SUMMARY
Objective
To summarize literature on the responsiveness and reliability of MRI-based measures of knee osteoarthritis (OA) structural change.
Methods
A literature search was conducted using articles published up to the time of the search, April 2009. 1338 abstracts obtained with this search were preliminarily screened for relevance and of these, 243 were selected for data extraction. For this analysis we extracted data on reliability and responsiveness for every reported synovial joint tissue as it relates to MRI measurement in knee OA. Reliability was defined by inter- and intra-reader intra-class correlation (ICC), or coefficient of variation, or kappa statistics. Responsiveness was defined as standardized response mean (SRM) - ratio of mean of change over time divided by standard deviation of change. Random-effects models were used to pool data from multiple studies.
Results
The reliability analysis included data from 84 manuscripts. The inter-reader and intra-reader ICC were excellent (range 0.8–0.94) and the inter-reader and intra-reader kappa values for quantitative and semi-quantitative measures were all moderate to excellent (range 0.52–0.88). The lowest value (kappa = 0.52) corresponded to semi-quantitative synovial scoring intra-reader reliability and the highest value (ICC = 0.94) for semi-quantitative cartilage morphology.
The responsiveness analysis included data from 42 manuscripts. The pooled SRM for quantitative measures of cartilage morphometry for the medial tibiofemoral joint was −0.86 (95% confidence intervals (CI) −1.26 to −0.46). The pooled SRM for the semi-quantitative measurement of cartilage morphology for the medial tibiofemoral joint was 0.55 (95% CI 0.47–0.64). For the quantitative analysis, SRMs are negative because the quantitative value, indicating a loss of cartilage, goes down. For the semi-quantitative analysis, SRMs indicating a loss in cartilage are positive (increase in score).
Conclusion
MRI has evolved substantially over the last decade and its strengths include the ability to visualize individual tissue pathologies, which can be measured reliably and with good responsiveness using both quantitative and semi-quantitative techniques.
Keywords: Osteoarthritis, Magnetic resonance imaging, Reliability, Responsiveness
Introduction
One proposed osteoarthritis (OA) treatment goal is modification of the underlying joint structure. Highly reproducible and responsive measures of the rate of disease progression are a prerequisite for assessing structural change in clinical trials. Conventional radiography (CR) has been the mainstay of assessing structural change in OA clinical trials and is currently part of FDA recommendations on how to conduct trials to assess structural progression. The focus of such evaluations has been on the radiographic joint space as a surrogate for hyaline cartilage assessment.
There has been a growing awareness that symptomatic OA represents a process involving all the tissues in the OA joint. Structure modification should therefore be considered in a broader context than that of cartilage alone. Modern imaging, especially magnetic resonance imaging (MRI), allows unparalleled direct visualization of all the tissues involved in OA joint pathology, including cartilage, menisci, subchondral bone and soft tissue. MRI is ideally suited for imaging arthritic joints as is it is free of ionizing radiation, and its tomographic viewing perspective obviates morphological distortion, magnification and superimposition. More importantly, MRI has a rich image contrast variability resulting in an ability to discriminate articular tissues and it therefore holds great potential as a tool for whole-organ imaging of the OA joint. The last 20 years has seen a rapid improvement in imaging technology and in the last decade this has translated into improved understanding of the importance of individual features, their relation to clinical outcome and disease pathogenesis and better data on the quantification of these pathologies1,2. There is a wealth of literature on the measurement properties of MRI in the setting of OA including responsiveness and reliability. Prior to considering the merits of MRI in the setting of potential disease modifying trials and trial guidance it is important to review this systematically.
The objective of this review was to summarize the literature on the responsiveness and reliability of MRI-based measures of knee OA structural change.
Material and methods
Systematic literature search details
An online literature search was conducted using the OVID MEDLINE (1945–), EMBASE (1980–) and Cochrane databases (1998–). The search was not limited by publication date and the last search occurred in April 2009, with the search entries “MRI”, and “osteoarthritis”, “osteoarthritides”, “osteoarthrosis”, “osteoarthroses”, “degenerative arthritis”, “degenerative arthritides”, or “osteoarthritis deformans”. The abstracts of the 1330 citations received with this search were then preliminarily screened for relevance by two reviewers (KH and DJH). Although review articles were not included (see Inclusion/exclusion criteria), citations found in any review articles which were not already included in our preliminary search were screened for possible inclusion in this study. This added seven more relevant studies to our search. One further article was added, before publication, by one of the authors of this meta-analysis bringing the preliminary total to 1338.
Inclusion/exclusion criteria
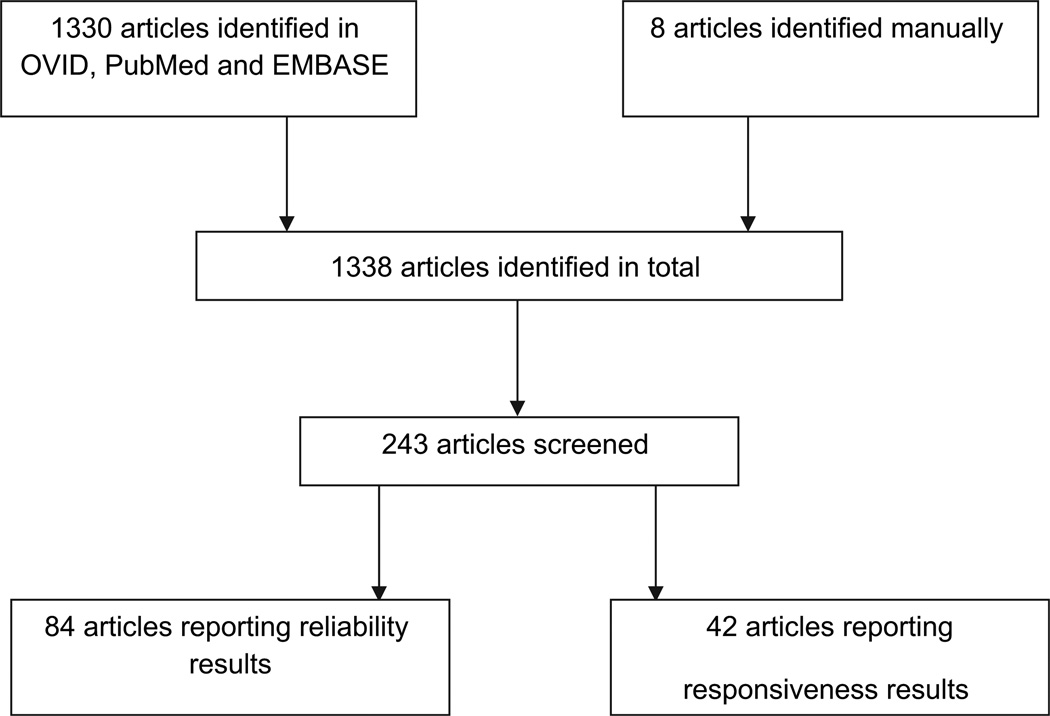
Only studies published in English were included. Studies presenting non-original data were excluded, such as reviews, editorials, opinion papers, case studies or letters to the editor. Studies with questionable clinical relevance and those using non-human subjects or specimens were excluded. Studies in which rheumatoid, inflammatory, or other forms of arthritis were included in the OA datasets were excluded, as well as general joint-pertinent MRI studies not focused on OA. Studies with no extractable, numerical data were excluded. Any duplicates which came up in the preliminary search were excluded. Of the preliminary 1338 abstracts, 243 were selected for data extraction (Fig. 1).
Fig. 1.
Flow chart of the screening process for articles included in the systematic review.
Data abstraction
We used a data abstraction tool constructed in EpiData (Entry version 2.0 Odense, Denmark). Two reviewers (KH and LM) independently abstracted the following data: (1) patient demographics; (2) MRI make (vendor and field strength), sequences and techniques used (see further description below), tissue types viewed; (3) study type and funding source; (4) details on rigor of study design to construct the Downs methodological quality score (see further description below)3; (5) MRI reliability/reproducibility data; (6) MRI diagnostic measures and performance; (7) gold standard measures against which the MRI measure was evaluated; (8) treatment and MRI measures (when appropriate).
Multiple techniques have been used to measure structural abnormality and change on MRI in OA. Broadly speaking these methods are divided into quantitative and semi-quantitative methods1. Quantitative measurements using computer-aided image processing to assess whole joint quantification (cartilage morphometry, bone volume, bone marrow lesion volume, meniscal position and volume, synovial volume, etc). The three-dimensional (3D) coverage of an entire cartilaginous region by MRI allows for the direct quantification of volumetric structures. Compositional measures of articular cartilage are also included within the quantitative measures as the measurement provides for a quantitative output. These methods include T2mapping, dGEMRIC and T1rho and are extensively reviewed elsewhere4,5.
In contrast to quantitative measures semi-quantitative image analysis is typically much more observer dependent and generates grades or scales rather than truly continuous output. Semi-quantitative scoring of MRI’s are a valuable method for performing multi-feature assessment of the knee using conventional MRI acquisitions6–8,98. Such approaches score, in an observer dependent semi-quantitative manner, a variety of features that are currently believed to be relevant to the functional integrity of the knee and/or potentially involved in the pathophysiology of OA. These articular features can include articular cartilage morphology, subarticular bone marrow abnormality, subarticular cysts, subarticular bone attrition, marginal and central osteophytes, medial and lateral meniscal integrity, anterior and posterior cruciate ligament integrity, medial and lateral collateral ligament integrity, synovitis/effusion, intra-articular loose bodies, and periarticular cysts/bursitis.
The Downs methodological quality score3 collects a profile of scores (quality of reporting, internal validity (bias and confounding), power, external validity so that the overall study quality score reflects all of these elements. Answers were scored 0 (No) or 1 (Yes), except for one item in the Reporting subscale, which scored 0–2 and the single item on power, which was scored 0–5. The possible range is from0–27 where 0 represents poor quality and 27 optimal quality.
The outcomes for psychometric properties on MRI were examined using the OMERACT filter10,11. The material pertinent to this manuscript is Discrimination: does the measure discriminate between situations that are of interest? The situations can be states at one time (for classification or prognosis) or states at different times (to measure change). This criterion captures the issues of reliability and responsiveness (sensitivity to change).
Statistical analysis
Reliability was defined by inter- and intra-reader measures of coefficient of variation (CV), or intra-class correlation (ICC), or kappa statistics.
Responsiveness was defined as standardized response mean (SRM) - ratio of mean of change over time divided by standard deviation of change. Whenever possible, both reliability measures and SRMs were stratified by measurement method (quantitative and semi-quantitative), tissue lesion (cartilage, synovium, bone, bone marrow lesions, meniscus and ligament) and plate/region for cartilage divisions.
For the quantitative analysis, a negative SRM expresses cartilage loss whereas a positive SRM would indicate cartilage gain. For the semi-quantitative analysis, positive SRMs indicate a loss in cartilage with higher scores reflecting greater lesions.
Random-effects models were used to summarize data from multiple studies. Since some studies reported more than one measure for each region, to avoid substantial skewness of results influenced by multiple observations from a single study and to ensure that the estimates included in the analysis came from independent studies, we repeated analyses 500 times. We did this by selecting one observation (estimate) from each study at random so that the number of observations in the model reflected the number of studies. We then ran a random-effects model to obtain the pooled summary measure and its standard error. The process was repeated 500 times to obtain the empirical distribution of the summary measure. The final pooled summary measure and its standard error were obtained by averaging the 500 summary measures and the 500 standard errors obtained from the random-effects models respectively. Ninety-five percent confidence intervals (CI) were obtained using a normal approximation for the final pooled summary measure and its standard error.
Results
Reliability
The reliability analysis included data from 84 manuscripts (Table I). The mean Downs criteria score for these manuscripts was 9.4 (range 4–16).
Table I.
Summary table of studies reporting data on reliability of MRI in knee OA
| Reference: Author, Journal, Year, PMID |
Whole sample size |
No. of cases |
No. of controls |
Age, yrs, mean (SD), range |
No. (%) of females |
Quantitative | Compositional techniques |
Semi- quantitative |
Cartilage | Synovium | Bone | Bone marrow lesions |
Meniscus | Ligament | Study design |
Downs criteria score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karvonen RL; Journal of Rheumatology; 1994; 796607513 |
92 | 52 | 40 | All OA Pts: 55(14), (Range: 25–86); Bilateral OA Pts: 53(13) (Range: 25–73); Control: 49(15), (Range: 22–78) |
All OA Pts: 35; Bilateral OA Pts: 19; Control: 25 |
X | X | X | Case control |
11 | ||||||
| Peterfy CG; Radiology; 1994; 802942014 |
8 | 5 | 3 | 62 (Range: 45–82) | 4(50%) | X | X | Cross- sectional |
4 | |||||||
| Marshall KW; Journal of Orthopaedic Research; 1995; 854401615 |
2 | 31 | X | X | Other | 6 | ||||||||||
| Disler DG; AJR Am J Roentgel.; 1996; 865935616 |
114 | 79 | 35 | 36 | 48 | X | X | Cross- sectional |
6 | |||||||
| Dupuy DE; Academic Radiology; 1996; 895918117 |
7 | 2 | 5 | TKA Pts: (Range: 64–75); Asymptomatic Pts: (Range: 25–35) |
TKA Pts: 1(50%); Asymptomatic Pts: 2 |
X | X | other | 6 | |||||||
| Trattnig S; Journal of Computer Assisted Tomography; 1998; 944875418 |
20 | 20 | 0 | 72.2 (Range: 62–82) | 18 | X | X | Other | 8 | |||||||
| Drape JL; Radiology; 1998; 964679219 |
43 | 43 | 0 | 63 (Range: 53–78) | 30 | X | X | Cross- sectional |
5 | |||||||
| Cicuttini F; Osteoarthritis & Cartilage; 1999; 1032930120 |
28 | Males: 41.4(14.8); Females: 31.2(8.6) |
11(39%) | X | X | Cross- sectional |
7 | |||||||||
| Pham XV; Revue du Rhumatisme; 1999; 1052638021 |
10 | 10 | 10 | 67.2(7.34), (Range: 57–80) |
6 | X | X | Cross- sectional |
13 | |||||||
| Gale DR; Osteoarthritis & Cartilage; 1999; 1055885022 |
291 | 233 | 58 | Men cases: 67(10); Men controls 65(10); Women cases: 66(10); Women controls: 66(8) |
61(21%) | X | Case control |
10 | ||||||||
| Hyhlik–Durr A; European Radiology; 2000; 1066376023 |
11 | 3 | 8 | OA group: (Range: 61–75); Healthy group: |
5(45.5%) | X | X | Cross- sectional |
6 | |||||||
| Jones G; Arthritis & Rheumatism; 2000; 1108327924 |
92 | 0 | 92 | Boys:12.8(2.7); Girls: 12.6(2.9) |
43(46.8%) | X | X | X | Cross- sectional |
13 | ||||||
| Wluka AE; Annals of the Rheumatic Diseases; 2001; 1124786125 |
81 | 42 | 39 | Cases: 58(6.1); Controls: 56(5.4) |
81(100%) | X | X | X | Case control |
16 | ||||||
| Felson DT; Annals of Internal Medicine; 2001; 1128173626 |
401 | 401 | 0 | 66.8 | X | X | Cross- sectional |
13 | ||||||||
| Hill CL; Journal of Rheumatology; 2001; 1140912727 |
458 | 433 | 25 | 67 | (34%) | X | X | Case control |
13 | |||||||
| Bergin D; Skeletal Radiology; 2002; 1180758728 |
60 | 30 | 30 | Cases: 50; Controls: 57 | X | X | X | Case control |
9 | |||||||
| Beuf O; Arthritis & Rheumatism; 2002; 1184044129 |
46 | 18 | 28 | Mild OA: 56.3(4.5); Severe OA: 70(6.3) |
17(37%) | X | Case control |
5 | ||||||||
| Wluka AE; Arthritis & Rheumatism; 2002; 1220951030 |
123 | 123 | 0 | 63.1(10.6) | 71 | X | X | X | Longitudinal Prospective |
14 | ||||||
| Gandy SJ; Osteoarthritis & Cartilage; 2002; 1246455331 |
16 | 16 | 0 | 6 | X | X | Longitudinal Prospective |
8 | ||||||||
| Bhattacharyya T; Journal of Bone & Joint Surgery - American Volume; 2003; 1253356532 |
203 | 154 | 49 | Cases: 65; Controls: 67 |
X | X | Case control |
9 | ||||||||
| Cicuttini FM; Clinical & Experimental Rheumatology; 2003; 1267389333 |
81 | 42 | 39 | ERT: 58(6.1); Controls: 56(5.4) |
81(100%) | X | X | X | Case control |
12 | ||||||
| Raynauld JP; Osteoarthritis & Cartilage; 2003; 1274494134 |
28 | 17 | 11 | Healthy subjects: (Range: 25–35); OA Pts: 63.5 |
X | X | Other | 7 | ||||||||
| Felson DT; Annals of Internal Medicine; 2003; 1296594135 |
256 | 256 | 0 | Followed: 66.2(9.4); t followed: 67.8(9.6) |
Followed: 41.7%; t followed: 15.2% |
X | X | Other | 11 | |||||||
| Hill CL; Arthritis & Rheumatism; 2003; 1455808936 |
451 | 427 | Knee pain/ROA/MALE: 68.3; Knee pain/ROA/ Female: 65; knee pain/ ROA/male: 66.8; knee pain/ROA/ female:66.1 |
X | X | Cross-s ectional |
10 | |||||||||
| Glaser C; Magnetic Resonance in Medicine; 2003; 1464857137 |
23 | 7 | 16 | Healthy subjects: (Range: 23–33); |
13(56.5%) | X | X | Cross- sectional |
5 | |||||||
| Lindsey CT; Osteoarthritis & Cartilage; 2004; 1472386838 |
74 | 33 | 21 | OA1(KL = 1/2):62.7(10.9); OA2(KL = 3/4):66.6(11.6); Controls: 34.2(12.5) |
39(52.7%) | X | X | X | Case control |
8 | ||||||
| Cicuttini FM; Arthritis & Rheumatism; 2004; 1473060439 |
117 | 117 | 63.7(10.2) | (58.1%) | X | X | Longitudinal Prospective |
9 | ||||||||
| Raynauld JP; Arthritis & Rheumatism; 2004; 1487249040 |
32 | 32 | 0 | 62.9(8.2) | (74%) | X | X | Longitudinal Prospective |
10 | |||||||
| Cicuttini F; Rheumatology; 2004; 1496320141 |
117 | 117 | 0 | 67(10.6) | (58.1%) | X | X | Longitudinal Prospective |
12 | |||||||
| Peterfy CG; Osteoarthritis & Cartilage; 2004; 149723356 |
19 | 19 | 0 | 61(8) | 4 | X | X | X | X | X | X | X | Other | 5 | ||
| Dashti M; Scandinavian Journal of Rheumatology; 2004; 1516310942 |
174 | 117 | 57 | 61.6(9.5) | 123(70.7%) | X | X | Case control | 11 | |||||||
| Cicuttini FM; Journal of Rheumatology; 2004; 1522995943 |
102 | 102 | 0 | 63.8(10.1) | (63%) | X | X | Longitudinal Prospective |
10 | |||||||
| Baysal O; Swiss Medical Weekly; 2004; 1524384944 |
65 | 65 | 0 | 53.1(7), (Range: 45–75) |
(100%) | X | X | X | X | Cross- sectional |
7 | |||||
| Kornaat PR; Skeletal Radiology; 2005; 154806499 |
25 | 25 | 0 | Median age = 63, (Range: 50–75) |
X | X | X | X | X | X | Other | 6 | ||||
| Yoshioka H; Journal of Magnetic Resonance Imaging; 2004; 1550332345 |
28 | 28 | 0 | 55.6 (Range: 40–73) |
10 | X | X | X | X | X | X | Other | 5 | |||
| Ding C; Osteoarthritis & Cartilage; 2005; 1572788546 |
372 | 162 | 210 | cartilage defects: 43.6(7.1); |
(58%) | X | X | X | Case control |
9 | ||||||
| Hill CL; Arthritis & Rheumatism; 2005; 1575106447 |
433 | 360 | 73 | Case males:68.2; Case females:65; Control males:66.8; Control females:65.8 |
143(33%) | X | X | Case control |
12 | |||||||
| Maataoui A; European Radiology; 2005; 1585624648 |
12 | 12 | 0 | median age = 70.5, (Range: 60–86) |
9 | X | X | Cross- sectional |
6 | |||||||
| Cicuttini F; Osteoarthritis & Cartilage; 2005; 1592263449 |
28 | 28 | 0 | 62.8(9.8) | (57%) | X | X | Longitudinal Prospective |
10 | |||||||
| Huh YM; Korean Journal of Radiology; 2005; 1596815150 |
94 | 73 | 21 | OA group: 57.8, (Range: 40–80), Median = 58; RA group:49.6, (Range: 37–76), Median = 48 |
73(80%) | X | X | Longitudinal Retropective |
7 | |||||||
| Wluka AE; Rheumatology; 2005; 1603008451 |
126 | 126 | 0 | 63.6(10.1) | 68(54%) | X | X | X | X | Longitudinal Prospective |
14 | |||||
| Eckstein, F; Annals of the Rheumatic Diseases; 2006; 1612679752 |
19 | 10 | 9 | 51 (Range: 40–71) | 12 | X | X | X | Other | 8 | ||||||
| Eckstein F; Arthritis & Rheumatism; 2005; 1620059253 |
30 | 15 | 15 | Cases: 49.6(Range: 37–76); Controls: 62.3(11.5) |
30(100%) | X | X | Cross- sectional |
7 | |||||||
| Sengupta M; Osteoarthritis & Cartilage; 2006; 1644231654 |
217 | 217 | 0 | 67.3(9.1) | (30%) | X | X | X | X | X | Cross- sectional |
7 | ||||
| Raynauld JP; Arthritis Research & Therapy; 2006; 1650711955 |
110 | 110 | 0 | 62.4(7.5) | (64%) | X | X | X | X | X | Longitudinal Prospective |
11 | ||||
| Hunter DJ; Arthritis & Rheumatism; 2006; 1650893056 |
257 | 257 | 0 | 66.6(9.2), (Range: 47–93) |
(41.6%) | X | X | X | Longitudinal Prospective |
10 | ||||||
| Brandt KD; Rheumatology; 2006; 1660665557 |
30 | 20 | 10 | 62 | 29 | X | Other | 10 | ||||||||
| Jaremko JL; Osteoarthritis & Cartilage; 2006; 1664424558 |
12 | 3 | 9 | OA: (Range: 59–71); ) Healthy: 37(8), (Range: 23–48) |
4(33.3%) | X | X | Cross- sectional |
8 | |||||||
| Hunter DJ; Osteoarthritis & Cartilage; 2007; 1685739359 |
127 | 127 | 67(9.05) | (46.7%) | X | X | Cross- sectional |
12 | ||||||||
| Boks SS; American Journal of Sports Medicine; 2006; 1686157560 |
134 | 136 | 132 | 40.8 (Range: 18.8–63.8) |
X | X | X | X | Cross- sectional |
7 | ||||||
| Brem MH; Skeletal Radiology; 2007; 1721923161 |
5 | 5 | 0 | 64.3 (Range: 40–73) |
2 | X | X | Other | 6 | |||||||
| Folkesson J; IEEE Transactions on Medical Imaging; 2007; 1724358962 |
139 | 56 (Range: 22–79) | (59%) | X | X | Other | 7 | |||||||||
| Dam EB; Osteoarthritis & Cartilage; 2007; 1735313263 |
139 | Evaluation set: 55(Range: 21–78); |
(55%) | X | X | Other | 9 | |||||||||
| Baranyay FJ; Seminars in Arthritis & Rheumatism; 2007; 1739173864 |
297 | 297 | 58(5.5) | (63%) | X | X | X | Cross- sectional |
16 | |||||||
| Hanna F; Mepause; 2007; 1741364965 |
176 | 0 | 176 | 52.3(6.6), (Range: 40–67) |
176(100%) | X | X | Cross- sectional |
13 | |||||||
| Hunter DJ; Annals of the Rheumatic Diseases; 2008; 174729958 |
71 | 67.9(9.3) | (28.2%) | X | X | X | X | X | X | X | Other | 8 | ||||
| Hill CL; Annals of the Rheumatic Diseases; 2007; 1749109666 |
270 | 270 | 0 | 66.7(9.2) | 112 | X | X | X | Longitudinal Prospective |
9 | ||||||
| Qazi AA; Osteoarthritis & Cartilage; 2007; 1749384167 |
X | X | Cross- sectional |
8 | ||||||||||||
| Guymer E; Osteoarthritis & Cartilage; 2007; 1756013468 |
176 | 0 | 176 | 52.3(6.6) | (100%) | X | X | X | X | Cross- sectional |
11 | |||||
| Eckstein F; Osteoarthritis & Cartilage; 2007; 1756081369 |
9 | 9 | 52.2(9.3) | 5 | X | X | Other | 9 | ||||||||
| Akhtar, S; Osteoarthritis & Cartilage; 2007; 1770766070 |
6 | (Range: 25–69) | 2(33%) | X | X | Other | 7 | |||||||||
| Raynauld JP; Annals of the Rheumatic Diseases; 2008; 1772833371 |
107 | 107 | 0 | 62.4(7.5) | (64%) | X | X | X | X | Longitudinal Retropective |
15 | |||||
| Felson DT; Arthritis & Rheumatism; 2007; 1776342772 |
330 | 110 | 220 | Cases: 62.9(8.3); Controls: 61.2(8.4) |
211(64%) | X | X | X | Case control | 12 | ||||||
| Lo GH; Osteoarthritis & Cartilage; 2008; 1782558673 |
845 | 170 | 63.6(8.8) | (58%) | X | X | Cross- sectional |
10 | ||||||||
| Davies-Tuck M; Osteoarthritis & Cartilage; 2008; 1786954674 |
100 | 100 | 0 | 63.6(10.2) | 61(61%) | X | X | Longitudinal Prospective |
11 | |||||||
| Folkesson J; Academic Radiology; 2007; 1788933975 |
. | 56 (Range: 22–79) | (59%) | Other | 7 | |||||||||||
| Sanz R; Journal of Magnetic Resonance Imaging; 2008; 1802285076 |
22 | 9 | Normal: 43.6(15); Chondromalacia: 33.3(11.8); OA Pts: 58.9(11.5) |
14(64%) | X | X | Case control | 6 | ||||||||
| Englund M; Arthritis & Rheumatism; 2007; 1805020177 |
310 | 102 | 208 | Cases: 62.9(8.3); Controls: 61.2(8.3) |
211(68%) | X | X | Case control | 15 | |||||||
| Hernandez-Molina G; Arthritis & Rheumatism; 2008; 1816348378 |
258 | 258 | 0 | 66.6(9.2) | (42.6%) | X | X | X | X | Longitudinal Prospective |
11 | |||||
| Amin S; Osteoarthritis & Cartilage; 2008; 1820362979 |
265 | 265 | 67(9) | (43%) | X | X | X | X | Longitudinal Prospective |
11 | ||||||
| Teichtahl AJ; Obesity; 2008; 1823965480 |
297 | 297 | 58(5.5) | 186 | X | X | X | X | Longitudinal Prospective |
14 | ||||||
| Anandacoomarasamy; Journal of Rheumatology; 2008; 1827883181 |
32 | 32 | Males: 64(11.5); Females: 66(9.5); Total: 65(Range: 42–87) |
17(53%) | X | X | X | Longitudinal Prospective |
11 | |||||||
| Eckstein F; Annals of the Rheumatic Diseases; 2008; 1828305482 |
158 | Mild to moderate OA2: 57.6(8.3); Controls: 56.1(8.7) |
158(100%) | X | X | Case control | 10 | |||||||||
| Reichenbach S; Osteoarthritis & Cartilage; 2008; 1836741583 |
964 | 217 | 747 | 63.3 | (57%) | X | X | X | Cross- sectional |
8 | ||||||
| Petterson SC; Medicine & Science in Sports & Exercise; 2008; 1837920284 |
123 | 123 | 0 | 64.9(8.5) | 67 | Case control |
11 | |||||||||
| Bolbos RI; Osteoarthritis & Cartilage; 2008; 1838782885 |
32 | Cases: 47.2(11.5), (Range: 29–72); Controls: 36.3(10.5), Range: (27–56) |
14(44%) | X | X | X | X | Case control |
7 | |||||||
| Pai A; Magnetic Resonance Imaging; 2008; 1850207386 |
10 | 0 | 10 | 27 (Range: 21–31) | 4(40%) | X | X | Other | 6 | |||||||
| Folkesson J; Magnetic Resonance in Medicine; 2008; 1850684587 |
143 | Healthy subjects: 48(Range: 21–78); KL1: 62(Range: 37–81); KL2: 67(Range: 47–78); KL3&4: 68(58–78) |
X | Other | 12 | |||||||||||
| Mills PM; Osteoarthritis & Cartilage; 2008; 1851515788 |
49 | 25 | 24 | APMM: 46.8(5.3); Controls: 43.6(6.6) |
18(36.7%) | X | X | X | Case control |
12 | ||||||
| Dore D; Osteoarthritis & Cartilage; 2008; 1851516089 |
50 | 50 | 64.5(7.1) | 23 | X | X | X | X | Cross- sectional |
9 | ||||||
| Pelletier JP; Osteoarthritis & Cartilage; 2008; 1867238690 |
27 | 1 | 64.1(9.6) | 14 | X | X | X | X | X | X | Other | 9 | ||||
| Englund M; New England Journal of Medicine; 2008; 1878410091 |
991 | 171 | 62.3(8.6), (Range: 50.1–90.5) |
565(57%) | X | X | Cross- sectional |
10 | ||||||||
| Rauscher I; Radiology; 2008; 1893631592 |
60 | 37 | 23 | Healthy controls: 34.1(10); Mild OA: 52.5(10.9); Severe OA: 61.6(11.6) |
32(53.3%) | X | X | X | Case control |
9 | ||||||
| Kijowski R.; Radiology; 2009; 1916412193 |
200 | 200 | 1.5T image group: 38.9(Range: 16–63); 3T image group: 39.1(Range: 15–65) |
87(43.5%) | X | X | Longitudinal Retropective |
10 |
Inter- and intra-reader CV and test-retest measures were confined to quantitative or compositional measures (Tables II and III). The pooled CV for quantitative cartilage was 3% for both inter- and intra-reader reliability.
Table II.
Results of random-effects pooling of intra-reader CV from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament)
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled CV (%) | 95% CI |
|---|---|---|---|---|
| Quantitative | ||||
| Cartilage | 32 (10) | 60 | 3 | −2, 7 |
| Synovium | 2 (1) | 94 | 8 | −6, 22 |
| Compositional | 6 (1) | 60 | 5 | −5, 15 |
Table III.
Results of random-effects pooling of inter-reader CV from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament)
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled CV (%) | 95% CI |
|---|---|---|---|---|
| Quantitative | ||||
| Cartilage | 42 (13) | 65 | 3 | −1, 6 |
| Synovium | 1 (1) | 94 | 5 | −15, 25 |
| Bone | 9 (5) | 119 | 2 | −4, 8 |
The inter-reader and intra-reader ICCs for quantitative and semi-quantitative measures were all excellent (range 0.8–0.94)(Tables IV and V). For quantitative measures the intra-reader ICC ranged from 0.87 (0.61–1.00) for synovium to 0.93 (0.82–1.00) for meniscus measurement. For quantitative measures the inter-reader ICC ranged from 0.81 (0.72–0.89) for meniscus to 0.90 (0.86–0.95) for cartilage morphometry measurement.
Table IV.
Results of random-effects pooling of intra-reader ICC from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament)
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled ICC | 95% CI |
|---|---|---|---|---|
| Quantitative | ||||
| Cartilage | 23 (9) | 108 | 0.92 | 0.88, 0.96 |
| Synovium | 2 (1) | 30 | 0.87 | 0.61, 1.00 |
| Meniscus | 1 (1) | 291 | 0.93 | 0.82, 1.00 |
| Semi-quantitative | ||||
| Cartilage | 7 (4) | 114 | 0.94 | 0.87, 1.00 |
| Synovium | 3 (2) | 26 | 0.88 | 0.66, 1.00 |
| Bone Marrow Lesion | 2 (2) | 178 | 0.93 | 0.83, 1.00 |
| Meniscus | 2 (1) | 25 | 0.77 | 0.49, 1.00 |
Table V.
Results of random-effects pooling of inter-reader ICC from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament)
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled ICC | 95% CI |
|---|---|---|---|---|
| Quantitative | ||||
| Cartilage | 10 (4) | 196 | 0.90 | 0.86, 0.95 |
| Meniscus | 2 (1) | 291 | 0.81 | 0.72, 0.89 |
| Semi-Quantitative | ||||
| Cartilage | 9 (7) | 88 | 0.85 | 0.77, 0.94 |
| Synovium | 5 (4) | 46 | 0.87 | 0.74, 1.00 |
| Bone | 3 (2) | 23 | 0.90 | 0.66, 1.00 |
| Bone Marrow Lesion | 2 (2) | 22 | 0.84 | 0.54, 1.00 |
| Meniscus | 5 (3) | 67 | 0.93 | 0.82, 1.00 |
| Ligament | 4 (2) | 105 | 0.80 | 0.56, 1.00 |
The inter-reader and intra-reader kappa values for quantitative and semi-quantitative measures were all moderate to excellent (range 0.52–0.88)(Tables VI and VII). For semi-quantitative measures the range for intra-reader kappa values extended from 0.52 (0.28–0.77) for synovium to 0.66 (0.54–0.78) for BML assessment. For semi-quantitative measures the range for inter-reader kappa values extended from 0.57 (0.44–0.71) for cartilage morphology to 0.88 (0.79–0.97) for BML assessment.
Table VI.
Results of random-effects pooling of intra-reader kappa values from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament)
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled Kappa | 95% CI |
|---|---|---|---|---|
| Quantitative | ||||
| Cartilage | 1 (1) | 158 | 0.66 | 0.50, 0.82 |
| Semi-Quantitative | ||||
| Synovium | 4 (2) | 317 | 0.52 | 0.28, 0.77 |
| Bone Marrow Lesion | 1 (1) | 256 | 0.66 | 0.54, 0.78 |
Table VII.
Results of random-effects pooling of inter-reader kappa values from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament)
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled Kappa | 95% CI |
|---|---|---|---|---|
| Semi-quantitative | ||||
| Cartilage | 15 (4) | 136 | 0.57 | 0.44, 0.71 |
| Bone marrow lesion | 2 (2) | 237 | 0.88 | 0.79, 0.97 |
| Meniscus | 3 (3) | 418 | 0.73 | 0.63, 0.84 |
| Ligament | 3 (3) | 209 | 0.80 | 0.69, 0.90 |
Responsiveness
The responsiveness analysis included data from 42 manuscripts (Table VIII). The mean Downs criteria score for these manuscripts was 11.2 (range 8–21). Table IX includes the summary responsiveness data for both types of measurement methods (quantitative and semi-quantitative). As some studies reported multiple estimates, random-effects model pooling was done to reduce potential bias from studies reporting multiple estimates. The pooled SRM for quantitative measures of cartilage morphometry for the medial tibiofemoral joint was −0.86 (95%CI −1.26 to −0.46), for lateral tibofemoral joint was −1.01 (95%CI −2.04 to 0.02), and for the patella was −0.63 (95%CI −0.90 to −0.37). The quantitative cartilage morphometry pooled SRM ranged from −0.21 (−0.48 to 0.05) for the lateral femoral plate to −1.01 (−2.04 to 0.02) for lateral tibiofemoral plate. The results for the compositional measures are from one study and should be interpreted with caution. The pooled SRM for semi-quantitative measures of cartilage for medial tibiofemoral joint was 0.55 (95%CI 0.47–0.64), for lateral tibofemoral joint was 0.37 (95%CI 0.18–0.57), and for the patella was 0.29 (95% CI 0.03–0.56). The semi-quantitative cartilage morphology SRMs ranged from −0.07 (−0.18 to 0.04) for the medial tibial region to 0.55 (0.47–0.64 for the medial tibiofemoral region. The pooled SRM for semi-quantitative measures of synovium was 0.47 (95%CI 0.18–0.77), and for BMLs was 0.43 (95%CI −0.17 to 1.03).
Table VIII.
Summary table of studies reporting data on responsiveness of MRI in OA
| Reference: Author, Journal, Year, PMID |
Whole sample size |
No. of cases |
No. of controls |
Age, yrs, Mean(SD), Range |
No. (%) of females |
Quantitative | Compositional technique |
Semi- quantitative |
Cartilage | Synovium | Bone | Bone marrow lesions |
Meniscus | Ligament | Study design |
Downs criteria score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wluka AE; Arthritis & Rheumatism; 2002; 1220951030 |
123 | 123 | 0 | 63.1(10.6) | 71 | X | X | X | Longitudinal Prospective |
14 | ||||||
| Cicuttini FM; Journal of Rheumatology; 2002; 1223389294 |
21 | 8 | 13 | Case:41. 3(13.2); Controls: 49.2(17.8) |
14(66.7%) | X | X | Longitudinal Prospective |
13 | |||||||
| Biswal S; Arthritis & Rheumatism; 2002; 124282287 |
43 | 4 | 39 | 54.4(Range 17–65) |
21 | X | X | Longitudinal Prospective |
8 | |||||||
| Gandy SJ; Osteoarthritis & Cartilage; 2002; 1246455331 |
16 | 16 | 0 | 63.4 (Range 52–70) |
6 | X | X | Longitudinal Prospective |
8 | |||||||
| Wluka AE; Journal of Rheumatology; 2002; 1246515795 |
136 | 136 | 0 | Vitamin E group: 64.3(11); Placebo group: 63.7(10) |
75(55%) | X | X | Randomized controlled trial |
21 | |||||||
| Cicuttini F; Journal of Rheumatology; 2002; 1246516296 |
110 | 110 | 0 | 63.2(10.2) | 66 | X | X | Longitudinal Prospective |
12 | |||||||
| Pessis E; Osteoarthritis & Cartilage; 2003; 1274494297 |
20 | 20 | 63.9(9) | 13 | X | X | X | Longitudinal Prospective |
12 | |||||||
| Cicuttini FM; Arthritis & Rheumatism; 2004; 1473060439 |
117 | 117 | 63.7(10.2) | (58.1%) | X | X | Longitudinal Prospective |
9 | ||||||||
| Raynauld JP; Arthritis & Rheumatism; 2004; 1487249040 |
32 | 32 | 0 | 62.9(8.2) | (74%) | X | X | Longitudinal Prospective |
10 | |||||||
| Wluka AE; Annals of the Rheumatic Diseases; 2004; 1496296098 |
132 | 132 | 0 | 63.1(Range: 41–86) |
71(54%) | X | X | Longitudinal Prospective |
10 | |||||||
| Cicuttini FM; Journal of Rheumatology; 2004; 1522995943 |
102 | 102 | 0 | 63.8(10.1) | (63%) | X | X | Longitudinal Prospective |
10 | |||||||
| Blumenkrantz G; Osteoarthritis & Cartilage; 2004; 1556406799 |
38 | 30 | 8 | 58(Range: 28–81) |
(39.5%) | X | X | X | Longitudinal Prospective |
9 | ||||||
| Zhai G; BMC Musculoskeletal Disorders; 2005; 15720725100 |
150 | 80 | 70 | TASOAC dataset: 62.3(7.6); KCV dataset: 42.8(6.1) |
79(52.7%) | X | X | Other | 9 | |||||||
| Wang Y; Arthritis Res Ther; 2005; 15899054101 |
126 | 126 | 63.6(10.1) | 68 | X | Longitudinal Prospective |
12 | |||||||||
| Cicuttini F; Osteoarthritis & Cartilage; 2005; 1592263449 |
28 | 28 | 0 | 62.8(9.8) | (57%) | X | X | Longitudinal Prospective |
10 | |||||||
| Wluka AE; Rheumatology; 2005; 1603008451 |
126 | 126 | 0 | 63.6(10.1) | 68(54%) | X | X | Longitudinal Prospective |
14 | |||||||
| Ding C; Arthritis & Rheumatism; 2005; 16320339102 |
325 | 45.2(6.5) | 190 | X | X | X | Longitudinal Prospective |
10 | ||||||||
| Raynauld JP; Arthritis Research & Therapy; 2006; 1650711955 |
110 | 110 | 0 | 62.4(7.5) | (64%) | X | X | Longitudinal Prospective |
11 | |||||||
| Hunter DJ; Arthritis & Rheumatism; 2006; 1650893056 |
257 | 257 | 0 | 66.6(Range: 47–93) |
(41.6%) | X | X | Longitudinal Prospective |
10 | |||||||
| Hunter, DJ; Osteoarthritis & Cartilage; 2006; 16678452103 |
150 | 150 | 0 | 58.9(Range: 44–81) |
(72%) | X | X | X | X | X | X | Longitudinal Prospective |
9 | |||
| Wluka AE; Arthritis Research & Therapy; 2006; 16704746104 |
105 | 105 | 0 | All eligible: 62.5(10.7); MRI at FU: 63.8(10.6); Lost to FU: 61.6(11.3) |
61(58.1%) | X | X | Longitudinal Prospective |
17 | |||||||
| Ding C; Rheumatology; 2007; 16861710105 |
325 | 45.2(6.4) | 190 | X | X | Longitudinal Prospective |
12 | |||||||||
| Hunter DJ; Arthritis & Rheumatism; 2006; 16868968106 |
264 | 264 | 0 | 66.7(9.2), (Range: 47–93) |
(40.9%) | X | X | X | Longitudinal Prospective |
9 | ||||||
| Bruyere O; Osteoarthritis Cartilage; 2007; 16890461107 |
62 | 64.9 (10.3) | (74%) | X | X | Longitudinal Prospective |
10 | |||||||||
| Stahl R; Osteoarthritis & Cartilage; 2007; 17561417108 |
18 | 8 | 10 | OA Pts: 55.7(7.3); Controls: 57.6(6.2) |
18(100%) | X | X | Case control | 10 | |||||||
| Pelletier JP; Arthritis Research & Therapy; 2007; 17672891109 |
110 | 110 | Q1greatestlossglobal: 63.7(7.2); Q4 least loss global: 61.3(7.5); Q1 greatest loss_med: 64.1(7.4); Q1 least loss_medial: 61.6(7.8) |
74(67.3%) | X | X | Longitudinal Prospective |
15 | ||||||||
| Raynauld JP; Annals of the Rheumatic Diseases; 2008; 1772833371 | 107 | 107 | 0 | 62.4(7.5) | (64%) | X | X | X | Longitudinal Retrospective |
15 | ||||||
| Davies-Tuck M; Osteoarthritis & Cartilage; 2008; 1786954674 |
100 | 100 | 0 | 63.3(10.2) | 61(61%) | X | X | Longitudinal Prospective |
11 | |||||||
| Hunter DJ; Arthritis Research & Therapy; 2007; 17958892110 |
160 | 80 | 80 | 67(9) | (46%) | X | X | Case control | 11 | |||||||
| Teichtahl AJ; Osteoarthritis & Cartilage; 2008; 18194873111 |
99 | 99 | 0 | 63(10) | (60%) | X | X | Longitudinal Prospective |
14 | |||||||
| Hunter DJ; Annals of the Rheumatic Diseases; 2009; 18408248112 |
150 | 150 | 60.9(9.9) | 76(51%) | X | X | Longitudinal Prospective |
8 | ||||||||
| Folkesson J; Magnetic Resonance in Medicine; 2008; 1850684587 |
288 | 143 | KL0(Healthy): 48(Range: 21–78); KL1: 62(Range: 37–81); KL2: 67(Range: 47–78); KL3&4: 68(Range: 58–78) |
(44%) | X | Other | 12 | |||||||||
| Sharma L; Arthritis & Rheumatism; 2008; 18512777113 |
153 | 153 | 0 | 66.4(11) | X | X | Longitudinal Prospective |
11 | ||||||||
| Teichtahl AJ; Osteoarthritis & Cartilage; 2009; 18590972114 |
78 | 63 (10.5) | (52%) | X | X | Longitudinal Prospective |
14 | |||||||||
| Raynauld JP; Annals Rheumatic Disease; 2009; 18653484115 |
154 | 60.3 (8.1) | 100 (65%) | X | X | Randomized controlled trial |
11 | |||||||||
| Pelletier JP; Osteoarthritis & Cartilage; 2008; 1867238690 |
27 | 1 | 64.1(9.6) | 14 | X | X | X | X | X | Other | 9 | |||||
| Wirth W; Osteoarthritis & Cartilage; 2009; 18789729116 |
79 | 60.3 (9.5) | 79 (100%) | X | X | Longitudinal Prospective |
14 | |||||||||
| Eckstein F; Arthritis & Rheumatism; 2008; 18975356117 |
174 | 174 | 0 | 66(11.1) | (76%) | X | X | Longitudinal Prospective |
8 | |||||||
| Hellio Le Graverand MP; Annals Rheumatic Diseases; 2008; 19103634118 |
180 | (100%) | X | X | Longitudinal Prospective |
19 | ||||||||||
| Eckstein F; Arthritis Research Therapy; 2009; 19534783119 |
79 | 60.3 (9.5) | 79 (100%) | X | X | Longitudinal Prospective |
15 | |||||||||
| Eckstein F; Arthritis Rheum; 2009: 19714595120 |
80 | 60.9 (9.1) | 48 (60%) | X | X | Longitudinal Prospective |
14 | |||||||||
| Hunter DJ; Osteoarthritis & Cartilage; 2009; 19744588121 |
150 | 150 | 60.9(9.9) | 76(51%) | X | X | Longitudinal Prospective |
18 |
Table IX.
Results of random-effects pooling of SRM from MRI studies stratified by measure (quantitative and semi-quantitative) and tissue (cartilage, synovium, bone, bone marrow lesion, meniscus, and ligament). Studies with multiple estimates had an estimate selected at random and a pooled analysis was performed. The process was repeated 500 times to obtain the empirical distribution of pooled SRMs
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled SRM |
95% CI |
|---|---|---|---|---|
| Quantitative cartilage* | ||||
| Medial femoral | 54 (12) | 118 | −0.51 | −0.74, −0.28 |
| Medial tibial | 55 (17) | 134 | −0.48 | −0.63, −0.34 |
| Medial tibiofemoral | 31 (12) | 92 | −0.86 | −1.26, −0.46 |
| Lateral femoral | 32 (8) | 151 | −0.21 | −0.48, 0.05 |
| Lateral tibial | 44 (14) | 152 | −0.56 | −0.72, −0.39 |
| Lateral tibiofemoral | 14 (5) | 110 | −1.01 | −2.04, 0.02 |
| Patella | 13 (9) | 131 | −0.63 | −0.90, −0.37 |
| Global | 5 (4) | 48 | −0.89 | −2.59, 0.80 |
| Quantitative other* | ||||
| Denuded area | 19 (2) | 114 | −0.20 | −0.85, 0.45 |
| Bone | 14 (2) | 167 | 0.12 | −0.46, 0.70 |
| Bone marrow lesion | 4 (1) | 107 | 0.11 | −0.08, 0.30 |
| Meniscus | 2 (1) | 264 | −0.24 | −0.36, −0.12 |
| Compositional | 3 (1) | 18 | −3.27 | −3.73, −2.81 |
| Semi-quantitative cartilage† | ||||
| Medial tibial | 1 (1) | 325 | −0.07 | −0.18, 0.04 |
| Medial tibiofemoral | 3 (3) | 224 | 0.55 | 0.47, 0.64 |
| Lateral tibial | 1 (1) | 325 | −0.05 | −0.15, 0.06 |
| Lateral tibiofemoral | 3 (3) | 224 | 0.37 | 0.18, 0.57 |
| Patella | 2 (2) | 238 | 0.29 | 0.03, 0.56 |
| Semi-quantitative other* | ||||
| Synovium | 3 (2) | 68 | 0.47 | 0.18, 0.77 |
| Osteophytes | 4 (1) | 150 | 0.36 | 0.20, 0.52 |
| Bone marrow lesion | 6 (2) | 130 | 0.43 | −0.17, 1.03 |
| Meniscus | 2 (1) | 264 | 0.27 | 0.15, 0.39 |
Analysis used re-sampling techniques.
Analysis did not use re-sampling techniques.
There has been some concern that some of the earlier literature for quantitative measures of cartilage morphometry was more responsive than more recent estimates. Table X reflects an effort to distil distinct time periods. In general, the earlier estimates demonstrate larger SRMs than more recent studies with the medial tibiofemoral estimates from 2002–2006 being −0.95 (−1.15, −0.76) and from more recent studies (2007–2009) being −0.84 (−1.35, −0.33).
Table X.
Results of random-effects pooling of SRM from MRI studies evaluating quantitative cartilage stratified by year of publication and plate region. Studies with multiple estimates had an estimate selected at random and a pooled analysis was performed. The process was repeated 500 times to obtain the empirical distribution of pooled SRMs
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled SRM | 95% CI |
|---|---|---|---|---|
| 2002–2006 | ||||
| Medial femoral | 3 (3) | 126 | −0.59 | −1.21, 0.03 |
| Medial tibial | 7 (7) | 123 | −0.58 | −0.81, −0.35 |
| Medial tibiofemoral* | 4 (3) | 51 | −0.95 | −1.15, −0.76 |
| Lateral femoral | 1 (1) | 117 | −0.01 | −0.19, 0.17 |
| Lateral tibial | 6 (6) | 139 | −0.55 | −0.82, −0.29 |
| Patella | 5 (5) | 141 | −0.68 | −1.04, −0.32 |
| Global | 2 (2) | 24 | −0.58 | −1.15, −0.02 |
| 2007–2009 | ||||
| Medial femoral | 51 (9) | 117 | −0.49 | −0.75, −0.22 |
| Medial tibial | 48 (10) | 135 | −0.42 | −0.62, −0.22 |
| Medial tibiofemoral | 27 (9) | 98 | −0.84 | −1.35, −0.33 |
| Lateral femoral | 31 (7) | 152 | −0.24 | −0.53, 0.05 |
| Lateral tibial | 38 (8) | 154 | −0.56 | −0.79, −0.33 |
| Lateral tibiofemoral | 14 (5) | 110 | −1.01 | −2.04, 0.02 |
| Patella | 8 (4) | 125 | −0.58 | −0.97, −0.18 |
| Global | 3 (2) | 63 | −1.24 | −4.42, 1.94 |
Note: All analyses of articles published in 2002–2006 did not use re-sampling techniques except for the medial tibial-femoral component. All analyses of articles published in 2007–2009 did use re-sampling techniques.
Table XI shows the results of random-effects pooling of SRM from MRI studies evaluating quantitative cartilage stratified by duration of study and plate region for studies published between 2007 and 2009. Studies with multiple estimates had an estimate selected at random and a pooled analysis was performed. In this analysis the pooled SRM for the medial tibiofemoral joint for studies of 1 year or less is −0.80 (−1.27, −0.33) and for studies of 1–2 years is −1.16 (−2.90, 0.58).
Table XI.
Results of random-effects pooling of SRM from MRI studies evaluating quantitative cartilage stratified by duration of study and plate region for studies published between 2007 and 2009. Studies with multiple estimates had an estimate selected at random and a pooled analysis was performed. The process was repeated 500 times to obtain the empirical distribution of pooled SRMs
| Stratification | Number of estimates (Studies) |
Mean sample size |
Pooled SRM | 95% CI |
|---|---|---|---|---|
| Quantitative cartilage | ||||
| 1 year or less | ||||
| Medial femoral | 27 (5) | 82 | −0.49 | −0.81, −0.17 |
| Medial tibial | 18 (6) | 93 | −0.33 | −0.53, −0.13 |
| Medial tibiofemoral | 16 (6) | 83 | −0.80 | −1.27, −0.33 |
| Lateral femoral | 7 (3) | 137 | −0.30 | −0.98, 0.38 |
| Lateral tibial | 8 (4) | 130 | −0.56 | −0.88, −0.24 |
| Lateral tibiofemoral | 3 (2) | 79 | −1.03 | −2.79, 0.73 |
| Patella | 7 (3) | 129 | −0.47 | −0.92, −0.02 |
| Global | 2 (1) | 18 | 0.45 | −0.01, 0.92 |
| 1–2 years | ||||
| Medial femoral | 6 (3) | 104 | −0.51 | −1.15, 0.13 |
| Medial tibial | 6 (3) | 104 | −0.63 | −1.14, −0.12 |
| Medial tibiofemoral | 5 (2) | 53 | −1.16 | −2.90, 0.58 |
| Lateral femoral | 6 (3) | 104 | −0.21 | −0.51, 0.09 |
| Lateral tibial | 6 (3) | 104 | −0.61 | −1.14, −0.08 |
| Lateral tibiofemoral | 5 (2) | 53 | −1.28 | −3.48, 0.91 |
| Patella | 1 (1) | 99 | −0.90 | −1.10, −0.71 |
| Global | 1 (1) | 154 | −2.85 | −3.01, −2.70 |
| Greater than 2 years* | ||||
| Medial femoral | 18 (1) | 174 | −0.32 | −0.47, −0.17 |
| Medial tibial | 24 (1) | 174 | −0.27 | −0.42, −0.12 |
| Medial tibiofemoral | 6 (1) | 174 | −0.41 | −0.56, −0.26 |
| Lateral femoral | 18 (1) | 174 | −0.22 | −0.37, −0.07 |
| Lateral tibial | 24 (1) | 174 | −0.42 | −0.57, −0.27 |
| Lateral tibiofemoral | 6 (1) | 174 | −0.43 | −0.57, −0.28 |
Represents results of one study117.
Discussion
The purpose of this study was to summarize the literature on the responsiveness and reliability of MRI-based measures of knee OA structural change. In general, this review provides clear evidence that structural change in OA can be measured both reliably and with good responsiveness on MRI.
The data from this review indicates that quantitative measures of joint structure have excellent reliability (ICC range 0.81–0.94). Similarly agreement for semi-quantitative measures is good to excellent (kappa range 0.52–0.88). Directly comparing the reliability between quantitative and semi-quantitative techniques is not possible given the extracted data comes from different studies and the statistical methods used are frequently distinct but an overarching view would suggest they are broadly comparable with a slight benefit in reliability for quantitative measures. This is not surprising given the continuous nature of these measures, the greater use of technology to automate processes and quality control vigilance in quantitative measures.
The aim of the systematic review is to provide a summary of the best evidence. However, as a result of issues related to the quality of research, findings of studies can sometimes be misleading or incorrect. To minimize these risks, the quality of the studies was critically appraised using Downs checklist3. The findings from our review indicate that in general this literature is of adequate quality. No studies were identified in our search prior to 1994.
Several studies have suggested that baseline clinical, biomarker and imaging features are predictive of progression of cartilage loss in the medial compartment of the knee and could be used to provide greater study power by selecting a population at greater risk for more rapid progression. Whilst the estimates included in this analysis reflect these studies we have not explicitly selected for these studies so the pooled estimates reflect all studies not just selected estimates for those at highest risk for progression.
This review does not include results focused upon using MRI to stage OA. Whilst MRI has been extensively used for measuring progression its use in staging OA as a disease is at this point quite limited. In an effort to shorten discovery and development timelines, clinical trial brevity is paramount. As OA is typically a very slowly progressive condition, one can optimize trial efficiency by finding more responsive endpoint/s. The results of the responsiveness data reaffirm the potential benefit of MRI compared to plain radiography that generally has SRMs in the 0.3–0.4 range12. For MRI there is quite a lot of variability between different regions within the knee, and with different measures of different tissues, yet the SRM of −0.86 (95%CI −1.26 to −0.46) for the medial tibiofemoral joint quantitative cartilage measure provides advantages with regards to adequately powering studies.
Interestingly there have been a number of concerns raised about what appears to be conflicting data from earlier studies that were more responsive than studies conducted more recently. This analysis confirms that more recent studies (2007–2009) have slightly more conservative SRMs than earlier studies (2002–2006). For example the SRM for the medial tibiofemoral joint quantitative cartilage measure is −0.95 (−1.15, −0.76) for studies from 2002–2006 and is −0.84 (−1.35, −0.33) for studies from 2007 to 2009. The CIs for both these periods overlap and while there may be some differences in techniques between the two time periods including routine blinding to sequence in more recent studies that may explain differences, identifying the reasons for these differences was not the focus of this analysis. We have also been able to clearly demonstrate that adequate responsiveness can be attained in periods as short as 12 months.
Semi-quantitative scoring of MRIs is a valuable method for performing multi-feature assessment of the knee using conventional MRI acquisitions6–8,98. The responsiveness of the semi-quantitative assessment of medial tibiofemoral cartilage morphology (SRM 0.55) is broadly consistent with quantitative assessment for the medial tibiofemoral joint. Semi-quantitative assessment of synovium also demonstrated good responsiveness (SRM 0.52). In addition the semi-quantitative assessment of BMLs, a structural target with good clinical and predictive validity was also adequately responsive (SRM 0.43).
In summary, OA changes on MRI can be measured reliably using both quantitative and semi-quantitative techniques. MRI can accurately and feasibly measure change in quantitative cartilage morphometry over 12 months for knee OA. Based upon extant literature these study findings strongly support inclusion of MRI structure in updated regulatory guidance statements for clinical trials of structure modifying agents in OA.
Acknowledgements
We recognize the invaluable support of Valorie Thompson for administrative and editorial support and OARSI for their invaluable support of this activity. This analysis and literature review was undertaken to facilitate discussions and development of recommendations by the Assessment of Structural Change Working group for the OARSI FDA Initiative. The members of the working group were: Philip Conaghan, MB, BS, PhD (Chair), David Hunter, MBBS, PhD, Jeffrey Duryea, PhD, Garry Gold, MD, Steven Mazzuca, PhD, Jean Francis Maillefert, MD, Timothy Mosher, MD, Hollis Potter, MD, David Felson, MD, Ali Guermazi, MD, Helen Keen, MD, Gayle Lester, PhD, Wayne Tsuji, MD, John Randle, PhD, Felix Eckstein, MD, Erika Schneider, PhD, Elena Losina, PhD, Sarah Kingsbury, PhD, William Reichman, PhD, Jean Pierre Pelletier, MD, Saara Totterman, MD, PhD, Rose Maciewicz, PhD, Bernard Dardzinski, PhD, Mona Wahba, MD, Marie Pierre Hellio Le Graverand-Gastineau, MD, PhD, DSc, Elisabeth Morris, DVM, Jeffrey Kraines, MD, Lucio Rovati, MD, Don Dreher, MD, PhD, James Huckle, PhD, Mary-Ann Preston, PhD, Brooks Story, PhD.
Declaration of funding and role of funding source
The OARSI FDA OA Initiative received financial support from the following professional organization:
American College of Rheumatology
Additionally the OARSI FDA OA Initiative received financial support from the following companies:
Amgen
ArthroLab
AstraZeneca
Bayer Healthcare
Chondrometrics
CombinatoRx
Cypress BioScience
DePuy Mitek
Expanscience 4QImaging
Genevrier/IBSA
Genzyme
King (Alpharma)
Merck
Merck Serono
NicOx
Pfizer
Rottapharm
Smith & Nephew
Wyeth
While individuals from pharmaceutical, biotechnology and device companies actively participated in on-going working group discussions, due to the conflict of interest policy enacted by OARSI, these individuals were not allowed to vote on the final recommendations made by OARSI to the Food and Drug Administration.
Footnotes
Author contributions
DJH conceived and designed the study, drafted the manuscript and takes responsibility for the integrity of the work as a whole, from inception to finished article. EL and WZ were also involved in the design of the study. All authors contributed to acquisition of the data. All authors critically revised the manuscript and gave final approval of the article for submission.
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest
David Hunter receives research or institutional support from DonJoy, NIH, and Stryker.
Other authors declared no conflict of interest.
References
- 1.Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, et al. Imaging in osteoarthritis. [Review] [183 refs] Rheum Dis Clin North Am. 2008;34:645–687. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. [Review] [238 refs] NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 3.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. [Review] [100 refs] Curr Opin Rheumatol. 2007;19:435–443. doi: 10.1097/BOR.0b013e328248b4be. [DOI] [PubMed] [Google Scholar]
- 5.Gray ML, Burstein D. Molecular (and functional) imaging of articular cartilage. [Review] [69 refs] J Musculoskelet Neuronal Interact. 2004;4:365–368. [PubMed] [Google Scholar]
- 6.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–2892. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 8.Hunter D, Gale D, Grainger G, Lo G, Guermazi A, Conaghan P. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 9.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)–inter-observer and intraobserver reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102. doi: 10.1007/s00256-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 10.Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for outcome measures in rheumatology. J Rheumatol. 1998;25:198–199. [PubMed] [Google Scholar]
- 11.Lassere M. A users guide to measurement in medicine. Osteoarthritis Cartilage. 2006;14(Suppl 1):10–14. doi: 10.1016/j.joca.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Emrani PS, Katz JN, Kessler CL, Reichmann WM, Wright EA, McAlindon TE, et al. Joint space narrowing and Kellgren–Lawrence progression in knee osteoarthritis: an analytic literature synthesis. Osteoarthritis Cartilage. 2008;16:873–882. doi: 10.1016/j.joca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karvonen RL, Negendank WG, Teitge RA, Reed AH, Miller PR, Fernandez-Madrid F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21:1310–1318. [PubMed] [Google Scholar]
- 14.Peterfy CG, van Dijke CF, Janzen DL, Gluer CC, Namba R, Majumdar S, et al. Quantification of articular cartilage in the knee with pulsed saturation transfer subtraction and fat-suppressed MR imaging: optimization and validation. Radiology. 1994;192:485–491. doi: 10.1148/radiology.192.2.8029420. [DOI] [PubMed] [Google Scholar]
- 15.Marshall KW, Mikulis DJ, Guthrie BM. Quantitation of articular cartilage using magnetic resonance imaging and three-dimensional reconstruction. J Orthop Res. 1995;13:814–823. doi: 10.1002/jor.1100130603. [DOI] [PubMed] [Google Scholar]
- 16.Disler DG, McCauley TR, Kelman CG, Fuchs MD, Ratner LM, Wirth CR, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167:127–132. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 17.Dupuy DE, Spillane RM, Rosol MS, Rosenthal DI, Palmer WE, Burke DW, et al. Quantification of articular cartilage in the knee with three-dimensional MR imaging. Acad Radiol. 1996;3:919–924. doi: 10.1016/s1076-6332(96)80299-6. [DOI] [PubMed] [Google Scholar]
- 18.Trattnig S, Huber M, Breitenseher MJ, Trnka HJ, Rand T, Kaider A, et al. Imaging articular cartilage defects with 3D fat-suppressed echo planar imaging: comparison with conventional 3D fat-suppressed gradient echo sequence and correlation with histology. J Comput Assist Tomogr. 1998;22:8–14. doi: 10.1097/00004728-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Drape JL, Pessis E, Auleley GR, Chevrot A, Dougados M, Ayral X. Quantitative MR imaging evaluation of chondropathy in osteoarthritic knees. Radiology. 1998;208:49–55. doi: 10.1148/radiology.208.1.9646792. [DOI] [PubMed] [Google Scholar]
- 20.Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis Cartilage. 1999;7:265–271. doi: 10.1053/joca.1998.0200. [DOI] [PubMed] [Google Scholar]
- 21.Pham XV, Monteiro I, Judet O, Sissakian JF, Plantin P, Aegerter P, et al. Magnetic resonance imaging changes in periarticular soft tissues during flares of medial compartment knee osteoarthritis. Preliminary study in 10 p.tients. Rev Rhum Engl Ed. 1999;66:398–403. [PubMed] [Google Scholar]
- 22.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 23.Hyhlik-Durr A, Faber S, Burgkart R, Stammberger T, Maag KP, Englmeier KH, et al. Precision of tibial cartilage morphometry with a coronal water-excitation MR sequence. Eur Radiol. 2000;10:297–303. doi: 10.1007/s003300050047. [DOI] [PubMed] [Google Scholar]
- 24.Jones G, Glisson M, Hynes K, Cicuttini F. Sex and site differences in cartilage development: a possible explanation for variations in knee osteoarthritis in later life. Arthritis Rheum. 2000;43:2543–2549. doi: 10.1002/1529-0131(200011)43:11<2543::AID-ANR23>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Wluka AE, Davis SR, Bailey M, Stuckey SL, Cicuttini FM. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann Rheum Dis. 2001;60:332–336. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [see comments.] [DOI] [PubMed] [Google Scholar]
- 27.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–1337. [PubMed] [Google Scholar]
- 28.Bergin D, Keogh C, O’Connell M, Rowe D, Shah B, Zoga A, et al. Atraumatic medial collateral ligament oedema in medial compartment knee osteoarthritis. Skeletal Radiol. 2002;31:14–18. doi: 10.1007/s002560100418. [DOI] [PubMed] [Google Scholar]
- 29.Beuf O, Ghosh S, Newitt DC, Link TM, Steinbach L, Ries M, et al. Magnetic resonance imaging of normal and osteoarthritic trabecular bone structure in the human knee. Arthritis Rheum. 2002;46:385–393. doi: 10.1002/art.10108. [DOI] [PubMed] [Google Scholar]
- 30.Wluka AE, Stuckey S, Snaddon J, Cicuttini FM. The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum. 2002;46:2065–2072. doi: 10.1002/art.10460. [DOI] [PubMed] [Google Scholar]
- 31.Gandy SJ, Dieppe PA, Keen MC, Maciewicz RA, Watt I, Waterton JC, et al. No loss of cartilage volume over three years in patients with knee osteoarthritis as assessed by magnetic resonance imaging. Osteoarthritis Cartilage. 2002;10:929–937. doi: 10.1053/joca.2002.0849. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. [comment] [DOI] [PubMed] [Google Scholar]
- 33.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL, Davis SR. Effect of estrogen replacement therapy on patella cartilage in healthy women. Clin Exp Rheumatol. 2003;21:79–82. [PubMed] [Google Scholar]
- 34.Raynauld JP, Kauffmann C, Beaudoin G, Berthiaume MJ, de Guise JA, Bloch DA, et al. Reliability of a quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthritis Cartilage. 2003;11:351–360. doi: 10.1016/s1063-4584(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 35.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 36.Hill CL, Gale DR, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Periarticular lesions detected on magnetic resonance imaging: prevalence in knees with and without symptoms. Arthritis Rheum. 2003;48:2836–2844. doi: 10.1002/art.11254. [DOI] [PubMed] [Google Scholar]
- 37.Glaser C, Burgkart R, Kutschera A, Englmeier KH, Reiser M, Eckstein F. Femoro-tibial cartilage metrics from coronal MR image data: technique, test-retest reproducibility, and findings in osteoarthritis. Magn Reson Med. 2003;50:1229–1236. doi: 10.1002/mrm.10648. [DOI] [PubMed] [Google Scholar]
- 38.Lindsey CT, Narasimhan A, Adolfo JM, Jin H, Steinbach LS, Link T, et al. Magnetic resonance evaluation of the interrelationship between articular cartilage and trabecular bone of the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:86–96. doi: 10.1016/j.joca.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL, Cicuttini FM, Wluka AE, et al. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum. 2004;50:94–97. doi: 10.1002/art.11483. [see comment] [DOI] [PubMed] [Google Scholar]
- 40.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 41.Cicuttini F, Wluka A, Hankin J, Wang Y, Cicuttini F, Wluka A, et al. Longitudinal study of the relationship between knee angle and tibiofemoral cartilage volume in subjects with knee osteoarthritis. Rheumatology. 2004;43:321–324. doi: 10.1093/rheumatology/keh017. [DOI] [PubMed] [Google Scholar]
- 42.Dashti M, Wluka AE, Geso M, Davis SR, Stuckey S, Cicuttini FM. Relationship between the area of cartilage shown on the magnetic resonance imaging middle-slice image of the medial and lateral tibial cartilages with cartilage volume and grade of osteoarthritis over time. Scand J Rheumatol. 2004;33:87–93. doi: 10.1080/03009740310004612. [DOI] [PubMed] [Google Scholar]
- 43.Cicuttini FM, Wluka AE, Hankin J, Stuckey S. Comparison of patella cartilage volume and radiography in the assessment of longitudinal joint change at the patellofemoral joint. J Rheumatol. 2004;31:1369–1372. [PubMed] [Google Scholar]
- 44.Baysal O, Baysal T, Alkan A, Altay Z, Yologlu S. Comparison of MRI graded cartilage and MRI based volume measurement in knee osteoarthritis. Swiss Med Wkly. 2004;134:283–288. doi: 10.4414/smw.2004.10546. [DOI] [PubMed] [Google Scholar]
- 45.Yoshioka H, Stevens K, Hargreaves BA, Steines D, Genovese M, Dillingham MF, et al. Magnetic resonance imaging of articular cartilage of the knee: comparison between fat-suppressed three-dimensional SPGR imaging, fat-suppressed FSE imaging, and fat-suppressed three-dimensional DEFT imaging, and correlation with arthroscopy. J Magn Reson Imaging. 2004;20:857–864. doi: 10.1002/jmri.20193. [DOI] [PubMed] [Google Scholar]
- 46.Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G, et al. Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage. 2005;13:198–205. doi: 10.1016/j.joca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52:794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 48.Maataoui A, Graichen H, Abolmaali ND, Khan MF, Gurung J, Straub R, et al. Quantitative cartilage volume measurement using MRI: comparison of different evaluation techniques. Eur Radiol. 2005;15:1550–1554. doi: 10.1007/s00330-005-2744-7. [DOI] [PubMed] [Google Scholar]
- 49.Cicuttini F, Hankin J, Jones G, Wluka A. Comparison of conventional standing knee radiographs and magnetic resonance imaging in assessing progression of tibiofemoral joint osteoarthritis. Osteoarthritis Cartilage. 2005;13:722–727. doi: 10.1016/j.joca.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Huh YM, Kim S, Suh JS, Song H, Song K, Shin KH. The role of popliteal lymph nodes in differentiating rheumatoid arthritis from osteoarthritis by using CE 3D FSPGR MR imaging: relationship of the inflamed synovial volume. Korean J Radiol. 2005;6:117–124. doi: 10.3348/kjr.2005.6.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wluka AE, Ding C, Jones G, Cicuttini FM. The clinical correlates of articular cartilage defects in symptomatic knee osteoarthritis: a prospective study. Rheumatology (Oxford) 2005;44:1311–1316. doi: 10.1093/rheumatology/kei018. [DOI] [PubMed] [Google Scholar]
- 52.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 54.Sengupta M, Zhang YQ, Niu JB, Guermazi A, Grigorian M, Gale D, et al. High signal in knee osteophytes is not associated with knee pain. Osteoarthritis Cartilage. 2006;14:413–417. doi: 10.1016/j.joca.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 57.Brandt KD, Mazzuca SA, Buckwalter KA. Acetaminophen, like conventional NSAIDs, may reduce synovitis in osteoarthritic knees. Rheumatology (Oxford) 2006;45:1389–1394. doi: 10.1093/rheumatology/kel100. [DOI] [PubMed] [Google Scholar]
- 58.Jaremko JL, Cheng RW, Lambert RG, Habib AF, Ronsky JL. Reliability of an efficient MRI-based method for estimation of knee cartilage volume using surface registration. Osteoarthritis Cartilage. 2006;14:914–922. doi: 10.1016/j.joca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Hunter DJ, LaValley M, Li J, Zhang Y, Bauer D, Nevitt M, et al. Urinary pentosidine does not predict cartilage loss among subjects with symptomatic knee OA: the BOKS study. Osteoarthritis Cartilage. 2007;15:93–97. doi: 10.1016/j.joca.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Boks SS, Vroegindeweij D, Koes BW, Hunink MM, Bierma-Zeinstra SM. Magnetic resonance imaging abnormalities in symptomatic and contralateral knees: prevalence and associations with traumatic history in general practice. Am J Sports Med. 2006;34:1984–1991. doi: 10.1177/0363546506290189. [DOI] [PubMed] [Google Scholar]
- 61.Brem MH, Pauser J, Yoshioka H, Brenning A, Stratmann J, Hennig FF, et al. Longitudinal in vivo reproducibility of cartilage volume and surface in osteoarthritis of the knee. Skeletal Radiol. 2007;36:315–320. doi: 10.1007/s00256-006-0208-z. [DOI] [PubMed] [Google Scholar]
- 62.Folkesson J, Dam EB, Olsen OF, Pettersen PC, Christiansen C. Segmenting articular cartilage automatically using a voxel classification approach. IEEE Trans Med Imaging. 2007;26:106–115. doi: 10.1109/TMI.2006.886808. [DOI] [PubMed] [Google Scholar]
- 63.Dam EB, Folkesson J, Pettersen PC, Christiansen C. Automatic morphometric cartilage quantification in the medial tibial plateau from MRI for osteoarthritis grading. Osteoarthritis Cartilage. 2007;15:808–818. doi: 10.1016/j.joca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Baranyay FJ, Wang Y, Wluka AE, English DR, Giles GG, Sullivan RO, et al. Association of bone marrow lesions with knee structures and risk factors for bone marrow lesions in the knees of clinically healthy, community-based adults. Semin Arthritis Rheum. 2007;37:112–118. doi: 10.1016/j.semarthrit.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Hanna F, Teichtahl AJ, Bell R, Davis SR, Wluka AE, O’Sullivan R, et al. The cross-sectional relationship between fortnightly exercise and knee cartilage properties in healthy adult women in midlife. Menopause. 2007;14:830–834. doi: 10.1097/gme.0b013e31802f316b. [DOI] [PubMed] [Google Scholar]
- 66.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qazi AA, Folkesson J, Pettersen PC, Karsdal MA, Christiansen C, Dam EB. Separation of healthy and early osteoarthritis by automatic quantification of cartilage homogeneity. Osteoarthritis Cartilage. 2007;15:1199–1206. doi: 10.1016/j.joca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Guymer E, Baranyay F, Wluka AE, Hanna F, Bell RJ, Davis SR, et al. A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage. 2007;15:1437–1442. doi: 10.1016/j.joca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Eckstein F, Kunz M, Schutzer M, Hudelmaier M, Jackson RD, Yu J, et al. Two year longitudinal change and test-retest-precision of knee cartilage morphology in a pilot study for the osteoarthritis initiative. Osteoarthritis Cartilage. 2007;15:1326–1332. doi: 10.1016/j.joca.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akhtar S, Poh CL, Kitney RI. An MRI derived articular cartilage visualization framework. Osteoarthritis Cartilage. 2007;15:1070–1085. doi: 10.1016/j.joca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis. 2008;67:683–688. doi: 10.1136/ard.2007.073023. [DOI] [PubMed] [Google Scholar]
- 72.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 73.Lo GH, Niu J, McLennan CE, Kiel DP, McLean RR, Guermazi A, et al. Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthritis Cartilage. 2008;16:261–267. doi: 10.1016/j.joca.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davies-Tuck M, Teichtahl AJ, Wluka AE, Wang Y, Urquhart DM, Cui J, et al. Femoral sulcus angle and increased patella facet cartilage volume in an osteoarthritic population. Osteoarthritis Cartilage. 2008;16:131–135. doi: 10.1016/j.joca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Folkesson J, Dam EB, Olsen OF, Christiansen C. Accuracy evaluation of automatic quantification of the articular cartilage surface curvature from MRI. Acad Radiol. 2007;14:1221–1228. doi: 10.1016/j.acra.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Sanz R, Marti-Bonmati L, Rodrigo JL, Moratal D. MR pharmacokinetic modeling of the patellar cartilage differentiates normal from pathological conditions. J Magn Reson Imaging. 2008;27:171–177. doi: 10.1002/jmri.21233. [DOI] [PubMed] [Google Scholar]
- 77.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007;56:4048–4054. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 78.Hernandez-Molina G, Guermazi A, Niu J, Gale D, Goggins J, Amin S, et al. Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum. 2008;58:130–136. doi: 10.1002/art.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amin S, Guermazi A, LaValley MP, Niu J, Clancy M, Hunter DJ, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:897–902. doi: 10.1016/j.joca.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teichtahl AJ, Wang Y, Wluka AE, Szramka M, English DR, Giles GG, et al. The longitudinal relationship between body composition and patella cartilage in healthy adults. Obesity (Silver Spring) 2008;16:421–427. doi: 10.1038/oby.2007.37. [DOI] [PubMed] [Google Scholar]
- 81.Anandacoomarasamy A, Bagga H, Ding C, Burkhardt D, Sambrook PN, March LM. Predictors of clinical response to intraarticular Hylan injections – a prospective study using synovial fluid measures, clinical outcomes, and magnetic resonance imaging. J Rheumatol. 2008;35:685–690. [PubMed] [Google Scholar]
- 82.Eckstein F, Buck RJ, Burstein D, Charles HC, Crim J, Hudelmaier M, et al. Precision of 3.0 tesla quantitative magnetic resonance imaging of cartilage morphology in a multicentre clinical trial. Ann Rheum Dis. 2008;67:1683–1688. doi: 10.1136/ard.2007.076919. [DOI] [PubMed] [Google Scholar]
- 83.Reichenbach S, Guermazi A, Niu J, Neogi T, Hunter DJ, Roemer FW, et al. Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage. 2008;16:1005–1010. doi: 10.1016/j.joca.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422–427. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bolbos RI, Zuo J, Banerjee S, Link TM, Ma CB, Li X, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage. 2008;16:1150–1159. doi: 10.1016/j.joca.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26:1215–1220. doi: 10.1016/j.mri.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Folkesson J, Dam EB, Olsen OF, Karsdal MA, Pettersen PC, Christiansen C. Automatic quantification of local and global articular cartilage surface curvature: biomarkers for osteoarthritis? Magn Reson Med. 2008;59:1340–1346. doi: 10.1002/mrm.21560. [DOI] [PubMed] [Google Scholar]
- 88.Mills PM, Wang Y, Cicuttini FM, Stoffel K, Stachowiak GW, Podsiadlo P, et al. Tibio-femoral cartilage defects 3–5 years following arthroscopic partial medial meniscectomy. Osteoarthritis Cartilage. 2008;16:1526–1531. doi: 10.1016/j.joca.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Dore D, Ding C, Jones G. A pilot study of the reproducibility and validity of measuring knee subchondral bone density in the tibia. Osteoarthritis Cartilage. 2008;16:1539–1544. doi: 10.1016/j.joca.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 90.Pelletier JP, Raynauld JP, Abram F, Haraoui B, Choquette D, Martel-Pelletier J. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage. 2008;16(Suppl 3):S8–S13. doi: 10.1016/j.joca.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 91.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rauscher I, Stahl R, Cheng J, Li X, Huber MB, Luke A, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249:591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kijowski R, Blankenbaker DG, Davis KW, Shinki K, Kaplan LD, De Smet AA. Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology. 2009;250:839–848. doi: 10.1148/radiol.2503080822. [DOI] [PubMed] [Google Scholar]
- 94.Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–1956. [PubMed] [Google Scholar]
- 95.Wluka AE, Stuckey S, Brand C, Cicuttini FM, Wluka AE, Stuckey S, et al. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29:2585–2591. [PubMed] [Google Scholar]
- 96.Cicuttini F, Wluka A, Wang Y, Stuckey S, Cicuttini F, Wluka A, et al. The determinants of change in patella cartilage volume in osteoarthritic knees. J Rheumatol. 2002;29:2615–2619. [PubMed] [Google Scholar]
- 97.Pessis E, Drape JL, Ravaud P, Chevrot A, Dougados M, Ayral X. Assessment of progression in knee osteoarthritis: results of a 1 year study comparing arthroscopy and MRI. Osteoarthritis Cartilage. 2003;11:361–369. doi: 10.1016/s1063-4584(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 98.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63:264–268. doi: 10.1136/ard/2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, et al. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:997–1005. doi: 10.1016/j.joca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 100.Zhai G, Ding C, Cicuttini F, Jones G. Optimal sampling of MRI slices for the assessment of knee cartilage volume for cross-sectional and longitudinal studies. BMC Musculoskelet Disord. 2005;6:10. doi: 10.1186/1471-2474-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, Wluka AE, Cicuttini FM, Wang Y, Wluka AE, Cicuttini FM. The determinants of change in tibial plateau bone area in osteoarthritic knees: a cohort study. Arthritis Res Ther. 2005;7:R687–R693. doi: 10.1186/ar1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding C, Cicuttini F, Scott F, Boon C, Jones G. Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum. 2005;52:3918–3927. doi: 10.1002/art.21474. [DOI] [PubMed] [Google Scholar]
- 103.Hunter DJ, Conaghan PG, Peterfy CG, Bloch D, Guermazi A, Woodworth T, et al. Responsiveness, effect size, and smallest detectable difference of Magnetic Resonance Imaging in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl A):A112–A115. doi: 10.1016/j.joca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 104.Wluka AE, Forbes A, Wang Y, Hanna F, Jones G, Cicuttini FM. Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res Ther. 2006;8:R90. doi: 10.1186/ar1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding C, Cicuttini F, Blizzard L, Scott F, Jones G, Ding C, et al. A longitudinal study of the effect of sex and age on rate of change in knee cartilage volume in adults. Rheumatology. 2007;46:273–279. doi: 10.1093/rheumatology/kel243. [DOI] [PubMed] [Google Scholar]
- 106.Hunter DJ, Zhang YQ, Tu X, LaValley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 107.Bruyere O, Genant H, Kothari M, Zaim S, White D, Peterfy C, et al. Longitudinal study of magnetic resonance imaging and standard X-rays to assess disease progression in osteoarthritis. Osteoarthritis Cartilage. 2007;15:98–103. doi: 10.1016/j.joca.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 108.Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau MP, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15:1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 109.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hunter DJ, Li J, LaValley M, Bauer DC, Nevitt M, DeGroot J, et al. Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee Study. Arthritis Res Ther. 2007;9 doi: 10.1186/ar2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Teichtahl AJ, Wluka AE, Cicuttini FM. Frontal plane knee alignment is associated with a longitudinal reduction in patella cartilage volume in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:851–854. doi: 10.1016/j.joca.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 112.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68:349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–1726. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 114.Teichtahl AJ, Davies-Tuck ML, Wluka AE, Jones G, Cicuttini FM. Change in knee angle influences the rate of medial tibial cartilage volume loss in knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:8–11. doi: 10.1016/j.joca.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 115.Raynauld JP, Martel-Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, et al. Protective effects of licofelone, a 5-lip-oxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68:938–947. doi: 10.1136/ard.2008.088732. [DOI] [PubMed] [Google Scholar]
- 116.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–1570. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 118.Hellio Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls - a multicenter study using 3.0 Tesla MRI and Lyon schuss radiography. Ann Rheum Dis. 2010;69:155–162. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- 119.Eckstein F, Wirth W, Hudelmaier MI, Maschek S, Hitzl W, Wyman BT, et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the osteoarthritis initiative. Arthritis Res Ther. 2009;11:R90. doi: 10.1186/ar2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–1225. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hunter DJ, Li L, Zhang YQ, Totterman S, Tamez J, Kwoh CK, et al. Region of interest analysis: by selecting regions with denuded areas can we detect greater amounts of change? Osteoarthritis Cartilage. 2010;18:175–183. doi: 10.1016/j.joca.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]