Abstract
Understanding the mechanisms that underlie successful human reproduction and development is an ambitious goal, given the many unique methodologic challenges surrounding such study. These challenges are well understood by reproductive and perinatal epidemiologists and include its conditional nature, unobservable yet informative outcomes such as conception, multi-scale missing data, correlated or non-independent outcomes, interval censoring, and a hierarchical data structure. Novel methodologies for overcoming these challenges and for answering critical data gaps are needed if we are to better understand the inefficiency that currently characterizes human reproduction with the goal of improving population health. The exposome is an emerging paradigm that offers promise for understanding the natural history of human reproduction and development, and its many associated impairments that develop later in child- or adult-hood. This novel paradigm recognizes the need to identify and measure the totality of environmental (non-genetic) exposures from preconception through sensitive windows, and to identify patterns associated with healthy and adverse outcomes. The exposome accommodates research focusing on unique subpopulations, such as couples undergoing assisted reproductive technologies, so that methodologic limitations such as unobservable and conditional outcomes can be better addressed. Reproductive and perinatal epidemiology is uniquely suited for proof-of-concept exposome research, given the intricate relations between fecundity, gravid health and later onset disease and the narrow and interrelated sensitive windows that characterize the conditional nature of human reproduction and development. Bold new conceptual frameworks such as the exposome are needed for designing research that may lead to discovery and improve population health.
Keywords: environment, exposome, exposure, fecundity, fertility, sensitive windows
Introduction – changing research paradigms
The genomic era has transformed how scientists formulate and empirically evaluate research questions aimed at understanding the genetic contributions to health and disease. This recent paradigm shift involves using technologies to consider the entire genome rather than focusing on a candidate gene approach to comprehensively investigate genetic factors of complex diseases and to discover novel pathways. The impact of the genomic revolution at the population level is limited for some chronic diseases as previously noted,1,2 at least partly given that such research occurred in isolation of the spectrum of environmental factors that characterize modern life. While environmental factors are estimated to account for approximately 70% – 90% of the attributable risk for common chronic diseases,3 much of the available evidence stems from narrowly defined research focusing on a particular exposure or class of exposures in isolation from the multitude of (un)measured exposures that characterize human life. This narrowly focused exposure strategy contrasts sharply with the untargeted or data driven approach implemented in contemporary genomics research. These contrasting research paradigms have prompted researchers to consider newer conceptual frameworks in which to ground epidemiologic research focusing on environmental exposures.
In 2005, Christopher Wild published an innovative and provocative paper that challenged investigators to rethink how we conceptualize, quantify and analyze environmental exposures irrespective of specialty area by moving toward an exposome framework.4 In this landmark paper, Wild defined the exposome “…as the totality of exposures from conception onwards” using the genome as an analogy. As such, the exposome includes all exposures that arise externally to the body, and also the many chemicals produced internally by physiologic processes aimed at maintaining homeostasis.5 For many investigators including reproductive and perinatal epidemiologists, the totality of exposures would include preconception exposures as well, given their ability to directly impact parental gametes amongst other pathways.
More recently, Wild further conceptualized the exposome paradigm with the designation of environmental exposures as internal, specific external and general external, while recognizing that some exposures may be over-lapping.6 Internal exposures are the so-called host or endogenous factors that impact the cellular environment such as inflammation, metabolism and oxidative stress, while specific external exposures arise from agents in the environment such as chemicals, diet, infectious agents and radiation. Internal exposures comprise a range of biomarkers that can be identified and quantified using laboratory and omics technologies, and generally reflect the body’s responses to external exposures. General external exposures include a host of macro level (e.g., climate) economic, educational and psychosocial factors, while specific external exposures represent the multitude of chemicals and other biological and physical agents that impact health and disease. Some general external exposures may not have biomarkers available for research purposes, which necessitate measurement via standardized data collection instruments. The categorization of exposures remains an evolving aspect of the exposome paradigm. One extreme view is to simply focus on internal exposures, given the marked technologic advances in measurement, spanning the transcriptome through the metabolome, to help inform about etiologic mechanisms. The characterization of possible mechanisms may, subsequently, identify external exposures for targeted research. Another perspective regarding the categorization of exposures is the measurement of external and internal exposures, and to analyze the data in an untargeted manner to detect potential associations.
The goal of the exposome is to delineate, quantify and profile exposures irrespective of their origin (external or internal to the body) to characterize healthy and diseased states, or to essentially characterize the humanome.7 Without question, this is an ambitious undertaking with a near infinite number of possible exposures. Another unique feature of the exposome is that it is not limited to exposures with biomarkers, as many external exposures have no measurable biomarker, at least none known to date.8 However, many external exposures can be measured with reliable proxies such as geocoding tools for air pollution or standardized instruments (e.g., depression scales; physical activity monitors). Again borrowing from the genome research paradigm, the totality of environmental exposures can be analyzed using environment-wide association study (EWAS) techniques as partly adapted from genome-wide association studies (GWAS). The utility and feasibility of using empirical EWAS techniques has been reported in two “proof-of-concept” research initiatives, which utilized the extensive chemical, clinical and questionnaire data from the NHANES surveys.9,10 This work corroborated earlier findings regarding associations between environmental factors, including select chemicals, and type 2 diabetes and serum lipids, respectively. While the authors accounted for multiple testing and performed extensive sensitivity analyses as a part of their investigation, the findings are limited by the cross-sectional nature of the NHANES surveys, relatively small number of external exposures considered and even fewer internal exposures. Future exposomic research is needed to help delineate the temporal relation between environmental factors, internal response and human health and disease using stored specimens. The promise of such discovery may facilitate our ability to design successful interventions at the population level, given the modifiable nature of many environmental exposures.
Exposome and the developmental origins of human reproduction and development
The exposome paradigm for reproductive and perinatal epidemiology is important and may have significant implications beyond reproductive and perinatal medicine in light of the growing weight of evidence that supports a periconception or developmental origin for human fecundity and fertility and, inadvertently, later onset gynecologic and urologic disorders associated with reproductive health and health, more generally. One well-established body of evidence is the innovative work of Skakkebaek and colleagues who synthesized the available environmental evidence focusing on male fecundity and fertility in developing the so-called testicular dysgenesis syndrome (TDS).11 The TDS hypothesis postulates that genital-urinary malformations, poor semen quality and testes cancer may have a shared in utero etiology.12 The exposome offers investigators an opportunity to explore and quantify the multitude of environmental exposures occurring during the periconception period onward that impact male fecundity, as measured by the spectrum of male reproductive outcomes that range from structural defects observed at birth to functional deficits such as impaired spermatogenesis to later onset adult cancers. Buck Louis and colleagues adapted the TDS hypothesis in relation to the available evidence focusing on an early environmental origin for female fecundity and fertility, or the so-called ovarian dysgenesis syndrome (ODS).13 This hypothesis posits an early origin for fecundity impairments such as polycystic ovarian syndrome that may adversely affect women’s ability for conception, while increasing their risk for gravid diseases such as gestational diabetes14 and later onset metabolic and cardiovascular diseases.15 Collectively, these evolving paradigms underscore the need to define the exposome during critical and sensitive windows of human development as we continue to understand the early origin of health and disease as it manifests across the lifespan.
While evidence supports both the TDS and ODS paradigms, a number of data gaps underlie these conceptual frameworks, which are largely a reflection of the limited exposure characterization beyond a single or few agents that characterize much of the available evidence. In addition, quantification of environmental chemicals or other environmental exposures often is not longitudinal or timed with critical or sensitive windows of human reproduction and development, and there have been limited attempts to consider both external and internal exposures relative to health outcomes. Furthermore, the couple-dependent nature of human reproduction requires consideration of both partners’ exposures in addition to menstrual cycle or day-level exposures, all of which generate a hierarchical data structure and opportunity for multi-scale missing data. The complexities of these methodologic issues become even greater when attempting to assess external and internal exposures and in the context of both partners when considering couple-dependent outcomes such as conception. Novel methodologies are needed that foster the identification, quantification and analysis of the multitude of time-varying exposures during sensitive windows for human reproduction and development, the hallmarks of reproductive and perinatal epidemiology, if discovery is to occur as described elsewhere.7
The exposome paradigm and reproductive and perinatal epidemiology
We propose that reproductive and perinatal epidemiology is well poised to take advantage of the transformative research and discovery that the exposome paradigm may offer for three overarching reasons. First, an evolving body of evidence suggests that human fecundity and fertility originate from exposures that occur during very narrow critical or sensitive windows, and that such exposures have implications across the lifespan as conceptualized in the developmental origins of health and disease hypothesis.16 For example, endocrine disrupting chemicals such as phthalates have been experimentally shown to produce the full-blown TDS in mice following environmentally relevant exposures during sensitive windows.17 Similarly, in utero androgen exposure at environmentally relevant concentrations during sensitive windows produced polycystic ovarian syndrome in rhesus monkeys and sheep.18,19 Other environmental chemical exposures such as bisphenol A have been associated with trans-generational adverse reproductive outcomes and, most recently, with child behavior.20,21 Thus, exposures during critical and sensitive windows of human reproduction and development have implications across the lifespan and, possibly, generations. Diethylstilbestrol is an excellent case study for in utero exposures and both life course and trans-generational effects in human and experimental animals.22,23 Collectively, these findings underscore the need for a more complete identification and quantification of exposures during sensitive windows of reproduction and development. The yield from such investigation may include a better understanding of disease etiology, and identification of prevention and therapeutic interventions across the lifespan.
A second reason supporting the suitability of the exposome paradigm for reproductive and perinatal epidemiology is the advantage of relatively short intervals, ranging from hours to days, for the many important critical and sensitive windows underlying successful human reproduction and development. For example, cell division occurs every 18–20 hours in normally developing cleavage stage (2 to 16 cell) embryos. Activation of the embryonic genome occurs during a 3-day period coinciding with the 4-and 8-cell stage. Delays or accelerations in embryonic development are associated with reduced viability, and can be informative for selecting embryos for assisted reproductive technologies (ART).24 Because of its unique ability to observe endpoints typically hidden at the population level, research focusing on the ART subpopulation is particularly well suited for exposomic research. This advantage includes characterizing the embryonic proteome and metabolome or transcription factors in relation to implantation success. Embryo culture media are an early all-inclusive environment impacting genomic imprinting and embryonic development, and all contain supplements such as salts, energy substrates, amino and fatty acids among other agents. Animal evidence suggests that type of culture media affects pregnancy outcomes such as birthweight, though limited study has been conducted with human populations and with equivocal results.25, 26 Recent research has isolated some environmental chemicals such as select perfluorochemicals and phthalates in IVF media, though often at trace levels.27,28 Thus, ART populations provide an excellent opportunity to compare the effects of different media as well as capture the secretome or the proteins and metabolites produced by the embryonic metabolome.29
Epidemiologists have the ability to capture biospecimens for relevant endpoints including highly select media such as follicular fluid, blastocyst cells or the spent IVF culture media dish. Following conception, unique biospecimens available during pregnancy also could be informative for capturing the maternal-fetal exposome, including amniotic fluid, cord blood and fetal and placental tissue. Examples of other key critical and sensitive windows for reproductive and perinatal epidemiology are presented in Table 1, along with their estimated length as recently summarized.30 From conception onwards, the developing organism can be sensitive to perturbations in its environmental milieu as evident by its limited ability for DNA repair, primitive to immature organ system development, lack of a blood brain barrier, and growing energy needs and metabolism required for supporting its developing state. Time-varying environmental exposures during these critical and sensitive windows are important aspects for determining epigenetic mechanisms, or chemical modifications of DNA and chromatin, that may affect genomic functions such as alterations in transcription, replication and recombination. Thus, capturing the totality of exposures to the extent possible during narrowly defined intervals is a first step in defining the exposomes for sensitive windows such as spermatogenesis, conception, and implantation, while allowing for the eventual comparison of exposomes by health or disease outcomes. Still, this is an ambitious undertaking as the intervals for many critical windows are imprecise. In addition, some windows may vary between and within study participants. Such examples include semen quality or the timing of ovulation and the fertile window across menstrual cycles. Finally, measuring the environment in the target tissue of interest also presents challenges.
Table 1.
Estimated duration of sensitive windows underlying human reproduction and development.
| Sensitive Window | Estimated Duration |
|---|---|
| Male Fecundity | |
| Steroidogenesis & spermatogenesis | ≈72 days |
| Female Fecundity | |
| Folliculogenesis (dominant follicle selection) | ≈4 months |
| Menstrual & ovarian cycles | ≈29 days |
| Ovulation (following LH surge) | ≈24–36 hours |
| Fertile window | <1–8 days |
| Fertilization | 3–4 hours |
| Implantation window | ≈6 days |
| Pregnancy (post-conception) | ≈266 days |
| Two-cell stage | ≈2 days |
| Eight-cell stage | ≈3 days |
| Blastocyst implantation | ≈7 days |
| Embryo | 3–8 weeks |
| Fetus | 8–40 weeks |
Adapted from Buck Louis 2011.30
A third overarching argument for considering the exposome is the highly timed, interrelated and conditional nature of human reproduction and development. For example, relatively narrow intervals exist for the timing of fertilization and implantation with the best quality embryos selectively undergoing continued development while others succumb. In keeping with the highly timed and interrelated nature of reproduction and development, delineating the exposome for one window such as steroidogenesis and spermatogenesis may not only be informative for an immediate study endpoint such as fecundity, but also for later onset health endpoints. For example, in utero exposures are believed responsible for cryptorchidism and hypospadias, two genital-urinary malformations often accompanied by reductions in birth size suggestive of a shared etiology.31 These malformations are often associated with diminished male fecundity as measured by alterations in semen qualityand testes cancer at later ages.32 Of note is the possible trans-generational effect of exposures in that fathers of boys born with hypospadias are reported to have poorer semen quality in comparison to fathers of unaffected boys.33 Moreover, recent evidence suggests that male fecundity as measured by semen quality is associated with longevity34 underscoring male fecundity’s relevancy for human health beyond the reproductive years. Semen quality is now recognized as an important factor when assessing couple-based outcomes such as infertility, and has been associated with fertilization, implantation and pregnancy outcomes among couples undergoing assisted reproductive technologies.35,36 Similar interrelated associations have been reported for women. For example, prospective cohorts of girls born small-for-gestational age have reported diminished fecundity, as measured by reduced size of reproductive organs, diminished responsiveness of follicle stimulating hormone and increased anovulation during adolescence in comparison to appropriately sized girls at birth.37–39 Women diagnosed with endometriosis are reported to track lean across childhood and adolescence suggesting an early origin for disease,40 and are at high risk for autoimmune disorders and reproductive site cancers later in life.41–43
Reproductive and perinatal epidemiology is unique in its substantive expertise in designing research during the earliest stages of human reproduction and development, during time intervals that may be important for many, if not all, facets of health. Figure 1 illustrates the highly interrelated and conditional nature of human development commencing with the fusion of gametes to form the zygote through adulthood. The declining sizes of the shapes and backward arrows represents the selective loss of individuals at each stage underscoring the conditional nature of human development. Also of note is the ability of the developing organism’s ability to influence the internal exposome of the mother, as illustrated by the double-arrow. This figure underscores the importance of starting early to delineate the exposome at critical windows.
Figure 1.
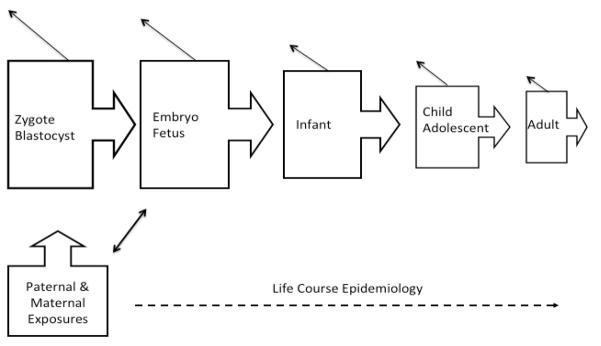
Illustration of the interrelated and conditional nature of human development across the life course.
Moving forward – to exposome or not?
The genome revolution during the past decade has spurred new avenues of research along with technologies for continued research and discovery as evident by advances in transcriptomics, proteomics, metabolomics and epigenetics. These advances enable us to characterize the ‘internal’ exposome or at least those exposures for which a biomarker is available, which eventually might identify external agents that impact health and disease. While some investigators suggest utilizing a post hoc or agnostic approach to explain observed molecular associations through the use of a series of existing cohorts during varying time periods in the life course or by taking cross-sectional measurements at key life stages,10,44 we propose that various approaches including the incorporation of external and internal exposures should be explored depending upon the research question and availability of data, biospecimens and other resources to foster a broader implementation of the exposome for understanding the humanome. Collectively, these efforts may help to identify risk factors for disease and, thereby, help improve population health.
Reproductive and perinatal epidemiology is well poised for exposomic research, given our longstanding history of successfully completing prospective cohort studies inclusive of various vulnerable populations. Such studies range from cohorts of women/couples recruited prior to conception45 or during pregnancy, particularly when interested in establishing birth cohorts in which to assess child growth and development.46 Other unique pregnancy cohorts allowed women to contribute more than one pregnancy allowing for the investigation of etiologic determinants associated with clustered perinatal outcomes.47 The latter type of study might be highly informative for establishing both normal and abnormal pregnancy exposomes, given that women serve as their own ‘control’, and for investigating adverse perinatal outcomes including in successive pregnancies. In addition, prospective cohort designs with longitudinal data and biospecimen collection that typify reproductive and perinatal epidemiology align well with the exposome paradigm, and many such datasets already exist. The longitudinal aspect of reproductive and perinatal epidemiologic data and biospecimen collection is an exceptional strength of our work, and is most relevant in light of recent findings that demonstrate the dynamic changes in gut microbiota across pregnancy, with possible implications for infant health.48 Leveraging existing resources is a timely and cost effective strategy for assessing the feasibility and utility of the exposome paradigm for discovery. Interested investigators might wish to pool resources in their quest to obtain funding for an exposome approach for understanding human reproduction and development.
Moving forward to implement proof-of-concept type research requires continued vigilance to basic epidemiologic methods in light of the many expected or unforeseen methodologic challenges that such novel exploration will generate in the context of trans-disciplinary research teams. Such challenges may generate new methods and analytic tools as evident by GWAS and related microarray techniques that were stimulated by defining the human genome. As opponents will be quick to point out, it will be necessary to validate exposures including those for which no biomarkers are available and to replicate findings across study populations before determining what represents normality, atypical physiologic variation or adverse outcomes. We argue that such considerations are already fundamental aspects of epidemiologic research, though we recognize the added complexity as we strive to measure and understand the dynamic nature of the humanome.
There are many possible ramifications of not considering emerging paradigms such as the exposome in reproductive and perinatal epidemiology, particularly as other disciplines move forward. These include continued publication of narrowly defined research findings with limited population impact, limited etiologic understanding or a young field that is seen as out of touch. The trans-disciplinary research teams that already characterize much of our work, coupled with the availability of tremendous resources that could be leveraged, put the opportunities for transformative research within our reach. Another strength of our field is the well-developed cadre of young investigators that are positioned for discovery with population impact. In fact, many young investigators grew up in the genomic era, and they may be less intimidated by the untargeted exploration that underlies the exposome paradigm. Believing the exposome paradigm merits exploration in the search for empirical evidence, the Division of Epidemiology, Statistics and Prevention Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development is implementing a proof-of-concept study aimed at assessing the feasibility and utility of using existing data and biospecimens with the goal of characterizing the normal pregnancy exposome. We encourage other investigators to share in this exploration and to consider such approaches using their existing resources so that we have empirical evidence from multiple sources and collective experiences to better inform how to exposome!
Conclusions
The exposome paradigm offers an exciting opportunity for characterizing exposures during sensitive windows of human reproduction and development that may impact population health and disease across the lifespan. As other disciplines continue to perfect the laboratory measurement of big to small omes (e.g., genomes, transcriptomes, proteomes, metabolomes), as well as develop appropriate methods to analyze such ome data, our contribution will be to collect the necessary data and biospecimens that are needed for analyzing the multi-scale hierarchy that characterizes the human exposome.
Acknowledgments
Supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institutes of Health. The authors acknowledge the input from members of the Exposome Working Group, Division of Epidemiology, Statistics and Prevention Research, NICHD for their helpful comments and suggestions regarding this paper. Members include: Drs. Nansi Boghossian, Alexander McLain (now at the University of South Carolina), Pauline Mendola, Sunni Mumford, Tonja Nansel, Neil Perkins, and Anna Pollack.
References
- 1.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proceedings of the National Academy of Science; May 27, 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer- analyses of cohorts of twins from Sweden, Denmark, and Finland. New England Journal of Medicine. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 3.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 4.Wild C. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiology Biomarkers and Prevention. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 5.Rappaport SM, Smith MT. Environment and disease risks. Science. 2010;330(6003):460–461. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wild CP. The exposome: from concept to utility. International Journal of Epidemiology. 2012;41:24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- 7.Buck Louis GM, Sundaram R. Exposome: Time for Transformative Research: Invited Commentary. Statistics and Medicine. 2012;31(22):2569–2575. doi: 10.1002/sim.5496. (Online doi:10.10021SIM.5496). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters A, Hoek G, Katsouyanni K. Understanding the link between environmental exposures and health: Does the exposome promise too much? Journal of Epidemiology and Community Health. 2012;66:103–105. doi: 10.1136/jech-2011-200643. [DOI] [PubMed] [Google Scholar]
- 9.Patel CJ, Bhattacharya J, Butte AJ. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One. 2010;5(5):e10746. doi: 10.1371/journal.pone.0010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel CJ, Cullen MR, Ioannidis JPA, Butte AJ. Systematic evaluation of environmental factors: Persistent Pollutants and nutrients correlated with serum lipid levels. International Journal of Epidemiology. 2012 Mar 15;2012 doi: 10.1093/ije/dys003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human Reproduction. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 12.Moller H. Trends in sex-ratio, testicular cancer and male reproductive hazards: are they connected? APMIS. 1998;106:232–238. doi: 10.1111/j.1699-0463.1998.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. Journal of Developmental Origins of Health and Disease. 2011;2(1):25–35. [Google Scholar]
- 14.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Human Reproduction Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 15.Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertility and Sterility. 2012;97(1):e1–25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Gluck PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 17.Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 18.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome – a hypothesis. Journal of Endocrinology. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 19.Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertility and Sterility. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- 20.Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, et al. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biology of Reproduction. 2011;84:79–86. doi: 10.1095/biolreprod.110.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, et al. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012 Jun 12; doi: 10.1210/en.2012-1195. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Human Reproduction. 2006;21(3):666–669. doi: 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- 23.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147(6 Suppl):S11–17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 24.Brown S. From genomics… to metabolomics. New technologies in embryo physiology. Focus on Reproduction. 2009 Jan;:22–29. [Google Scholar]
- 25.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. effect of in vitro culture of human embryos on birthweight of newborns. Human Reproduction. 2010;25:605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 26.Eaton JL, Lieberman ES, Stearns C, Chinchilla M, Racowsky C. Embryo culture media and neonatal birthweight following IVF. Human Reproduction. 2012;27:375–379. doi: 10.1093/humrep/der381. [DOI] [PubMed] [Google Scholar]
- 27.Takatori S, Akutsu K, Kondo F, Ishii R, Nakazawa H, Makino T. Di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate in media for in vitro fertilization. Chemosphere. 2012;86(5):454–459. doi: 10.1016/j.chemosphere.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki Y, Terayama E, Kato A, Ito R, Saito K, Makino T, et al. Quantitative analysis of perfluorinated chemicals in media for in vitro fertilization and related samples. Chemosphere. 2012;88:445–449. doi: 10.1016/j.chemosphere.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 29.Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environment and epigenetic effects upon preimplantation embryo metabolism and development. Trends in Endocrinology and Metabolism. 2011;22(10):412–420. doi: 10.1016/j.tem.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buck Louis GM. Fecundity and Fertility. In: Buck Louis GM, Platt RW, editors. Reproductive and Perinatal Epidemiology. New York: Oxford University Press; 2011. pp. 16–61. [Google Scholar]
- 31.Jensen MS, Wilcox AJ, Olsen J, Bonde JP, Thulstrup AM, Ramlau-Hansen CH, et al. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. American Journal of Epidemiology. 2012;175(9):917–925. doi: 10.1093/aje/kwr421. [DOI] [PubMed] [Google Scholar]
- 32.Raman JD, Nobert CF, Goldstein M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. Journal of Urology. 2005;174(5):1819–1822. doi: 10.1097/01.ju.0000177491.98461.aa. [DOI] [PubMed] [Google Scholar]
- 33.Asklund C, Jorgensen N, Skakkebaek NE, Jensen TK. Increased frequency of reproductive health problems among fathers of boys with hypospadias. Human Reproduction. 2007;22(10):2639–2646. doi: 10.1093/humrep/dem217. [DOI] [PubMed] [Google Scholar]
- 34.Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. American Journal of Epidemiology. 2009;170(5):559–565. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]
- 35.Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clinical Endocrinology. 2009;71 (4):459–465. doi: 10.1111/j.1365-2265.2009.03545.x. [DOI] [PubMed] [Google Scholar]
- 36.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertility and Sterility. 2004;81(5):1289–1295. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 37.Ibáñez L, Potau N, Enríquez G, de Zegher F. Reduced uterine and ovarian size in adolescent girls born small for gestational age. Pediatric Research. 2000;47:575–577. doi: 10.1203/00006450-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Ibáñez L, Potau N, de Zegher F. Ovarian hyporesponsiveness to follicle stimulating hormone in adolescent girls born small for gestational age. Journal Clinical Endocrinology and Metabolism. 2000;85:2624–2626. doi: 10.1210/jcem.85.7.6765. [DOI] [PubMed] [Google Scholar]
- 39.Ibáñez L, Potau N, Ferrer A, Rodriguez-Hierro F, Marcos MV, de Zegher F. Reduced ovulation rate in adolescent girls born small for gestational age. Journal Clinical Endocrinology and Metabolism. 2002;87:3391–3393. doi: 10.1210/jcem.87.7.8657. [DOI] [PubMed] [Google Scholar]
- 40.Hediger ML, Hartnett JH, Louis GM. Association of endometriosis with body size and figure. Fertility and Sterility. 2005;84(5):1366–1374. doi: 10.1016/j.fertnstert.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nina Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Human Reproduction. 2002;17:2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 42.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. American Journal of Obstetrics and Gynecology. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 43.Brinton LA, Sakoda LC, Sherman ME, Frederiksen K, Kjaer Sk, Graubard BI, et al. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiology Biomarkers Prevention. 2005;14:2929–2935. doi: 10.1158/1055-9965.EPI-05-0394. [DOI] [PubMed] [Google Scholar]
- 44.Rappaport SM. Biomarkers intersect with the exposome. Biomarkers. 2012;17 (6):483–489. doi: 10.3109/1354750X.2012.691553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buck Louis GM, Lynch CD, Stanford JB, Sweeney AM, Schieve LA, Rockett JC, et al. Prospective pregnancy study designs for assessing reproductive and developmental toxicants. Environmental Health Perspectives. 2004;112:79–86. doi: 10.1289/ehp.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golding J, Birmingham K, Jones R, editors. A guide to undertaking a birth cohort study: Purposes, pitfalls and practicalities. Paediatric and Perinatal Epidemiology. 2009;23(suppl 1):1–229. [Google Scholar]
- 47.Niswander KR, Gordon M. The women and their pregnancies. Philadelphia, PA: W.B. Saunders; 1972. [Google Scholar]
- 48.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Kling Backhed H, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


