point to consider in interpretation of these data is that progestins’ trophic effects may confer a benefit and underlie enhanced cognitive performance. Indeed, our laboratory and others have shown that progestins, in particular 3α, 5α-THP, can have neuroprotective/trophic effects in the hippocampus in several models of neurodegeneration (Ciriza, Azcoitia, & Garcia-Segura, 2004; Djebaili, Hoffman, & Stein, 2004, 2005; Frye & Rhodes, 2005; Frye & Scalise, 2000; Garcia-Estrada, Del Rio, Luquin, Soriano, & Garcia-Segura, 1993; He, Hoffman, & Stein, 2004a, He, Evans, Hoffman, Oyesiku, & Stein, 2004b; Rhodes & Frye, 2004b; Rhodes, McCormick, & Frye, 2004; Vongher & Frye, 1999). Together, the present data extend previous findings on the role of progestins to suggest that enhanced performance in the object recognition task may be related to levels of 3α, 5α-THP in the cortex and hippocampus.
One criticism of this study may be that having hormones present during testing may have contributed to other behavioral changes in rodents that masked the potential mnemonic effects of these compounds. We used a 4 h-inter-trial interval in this study. This testing procedure was utilized so that rats would be in the same estrous cycle phase when trained and tested in Experiment 1. When steroids were systemically administered to rats immediately after training, we found similar levels of steroids that were within a physiological range at testing time (4 h later) were produced concomitant with increased percentage of time spent exploring the novel object. We used this post-training steroid administration procedure to minimize potential effects of steroids interfering with training. We also investigated whether there was a critical time frame in which steroids had to be acting with the inclusion of Experiment 5. In this experiment, rats were administered vehicle or steroid hormone 1 h after training (i.e., after consolidation of memory occurs) and were tested 4 h after training. Although rats had similar levels of these steroids at testing in this experiment as in other experiments, performance in this task was not enhanced with a 1 h delay in post-training administration. Thus, these data suggest that ovarian steroids may have effects to enhance cognitive performance in this task that can be parsed out from their other effects during training.
There are other critical factors to consider when interpreting effects of estrogens and progestins for cognitive performance. Given that these effects have been investigated more thoroughly for estrogens (as reviewed in Korol, 2004), specific examples of each are discussed as follows, but similar factors likely influence effects of progestins for cognitive performance. These include the timing of E2 administration, E2 regimen, type of E2 administered, and factors related to the history of the individual. First, effects of E2 may be clearer when E2 is administered after training in a learning task, because this precludes effects of E2 interfering with training. E2 has effects on motor behavior (Joyce & Van Hartesveldt, 1984; Morgan & Pfaff, 2001, 2002), and these effects could alter training. Second, E2’s effects may also be concentration-dependent. There is a biphasic relationship between E2 and cognitive performance, such that beneficial effects are clearest when E2 levels are low and physiological (Frye & Rhodes, 2002; Gibbs, 1997; Gibbs, Burke, & Johnson, 1998; Luine et al., 1998; Wide, Hanratty, Ting, & Galea, 2004). Whereas, high levels and concentrations at nadir can be disruptive. Indeed, in the present study, higher cortical levels of E2 appear to be disruptive to performance among cycling females in this task, which may be related to these regimen-dependent effects of E2. Third, the type of E2 will also alter its effects. For example, conjugated equine estrogens that are typically used in hormone therapies for postmenopausal women are primarily composed of estrone, rather than the neuroactive E2, 17β-E2. We, and others, have demonstrated that various SERMs alter cognitive performance (Garey, Morgan, Frohlich, McEwen, & Pfaff, 2001; Gibbs et al., 2004; Luine et al., 2003; Lund & Lephart, 2001; Rhodes & Frye, in press). Specifically, as in the present study, both ERα and ERβ-specific SERMs have been shown to enhance performance in the object recognition task (Luine et al., 2003). Fourth, an individual’s history, such as environment and/or the duration of time spent in an E2-deficient state, may alter the response to subsequent E2 exposure. In one study, long-term (6+ months) environmental enrichment, compared to standard housing, attenuated the effects of E2 to enhance object memory, and impaired working memory in the water radial arm maze (Gresack & Frick, 2004). Length of time in an E2-deficient state before E2 is reintroduced may also alter response to exogenous E2. For instance, long-term treatment with E2 and/or P4 was only effective at enhancing acquisition in the delayed matching-to-position tasks, when initiated 3 months, but not 10 months, post-OVX (Gibbs, 2000). Thus, there are many factors that can contribute to the effects of ovarian steroids for cognitive performance.
Further characterization of ovarian steroids’ effects and putative mechanisms for cognitive performance are important for greater understanding of how estrogens and progestins may act in concert in brain areas, such as the PFC and hippocampus, to produce functional cognitive effects. For instance, our regression analyses suggest that the line of best fit explains only some of the unexplained error (i.e., 23% for 3α, 5α-THP in the cortex, 36% for E2 in the hippocampus, and 46% for 3α, 5α-THP in the hippocampus). These results suggest that there are other variables strongly affecting performance in the object recognition task. Notably, other steroids that alter cognitive performance, such as glucocorticoids and androgens, may have produced some of this variance (as reviewed in Rhodes & Frye, 2002). Future studies could investigate effects of glucocorticoids and/or androgens as well as the mechanisms of E2 and P4 for modulation of learning and memory. There is an extensive literature on the pharmacology of memory consolidation, which supports interplay between GABAergic, glutamatergic, noradrenergic, dopaminergic, and serotonergic systems and their downstream signal transduction pathways in the hippocampus (as reviewed in Izquierdo & Medina, 1997). Other studies using cognitive performance or sexual receptivity as behavioral assays have demonstrated the importance of ovarian steroids’ effects through these pathways, which may also underlie the effects observed in the present study. For instance, E2, which can be pro-excitatory, enhances NMDA receptor function for some of its cognitive performance effects (Bi, Foy, Vouimba, Thompson, & Baudry, 2001; El-Bakri et al., 2004; Gureviciene et al., 2003). P4’s effects to enhance sexual receptivity of female rodents requires its metabolism to 3α, 5α-THP in the ventral tegmental area and its subsequent actions at GABAA receptors (reviewed in Frye, 2001; Frye et al., 2006). Future studies could investigate these and downstream pathways that underlie the effects of ovarian steroids to enhance object recognition.
In summary, this study demonstrated that administration of 17β-E2 and/or P4, or 3α, 5α-THP to OVX rats produced similar results to enhance performance in the object recognition task and increase levels of these steroids in serum, the cortex, or hippocampus as was observed in behavioral estrous rats. Further, administration of ERα- or β-specific ligands similarly enhanced performance, suggesting involvement of these receptors for these effects. Lastly, similar behavioral responses of rats administered vehicle, 17β-E2, P4, or 17β-E2 and P41 h after training suggest that these ovarian steroids need to be present during memory consolidation for their beneficial effects for object recognition to occur. Thus, these data suggest that ovarian steroids may have actions in the cortex and/or hippocampus to enhance object recognition memory.
Acknowledgments
This research was supported by the National Science Foundation (IBN03-16083) and National Institute of Mental Health (MH0676980). The technical assistance provided by Alicia Babson, Kassandra Edinger, Bomi Lee, and Jason Paris is greatly appreciated.
References
- Arnaiz SL, D’Amico G, Paglia N, Arismendi M, Basso N, del Rosario Lores Arnaiz M. Enriched environment, nitric oxide production and synaptic plasticity prevent the aging-dependent impairment of spatial cognition. Molecular Aspects of Medicine. 2004;25:91–101. doi: 10.1016/j.mam.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proceedings of the National Academy of Science. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behavioral Neuroscience. 2004;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J. Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nature Neuroscience. 2003;6:911–912. doi: 10.1038/nn1101. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Experimental biology and medicine. 2004;229:977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3, 20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Hormones and Behavior. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. Journal of Neuroendocrinology. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of Learning and Memory. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiology and Behavior. 1991;50:61–65. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. Journal of Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behavioral Neuroscience. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, et al. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: A relationship to Morris water maze performance. Journal of cellular and molecular medicine. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA. Induction of estrus in female mice: Estrogen-progesterone interactions. Hormones and Behavior. 1970;1:299–304. [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intra-hippocampally. Neurobiology of Learning and Memory. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiology and Behavior. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain research Brain research reviews. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α, 5α-THP and 3α-Diol. Journal of Neuroendocrinology. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duncan JE. Estradiol benzoate potentiates neuroactive steroids’ effects on pain sensitivity. Pharmacology, Biochemistry and Behavior. 1996;53:27–32. doi: 10.1016/0091-3057(95)00194-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 2001;28:550–563. [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intra-cellular estrogen receptors in the hippocampus. Brain Research. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Estrogen-priming can enhance progesterone’s anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacology, Biochemistry, and Behavior. 2005;81:907–916. doi: 10.1016/j.pbb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Exploratory, anti-anxiety, and pro-social behaviors of female rats are mediated, in part, by progestins in the ventral tegmental area. Behavioral Neuroscience. 2006 (submitted) [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Research. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α, 5α-THP in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2005.06.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ. Anti-seizure effects of progesterone and 3α, 5α-THP in kainic acid and perforant pathway models of epilepsy. Psychoneuroendocrinology. 2000;25:407–420. doi: 10.1016/s0306-4530(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiology of Learning and Memory. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Research. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP, Hampson E. Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Hormones and Behavior. 1995;29:106–125. doi: 10.1006/hbeh.1995.1008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioural Brain Research. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Galea LA, Lee TT, Kostaras X, Sidhu JA, Barr AM. High levels of estradiol impair spatial performance in the Morris water maze and increase ‘depressive-like’ behaviors in the female meadow vole. Physiology and Behavior. 2002;77:217–225. doi: 10.1016/s0031-9384(02)00849-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Research. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- Garey J, Morgan MA, Frohlich J, McEwen BS, Pfaff DW. Effects of the phytoestrogen coumestrol on locomotor and fear-related behaviors in female mice. Hormones and Behavior. 2001;40:65–76. doi: 10.1006/hbeh.2001.1660. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Research. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones and Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Burke AM, Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Hormones and Behavior. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behavioral Neuroscience. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureviciene I, Puolivali J, Pussinen R, Wang J, Tanila H, Ylinen A. Estrogen treatment alleviates NMDA-antagonist induced hippocampal LTP blockade and cognitive deficits in ovariectomized mice. Neurobiology of Learning and Memory. 2003;79:72–80. doi: 10.1016/s1074-7427(02)00012-6. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restorative neurology and neuroscience. 2004a;22:19–31. [PubMed] [Google Scholar]
- He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Experimental Neurology. 2004b;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Experimental Gerontology. 2004;39:1277–1283. doi: 10.1016/j.exger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behavioral Neuroscience. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiology of Learning and Memory. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Van Hartesveldt C. Estradiol application to one striatum produces postural deviation to systemic apomorphine. Pharmacology, Biochemistry, and Behavior. 1984;20:575–581. doi: 10.1016/0091-3057(84)90307-1. [DOI] [PubMed] [Google Scholar]
- Kiray M, Uysal N, Sonmez A, Acikgoz O, Gonenc S. Positive effects of deprenyl and estradiol on spatial memory and oxidant stress in aged female rat brains. Neuroscience Letters. 2004;354:225–228. doi: 10.1016/j.neulet.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of Learning and Memory. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Long JA, Evans HM. Oestrus cycle in the rat and its associated phenomena. Memoirs of the University of California. 1922;6:1–22. [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neuroscience. 2001;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz LK. Sex differences in adrenocortical structure and function. III. The effects of postpubertal gonadectomy and gonadal hormone replacement on adrenal cholesterol sidechain cleavage activity and on steroids biosynthesis by rat adrenal homogenates. Endokrinologie. 1976;67:26–35. [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiology of Learning and Memory. 2003;80:315–322. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Martini L, Celotti F, Melcangi RC. Testosterone and progesterone metabolism in the central nervous system: cellular localization and mechanism of control of the enzymes involved. Cellular Molecular Neurobiology. 1996;16:271–282. doi: 10.1007/BF02088095. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Cheng AY, Gilmartin MR. Trace fear conditioning is reduced in the aging rat. Neurobiology of Learning and Memory. 2004;82:71–76. doi: 10.1016/j.nlm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. Journal of medicinal chemistry. 2001;44:4230–4235. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Hormones and Behavior. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behavioural Brain Research. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience and Biobehavioral Reviews. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Hormones and Behavior. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, et al. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Resko JA, Stadelman HL, Handa RJ. Control of 5α-reduction of testosterone in neuroendocrine tissues of female rats. Biology of Reproduction. 1986;34:870–877. doi: 10.1095/biolreprod34.5.870. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Hormones influence cognitive performance. In: Flint R, editor. Forget it? -Sources, Theories, and Mechanisms of Alterations in Mnemonic Function. North Chelmsford, MA: Erudition Books; 2002. pp. 175–196. [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic enhancing effects in the inhibitory avoidance task. Pharmacology, Biochemistry, and Behavior. 2004a;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia. 2004b;45:1531–1538. doi: 10.1111/j.0013-9580.2004.16504.x. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiology of Learning and Memory. doi: 10.1016/j.nlm.2005.10.003. (in press) [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3α, 5α-THP mediates progestins’ effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacology, Biochemistry, and Behavior. 2004;78:505–512. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proceedings of the National Academy of Sciences. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Hormones and Behavior. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behavioral Brain Research. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiology of Learning and Memory. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, et al. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. Journal of medicinal chemistry. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, et al. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiology of Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Vazquez-Pereyra F, Rivas-Arancibia S, Loaeza-Del Castillo A, Schneider-Rivas S. Modulation of short term and long term memory by steroid sexual hormones. Life Science. 1995;56:PL255–PL260. doi: 10.1016/0024-3205(95)00067-g. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacology, Biochemistry, and Behavior. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiology of learning and Memory. 2000;74:229–240. doi: 10.1006/nlme.1999.3948. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behavioral Neuroscience. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behavioral Brain Research. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]







