Abstract
OBJECTIVES
To assess the association between angiotensin converting enzyme inhibitors (ACEis) and improvements in the physical function of older adults in response to chronic exercise training.
DESIGN
Secondary analysis of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study, a multisite randomized clinical trial to evaluate the effects of chronic exercise on the physical function of older adults at risk for mobility disability.
SETTING
Four academic research centers within the United States.
PARTICIPANTS
Four hundred twenty-four individuals aged 70 to 89 with mild to moderate functional impairments categorized for this analysis as ACEi users, users of other antihypertensive drugs, or antihypertensive nonusers.
INTERVENTION
A 12-month intervention of structured physical activity (PA) or health education promoting successful aging (SA).
MEASUREMENTS
Change in walking speed during a 400-m test and performance on a battery of short-duration mobility tasks (Short Physical Performance Battery (SPPB)).
RESULTS
Physical activity significantly improved the adjusted walking speed of ACEi users (P < .001) but did not of nonusers. PA improved the adjusted SPPB score of ACEi users (P < .001) and of persons who used other antihypertensive drugs (P = .005) but not of antihypertensive nonusers (P = .91). The percentage of ACEi users deriving clinically significant benefit from exercise training for walking speed (30%) and SPPB score (48%) was dramatically higher than for nonusers (14% and 12%, respectively).
CONCLUSION
For older adults at risk for disability, exercise-derived improvements in physical function were greater for ACEi users than users of other antihypertensive drugs and antihypertensive nonusers.
Keywords: aging, exercise, physical function, LIFE Study, ACE inhibitors
As the number of older men and women continues to rise worldwide, the maintenance of their physical independence is an important public health challenge. The inability of older adults to perform basic physical tasks marks a serious decline in functional health, conferring increased risk of hospitalization1 and death.2 In recent years, investigators have suggested that use of angiotensin-converting enzyme inhibitors (ACEis) may attenuate declines in physical function in older adults.3,4 Epidemiological studies have suggested that use of ACEis can slow functional decline in older adults,5,6 possibly by increasing muscle mass and strength,6,7 but the results of subsequent randomized controlled trials (RCTs) have been mixed, with studies reporting that ACEis do8,9 and do not10,11 improve physical performance. Although it remains possible that the efficacy of ACEis as a therapy for physical function may vary according to drug and patient population, recent evidence suggests that the greatest benefit of ACEis may be observed when combined with regular exercise.
Others have proposed that exercise may be used to stimulate certain adaptations to pharmaceuticals that may not be observed in response to the drug alone.12 For example, an oral peroxisome proliferator-activated receptor delta agonist was shown to improve exercise tolerance in mice only when combined with exercise.13 Similarly, it was recently reported that the addition of ACEis potentiated exercise-mediated improvements in physical performance in older rats.14,15 Both of these studies reported that the addition of ACEis potentiated the beneficial effects of aerobic training on exercise tolerance determined according to treadmill endurance. These preclinical findings add to an extensive literature from genetic studies of humans indicating an important interaction between the renin–angiotensin system and physical performance.16–21 Although the mechanism of the potential interaction is not fully understood, other animal studies suggest that local skeletal muscle changes may modulate improvements in exercise tolerance resulting from the combination of exercise and ACEi usage because the addition of perindopril to treadmill running results in significantly greater increases in muscle capillary density and the proportion of type I fibers in older rats.22,23 Collectively, these studies suggest that ACEis may have the most potential for preserving the physical capabilities of older adults when combined with regular exercise, but no study has examined the effects of ACEi usage on the functional adaptations of older adults in response to chronic exercise training.
The present study is a secondary analysis of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. LIFE-P was a multisite clinical trial that compared the effects of a 1-year structured physical activity (PA) intervention with a successful aging (SA) health education program in older adults at risk for physical disability. Two of the primary outcomes of the trial were walking speed during a 400-m test and performance on the Short Physical Performance Battery (SPPB), a battery of short-duration mobility tasks validated for use in older adults.24 The present analysis examined the effects of the interventions on SPPB score and walking speed in 424 participants in LIFE-P who did not use antihypertensive medications, used antihypertensive medications including ACEis, and used antihypertensive medications other than ACEis.
METHODS
Participant Recruitment
Details about specific study inclusion and exclusion criteria of LIFE-P have been reported previously.25,26 Briefly, individuals were eligible for the study if they were aged 70 to 89, were sedentary (<20 min/wk of structured exercise in prior month), had a SPPB score of 9 or less, and were able to walk 400 m within 15 minutes. Four hundred twenty-four participants were randomized into the PA and SA study arms at four sites (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University) and followed for at least 12 months. All participants provided written informed consent, and the institutional review boards of all participating institutions approved the study protocol.
Medical Screening
To ensure the safety of the exercise program for potential participants, a thorough medical screening was performed.27 The screening procedure included the completion of a medical history questionnaire, an electrocardiogram, a physical examination performed by a study physician, and completion of the Community Healthy Activities Model Program for Seniors physical activity questionnaire.28 Participants were also asked to bring all prescription and nonprescription medications they had taken in the previous 2 weeks to this screening visit. Drug names and whether the medication was prescribed were recorded. Medications were later coded to reflect their function (e.g., antihypertensive) and drug class (e.g., ACEi, diuretic, beta-blocker).
Successful Aging Intervention
The SA intervention (serving as an attention control group) was designed to provide health education and maintain contact with participants. Those randomly assigned to SA attended group presentations weekly for the first 26 weeks and then monthly until the end of the trial. Presentations were given on health topics relevant to older adults, such as nutrition, medication use, foot care, and preventive medicine. All SA participants received basic information about physical activity participation, and each class concluded with upper extremity stretching. Regular telephone contact was made to encourage participation.
Physical Activity Intervention
Participants randomized to the PA intervention performed walking, strength, flexibility, and balance training in center- and home-based settings. In the adoption phase (Weeks 1–8), three supervised center-based physical activity sessions per week were conducted. These sessions were 40 to 60 minutes in length and were used to initiate the walking program and introduce participants to the strength, stretching, and balance exercises in a safe and effective manner. The strength exercises included standing chair squats, toe stands, leg curl, knee extensions, and side hip raises with ankle weights. The balance exercises involved a series of dual- and single-leg standing movements.
The intensity of training was gradually increased over the first 2 to 3 weeks. Moderate-intensity exercise was promoted and assessed using the Borg scale,29 a numerical scale indicating effort from minimal6 to maximal. 20 Participants were asked to walk at a target intensity of 13 (somewhat hard) and perform strength training at an intensity of 15 to 16 (hard). In the transition phase (Weeks 9–24), the number of center-based sessions was reduced to two times per week, and home-based activities were increased. In the maintenance phase (Week 25 to the end), participants were encouraged to perform home-based physical activity a minimum of 5 days per week, and one weekly center-based session was offered. The maintenance phase was continued until the final closeout assessment visits. Staff members monitored the volume and intensity of exercise throughout the study by recording the completed walking time and overall rating of perceived exertion (RPE) for each training session.
Measures of Physical Function
The primary measures of physical function for this analysis were walking speed during a 400-m test (long-distance walking speed) and score on the SPPB. During the 400-m test, participants were asked to walk 10 laps of a 20-m course (1 lap = 40m) at their usual pace. Participants were allowed to stop and rest if necessary but without sitting. If a participant did not complete the course, the time and distance completed were recorded, and walking speed was determined accordingly. The SPPB is a well-validated battery of tasks designed to examine lower-extremity function in older adults. The test is based on timed measures of standing balance, short-duration walking (4 m), and ability to rise from a chair. Each task was scored on a scale from 0 to 4 depending on the ability and time needed to complete each task. A summary score (range 0–12) was subsequently calculated by summing the three scores.24
Statistical Analysis
For this analysis, participants were categorized according to antihypertensive medication status as nonusers of antihypertensive medications, users of ACEis, or users of other antihypertensive medications. If an individual used ACEi and another antihypertensive medication, they were considered to be an ACEi user. Separate analyses indicated that the use of angiotensin receptor blockers (ARBs) was not associated with differential effects of exercise, so these medications were not segregated for the present analysis. Sensitivity analyses were also performed to determine the estimated effect of the intervention on potentially confounding demographic factors and indications.
An a priori alpha level of 0.05 was established to determine statistical significance. Baseline characteristics according to medication stratum were compared using analysis of variance for continuous variables and chi-square tests for discrete variables. When continuous values were nonnormally distributed, the Kruskal–Wallis test was used for comparisons. Medians (25th and 75th percentiles) were reported for these comparisons. Post hoc tests were performed as appropriate to demonstrate pairwise comparisons between strata. Repeated-measures analysis of covariance (ANCOVA) was used to investigate differences in long-distance walking speed and SPPB scores. To adjust for potentially confounding factors, covariates included in the model were age, sex, study site, body mass index, self-reported health status, baseline outcome measure, and a comorbidity index. The comorbidity index was calculated as a composite score based on the presence of 10 prevalent comorbidities: hypertension, heart attack, heart failure, stroke, cancer, diabetes mellitus, broken hip, arthritis, liver disease, and lung disease. Intervention assignment, visit, medication status, and all interactions between visit, medication, and intervention assignment were checked. Only interaction between intervention assignment and visit was adjusted in the model. If the interaction between medication groups and intervention assignment was significant or showed a trend toward significance, the analysis was stratified according to medication group. For each medication stratum, hypothesis tests for intervention effects at the 6- and 12-month assessment visits were performed using contrasts of the 6- and 12-month intervention means in the ANCOVA model. Overall comparisons between groups across follow-up visits were obtained using a contrast to compare effects across both follow-up visits
To examine the clinical significance of ACEi use, the proportion of participants who obtained clinically significant benefits at 12 months was computed for ACEi users and all nonusers (nonusers included antihypertensive nonusers and users of other antihypertensive medications) for long-distance walking speed and SPPB score. Participants were coded as displaying clinically significant improvement, no clinically significant change, or clinically significant decline in each measure based on previously established cut points (SPPB, ±1 point; walking speed, ±0.05 m/s).30,31 For each measure, the number needed to treat (NNT)—an important measure of clinical meaningfulness 32,33—was then determined for improvement, as well as prevention of decline. The NNT was calculated using the formula [1/(proportion of PA benefiting) − (proportion of SA benefiting)]. Finally, the proportion of ACEi users and all nonusers with major mobility disability—operationally defined as inability to complete the 400-m walk—was calculated. Because the number of failures was small, walk failures from the 6- and 12-month follow-up visits were combined for this analysis.
RESULTS
The mean age of the 424 participants was 76.8 ± 4.2; 68.9% were female, 25.5% were racial or ethnic minorities, and 41.7% had a SPPB score of 7 or less. Participant demographic characteristics are listed in Table 1 according to medication status. Antihypertensive nonusers had lower body mass index, better self-reported health, and fewer health conditions than those who used antihypertensive medications, but the PA and SA groups were largely homogenous within each medication stratum, with the exception of diabetes mellitus prevalence and diastolic blood pressure in ACEi users (Table 2).
Table 1.
Baseline Characteristics of Lifestyle Interventions and Independence for Elders Pilot Participants According to Hypertension Medication Status
| Characteristic | P-value | ||||||
|---|---|---|---|---|---|---|---|
| AHT− (n = 107) | ACEi+ (n = 123) | ACEi− (n = 194) | Overall | AHT− vs ACEi+ | ACEi+ vs ACEi− | AHT− vs ACEi− | |
| Age, mean ± SD | 76.3 ± 4.1 | 75.9 ± 3.9 | 77.6 ± 4.3 | <.001 | .40 | <.001 | .009 |
| Body mass index, kg/m2, mean ± SD | 28.3 ± 6.2 | 31.4 ± 5.6 | 30.5 ± 6.0 | <.001 | <.001 | .18 | .002 |
| Systolic blood pressure, mmHg, mean ± SD | 130.5 ± 18.1 | 130.0 ± 16.2 | 134.2 ± 18.0 | .07 | .76 | .07 | .19 |
| Diastolic blood pressure, mmHg, mean ± SD | 71.0 ± 10.9 | 69.0 ± 9.0 | 69.1 ± 11.3 | .26 | .15 | .77 | .12 |
| Moderate-intensity physical activity, min/wk, median (25th–75th percentile) | 117 (0–135) | 113 (0–210) | 119 (0–135) | .48 | .22 | .64 | .41 |
| Female, n (%) | 81 (75.7) | 69 (56.1) | 142 (73.2) | .001 | .002 | .002 | .63 |
| Minority (nonwhite), n (%) | 23 (21.5) | 34 (27.6) | 52 (26.8) | .51 | .23 | .87 | .24 |
| Fair to poor health self-rated, n (%) | 12 (11.2) | 30 (24.4) | 44 (51.2) | .02 | .01 | .73 | .01 |
| Number of health conditions, mean ± SD | 0.9 ± 1.0 | 2.1 ± 1.0 | 2.1 ± 1.2 | <.001 | <.001 | .38 | <.001 |
| Prevalent health conditions, n (%) | |||||||
| Myocardial infarction | 2 (1.9) | 13 (10.6) | 24 (12.4) | .009 | .004 | .55 | .001 |
| Congestive heart failure | 0 (0) | 10 (8.1) | 14 (7.2) | .01 | .002 | .82 | .004 |
| Lung disease | 16 (15.0) | 16 (13.0) | 26 (13.4) | .90 | .85 | .82 | .82 |
| Cancer | 14 (13.1) | 25 (20.3) | 35 (18.0) | .34 | .24 | .84 | .41 |
| Diabetes mellitus | 10 (9.3) | 40 (32.5) | 42 (21.6) | <.001 | <.001 | .02 | .006 |
| Stroke | 2 (1.9) | 8 (6.5) | 10 (5.2) | .24 | .14 | .82 | .27 |
| Osteoarthritis | 21 (19.6) | 17 (13.8) | 55 (28.4) | .008 | .23 | .004 | .13 |
AHT− = not taking antihypertensive medications; ACEi+ = taking angiotensin-converting enzyme inhibitors (ACEis); ACEi− = taking antihypertensive medications except ACEis; SD = standard deviation.
Table 2.
Baseline Characteristics of Participants According to Medication Status and Randomized Group
| Characteristic | AHT− (n = 107)
|
ACEi+ (n = 123)
|
ACEi− (n = 194)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PA (n = 48) | SA (n = 59) | P-value | PA (n = 67) | SA (n = 56) | P-value | PA (n = 98) | SA (n = 96) | P-value | |
| Age, mean ± SD | 76.5 ± 4.6 | 76.1 ± 3.7 | .63 | 75.3 ± 3.8 | 76.5 ± 4.1 | .10 | 77.4 ± 4.0 | 77.9 ± 4.7 | .42 |
|
| |||||||||
| Body mass index, kg/m2, mean±SD | 28.3 ± 6.2 | 28.3 ± 6.2 | .98 | 31.9 ± 5.1 | 30.9 ± 6.2 | .34 | 31.1 ± 6.7 | 30.0 ± 5.1 | .18 |
|
| |||||||||
| Systolic blood pressure, mmHg, mean ± SD | 134.2 ± 18.9 | 127.6 ± 17.0 | .06 | 129.7 ± 17.1 | 130.5 ± 15.2 | .80 | 132.4 ± 16.6 | 136.0 ± 19.2 | .16 |
|
| |||||||||
| Diastolic blood pressure, mmHg, mean ± SD | 71.2 ± 12.6 | 70.9 ± 9.4 | .88 | 67.2 ± 7.9 | 71.2 ± 9.8 | .01 | 69.6 ± 11.1 | 68.6 ± 11.5 | .53 |
|
| |||||||||
| Moderate-intensity physical activity, min/wk, median (25th–75th percentile) | 113 (0–105) | 119 (0–135) | .27 | 113 (0–210) | 113 (0–176) | .91 | 124 (0–176) | 114 (0–105) | .76 |
|
| |||||||||
| Female, n (%) | 36 (75.0) | 45 (76.3) | .88 | 39 (58.2) | 30 (53.6) | .61 | 71 (72.4) | 71 (74.0) | .81 |
|
| |||||||||
| Minority (nonwhite). n (%) | 9 (18.8) | 14 (23.7) | .53 | 18 (26.9) | 16 (28.6) | .83 | 26 (26.5) | 26 (27.1) | .93 |
|
| |||||||||
| Fair-to-poor health self-rated, n (%) | 6 (12.5) | 6 (10.1) | .70 | 13 (19.4) | 17 (30.4) | .16 | 20 (20.4) | 24 (25) | .44 |
|
| |||||||||
| Number of health conditions, mean ± SD | 1.0 ± 1.0 | 0.8 ± 0.9 | .21 | 2.2 ± 1.1 | 2.1 ± 1.0 | .71 | 2.1 ± 1.3 | 2.1 ± 1.1 | .73 |
|
| |||||||||
| Prevalent health conditions, n (%) | |||||||||
|
| |||||||||
| Myocardial infarction | 2 (4.2) | 0 (0) | .11 | 6 (9.0) | 7 (12.5) | .52 | 16 (16.3) | 8 (8.3) | .09 |
|
| |||||||||
| Congestive heart failure | 0 (0) | 0 (0) | — | 3 (4.5) | 7 (12.5) | .10 | 8 (8.2) | 6 (6.3) | .61 |
|
| |||||||||
| Lung disease | 9 (18.8) | 7 (11.9) | .32 | 7 (10.4) | 9 (16.1) | .36 | 13 (13.3) | 13 (13.5) | .95 |
|
| |||||||||
| Cancer | 7 (14.6) | 7 (11.9) | .68 | 15 (22.4) | 10 (17.9) | .53 | 16 (16.3) | 19 (19.8) | .53 |
|
| |||||||||
| Diabetes mellitus | 7 (14.6) | 3 (5.1) | .09 | 27 (40.3) | 13 (23.2) | .04 | 24 (24.5) | 18 (18.8) | .33 |
|
| |||||||||
| Stroke | 0 (0) | 2 (3.4) | .20 | 4 (6.0) | 4 (7.1) | .79 | 4 (4.1) | 6 (6.3) | .49 |
|
| |||||||||
| Osteoarthritis | 11 (22.9) | 10 (16.9) | .44 | 9 (13.4) | 8 (14.3) | .89 | 30 (30.6) | 25 (26.0) | .48 |
PA = physical activity; SA = successful aging; AHT− = not taking antihypertensive medications; ACEi+ = taking angiotensin-converting enzyme inhibitors (ACEis); ACEi− = taking antihypertensive medications except ACEis; SD = standard deviation.
Seventy-five percent of study participants were taking at least one antihypertensive drug. Of the antihypertensive medication users (n = 317), 39% (30% of the total sample) were using ACEis. Study participants used eight different ACEi, with the most commonly used drugs being lisinopril (n = 62), benazepril (n = 17), and enalapril (n = 15). Of those who did not use antihypertensive medications (n = 107), approximately one-third (n = 35) had systolic blood pressure greater than 140 mmHg or diastolic pressure greater than 90 mmHg at baseline.
Attendance to (P = .73) and volume of exercise performed during (P = .26) the PA intervention sessions did not differ between medication strata, nor did median RPE of the sessions (P = .19). Over the course of the study, median session attendance was 72% (interquartile range (IQR) 45–84) for ACEi users, 68% (IQR 49–82) for users of other antihypertensive medications, and 70% (IQR 50–78) for antihypertensive nonusers. The median center-based walk time recorded by ACEi users over the course of the study was 1,885 minutes (IQR 1,144–2,708), slightly higher than that of antihypertensive nonusers (1,613; IQR 967–2,265 min) and users of other antihypertensive medications (1,660; IQR 914–2,457). ACEi users reported a median RPE of 12.2 (IQR 11.6–12.8), compared with 12.0 (IQR 11.4–12.5) for antihypertensive nonusers and 12.5 (IQR 11.8–13.1) for users of other antihypertensive medications.
For SPPB performance, a significant intervention according to medication interaction was observed (P = .02). The PA intervention did not improve SPPB performance in those who did not use antihypertensive medications any more than the SA intervention did (Figure 1A). The adjusted difference between PA and SA interventions for nonusers was 0.15 points (P = .63) at 6 months and −0.08 points at 12 months (P = .83). In contrast, ACEi users displayed dramatic improvements in SPPB score in response to PA (Figure 1B). ACEi users in the PA intervention improved by an adjusted 1.35 more points at 6 months (P < .001) and 0.95 more points at 12 months (P = .003) than those in the SA intervention. Likewise, users of other antihypertensive medications displayed improvements in SPPB in response to the PA intervention, although these improvements were more modest (Figure 1C). The adjusted differences for this strata were 0.68 points at 6 months (P = .008) and 0.77 points at 12 months (P = .01).
Figure 1.
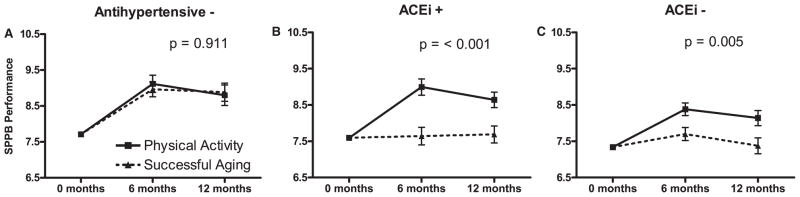
Performance on the Short Physical Performance Battery (SPPB) of participants who were (A) not taking antihypertensive medications (AHT−), (B) taking angiotensin-converting enzyme inhibitors (ACEi+), and (C) taking antihypertensive medications excluding ACEis (ACEi−). The data are also stratified according to physical activity (PA) and successful aging (SA) intervention groups. Values are predicted from analysis of covariance models adjusted for age, sex, study site, body mass index, self-reported health status, a comorbidity index, baseline SPPB score, and intervention-by-visit interaction. Bars represent standard errors of the estimated least squares means, and P-values reflect significance of the mean intervention effect across both follow-up visits within each medication stratum.
For long distance walking speed, the medication-by-intervention interaction did not reach statistical significance (P = .18), nor did the medication-by-intervention-by-visit interaction (P = .09). In the stratified analysis, a significant effect across all study visits was observed only for ACEi users. For participants who did not use antihypertensive medications, the adjusted difference between the PA and SA interventions was 0.01 m/s at 6 months (P = .58) and 0.05 m/s (P = .048) at 12 months (Figure 2A). For ACEi users, the adjusted difference between the PA and SA groups was 0.07 m/s at 6 months (P < .001) and 0.06 m/s (P = .009) at 12 months (Figure 2B). For users of other antihypertensive medications, the adjusted difference between PA and SA groups was 0.03 m/s at 6 months (P = .03) and 0.02 m/s at 12 months (P = .23) (Figure 2C). Sensitivity analyses for each outcome indicated that included covariates did not appear to be responsible for the effects of exercise in ACEi users (Figure S1, supporting information). The results for long-distance walking speed were similar to those of short-distance (4 m) walking speed measured during the SPPB. For short-duration walking speed, exercise significantly improved walking speed in ACEi users (P = .049) but not users of other antihypertensive medications (P = .86) or antihypertensive nonusers (P = .16).
Figure 2.
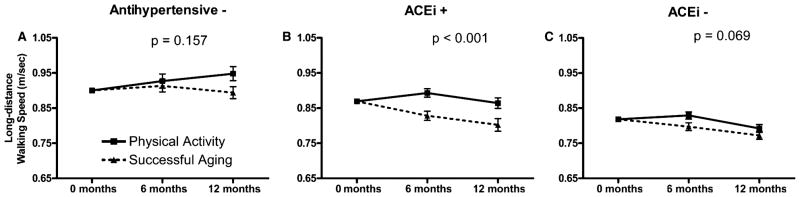
Walking speed during a 400-m test of participants who were (A) not taking antihypertensive medications (AHT−), (B) taking angiotensin-converting enzyme inhibitors (ACEi+), and (C) taking antihypertensive medications excluding ACEis (ACEi−). The data are also stratified according to physical activity (PA) and successful aging (SA) intervention groups. Values are predicted from analysis of covariance models adjusted for age, sex, study site, body mass index, self-reported health status, a comorbidity index, baseline walking speed, and intervention-by-visit interaction. Bars represent standard errors of the estimated least squares means, and P-values reflect significance of the mean intervention effect across both follow-up visits within each medication strata.
With respect to promoting a clinically significant improvement in SPPB performance, the percentage of ACEi users who benefited from PA at 12 months (percentage of PA participants − percentage of SA participants who experienced clinically significant improvements) was much higher in ACEi users than nonusers (Figure 3A). As a result of these differences, the NNT to achieve a clinically significant improvement in one participant was 4.3 for ACEi users and 14.7 for nonusers. The disparity between users and nonusers was even more dramatic for preventing decline (Figure 3B). The NNT to prevent one clinically significant decline was 4.0 for ACEi users and 20.8 for nonusers. Similar to SPPB, the proportion of ACEi users making clinically significant improvements in 400-m walking speed was significantly higher than of nonusers (Figure 3A), resulting in a NNT of 5.6 for ACEi users and 8.1 for nonusers. The disparity between ACEi users and nonusers was consistent for preventing decline in 400-m walking speed (Figure 3B), with a NNT of 8.3 for ACEi users and 52.6 for nonusers. The results were similar for the frequency of 400-m walk failures. Essentially no difference existed between interventions for ACEi nonusers. Of nonusers, 10.3% of participants in PA failed a walk during the study, compared with 11% of those in the SA intervention (P = .86). In contrast, ACEi users in the PA group had a lower rate of walk failure (9.2%) than those in the SA group (21.4%), although the comparison narrowly missed reaching statistical significance (P = .06).
Figure 3.
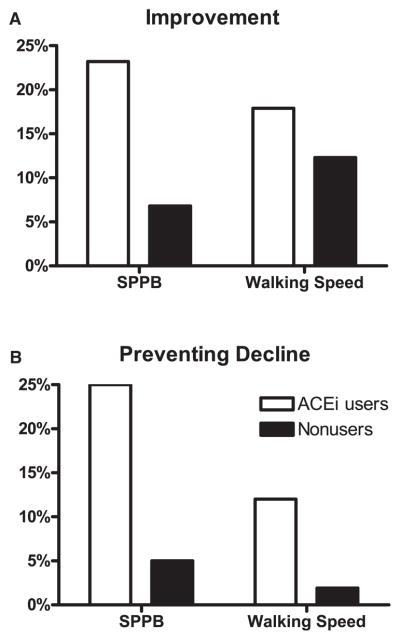
Percentage of study participants receiving clinically significant benefits in Short Physical Performance Battery (SPPB) score and 400-m walk speed from 12 months of exercise. (A) Clinically significant improvement and (B) prevention of clinically significant decline expressed as percentage of participants in the PA intervention receiving the benefit minus the percentage in the SA intervention. Data are dichotomized by for angiotensin-converting inhibitor (ACEi) users and all persons not using ACEis. The chosen clinically significant changes were 1 point for SPPB performance and 0.05 m/s for 400-m walking speed, as established in the literature.30,31
DISCUSSION
Angiotensin converting enzyme inhibitors are widely used in older adults for the treatment of hypertension. In recent years, investigators have also ascribed other potential benefits to ACEis, including improvement in physical function.3,4 The present study is the first to report that physically limited older men and women using ACEis derived more functional benefit from a 12-month exercise intervention than nonusers. Persons taking ACEis were more responsive to exercise than nonusers with respect to performance on short-duration mobility tasks and changes in 400-m walk speed.
Previously, epidemiological evidence from the Women’s Health and Aging Study and the Systematic Assessment of Geriatric drug use via Epidemiology Study reported that ACEi use was associated with attenuated declines in muscle strength, walking speed, and overall physical function according to self-reported activities of daily living.5,6 Despite these promising findings, results of subsequent RCTs were mixed regarding the effects of ACEis on physical performance.8–11 Although several factors may be responsible for explaining the disparate results of these trials, it may be that the results of these RCTs were limited because participants were in an untrained state. Taking the data from these trials in the context of the present findings related to the effectiveness of exercise, it may be that ACEi usage lays a foundation of improved exercise tolerance on which chronic training builds. It has previously been reported that, in older rats, combining exercise with pharmacological ACE inhibition improved exercise tolerance significantly more than exercise alone.14,15 These findings are consistent with genetic findings indicating that the insertion (I) of a 287 bp fragment in the ACE gene locus, resulting in low ACE circulating activity, is associated with enhanced cardiovascular performance.21
Local morphological changes within the activated skeletal muscle may modulate improvements in exercise tolerance resulting from the combination of exercise and ACEi usage. Previous studies have reported that the addition of perindopril to treadmill running results in significantly greater increases in muscle capillary density and the proportion of type I fibers in older rats.22,23 Low ACE activity has also been associated with greater insulin sensitivity after training.34 These adaptations stimulate oxygen delivery and metabolic exchange of the muscle, allowing the activated muscle to respond better to the increased energy demand of exercise. These changes could allow for an increased workload during each training session, resulting in improved adaptation over time. Although not statistically significant, ACEi users logged the most walking time in the present study—even more than the healthier group of participants not taking antihypertensive medications.
Meanwhile, ACEi users in the PA intervention also displayed the greatest improvement in performance on the SPPB—a battery of short-duration tasks. Although the 400-m walk and SPPB are each important tests of function in older adults, each test captures a different aspect of physical function. The 400-m walk involves cardiovascular function, whereas the SPPB more directly captures abilities such as muscle strength and balance. The present findings support those of a previous study that found that continuous ACEi usage in older women was associated with attenuated declines in muscle strength and 4-m walk speed.6 Because the PA intervention included resistance exercises, it is possible that greater aerobic capacity allowed for enhanced performance of these resistance exercises during each training session, although this interpretation is only speculative. In addition, the SPPB improvement was marked in the PA group in antihypertensive nonusers, although the exercise intervention did not improve SPPB score beyond that seen in the SA group, meaning that the overall efficacy of the exercise intervention was minimal in these individuals. Because the individuals in this stratum largely represent the healthiest segment of the study population, it is possible that an educational intervention is sufficient for maintaining function in these individuals, although it remains to be seen whether adjuvant therapy may enhance the benefits of exercise in these individuals.
These findings are in contrast to genetic studies that—although not entirely consistent—have largely reported that low ACE activity is associated with low muscle strength.20,35,36 A recent study reported that older adults who were homozygous for the I allele (I/I) displayed lesser gains in knee extensor strength in response to training than those with the deletion (D)/D genotype.17 Another study previously reported that physically active older adults with the I/I genotype had a greater risk of incident mobility limitation than those with the I/D and D/D genotypes.16 The cause of this apparent discrepancy between the literature regarding pharmacological ACE inhibition and genetically low ACE activity remains to be determined. One possible explanation is that ACEis have effects that extend beyond regulating the activities of ACE and bradykinin.37 Future studies may also investigate whether an interaction exists between ACE genotype and ACEi usage with regard to functional adaptations to exercise.
The primary strengths of this study are a clinically relevant population, multisite design, large sample size, and well-tracked exercise participation over a relatively long period, although the results of this study should not be overinterpreted because the findings are based on a secondary analysis, and participants were not randomly assigned to medication usage.38 The analyses were adjusted for several potentially confounding factors, and sensitivity analyses suggested that it is unlikely these factors caused the observed effects of ACEis. For example, the ACEi user stratum had the highest proportion of participants with diabetes mellitus yet people with diabetes mellitus had a slightly worse response than those without. Likewise, those with osteoarthritis had a strong improvement in SPPB, yet the ACEi users had the lowest proportion of individuals with osteoarthritis. Still, residual confounding remains a possibility and a potential source of bias in the direction or magnitude of effect observed for ACEi use. Subsequently, investigation using a randomized controlled design is still needed to determine conclusively whether ACEi use truly enhances exercise responsiveness in older men and women. Questions also remain regarding whether ACEis would provide such a benefit to individuals without hypertension. Older adults with hypertension have accelerated declines in physical function and are therefore the prime candidates for targeted interventions.39–42 The preclinical data support the hypothesis that ACEi use can improve the responsiveness of individuals without hypertension to chronic exercise training, but studies are needed to determine this.14
In conclusion, this study is the first to report that older persons at risk of physical disability who use ACEis are the greatest beneficiaries of exercise training. These findings add to those of previous studies in indicating that tremendous heterogeneity exists in the relative effects of exercise on the physical function of older adults.43 Thus, exercise alone may not be sufficient to prevent physical disability in many older men and women. If confirmed, these findings will have important implications treatment of older adults at risk of becoming physically disabled.
Supplementary Material
Estimated effects of the intervention on Short Physical Performance Battery (SPPB) performance and 400 m usual-paced walking speed expressed as mean change in the physical activity group minus mean change in the physical activity group from baseline to 12 months according to selected baseline characteristics. Mean changes and their 95% CI are illustrated by the bars.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health, National Institute on Aging: Lifestyle Interventions and Independence for Elders Pilot (U01AG22376), Claude D. Pepper Older Americans Independence Centers (University of Florida, P30AG028740; Wake Forest, P30AG21332; Pittsburgh, P30AG024827). TWB’s contribution was partially supported by the University of Florida Clinical and Translational Science Institute (National Center for Research Resources, UL1RR029890, KL2RR029888).
Sponsor’s Role: NIA scientists had substantial involvement in the study design and collection and management of data.
LIFE-P Investigators
Cooper Institute, Dallas, TX: Steven N. Blair, PED, Field Center Principal Investigator; Timothy Church, MD, PhD, MPH, Field Center Co-Principal Investigator; Jamile A. Ashmore, PhD; Judy Dubreuil, MS; Georita Frierson, PhD; Alexander N. Jordan, MS; Gina Morss, MA; Ruben Q. Rodarte, MS; Jason M. Wallace, MPH.
National Institute on Aging: Jack M. Guralnik, MD, PhD, Co-Principal Investigator of the Study; Evan C. Hadley, MD; Sergei Romashkan, MD, PhD.
Stanford University, Palo Alto, CA: Abby C. King, PhD, Field Center Principal Investigator; William L. Haskell, PhD, Field Center Co-Principal Investigator; Leslie A. Pruitt, PhD; Kari Abbott-Pilolla, MS; Karen Bolen, MS; Stephen Fortmann, MD; Ami Laws, MD; Carolyn Prosak, RD; Kristin Wallace, MPH.
Tufts University, Boston, MA: Roger Fielding, PhD; Miriam Nelson, PhD.
University of California at Los Angeles, Los Angeles, CA: Robert M. Kaplan, PhD, MA.
Veterans Affairs San Diego Healthcare System and University of California at San Diego, San Diego, CA: Erik J. Groessl, PhD.
University of Florida, Gainesville, FL: Marco Pahor, MD, Principal Investigator of the Study; Michael Perri, PhD; Connie Caudle; Lauren Crump, MPH; Sarah Hayden; Latonia Holmes; Cinzia Maraldi, MD; Crystal Quirin.
University of Pittsburgh, Pittsburgh, PA: Anne B. Newman, MD, MPH, Field Center Principal Investigator; Stephanie Studenski, MD, MPH, Field Center Co-Principal Investigator; Bret H. Goodpaster, PhD, MS; Nancy W. Glynn, PhD; Erin K. Aiken, BS; Steve Anthony, MS; Judith Kadosh, BSN, RN; Piera Kost, BA; Mark Newman, MS; Christopher A. Taylor, BS; Pam Vincent, CMA.
Wake Forest University, Winston-Salem, NC: Stephen B. Kritchevsky, PhD, Field Center Principal Investigator; Peter Brubaker, PhD; Jamehl Demons, MD; Curt Furberg, MD, PhD; Jeffrey A. Katula, PhD, MA; Anthony Marsh, PhD; Barbara J. Nicklas, PhD; Jeff D. Williamson, MD, MPH; Rose Fries, LPM; Kimberly Kennedy; Karin M. Murphy, BS, MT (ASCP); Shruti Nagaria, MS; Katie Wickley-Krupel, MS.
Data Management, Analysis and Quality Control Center (DMAQC): Michael E. Miller, PhD, DMAQC Principal Investigator; Mark Espeland, PhD, DMAQC Co-Principal Investigator; Fang-Chi Hsu, PhD; Walter J. Rejeski, PhD; Don P. Babcock, Jr., PE; Lorraine Costanza; Lea N. Harvin; Lisa Kaltenbach, MS; Wei Lang, PhD; Wesley A. Roberson; Julia Rushing, MS; Scott Rushing; Michael P. Walkup, MS.
Yale University, New Haven, CT: Thomas M. Gill, MD.
Footnotes
Conflict of Interest: None to disclose.
Author Contributions: Study concept and design: Church, Pahor. Acquisition of data: Church, Pahor. Analysis and interpretation of data: Buford, Manini, Hsu, Cesari, Anton, Nayfield, Stafford, Church, Pahor, Carter. Drafting of the manuscript: Buford, Manini, Carter. Critical revision of the manuscript for important intellectual content: Buford, Manini, Hsu, Cesari, Anton, Nayfield, Stafford, Church, Pahor, Carter. Statistical analysis: Buford, Hsu. Obtained funding: Pahor. Study supervision: Pahor.
References
- 1.Penninx BW, Ferrucci L, Leveille SG, et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55A:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 2.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter CS, Onder G, Kritchevsky SB, et al. Angiotensin-converting enzyme inhibition intervention in elderly persons: Effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60A:1437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- 4.Di Bari M, Pahor M, Franse LV, et al. Dementia and disability outcomes in large hypertension trials: Lessons learned from the Systolic Hypertension in the Elderly Program (SHEP) Trial. Am J Epidemiol. 2001;153:72–78. doi: 10.1093/aje/153.1.72. [DOI] [PubMed] [Google Scholar]
- 5.Gambassi G, Lapane KL, Sgadari A, et al. Effects of angiotensin-converting enzyme inhibitors and digoxin on health outcomes of very old patients with heart failure. SAGE study group. Systematic Assessment of Geriatric drug use via Epidemiology. Arch Intern Med. 2000;160:53–60. doi: 10.1001/archinte.160.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: An observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 7.Di Bari M, van de Poll-Franse LV, Onder G, et al. Antihypertensive medications and differences in muscle mass in older persons: The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- 8.Hutcheon SD, Gillespie ND, Crombie IK, et al. Perindopril improves six minute walking distance in older patients with left ventricular systolic dys-function: A randomised double blind placebo controlled trial. Heart. 2002;88:373–377. doi: 10.1136/heart.88.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumukadas D, Witham MD, Struthers AD, et al. Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. Can Med Assoc J. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesari M, Pedone C, Incalzi RA, et al. ACE-inhibition and physical function: Results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) Study. J Am Med Dir Assoc. 2010;11:26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther. 2003;17:133–139. doi: 10.1023/a:1025387702212. [DOI] [PubMed] [Google Scholar]
- 12.Booth FW, Laye MJ. The future: Genes, physical activity and health. Acta Physiol (Oxf) 2010;199:549–556. doi: 10.1111/j.1748-1716.2010.02117.x. [DOI] [PubMed] [Google Scholar]
- 13.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter CS, Marzetti E, Leeuwenburgh C, et al. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67A:17–27. doi: 10.1093/gerona/glr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habouzit E, Richard H, Sanchez H, et al. Decreased muscle ACE activity enhances functional response to endurance training in rats, without change in muscle oxidative capacity or contractile phenotype. J Appl Physiol. 2009;107:346–353. doi: 10.1152/japplphysiol.91443.2008. [DOI] [PubMed] [Google Scholar]
- 16.Kritchevsky SB, Nicklas BJ, Visser M, et al. Angiotensin-converting enzyme insertion/deletion genotype, exercise, and physical decline. JAMA. 2005;294:691–698. doi: 10.1001/jama.294.6.691. [DOI] [PubMed] [Google Scholar]
- 17.Giaccaglia V, Nicklas B, Kritchevsky S, et al. Interaction between angiotensin converting enzyme insertion/deletion genotype and exercise training on knee extensor strength in older individuals. Int J Sports Med. 2008;29:40–44. doi: 10.1055/s-2007-964842. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery HE, Clarkson P, Dollery CM, et al. Association of angiotensin- converting enzyme gene I/D polymorphism with change in left ventricular mass in response to physical training. Circulation. 1997;96:741–747. doi: 10.1161/01.cir.96.3.741. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery H, Clarkson P, Barnard M, et al. Angiotensin-converting-enzyme gene insertion/deletion polymorphism and response to physical training. Lancet. 1999;353:541–545. doi: 10.1016/S0140-6736(98)07131-1. [DOI] [PubMed] [Google Scholar]
- 20.Folland J, Leach B, Little T, et al. Angiotensin-converting enzyme genotype affects the response of human skeletal muscle to functional overload. Exp Physiol. 2000;85:575–579. [PubMed] [Google Scholar]
- 21.Myerson S, Hemingway H, Budget R, et al. Human angiotensin I-converting enzyme gene and endurance performance. J Appl Physiol. 1999;87:1313–1316. doi: 10.1152/jappl.1999.87.4.1313. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa M, Kawamura T, Li L, et al. Combination of exercise and enalapril enhances renoprotective and peripheral effects in rats with renal ablation. Am J Hypertens. 2006;19:80–86. doi: 10.1016/j.amjhyper.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Guo Q, Minami N, Mori N, et al. Effects of estradiol, angiotensin-converting enzyme inhibitor and exercise training on exercise capacity and skeletal muscle in old female rats. Clin Exp Hypertens. 2010;32:76–83. doi: 10.3109/10641960902993046. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Rejeski WJ, Fielding RA, Blair SN, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Pilot Study: Design and methods. Contemp Clin Trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Pahor M, Blair SN, et al. LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61A:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 27.Katula JA, Kritchevsky SB, Guralnik JM, et al. Lifestyle Interventions and Independence for Elders Pilot Study: Recruitment and baseline characteristics. J Am Geriatr Soc. 2007;55:674–683. doi: 10.1111/j.1532-5415.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 28.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Borg G. Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 30.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P Study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Juniper EF, Walter SD, et al. Interpreting treatment effects in randomised trials. BMJ. 1998;316:690–693. doi: 10.1136/bmj.316.7132.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 34.Dengel DR, Brown MD, Ferrell RE, et al. Exercise-induced changes in insulin action are associated with ACE gene polymorphisms in older adults. Physiol Genomics. 2002;11:73–80. doi: 10.1152/physiolgenomics.00048.2002. [DOI] [PubMed] [Google Scholar]
- 35.Charbonneau DE, Hanson ED, Ludlow AT, et al. ACE genotype and the muscle hypertrophic and strength responses to strength training. Med Sci Sports Exerc. 2008;40:677–683. doi: 10.1249/MSS.0b013e318161eab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AG, Day SH, Folland JP, et al. Circulating angiotensin converting enzyme activity is correlated with muscle strength. Med Sci Sports Exerc. 2005;37:944–948. [PubMed] [Google Scholar]
- 37.Diz DI. Future directions in cardiovascular pharmacology: Examples from the renin-angiotensin system. Mol Interv. 2008;8:222–225. doi: 10.1124/mi.8.5.5. [DOI] [PubMed] [Google Scholar]
- 38.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 39.Hajjar I, Lackland DT, Cupples LA, et al. Association between concurrent and remote blood pressure and disability in older adults. Hypertension. 2007;50:1026–1032. doi: 10.1161/HYPERTENSIONAHA.107.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balzi D, Lauretani F, Barchielli A, et al. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010;39:92–98. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosano C, Longstreth WT, Jr, Boudreau R, et al. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc. 2011;59:390–397. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumurgier J, Elbaz A, Dufouil C, et al. Hypertension and lower walking speed in the elderly: The Three-City Study. J Hypertens. 2010;28:1506–1514. doi: 10.1097/HJH.0b013e328338bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manini TM, Newman AB, Fielding R, et al. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010;18:1168–1175. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimated effects of the intervention on Short Physical Performance Battery (SPPB) performance and 400 m usual-paced walking speed expressed as mean change in the physical activity group minus mean change in the physical activity group from baseline to 12 months according to selected baseline characteristics. Mean changes and their 95% CI are illustrated by the bars.


