Abstract
Significant advances have recently been made in understanding the mechanisms involved in noncontact anterior cruciate ligament (ACL) injury. Most ACL injuries involve minimal to no contact. Female athletes sustain a two- to eightfold greater rate of injury than do their male counterparts. Recent videotape analyses demonstrate significant differences in average leg and trunk positions during injury compared with control subjects. These findings as well as those of cadaveric and MRI studies indicate that axial compressive forces are a critical component in noncontact ACL injury. A complete understanding of the forces and risk factors associated with noncontact ACL injury should lead to the development of improved preventive strategiess for this devastating injury.
Introduction
Nearly three quarters of anterior cruciate ligament (ACL) injuries are noncontact injuries.1 Understanding the mechanism of injury is critical to optimizing prevention strategies. Several theories and risk factors have been proposed to explain the mechanism of noncontact ACL injury, including impingement on the intercondylar notch,2 quadriceps contraction,3 the quadriceps-hamstring force balance and, more recently, axial compressive forces on the lateral aspect of the joint.4,5 Female athletes have been reported to sustain noncontact ACL injuries at a rate two- to eightfold greater than their male counterparts.6 Many explanations for the increased risk of injury to female athletes have been proposed, including increased knee valgus or abduction moments, generalized joint laxity,2 knee recurvatum,1 ACL size8, and the hormonal effects of estrogen on the ACL.8 This article explores the theory that axial impulsive forces (ie, force applied for a very short period) lead to ACL disruption by buckling the leg and that valgus forces and the compressiveanterior force of the quadriceps also contribute, perhaps by lowering the force threshold required for this disruption.
Impingement
Impingement of the ACL against the medial border of the intercondylar notch has been proposed as a possible anatomic cause of ACL injury.2 The literature on intercondylar notch stenosis as a predictor of ACL injury is controversial and is limited by radiographic techniques used to measure the dimensions of the intercondylar notch. Although it is plausible that ACL impingement might occur with the knee in hyperextension, most ACL injuries are known to occur with the knee in partial flexion.1,5 Further refuting the likelihood that impingement causes ACL injury is the fact that most noncontact ACL injuries occur close to the femoral attachment site. An impingement mechanism would be expected to cause midsubstance injury instead.
Quadriceps Force
Previous reports have postulated that the anterior vector of the quadriceps is the primary contributing force to ACL injury3,8 because the quadriceps is one of the primary producers of anterior knee force with the knee at or near full extension.9 However, the angle of the patellar tendon is shallow—10° to 25° at full extension10,11—and the quadriceps primarily generates a compressive tibiofemoral joint force; the anterior force is a minor component (Figure 1). Thus, the compressive vector of a quadriceps contraction is at least twice that of the anterior shear vector at 0°.11 The one study to date that demonstrated rupture of the ACL with isolated quadriceps force produced failure in only 6 of the 11 cadavers and used an unrealistically high level of quadriceps force (4,500 N).3
Figure 1.
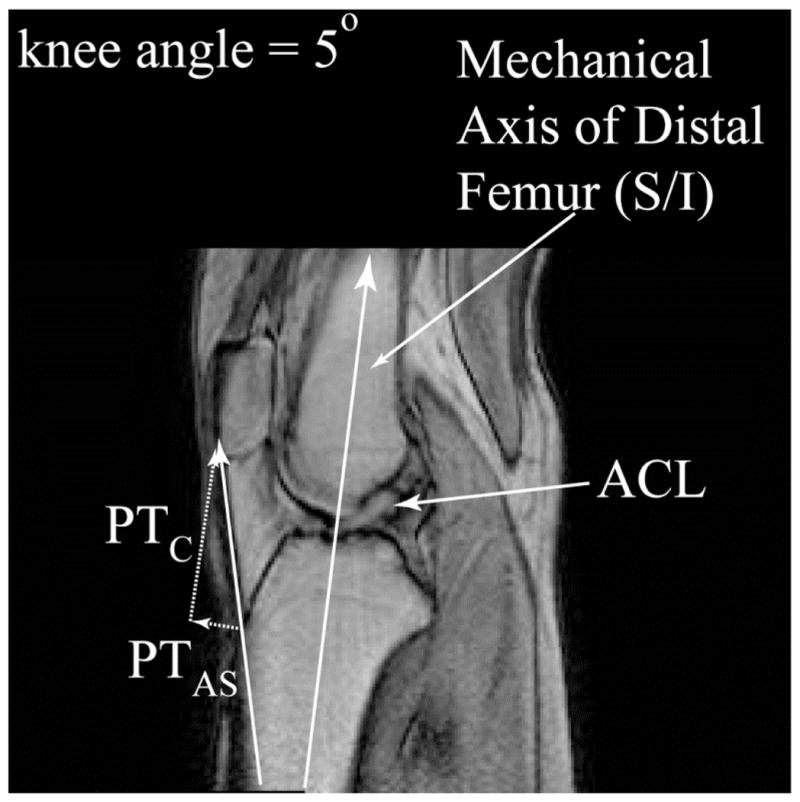
Sagittal magnetic resonance image with the knee at an angle of 5°, demonstrating the angle of the patellar tendon (solid white arrow) at its attachment to the tibial tubercle. Because the angle is low (<45°), the compressive vector (PTC) is larger than the anterior shear vector (PTAS). ACL = anterior cruciate ligament.
Bone bruises seen on magnetic resonance images after noncontact ACL injury are more consistent with impaction injury than with excessive anterior force.12 Numerous in vivo,13 in vitro,14 and modeling15 studies have demonstrated an increase in ACL strain at or near full extension with isolated quadriceps activity. These findings have been used to support the theory that the anterior thrust of the quadriceps is the predominant factor in ACL rupture. However, numerous studies have clearly demonstrated ACL strain resulting from isolated quadriceps activity at knee angles at which the quadriceps creates posterior force.15 Thus, it is more likely that quadriceps contraction contributes to ACL injury by increasing the compressive loads on the tibiofemoral joint16 rather than by introducing a large anterior force.
Hamstrings compensation
Co-contraction of the hamstrings has been proposed as a protective mechanism for the ACL.17 However, Pandy and Shelburne15 demonstrated that the architecture of the hamstrings precludes them from providing protection of the ACL at full extension. The inability of the hamstrings to protect the ACL was further supported in the experimental work of Simonsen et al.18 Thus, hamstring co-contraction likely contributes to tibiofemoral joint compression forces with minor posterior protective forces.
Axial/Compressive Forces
Until recently, it was assumed that an axial compressive force on the tibiofemoral joint would not increase strain at the ACL. However, literature published since the mid 1990s supports the concept that adding a compressive force to the knee joint,4,14,16,19,20 such as during the transition from non–weight bearing to weight bearing,13 does cause anterior translation of the tibia.
Meyer and colleagues4,20 were the first to demonstrate that excessive joint compressive loads and internal torque can lead to complete ACL rupture in human cadaver knees. They reported peak compression loads at failure ranging from 2,900 N to 7,800 N at knee extension angles ranging from 30° to 120°. Similar results were reported in a porcine study, with ACL failure occurring at compressive loads of 1,812 N to 2,659 N.19 In a study by Meyer et al,21 occult microcracks at the interface between cartilage and subchondral bone caused by compression forces were consistent with ACL bone bruises found on MRI after injury. The authors hypothesized that the dominant factor leading to ACL failure is a compressive force acting on the posterior tibial slope, which results in posterior displacement of the femoral condyle on the tibial plateau. These compressive forces result primarily from inadequate absorption of ground reaction forces (GRFs) by the lower leg; however, both quadriceps and hamstring contraction can contribute to the compressive force, as well.
Several authors have demonstrated that an axial weight-bearing compressive force combined with increased tibial posterior slope produces an anterior tibial force in knees with an intact or deficient ACL.22,23 Dejour and Bonnin23 reported a 6-mm increase in anterior tibial translation with monopodal stance for every 10° increase in tibial slope. Torzilli et al16 reported that compressive forces resulted in an anterior force vector on the proximal tibia due to the posterior slope of the tibial plateau. Giffin et al22 observed that anterior tibial translation increased following osteotomy to enhance the posterior tibial slope. Adding an isolated compressive force postosteotomy further increased anterior tibial placement, whereas the addition of an isolated AP load postosteotomy resulted in no further tibial anterior shift or increase in in situ forces in the cruciate ligaments.
Whole Body Dynamics
The hip, knee, ankle, and foot help absorb GRFs during normal landing and deceleration. The hip muscles assist in the absorption of reaction forces from the upper body weight, while the knee, ankle, and foot absorb the GRFs. In the ACL injury position, the joint segments of the leg are not effective in synergistically dampening GRFs in an accordion like fashion. In particular, during noncontact ACL injury, the foot lands at or near the flat-footed position,5 with the result that the lower leg and foot act as a single segment. The position of the foot and ankle reduces the ability of the calf muscles to absorb GRFs, and the leg is converted into a two-segment column (above the knee and below the knee) that may be incapable of adequately absorbing the energy from the GRFs,24 resulting in column buckling.
Ankle/Foot
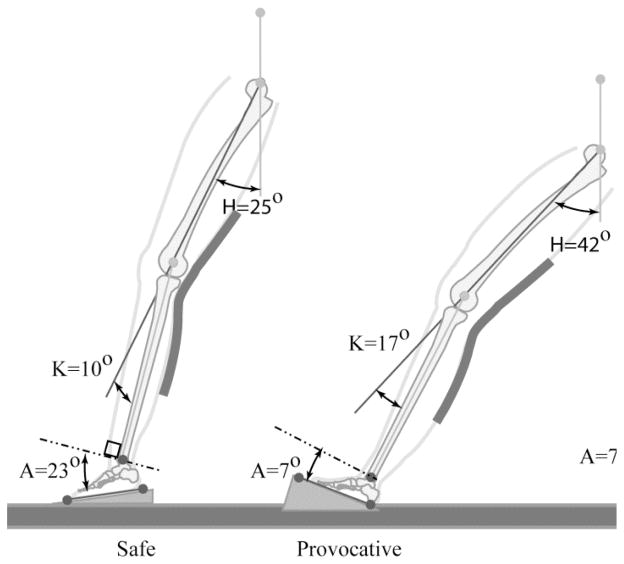
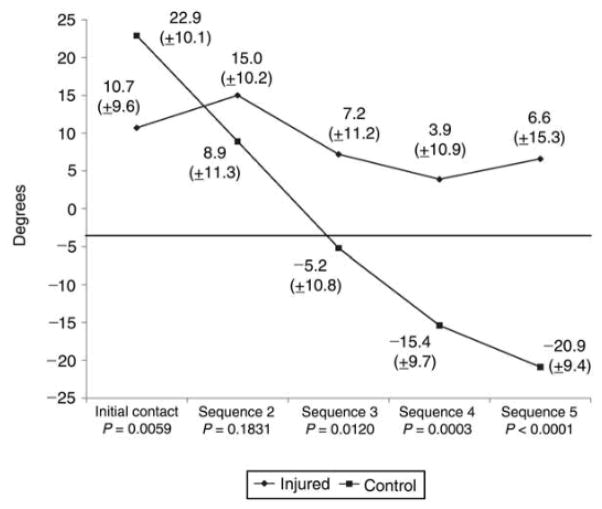
A recent video-based analysis by Boden et al5 demonstrated key dynamic elements associated with noncontact ACL rupture during competitive sports. The authors identified a safe (ie, control) and a provocative single-leg landing position (Figure 2). Subjects who experienced ACL rupture initially came into contact with the ground with the hindfoot or with foot flat, whereas the control subjects, those who did not rupture their ACL during a similar activity and maneuver, landed on the forefoot. The injured athletes had significantly less ankle plantar flexion than did uninjured control athletes at the point of initial ground contact (10.7° and 22.9°, respectively; P = 0.0059) (Figure 3), with minimal change from initial contact (frame 1) to frame 5 (4.1° versus 43.8°, respectively) at a rate of 30 frames per second (30 Hz). Persons with ACL rupture reached the flatfoot position 50% sooner than did controls (average difference, 1.6 frames).
Figure 2.
Initial foot contact with the ground in a safe (A) and an injured (B) athlete demonstrating safe (A) and dangerous (B) landing posture. (Reproduced with permission from Boden BP, Torg JS, Knowles SB, Hewett TE: Video analysis of anterior cruciate ligament injury: Abnormalities in hip and ankle kinematics. Am J Sports Med 2009;37:252–259.)
Figure 3.
Sagittal ankle angles in injured and uninjured athletes for the first five frames of videotape analysis, beginning with initial ground contact (30 Hz). (Reproduced with permission from Boden BP, Torg JS, Knowles SB, Hewett TE: Video analysis of anterior cruciate ligament injury: Abnormalities in hip and ankle kinematics. Am J Sports Med 2009;37:252–259.)
This shorter time span reduces the ability of the calf muscles to contract and absorb forces, resulting in increased impulsive forces. The impulse force is directly related to change in velocity and inversely related to the time required for the change. Assuming that injured and control athletes experience the same change in velocity, then the shorter stopping time for the injured athletes results in a higher impulsive force. A head-on car accident provides an analogous situation. The crumple zone of a car and a deployed airbag serve to slow the time of impact, resulting in lower forces on the driver. Likewise, the gastrocnemius-soleus complex slows the speed with which the GRFs travel to the knee. The ab- normal mechanics measured on videotapes of injured athletes5 imply that the calf muscles do not have sufficient time to absorb the GRFs. This results in transmission of the GRFs to the knee, which increases the compressive or impulse force. Thus, the plantarflexed ankle in the safe position protects the ACL by giving the forces more time to dissipate. Maximum peak vertical GRFs experienced by one-leg landings after jumping maneuvers have been estimated to range from 2 to 18 times body weight.25 For an individual weighing 70 kg (154 lb) and with a GRF five times body weight, the body must absorb 3,430 N of force (1 lb = 4.4545 N). The threshold for ACL tear (ie, 2,160 N) can easily be exceeded if the calf muscles do not absorb a large portion of these forces. The provocative position places the tibia in an unstable position in which subluxation is more likely than rolling.26 High axial loads placed on the tibia result in buckling of the knee, along with anterior displacement of the tibia and ACL disruption. The shortened time of the impulsive force reduces the ability of the soft-tissue components of the leg to dampen the force. Thus, landing on the forefoot may be one of the most crucial aspects of preventing ACL injury.
Hip/Trunk
Videotape analysis also revealed that subjects with ACL rupture had significantly higher hip flexion angles at initial ground contact (50.1° versus 25.8°; P = 0.0003).5 Qualitatively, this would place the torso farther posterior to the knee in subjects with ACL rupture, and hip flexion and knee extension torque would be required to stabilize the torso during landing. Activation of the rectus femoris would provide such torque, but it also would increase ACL strain by adding a compressive and an anterior force on the tibia.
Tibiofemoral Kinematics
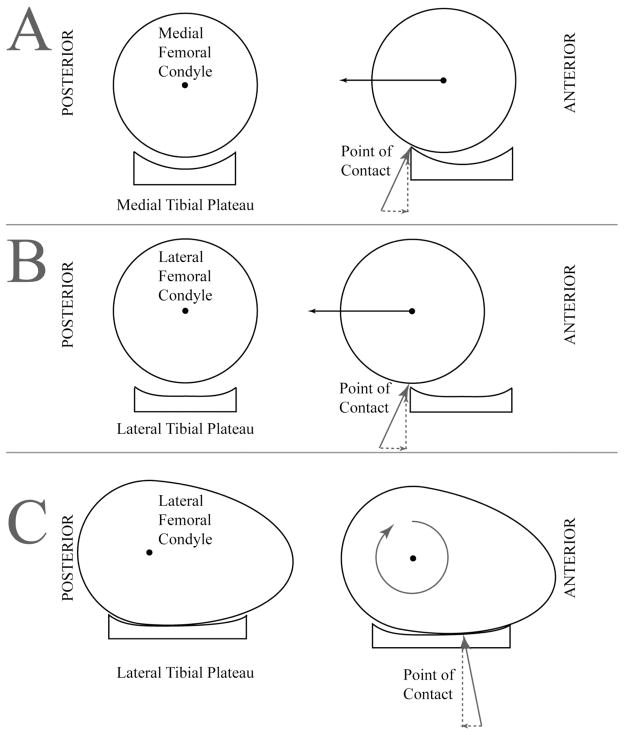
Using MRI, Boden et al26 evaluated noninjured athletes. Differences in limb alignment between the provocative position (that which resulted in ACL rupture) and the safe position (that which did not result in ACL rupture) tended to place the tibiofemoral joint in a position in which ACL injury is more likely. Specifically, in the provocative position, the tibial slope relative to the femur was significantly more vertical (P < 0.001), the point of contact on the lateral compartment was closer to the sulcus on the lateral femoral condyle, and the lateral femoral condyle came into contact with the tibial plateau on its flatter anterior surface rather than on its rounder posterior surface. These findings indicate that the knee is vulnerable in the provocative position, which is closer to the subluxated orientation in which bone bruises occur. As knee extension and hip flexion increase in the provocative position, the angle between the tibial plateau and the femoral shaft increases. That is, it changes from horizontal to more vertical (Figure 4). This increase in the slope of the posterior tibial plateau in the provocative positions may promote anterior tibial shift, thereby causing strain on and potential tearing of the ACL. The risk of ACL injury with greater tibial slope is compounded by the higher impulse forces applied to the limb during landing.
Figure 4.
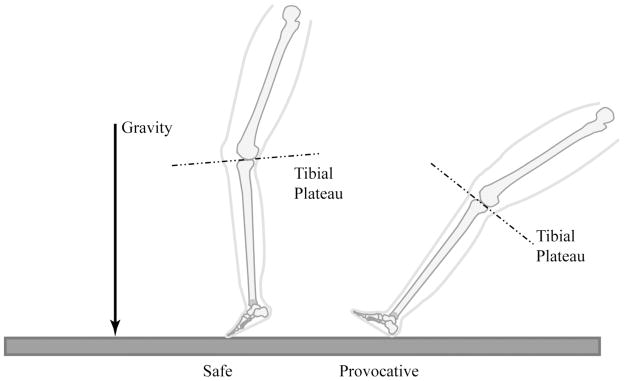
Illustration of the tibial plateau as the leg transitions from a safe to a provocative position. The tibial plateau has a more vertical orientation in the provocative position. (Adapted with permission from Boden BP, Breit I, Sheehan FT: Tibiofemoral alignment: Contributing factors to noncontact anterior cruciate ligament injury. J Bone Joint Surg Am 2009;91:2381–2389.)
Alteration of the posterior tibial slope with tibial osteotomy has beenstudied in canines with deficient ACLs. An anterior tibial shift can be produced by combining a weight-bearing axial compressive force with an increased posterior tibial slope. Surgical treatment of ACL-deficient knees in canines often involves an osteotomy to reduce the posterior tibial slope.27 Decreasing the posterior tibial slope has been demonstrated to convert an anterior tibial thrust to a posterior tibial shift with an axial weight-bearing load.27 The amount of anterior or posterior translation is dependent on the amount of axial force applied as well as the tibial slope.27 As the leg moves toward the more provocative position,26 the point of contact moves from the rounder, posterior portion of the lateral femoral condyle to the flatter, anterior portion of the lateral femoral condyle, which has a greater radius of curvature. The contact area between the articular surfaces is greater on the medial side of the joint than on the lateral side.28 Thus, in the provocative position, when contact occurs between the flatter anterior portion of the lateral femoral condyle and the convex lateral tibial plateau,26,29 sliding (ie, pivot shift) is favored over rolling (Figure 5). In the safe position and with the knee in greater flexion, the contact point moves to the rounder posterior aspect of the lateral femoral condyle, and rolling is favored over sliding. The anatomic configuration of the medial compartment of the knee (ie, round condyle on a concave tibial plateau) is inherently more stable than the lateral compartment and favors rolling over sliding.
Figure 5.
A, Schematic representation of the medial compartment, demonstrating the concave shape of the medial tibial plateau. Left, This shape enables the femur to fit with the tibia as a ball fits into a cup. Right, The femur has the potential to slide relative to the tibia, but it runs up against the cup, so it tends to roll instead. The femur also needs to go uphill, which costs energy. B, The cup is flatter on the lateral tibial plateau (left), resulting in lesser forces when the femur tries to slide (right). The assumption that the lateral femoral condyle is circular is incorrect. C, In full extension, the anterior portion of the lateral femoral condyle, which is much flatter than the posterior aspect, is in contact with the tibial plateau (left). Thus, there are two “flat” surfaces riding against each other. For the femur to roll on the tibia, its posterior side must rise, which costs energy. Thus, it is more likely to slide (right).
Knee Abduction (valgus)
The contribution of valgus forces to ACL disruption is controversial. Numerous studies have shown that a valgus moment does not significantly load the ACL and that valgus rotation is not associated with ACL injury.19,30 Further, valgus rotation associated with noncontact ACL injury may only occur after injury.1,4,5 However, Hewett et al31 have stated that landing with the knee in abduction (ie, valgus) is a risk factor for noncontact ACL injury. Training programs that reduce knee abduction moments have been shown to reduce the risk of ACL injury.32 In addition, Chaudhari and Andriacchi33 demonstrated that valgus alignment compounds the effect of axial compressive loading with regard to ACL disruption. By shifting the valgus alignment by as little as 2°, the compressive load threshold for ACL injury was lowered by the equivalent of 1 body weight. Both modeling34 and in vitro35 studies have been able to produce ACL strain with pure valgus torque. Increased knee abduction places greater axial forces on the lateral side of the knee than on the medial side. This magnifies the lateral compressive forces and may contribute to a greater internal rotation component. In addition, with knee abduction, the ligaments on the lateral side of the knee may become relaxed, while the medial ligaments become taut. The combination of a constrained medial compartment and a relatively loose lateral compartment may allow the lateral tibial plateau to shift anteriorly with internal rotation, which can dramatically increase strain on the ACL.30
Historically, little attention was focused on the position of the trunk during noncontact ACL injury. In a videotape study assessing the trunk, Hewett et al36 reported that the mean lateral trunk angle relative to the vertical plane was higher in female athletes than in male athletes during ACL injury. Lateral trunk lean in female athletes displaces the center of mass to the lateral side of the knee joint, thereby significantly increasing the axial force on the lateral knee compartment and the knee abduction or valgus moment during the weight acceptance phase.
Female ACL Non-contact Injuries
Female athletes who participate in high-risk sports such as basketball, soccer, and volleyball have a two- to eightfold greater rate of ACL injury than do male athletes.6 Knee abduction appears to be the predominant risk factor for ACL injury in female athletes.31 History of ACL reconstruction is a risk factor for subsequent ACL injury on the contralateral knee.37 Other potential risk factors include joint laxity,38 knee recurvatum1 or hyperextension, increased posterior tibial slope,29 and changes in estrogen levels.7
Descriptive and analytic videotape reviews of female athletes with ACL injury found that these athletes were commonly injured during a simple deceleration maneuver, whereas male athletes were usually injured during more strenuous jumping maneuvers.5 Thus, female athletes may injure their ACL at lower GRFs than do male athletes. Female athletes often land with higher knee abduction moments (ie, valgus torque), which are primary predictors of future ACL injury risk.31 In a recent prospective study, Zazulak et al39 reported core proprioception deficits and excessive lateral trunk displacement to be strong predictors of knee, ligament, and ACL injury risk in female athletes; however, these were not strong predictors in male athletes. This report indicated that inadequate neuromuscular control of the trunk or core in the coronal plane of female athletes may increase abduction torque at the knee, predisposing these athletes to ACL injury. Even alteration of arm position relative to the center line of the body can increase the external knee abduction load by 29% to 60%.33 In addition, proprioception deficits in female subjects have been demonstrated to lead to a quadriceps-dominant activation at landing, with delays in hamstring activation; this is not seen in male subjects.7
Schmitz et al38 demonstrated that women exhibit less knee stiffness than do men. The increased stiffness of male knees may be partially protective against ACL injury, especially when impulsive loads are transmitted across the knee joint. Knee recurvatum may be another risk factor in female athletes. Boden et al1 initially reported significantly more knee recurvatum at 10° and 90° of hip flexion in patients with ACL injury. This finding was confirmed by Ramesh et al,40 who reported knee hyperexten-sion in 79% of athletes with ACL injury, compared with 37% of control subjects. Knee recurvatum may allow the knee to be closer to full extension or the provocative position during athletic activities. It has also been demonstrated that female athletes who sustain an ACL injury have a greater association with knee recurvatum than do uninjured controls.40 In an MRI study assessing the geometry of the tibial plateau, female subjects were found to have a steeper posteriorly directed tibial slope laterally (by 30%) than do male subjects (7.0° versus 5.4°).29 This may increase the risk that the lateral femoral condyle will slide posteriorly on the lateral tibial plateau and injure the ACL. Although there is no good evidence that impingement of the ACL on the intercondylar notch causes ACL injury, it has been proposed that a narrower notch corresponds to a smaller ACL, which is more predisposed to tear.7 Because of difficulties in measuring notch width, this theory requires further research. The data on estrogen indicate that there may be an increased risk of ACL injury during the preovulatory phase, the time of peak estrogen level. Estrogen has been proposed to decrease ACL strength by reducing the tensile properties of the ligament.7 In addition, estrogen has been shown to affect the central nervous system, possibly leading to a decrease in motor skills in the premenstrual phase. However, the reports on estrogen are conflicting, and further research is needed to substantiate whether higher estrogen levels lead to increased risk of injury.7
Summary and Conclusions
We propose that a combination of forces contributes to noncontact ACL injury. It is likely that an external impulsive axial force is the primary force resulting in noncontact ACL injury. The provocative position of initial ground contact in or close to a flatfooted position (ie, reduced ankle plantar flexion) and increased hip flexion predispose the knee to ACL disruption by reducing the dampening capabilities of the leg and by placing the lateral tibial compartment closer to the subluxated position. Knee abduction (ie, valgus) also may play an important role, especially in female athletes, by potentially reducing the compression force threshold needed to produce a noncontact ACL injury. Anterior shear forces from the quadriceps may play a role in ACL disruption. However, it is more likely that quadriceps contraction lowers the axial threshold of injury by increasing the compressive force on the knee. Thus, it is probable that the mechanism causing noncontact ACL injury simulates the pivot-shift test in patients with ACL deficiency. This test has been reported to involve both an axial loading force on the lateral compartment and a valgus force.41 The pivot-shift test produces anterior and internal rotation subluxation of the lateral tibia on the femur. Further research is necessary to understand the importance of the various components of ACL injury. However, the mechanism of noncontact ACL injury is becoming clearer, which should lead to enhanced preventive strategies.
Acknowledgments
This work is supported in part by NIH/NIAMS R01 AR049735, R01-AR05563, R01 AR056259 (TEH) and the intramural program of the National Institutes of Health
References
- 1.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23:573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 2.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St PP, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 3.DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32:477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- 4.Meyer EG, Baumer TG, Slade JM, Smith WE, Haut RC. Tibiofemoral contact pressures and osteochondral microtrauma during anterior cruciate ligament rupture due to excessive compressive loading and internal torque of the human knee. Am J Sports Med. 2008;36:1966–1977. doi: 10.1177/0363546508318046. [DOI] [PubMed] [Google Scholar]
- 5.Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37:252–259. doi: 10.1177/0363546508328107. [DOI] [PubMed] [Google Scholar]
- 6.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 7.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am J Sports Med. 2006;34:299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- 8.Chappell JD, Creighton RA, Giuliani C, Yu B, Garrett WE. Kinematics and electromyography of landing preparation in vertical stop-jump: risks for noncontact anterior cruciate ligament injury. Am J Sports Med. 2007;35:235–241. doi: 10.1177/0363546506294077. [DOI] [PubMed] [Google Scholar]
- 9.Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31:519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 10.van Eijden TM, de BW, Weijs WA. The orientation of the distal part of the quadriceps femoris muscle as a function of the knee flexion-extension angle. J Biomech. 1985;18:803–809. doi: 10.1016/0021-9290(85)90055-7. [DOI] [PubMed] [Google Scholar]
- 11.Nisell R. Mechanics of the knee. A study of joint and muscle load with clinical applications. Acta Orthop Scand Suppl. 1985;216:1–42. [PubMed] [Google Scholar]
- 12.Viskontas DG, Giuffre BM, Duggal N, Graham D, Parker D, Coolican M. Bone bruises associated with ACL rupture: correlation with injury mechanism. Am J Sports Med. 2008;36:927–933. doi: 10.1177/0363546508314791. [DOI] [PubMed] [Google Scholar]
- 13.Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20:332–337. doi: 10.1016/S0736-0266(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Rudy TW, Allen C, Sakane M, Woo SL. Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: a porcine study. J Orthop Res. 1998;16:122–127. doi: 10.1002/jor.1100160121. [DOI] [PubMed] [Google Scholar]
- 15.Pandy MG, Shelburne KB. Dependence of cruciate-ligament loading on muscle forces and external load. J Biomech. 1997;30:1015–1024. doi: 10.1016/s0021-9290(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 16.Torzilli PA, Deng X, Warren RF. The effect of joint-compressive load and quadriceps muscle force on knee motion in the intact and anterior cruciate ligament-sectioned knee. Am J Sports Med. 1994;22:105–112. doi: 10.1177/036354659402200117. [DOI] [PubMed] [Google Scholar]
- 17.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;90:815–823. doi: 10.2106/JBJS.F.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsen EB, Magnusson SP, Bencke J, Naesborg H, Havkrog M, Ebstrup JF, Sorensen H. Can the hamstring muscles protect the anterior cruciate ligament during a side-cutting maneuver? Scand J Med Sci Sports. 2000;10:78–84. doi: 10.1034/j.1600-0838.2000.010002078.x. [DOI] [PubMed] [Google Scholar]
- 19.Yeow CH, Cheong CH, Ng KS, Lee PV, Goh JC. Anterior cruciate ligament failure and cartilage damage during knee joint compression: a preliminary study based on the porcine model. Am J Sports Med. 2008;36:934–942. doi: 10.1177/0363546507312645. [DOI] [PubMed] [Google Scholar]
- 20.Meyer EG, Haut RC. Excessive compression of the human tibio-femoral joint causes ACL rupture. J Biomech. 2005;38:2311–2316. doi: 10.1016/j.jbiomech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Meyer EG, Villwock MR, Haut RC. Osteochondral microdamage from valgus bending of the human knee. Clin Biomech (Bristol, Avon ) 2009;24:577–582. doi: 10.1016/j.clinbiomech.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Giffin JR, Stabile KJ, Zantop T, Vogrin TM, Woo SL, Harner CD. Importance of tibial slope for stability of the posterior cruciate ligament deficient knee. Am J Sports Med. 2007;35:1443–1449. doi: 10.1177/0363546507304665. [DOI] [PubMed] [Google Scholar]
- 23.Dejour H, Bonnin M. Tibial translation after anterior cruciate ligament rupture. Two radiological tests compared. J Bone Joint Surg Br. 1994;76:745–749. [PubMed] [Google Scholar]
- 24.Hsu V, Stearne D, Torg JS. Elastic instability, columnar buckling, and non-contact anterior cruciate ligament ruptures: a preliminary report. Temple University Journal of Orthopaedic Surgery and Sports Medicine. 2006;1:21–23. [Google Scholar]
- 25.McNitt-Gray JL. Kinetics of the lower extremities during drop landings from three heights. J Biomech. 1993;26:1037–1046. doi: 10.1016/s0021-9290(05)80003-x. [DOI] [PubMed] [Google Scholar]
- 26.Boden BP, Briet I, Sheehan F. Tibiofemoral alignment:contributing factors to noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2009 doi: 10.2106/JBJS.H.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reif U, Hulse DA, Hauptman JG. Effect of tibial plateau leveling on stability of the canine cranial cruciate-deficient stifle joint: an in vitro study. Vet Surg. 2002;31:147–154. doi: 10.1053/jvet.2002.31041. [DOI] [PubMed] [Google Scholar]
- 28.Kettelkamp DB, Jacobs AW. Tibiofemoral contact area--determination and implications. J Bone Joint Surg Am. 1972;54:349–356. [PubMed] [Google Scholar]
- 29.Hashemi J, Chandrashekar N, Gill B, Beynnon BD, Slauterbeck JR, Schutt RC, Jr, Mansouri H, Dabezies E. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724–2734. doi: 10.2106/JBJS.G.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13:930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 31.Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 32.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhari AM, Andriacchi TP. The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. J Biomech. 2006;39:330–338. doi: 10.1016/j.jbiomech.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 34.McLean SG, Huang X, Su A, Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech (Bristol, Avon ) 2004;19:828–838. doi: 10.1016/j.clinbiomech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Withrow TJ, Huston LJ, Wojtys EM, shton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon ) 2006;21:977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43:417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz RJ, Ficklin TK, Shimokochi Y, Nguyen AD, Beynnon BD, Perrin DH, Shultz SJ. Varus/valgus and internal/external torsional knee joint stiffness differs between sexes. Am J Sports Med. 2008;36:1380–1388. doi: 10.1177/0363546508317411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35:368–373. doi: 10.1177/0363546506297909. [DOI] [PubMed] [Google Scholar]
- 40.Ramesh R, Von Arx O, Azzopardi T, Schranz PJ. The risk of anterior cruciate ligament rupture with generalised joint laxity. J Bone Joint Surg Br. 2005;87(6):800–803. doi: 10.1302/0301-620X.87B6.15833. [DOI] [PubMed] [Google Scholar]
- 41.Losee RE. Concepts of the pivot shift. Clin Orthop Relat Res. 1983:45–51. [PubMed] [Google Scholar]