Abstract
Fetal exposure to diazepam (DZ), a positive modulator of GABAA receptors and an agonist at mitochondrial benzodiazine receptors, induces long-term neural and behavioral effects. This study evaluated whether the early manipulation influenced the normal development of brain levels of neurosteroids or altered steroid action at GABAA receptors. Pregnant dams were injected over gestation days 14 through 20 with DZ (2.5 mg/kg) or the vehicle. Male and female offspring were analyzed at five postnatal ages. The levels of progesterone (P), dihydroprogesterone (DHP), 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP), testosterone (T), dihydrotestosterone, and 5α-androstan-3α,17β diol were measured in the cerebral cortex and diencephalon. The results indicated that development of brain steroid levels and the impact of fetal DZ exposure were region- and sex-specific. Age-related changes in brain steroids did not mirror associated changes in circulating P and T. Age regulated the levels of all 3 progestins in the cerebral cortex, and fetal DZ exposure interacted with the development of P and DHP. The development of 3α,5α-THP in the cortex was markedly influenced by sex, with levels in males decreasing over postnatal development whereas they increased over postpubertal development in females. An adolescent surge in T levels was observed in male cortex and fetal DZ exposure prevented that surge. Steroid levels in the diencephalon were altered by age mainly in females, and DZ exposure had little effect in this region. The data support region-specific regulation of brain steroid synthesis. Only in the cerebral cortex are relevant mechanisms readily modifiable by fetal DZ exposure. However, neither sex nor fetal DZ exposure altered the response of GABAA receptors in adult cortex to neurosteroid.
Keywords: Progesterone, Testosterone, 3α, 5α-THP, GABAA receptor, Cerebral cortex, Diencephalon
1. Introduction
Experimental studies have demonstrated that perturbation of the developing brain can lead to lasting consequences, and that the effects of early perturbations often do not become apparent until much later in the life of the organism (Zagon and Slotkin, 1992). Furthermore, several clinical neurobehavioral disorders that are expressed in adulthood have come to be regarded as neurodevelopmental disorders (Keshavan and Murray, 1997). Basic research designed to define principles of neurodevelopment and the effects of interference in brain development can help elucidate understanding of developmental psychopathology.
We have examined the effects of late fetal exposure of rats to the benzodiazepine (BZD) diazepam (DZ) in order to evaluate how an early developmental manipulation could influence brain organization and lead to later altered function. DZ binds to a specific site on GABAA receptors (the central BZD binding site), and it is a positive modulator of GABA action at these receptors (Möhler et al., 2000). GABA is now thought to play an important trophic role in brain development (Barker et al., 1998), and GABA action at GABAA receptors in fetal brains results in depolarization, whereas hyperpolarization is characteristic of GABA action in mature brains (Cherubini et al., 1991). Interference in functioning of this receptor complex during fetal development should alter brain organization and could lead to later dysfunction. Indeed, we have described diverse long-term consequences of fetal DZ exposure and have defined developmental neural changes that may affect brain organization (Kellogg, 1998, 1999; Kellogg et al., 2000; Roberts et al., 2001). Many of the latent effects of fetal exposure to DZ can be prevented by co-exposure during fetal development to a BZD central site antagonist, implicating in utero action at GABAA receptors in mediation of the long-term effects (Kellogg, 1992). However, clearly, there are some latent effects that are not prevented by co-exposure to the central site antagonist. Furthermore, DZ not only binds at the BZD site on GABAA receptors, but it can also bind to a site on mitochondria referred to as the mitochondrial BZD receptor (MBR), a site that can influence the synthesis of neurosteroids from cholesterol (Plassart-Schiess and Baulieu, 2001).
Steroids can influence neuronal function either via action at intracellular receptors that translocate to the nucleus when bound by steroids and regulate gene expression or by interaction with certain cell membrane neurotransmitter receptors. Steroids exert a broad spectrum of effects on the brain (Rupprecht et al., 2001). Important steroids shown to affect GABAA receptor function include reduced metabolites of progesterone and testosterone (Bitran et al., 2000; Rosellini et al., 2001). We have reported very high levels of the progesterone metabolite, 3α,5α-tetrahydroprogesterone (3α,5α-THP), in rat fetal brain during the last week of gestation, the developmental period during which we expose animals in utero to DZ. The conversion of progesterone to its reduced metabolites appeared to be many fold greater in fetal than in adult brains (Kellogg and Frye, 1999). We have also demonstrated that neurosteroids influence function at GABAA receptors in fetal brain (Kellogg et al., 1998b). These observations led us to evaluate the hypothesis that the presence of DZ in fetal brains during late gestation could interact with the fetal action of neurosteroids. Consequences of such an interaction could include effects on development of brain neurosteroid levels or long-term effects on GABAA receptor function.
While levels of 3α,5α-THP have been reported to be developmentally regulated in the cerebral cortex (Grobin and Morrow, 2001), and fetal exposure to alcohol has been reported to alter the development of the neurosteroid, pregnenolone sulfate (Caldeira et al., 2004), few studies have addressed the development of brain steroids in a systematic way. In the present study, we measured levels of progesterone (P) and its reduced metabolites, dihydroprogesterone (DHP) and 3α,5α-THP as well as levels of testosterone (T) and its reduced metabolites, dihydrotestosterone (DHT) and 5α-androstan-3α,17β diol (3α-diol) in the cerebral cortex and diencephalon from neonatal to adult ages in both male and female rats. We selected these two regions for analysis as we have previously reported changes in neurotransmitter function in both of these regions in adult rats exposed in utero to DZ (Kellogg, 1992, 1998, 1999). Also, we have observed the effects of fetal exposure to DZ to be more pronounced in males than in females. Steroid levels were measured at postnatal ages corresponding to neonatal, early juvenile, late juvenile, adolescent and young adult periods of brain development. The results indicated that developmental regulation of brain steroid levels, as well as the consequences of fetal DZ exposure on neurosteroid development, are region- and sex-specific. We also measured 3α,5α-THP-facilitation of GABA-mediated chloride uptake in the cortex of adult male and female rats to determine whether fetal DZ exposure altered sensitivity to this neurosteroid. The results indicated that neither sex nor prenatal exposure affected facilitation by 3α,5α-THP in adult cortex.
2. Results
2.1. Analysis of brain steroids
2.1.1. Cerebral cortex
2.1.1.1. Progestins
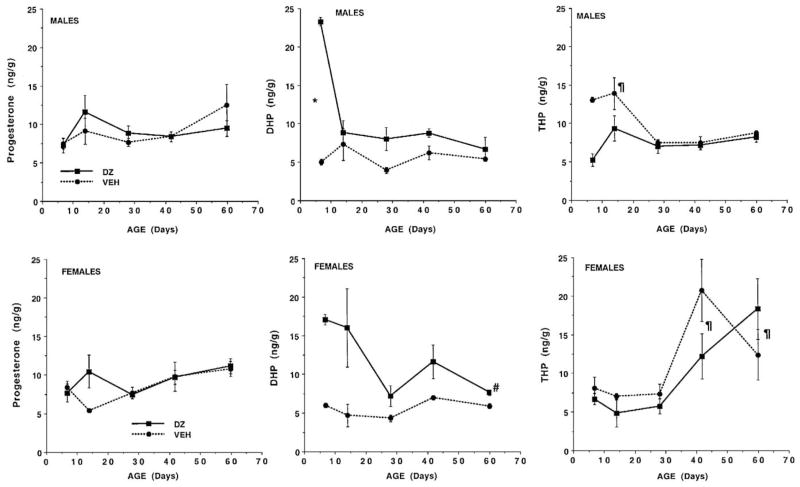
The levels of the different progestins measured in male and female cortex at different ages are illustrated in Fig. 1. A 3-way ANOVA of progesterone levels indicated that the levels varied significantly with age (P < 0.01) and that age interacted significantly with treatment (P < 0.05). Probing this interaction with 1-way ANOVA of each treatment group indicated that in the control group (vehicle and uninjected, collapsed over sex) P levels changed significantly with age (P < 0.03), with the level at PD 60 significantly greater than levels at PD 7, 14, and 28. Hence, in control rats, irrespective of sex, P levels in the cortex increase after the onset of puberty (which occurs around PD 30–32). ANOVA of P levels in DZ-exposed rats also indicated that the levels varied significantly with age (P < 0.05), with levels at PD 7 less than those at PD 60. However, the analysis also indicated that in DZ-exposed animals, P levels at PD 14 were significantly greater than at PD 7 and PD 28. This indicates a transient surge in P levels after the first week of life in the cortex of DZ-exposed pups, irrespective of sex.
Fig. 1.
Levels (ng/g) of progesterone, DHP, and 3α,5α-THP (THP) measured in the cerebral cortex of male and female rats at different postnatal ages from PD 7 to PD 60. Steroids were measured in Long–Evans rats exposed in utero to DZ (2.5 mg/kg) over gestational days 14–20 and in controls (VEH). The control group consisted of animals whose dams had either not been injected in utero or were injected with the vehicle. Data are expressed as mean ± SEM. Significance is designated as follows: *significant difference between treatment groups in respective sex at indicated age; #significantly different from control group, respective sex (collapsed across age); ¶significantly different from unmarked ages, respective sex (collapsed over treatment) and significantly different from other sex, same age. See text for other significant results.
Three-way ANOVA of DHP levels in the cortex indicated that the levels varied significantly with treatment (P < 0.0001) and age (P < 0.0001) and that treatment interacted significantly with age (P < 0.001). Furthermore, there was a significant 3-way interaction between sex, age, and treatment (P < 0.03). A separate 2-way ANOVA of DHP levels in each sex indicated that DHP levels in females varied significantly only with treatment (P < 0.0001). DHP levels in females, collapsed across postnatal age, were higher in DZ-exposed animals than in controls. Two-way ANOVA of DHP levels in males indicated that levels varied significantly with age and treatment and that age interacted significantly with treatment (P < 0.0001). Probing this with 1-way ANOVA of DHP levels in each of the two treatment groups indicated that DHP levels in the vehicle (control) group did not vary with age. However, DHP levels varied significantly with age (P < 0.0002) in DZ-exposed animals. The level of DHP at PD 7 in this group was significantly higher than at all other ages as determined by Fisher’s PLSD (P < 0.0001). The levels of DHP in the cortex at PD 7 differed significantly between the DZ and control group (P < 0.05; Student’s ‘t’ test). The high level of DHP in the cortex of DZ-exposed male rats at PD 7 seems to account for the results of the 3-way ANOVA.
Analysis of 3α,5α-THP levels in the cortex indicated that the levels varied significantly with sex (P < 0.04) and age (P < 0.0001), and that sex interacted significantly with age (P < 0.0001). The 3-way interaction between age, sex, and treatment indicated a trend (P < 0.09). To probe the significant 2-way interaction, 3α,5α-THP levels in males and females (collapsed over treatment) were compared at each age using Student’s ‘t’ test. This indicated that the levels in males were higher than in females at PD 14 (P < 0.004), while the levels in females were higher than in males at PD 42 (P < 0.01) and PD 60 (P < 0.004). 3α,5α-THP levels also were analyzed using separate 1-way ANOVA of levels in each sex. Analysis of levels in females supported a postpubertal increase in 3α,5α-THP levels; the levels at PD 7, 14 and 28 were all significantly lower than levels at PD 42 and PD 60 (P < 0.02–0.001, Fisher PLSD). Conversely, analysis in males supported high 3α,5α-THP levels at early juvenile ages. 3α,5α-THP levels at PD 14 were significantly higher than at all other ages (P < 0.004–0.0001, Fisher PLSD).
In summary, the levels of all 3 progestins measured in the cerebral cortex were found to vary significantly with age, and the developmental profiles of P and DHP were influenced by fetal DZ exposure. Sex interacted with fetal drug exposure to influence the development of DHP levels. A main impact of sex was observed for the age-related changes in 3α,5α-THP levels with very different profiles observed between males and females.
2.1.1.2. Androgens
The levels of the different androgens measured in male and female cortex at different postnatal ages are illustrated in Fig. 2. Three-way ANOVA of T levels indicated that T levels varied significantly with age (P < 0.03), treatment (P < 0.001), and that there was a significant interaction between sex, age, and treatment (P < 0.05). Separate 2-way ANOVA of T levels in each sex revealed a significant interaction between age and treatment (P < 0.03) in male cortex. This interaction was then probed with 1-way ANOVA of T levels over age in the respective groups. T levels in vehicle males varied significantly with age (P < 0.04), with the T level at PD 42 significantly higher than at all other ages (Fisher PLSD). These results support a mid-adolescent surge in T levels in the cortex of control males. Analysis of T levels in DZ-exposed males indicated that in this group T levels did not vary with age. Hence fetal DZ exposure prevented the mid-adolescent surge in T levels observed in control male rats. Furthermore, T levels at both PD 7 and PD 42 differed significantly between the two groups (Student’s ‘t’ test). Two-way ANOVA of T levels in female cortex indicated that the levels varied only with treatment (P < 0.003), with levels in the vehicle group higher than in the DZ-exposed group.
Fig. 2.
Levels (ng/g) of testosterone, DHT, and 3α-diols (Diols) measured in the cerebral cortex of male and female rats at different postnatal ages from PD 7 to PD 60. Steroids were measured in Long–Evans rats exposed in utero to DZ (2.5 mg/kg) over gestational days 14–20 and in controls (VEH). The control group consisted of animals whose dams had either not been injected in utero or were injected with the vehicle. Data are expressed as mean ± SEM. Significance is designated as follows: *significant difference between treatment groups in respective sex at indicated age, #significantly different from control group (collapsed over age), respective sex, ^significantly different from the other sex, collapsed over age and treatment group. See text for other significant results.
ANOVA of DHT levels in the cortex revealed only a significant effect of sex (P < 0.01) with levels overall higher in males than in females. There was a trend toward an effect of treatment (P < 0.09). There were no significant interactions.
Analysis of 3α-diol levels indicated significant main effects of sex (P < 0.01), treatment (P < 0.01), and age (P < 0.04). Levels were higher in males than in females, overall higher in DZ-exposed than in control animals, and levels at PD 14 were greater than at PD 28 and PD 60. There were no significant interactions.
In summary, the only androgen in the cortex to show marked changes with postnatal age was T, and T was developmentally regulated only in males. Fetal exposure to DZ altered the developmental profile of T in male cortex and led to overall lower levels of T in female cortex.
2.1.2. Diencephalon
2.1.2.1. Progestins
The levels of the 3 progestins measured in male and female diencephalon are shown in Fig. 3. A 3-way ANOVA of P levels indicated a significant effect of age (P < 0.001) with P levels at PD 7 less than those at PD 14 and P levels at PD 7, 14, and 28 less than those at PD 60. These results indicate an initial increase in P levels in the diencephalon over the second week of postnatal life and a later postpubertal increase, irrespective of sex or treatment group.
Fig. 3.
Levels (ng/g) of progesterone, DHP, and 3α,5α-THP (THP) measured in the diencephalon of male and female rats at different postnatal ages from PD 7 to PD 60. Steroids were measured in Long–Evans rats exposed in utero to DZ (2.5 mg/kg) over gestational days 14–20 and in controls (VEH). The control group consisted of animals whose dams had either not been injected in utero or were injected with the vehicle. Data are expressed as mean ± SEM. Significance is designated as follows: *significant difference between treatment groups in respective sex at indicated age, ¶significantly different from unmarked ages, respective sex (collapsed over treatment) and significantly different from other sex, same age.
A 3-way ANOVA of DHP levels indicated a significant effect of treatment (P < 0.002), age (P < 0.002), a significant age by treatment interaction (P < 0.0002) and a significant 3-way interaction between treatment, age, and sex (P < 0.02). This latter interaction was probed with separate 2-way ANOVA of DHP levels in each sex. The results indicated a significant age by treatment interaction in males (P < 0.05), but when this was probed with 1-way ANOVA of each treatment group, DHP levels did not vary with age in either treatment group. Hence, the analyses do not support changes with postnatal age in DHP levels in male diencephalon. However, 2-way ANOVA of DHP levels in females indicated a significant effect of age (P < 0.002), treatment (P < 0.008), and a significant age by treatment interaction (P < 0.003). Probing this interaction with 1-way ANOVA of DHP levels in each treatment group indicated that the levels varied significantly with age in the vehicle group (P < 0.04), with the levels at PD 7 less than those at PD 28 and 42. Also, the levels at PD 14 were less than at PD 42. These results support a late juvenile, early adolescent increase in DHP levels in the diencephalon of control females. Analysis of DHP levels in DZ-exposed females also indicated a significant effect of age (P < 0.006), with levels at PD 60 greater than at all other ages. Fetal exposure to DZ seemed to delay the increase in DHP levels in female diencephalon until after mid-adolescence. DHP levels in females were found to differ significantly between treatment groups at both PD 14 and PD 60 (Student’s ‘t’ test).
Analysis of 3α,5α-THP levels indicated a significant effect of age (P < 0.02) and a significant age by sex interaction (P < 0.002). Comparing 3α,5α-THP levels in males and females (collapsed over treatment) at each age indicated higher levels in females only at PD 60. Probing the significant 2-way interaction with separate 1-way ANOVA in males and females revealed that 3α,5α-THP levels did not vary with age in males but did vary significantly with age in females (P < 0.007). Irrespective of treatment, 3α,5α-THP levels in female diencephalon at PD 60 were significantly greater than at all other ages, and the levels at PD 7 were also less than at PD 42. These results suggest that 3α,5α-THP levels increase during late adolescence in female diencephalon, somewhat later than was observed for changes in DHP levels in control females.
In summary, with the exception of age-related changes in P, age regulated progestin levels primarily in females. Fetal exposure to DZ only influenced developmental of DHP levels in females.
2.1.2.2. Androgens
The levels of the 3 androgens measured in male and female diencephalon are illustrated in Fig. 4. A 3-way ANOVA of T levels indicated only a significant effect of age (P < 0.03), with T levels overall less at PD 7 than at PD 28 and PD 60. T levels in the diencephalon appear to increase across juvenile and adolescent ages, irrespective of sex or fetal drug exposure.
Fig. 4.
Levels (ng/g) of testosterone, DHT, and 3α-diols (Diols) measured in the diencephalon of male and female rats at different postnatal ages from PD 7 to PD 60. Steroids were measured in Long–Evans rats exposed in utero to DZ (2.5 mg/kg) over gestational days 14–20 and in controls (VEH). The control group consisted of animals whose dams had either not been injected in utero or were injected with the vehicle. Data are expressed as mean ± SEM. Significance is designated as follows: #significantly different from control group, respective sex (collapsed across age).
A 3-way ANOVA of DHT levels indicated a significant effect of treatment (P < 0.02), with levels in DZ-exposed animals greater than in vehicle controls, and a significant effect of age (P < 0.007), with levels at PD 7 less than at all other ages. There was also a significant interaction between treatment and sex (P < 0.02), and this interaction was probed using Student’s t test. In males, DHT levels were higher in DZ-exposed than in control males (P < 0.005), but there was no significant difference noted between groups for females. The interaction between sex and age indicated a trend (P < 0.08).
Analysis of 3α-diol levels indicated a significant effect of age (P < 0.003), with levels at PD 7 less than at all other ages (collapsed over sex and treatment). There was a trend towards a treatment by sex interaction (P < 0.06).
In summary, androgens were only modestly altered in the diencephalon by any of the independent factors. With the data collapsed over treatment group and sex, both levels of T and 3α-diol increased after the first postnatal week. Fetal exposure to DZ only altered DHT levels in males.
2.2. Analysis of GABA-stimulated chloride uptake
Net GABA-mediated chloride uptake (at 10 μM GABA), the maximal facilitation induced by 3α,5α-THP and the EC50 for facilitation were calculated for each experiment, and the averaged data are shown in Table 1. Analysis of these characteristics using 2-way ANOVA indicated that neither sex nor prenatal DZ exposure altered any of the characteristics of GABAA receptor responsiveness in adult cerebral cortex.
Table 1.
GABA-stimulated chloride uptake in cerebral cortex of adult rats
| Sex | Prenatal exposure | Net GABA-mediated uptakea nmol/mg protein | 3α,5α-THP Facilitation
|
|
|---|---|---|---|---|
| Maximal increase nmoles/mg protein | EC50 μM | |||
| Male | Vehicle | 11.32 ± 1.45 | 16.20 ± 1.13 | 0.384 ± 0.171 |
| Male | DZb | 13.24 ± 0.78 | 16.05 ± 1.31 | 0.387 ± 0.081 |
| Female | Vehicle | 12.67 ± 0.94 | 18.59 ± 1.61 | 0.300 ± 0.045 |
| Female | DZb | 13.23 ± 1.58 | 16.30 ± 1.28 | 0.304 ± 0.075 |
At 10 μM GABA.
2.5 mg/kg daily over gestation days 14 through 20.
3. Discussion
The results demonstrated that fetal exposure to DZ can influence brain steroid levels at postnatal ages. This study also provided needed information on postnatal development of steroid levels in the cerebral cortex and diencephalon in control animals. Age regulation of brain steroids and the impact of fetal DZ exposure were clearly shown to be region-and sex-specific. Neither fetal DZ exposure nor sex influenced 3α,5α-THP facilitated or GABA-mediated chloride uptake in adult cortex.
3.1. Developmental regulation of brain steroids
In control animals, P increased in the cerebral cortex after the onset of puberty regardless of sex, but 3α,5α-THP levels were altered by age in a sex-specific manner (regardless of treatment). In males, 3α,5α-THP levels were higher at an early juvenile age than at later ages, whereas in females, 3α,5α-THP levels increased over postpubertal ages. This sex-specific difference in developmental profile resulted in 3α,5α-THP levels that were higher in males than in females at PD 14, whereas the converse was observed at PD 42 and PD 60. Grobin and Morrow (2001) reported higher levels of 3α,5α-THP in female than in male cortex at PD 25 and PD 33 in Sprague–Dawley rats. However, since levels at later ages were not reported in that study, it is unclear whether the sex differences persisted. Higher levels in female vs. male cortex were not observed in the present study until PD 42. The earlier appearance of a sex difference in the other study may relate to the different strain of rat used (Sprague–Dawley vs. Long–Evans). Grobin and Morrow (2001) also reported higher 3α,5α-THP levels (collapsed over sex) at PD 10 and PD 14 than at PD 7 or PD 8 in the cerebral cortex. This observation is similar to the higher level of 3α,5α-THP observed only in males at PD 14 in the present study. The authors suggested that the higher 3α,5α-THP levels they observed at PD 10 and PD 14, regardless of sex, might have contributed to the observed reduced in vitro responsiveness of GABAA receptors at PD 12 to GABAA ligands. However, we previously reported no difference in the responsiveness of GABAA receptors to GABA or to DZ potentiation across postnatal age (Kellogg and Pleger, 1989), indicating no age-related differences in sensitivity to GABAA ligands. Furthermore, the results of the present study indicate no difference in facilitation of GABA-stimulated chloride uptake by 3α,5α-THP in adult female vs. male rats despite the higher endogenous levels of 3α,5α-THP observed in females at PD 60. Hence, it does not seem that endogenous levels of steroids necessarily dictate the in vitro sensitivity of GABAA receptors to steroids in synaptoneurosomal preparations.
Certainly though, the different in vivo steroid levels observed between the two sexes at different ages could contribute to functional differences. Currently, it is unclear what role high 3α,5α-THP levels in males at early juvenile ages or in females at late adolescent, early adult ages may have. Early postnatal exposure to 3α,5α-THP reportedly influenced GABAergic interneuron localization in mature prefrontal cortex (Grobin et al., 2003). Since males have higher levels of 3α,5α-THP in the cortex than females, most evident during an important period for cortical organization (Blue and Parnavelas, 1983), males may then acquire different interneuorn localization compared to females. Higher 3α,5α-THP levels in adult female cortex may contribute to sex differences in responses to anxiogenic stimuli.
The age-related changes in 3α,5α-THP levels in the cortex of either sex did not correspond clearly to changes in cortical levels of the parent steroid, progesterone. A postpubertal increase in P was observed irrespective of sex. Furthermore, in control animals, the levels of DHP, the immediate precursor of 3α,5α-THP, did not change with age in the cortex of either sex. Progesterone can be converted to several compounds, only one of which is DHP (Mellon et al., 2001). The age-related changes in 3α,5α-THP levels in each sex may reflect sex- and age-specific local endogenous conditions that favor either reduction or oxidation, since the enzyme 3α-hydroxysteroid dehydrogenase catalyzes both reactions.
Progesterone levels in the diencephalon were also regulated by age irrespective of sex, however, the age-related profile was somewhat different from that observed in the cortex. Levels in the diencephalon showed a two-phase increase over age, with an early juvenile increase in P followed by a later postpubertal increase. DHP and 3α,5α-THP levels were regulated by age only in female diencephalon, with increases in DHP levels occurring at an earlier age than increases in 3α,5α-THP levels. Age then exerted a greater influence on the level of progestins in female than in male diencephalon.
Considering androgens in the cortex, the primary impact of age was on T levels in male cortex. A postpubertal surge was observed in males with peak T levels occurring at mid-adolescence. This striking change in cortical levels of T over adolescence may be important to the acquisition of male-specific behaviors that takes place over adolescence. We have observed that male-specific environment-related social interaction (SI) is acquired over adolescence (Primus and Kellogg, 1989) and have shown that the acquisition of the mature behavior can be prevented either by juvenile gonadectomy (Primus and Kellogg, 1990) or by fetal exposure to DZ (Kellogg et al., 1991). This age-related profile of T observed in the cortex of males was not observed in the diencephalon. Also, the mid-adolescent surge in T in male cortex was not mirrored by similar changes in DHT or 3α-diol. We have previously demonstrated that aromatization of T is critical to the acquisition of mature male-typical SI (Kellogg and Lundin, 1999); therefore, the adolescent increase in T may serve as a substrate for aromatase rather than for 5α-reductase. Age-related changes in cortical T levels may influence pubertal changes in cortical organization in the male rat.
These age-related changes in brain levels of T and P raise the question of whether the changes in these brain steroids are driven by age-related changes in circulating parent compounds (T and P). Several facts argue against the importance of circulating hormones to age-related changes in brain levels of the same hormones. Circulating T levels in male rats begin to increase around the fifth postnatal week and continue to increase until around postnatal weeks 8–10 (Corpechot et al., 1981). If brain levels of T reflect solely circulating levels of T, then cortical T levels would not be expected to decrease after 6 weeks of age. Furthermore, the adolescent surge in T observed in the cerebral cortex of male rats was not observed in the diencephalon. If circulating T levels drive brain T levels, then age-related profiles of T levels should be similar in different brain regions. Additionally, there was no main effect of sex nor an age × sex interaction for T levels in either the cortex or diencephalon. Similarly, P levels did not differ between sex in either of the two regions, although they varied with age in both regions. Certainly, circulating T and P levels differ between males and females over postpubertal ages, yet this difference was not reflected in these steroid levels in the brain. The current observation is consistent with our previous observation that neither sex nor gestational age influenced brain neurosteroid levels across the last week of gestation (Kellogg and Frye, 1999), despite known sex and age differences in the supply of parent hormones to the fetal brain.
These observations seem to support age-related regulation of in situ production of both brain T and P. Progesterone can be produced in brain cells from cholesterol (Mellon, 2001). Testosterone can also be produced from a metabolite of cholesterol,17-OH-pregnenolone; however, the enzyme, P450c17, responsible for converting this compound to dihydroepiandrosterone (DHEA), a precursor of T, is developmentally regulated in the brain (Mellon, 2001). The enzyme is expressed in developing brain but not in the adult. Therefore, in situ synthesis of T from 17-OH-pregnenelone during adolescence does not seem likely. However, DHEA entering via the circulation could be a likely precursor for the in situ synthesis of T. The region-specific changes in T and P levels seem to argue for region- and age- and sex-specific regulation of in situ steroid synthesis, regardless of the source of precursor.
Because gonad removal profoundly affects many behaviors, the importance of circulating hormones to brain function cannot be dismissed. However, there is currently little understanding of how the influences of circulating vs. in situ-produced hormones in the brain are coordinated. Observations made following prenatal exposure to DZ, however, suggest that for certain brain functions, the action of circulating steroid hormones may be essential but not sufficient (see following discussion).
3.2. Effect of fetal DZ exposure
Exposure to DZ during the last week of gestation in the rat induced effects on postnatal levels of the various steroids in the brain in several ways. For certain steroids, fetal DZ exposure interacted with developmental regulation but not in a sex-specific manner, whereas for other steroids, the developmental impact of the exposure was dependent on sex of the animal. For still other steroids, fetal DZ exposure significantly affected postnatal steroid levels irrespective of age. The postnatal development of the different steroids measured in the two regions must be regulated by quite select signals. Overall, fetal DZ exposure exerted a much greater influence on brain steroid levels in the cerebral cortex than in the diencephalon.
Fetal DZ exposure induced an early juvenile surge in P levels in the cortex regardless of sex, with a peak observed in the exposed animals at PD 14. This DZ-induced profile was not observed in the level of P metabolites measured nor was it observed in the diencephalon. The exposure did significantly increase DHP levels in the cortex in both sexes, but in females, the effect was observed across all ages whereas in males, the effect was apparent only at PD 7. The implications of DZ-induced changes in these progestins are not clear. But since DHP can bind to P receptors (Rupprecht et al., 1993) as well as serve as a precursor to 3α,5α-THP (Mellon, 2001), enhanced levels at any age could have multiple effects on cellular function.
Interestingly, the fetal exposure had no effect on brain levels of 3α,5α-THP, a neurosteroid that is a potent modulator of GABAA receptors. Likewise, the exposure did not alter the sensitivity of GABAA receptors in adult cortex to 3α,5α-THP nor did it change the maximal facilitation of GABA-mediated chloride uptake by the neurosteroid. While previous work demonstrated that fetal DZ exposure induces a change in sensitivity of GABAA receptors in adult male (but not female) cortex to GABA (Kellogg et al., 1991), that observation was made when chloride uptake was evaluated over several concentrations of GABA. Measuring uptake at a single concentration of GABA did not disclose this effect of prenatal DZ exposure. The overall impact of fetal DZ exposure on long-term GABAA receptor function may be modest. The long-term consequences of a potential interaction between DZ and neurosteroids during fetal development may involve targets other than GABAA receptors.
Fetal DZ exposure had a marked influence on development of T levels in male cortex, and this effect corresponds to the effect of the exposure on the development of environment-related SI in male rats. The present study showed that the exposure prevented the adolescent surge in T levels observed in control males. We have previously observed that prenatal exposure to DZ in gonadally intact animals prevented the adolescent acquisition of environment-specific SI in male rats (Kellogg et al., 1991). The effect of the fetal drug exposure resembled the effect of juvenile gonadectomy. Interestingly, fetal DZ exposure does not alter circulating levels of T nor does it alter male reproductive behavior (unpublished observations). Hence, intact gonadal function alone does not seem sufficient for the adolescent organization of adult male SI behavior. The effect of prenatal DZ exposure on levels of T in the cerebral cortex likewise does not appear to reflect an impact of the exposure on the gonadal production of steroids. As presented above, circulating levels of DHEA may be critical to the adolescent production of T in the cortex. This raises the possibility that fetal DZ may have altered the in situ regulation of synthesis of T from DHEA in the cortex, but not diencephalon. While speculative, this possibility can be evaluated.
In addition to the acquisition of environment-related SI during adolescence in male rats, we have reported other changes in environmental impact on the brain over the same developmental period, and many of these changes are affected by fetal DZ exposure. For example, while environmental experience influences function of GABAA receptors in the cortex of adult males, environmental stimuli did not alter cortical GABAA receptor responses at late juvenile (Primus and Kellogg, 1991a,b) or early adolescent ages (Primus and Kellogg, 1993). Gonadal hormones influence the maturation of GABAA receptor responsiveness over this time period (Primus and Kellogg, 1991b). Hence, responsiveness of cortical GABAA receptors to environmental stimuli emerges during development over the same time period as environment-specific SI, and both the maturation of the behavioral response and of the responsiveness of the GABAA receptor were prevented by fetal DZ exposure (Kellogg et al., 1991, 1993). Furthermore, activation of specific cortical areas by environmental stressors emerges over adolescence, as indicated by cfos immunocytochemistry (Kellogg et al., 1998a). Whether the cortical organization that appears to take place over adolescence is driven by the mid-adolescent surge in cortical T levels is not known. Clearly though, fetal DZ exposure prevents this surge, interferes with the acquisition of mature SI behavior, and prevents maturation of stressor responsiveness of cortical GABAA receptors, a maturation that is influenced by gonadal hormones.
Fetal DZ exposure induced a decrease in T in the cortex of females that was observed across age. While we have observed fetal DZ exposure to more profoundly affect tested behaviors in males than in females, others have reported that early postnatal DZ exposure facilitates maternal behavior in female rats and induces it in males (Segovia et al., 1996). Furthermore, postnatal DZ exposure did not alter circulating levels of gonadal hormones in adults of either sex (Segovia et al., 1996), supporting our observations that the effects of fetal DZ exposure do not relate to an effect of the exposure on gonadal steriodogenesis. Whether postnatal DZ exposure would exert a similar effect on cortical levels of T during adolescence as seen following fetal exposure has not been examined, but such an impact could relate to the effects of the exposure on maternal behavior. The present study indicated that exposure to DZ more profoundly affected cortical levels of parent hormones rather than of their metabolites. It seems critical to understand the driving force for developmental changes in cortical levels of the parent hormones, P and T, in order to understand how developmental perturbations can affect these levels and to understand the functional implications of any changes.
Fetal exposure to DZ exerted very little impact on steroids in the diencephalon. The only effects noted were an age-related increase in DHP levels in females and an overall increase in DHT in males. And as discussed above, age influenced steroid levels in the diencephalon mainly in female rats. Again, the data seem to support region-specific regulation of steroid synthesis, and only in the cerebral cortex are these mechanisms readily modifiable by in utero exposure to DZ.
The major impact of the fetal manipulation on brain steroid levels was not seen at adult ages when functional changes have been most apparent. Clearly though, changing steroid function in the brain at earlier developmental time points could influence overall brain organization and later function. It is critical to examine the effect of fetal DZ exposure on other brain steroids and in other brain regions.
4. Experimental procedures
4.1. Animals
Adult female rats (Long–Evans [Blue Spruce strain], Harlan Sprague–Dawley, Altamont, NY) were mated overnight with male rats of the same strain. Vaginal lavage determined the presence of sperm on the day after mating with the presence of sperm designating gestation day 0. All animals were maintained under a 12–12-h light–dark cycle (lights on at 0600 h) with ad lib access to food and water. For steroid analysis, pregnant females were injected subcutaneously with either DZ (2.5 mg/kg, 6 dams) or its vehicle (40% propylene glycol, 10% ethanol, 4 dams) once daily over gestation days 14 through 20. Three pregnant dams remained uninjected. Offspring from both vehicle-exposed and uninjected litters comprised the control group as no differences were detected between these groups. Injection volumes were based on each pregnant animal’s weight on gestation day 13 and were held constant over the injection period. Parturition occurred on gestation day 22 (designated postnatal day 0). All litters were weaned at 28 days postnatal age and group housed according to sex and prenatal treatment. For steroid analysis, male and female pups were removed from a litter between 0900 and 1200 h and killed by rapid decapitation at postnatal days (PD) 7 (neonatal), 14 (early juvenile), 28 (late juvenile), 42 (adolescent) and 60 (young adult). Three animals of each sex were analyzed at each age in each group (DZ-exposed and control), and the 3 animals were selected from 3 different litters. Estrous stage was not determined in postpubertal females (PD 42 and 60). Reports have shown, however, that circulating progesterone levels (which could influence levels of brain progestins) are similar across the estrous cycle when measurements are made at the time in the light–dark cycle that tissue was collected in the present study (Smith et al., 1989). Furthermore, we have measured circulating progesterone and estradiol levels in adult females at different stages of the estrous cycle at a similar time in the light–dark cycle following a similar in utero protocol. While we observed slightly higher levels of P at diestrus 1 (similar to other reports (Smith et al., 1989)) than at other stages, we observed no effect of prenatal DZ exposure (unpublished observations). Assessing estrous stage in females at P42 and P60 would have exposed these two groups of animals to different environmental conditions than experienced by all other groups. Therefore, we elected to analyze animals at the point in the light–dark cycle when variations in circulating gonadal hormones in females would be minimal. For analysis of GABAA receptor function in adult cortex, male and females were killed by rapid decapitation at 70–90 days of age following prenatal exposure as described above. The University Animal Care Committee approved all animal procedures.
4.2. Brain dissection
For steroid analysis, the brains were rapidly removed and dissected over ice into the cerebral cortex and diencephalon. A single cortical hemisphere (with hippocampus removed) was taken from each animal for steroid analysis, and the average hemisphere weight/sample was 386.62 ± 15 mg. Hemisphere weight did not vary significantly with postnatal age. The diencephalon contained the thalamus, hypothalamus, and septum and was selected for steroid analysis rather than the hypothalamus alone in order to obtain sufficient tissue for steroid analysis. Diencephalon weight varied significantly from 92–166 mg depending upon postnatal age. The regions were quickly frozen over dry ice and stored at −70 °C until analysis.
For analysis of GABAA receptor function, the meninges and superficial blood vessels were stripped away following removal of the brain from the animal, and the cerebral cortex was separated from the rest of the brain and the underlying white matter was peeled away. Tissue was freshly prepared on the day of experiment.
4.3. Steroid analysis
Steroids were measured according to previously reported procedures using radioimmunoassay following prior separation with column chromatography (Frye and Bayon, 1999; Kellogg and Frye, 1999). Briefly, brain regions were homogenized with a glass/teflon homogenizer in 2 ml of distilled water containing 800 cpm of tritiated compounds. Steroids were extracted from the homogenate with diethyl ether and dried down in an evaporator drier. The resulting pellet was reconstituted in Trimethyl Pentane (TMP) to half the original homogenate volume. Reconstituted extracts were separated using Celite column chromatography. Solvents of increasing polarity were used to elute respective steroids: progestins (100% TMP), DHT (5% ethyl acetate/TMP), T and 3α-diol (15% ethyl acetate/TMP). Progestins were further separated on Sepak cartridges with increasing concentrations of methanol. Fractions were then dried using a Savant evaporator drier and reconstituted in phosphate assay buffer.
The antibodies used were obtained as follows: P antibody (#337) from Dr. G.D. Niswender (Colorado State University), DHP (X-947) and 3α,5α-THP (921412-5) antibodies from Dr. Robert Purdy (Veteran’s Medical Center, La Jolla, CA), T and DHT from Endocrine Science (Calabase Hills, CA), and 3α-diol (X-144) from Dr. P.N. Rao (Southwest Foundation for Biomedical Research, San Antonio, TX). Antibody concentrations were as follows: P-1:30,000; 3α,5α-THP and DHP-1:5000; T and 3α-diol-1:20,000; DHT-1-10,000. All standard curves were prepared in duplicate (progesterone, range = 5–800 pg; 3α,5α-THP, range = 10–4000 pg; DHP range = 5–800 pg, T, DHT, and 3α-diol range = 50–2000 pg). The standards were added to BSA assay buffer, followed by addition of the appropriate antibody and tritiated steroid. The following radioactive compounds were purchased from New England Nuclear: Tritiated P (NET-208, specific activity = 47.5 Ci/mmol), 3α,5α-THP (used for detection of both 3α,5α-THP and DHP, NET-1047, specific activity = 65 Ci/mmol), T (NET-387, specific activity = 51 Ci/mmol), DHT (NET-302, specific activity = 43.5 Ci/mmol), and 3α-diol (NET-806, specific activity = 41 Ci/mmol). Total assay volumes were as follows: 800 μl for P, 950 μl for DHP and 3α,5α-THP, 1200 μl for T, DHT, and 3α-diol.
All assays were incubated overnight at 4 °C except for 3α-diol, which was incubated at room temperature. Separation of bound and free steroid was done by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 1200 × g, and the supernatant was transferred to a glass scintillation vial and scintillation cocktail was added. Sample tube concentrations were calculated using the logit-log method of Robard and Hutt (1974) with interpolation of the standards and correction for recovery. The intra-assay and inter-assay coefficients of variance for P were 10 and 9%, DHP and 3α,5α-THP, 12 and 15%; T, 5 and 15%, DHT, 2 and 15%, and 3α-diol, 12 and 9% respectively.
4.4. Analysis of GABA-stimulated chloride flux
Cortex from an individual animal was homogenized in HEPES–Tris buffer (pH 7.4), and synaptoneurosomes were prepared and GABA stimulation of 36chloride uptake was measured as previously described (Kellogg and Pleger, 1989). Briefly, uptake was assessed in a total volume of 0.5 ml containing 0.5 μCi 36chloride, GABA (10 μM, around the EC50 for GABA stimulation), and varying concentrations of 3α,5α-THP (0.01 to 5 μM). 3α,5α-THP was dissolved in an ethanol solution; the final concentration of ethanol in the incubation tubes was 0.5% for all concentrations of steroid, an ethanol concentration that did not influence GABA-stimulated or basal chloride uptake in adult preparations. The reaction was initiated by the addition of pre-incubated (at 30 °C) freshly prepared synaptoneurosomes to pre-incubated tubes (protein per tube was 1.8–2.2 mg). The reaction was terminated after 10 s by adding cold HEPES–Tris buffer containing picrotoxin (100 μM) and immediately filtering the contents through GF/C filters that had been presoaked in 0.05% polyethyelenimine. The filters were counted using liquid scintillation spectrophotometry. Basal chloride uptake was determined in the absence of either GABA or steroid. The potentiation of net GABA-mediated chloride uptake (uptake in the presence of GABA minus basal uptake) by 3α,5α-THP was calculated and expressed as both percent facilitation and nmol of chloride/mg protein. Protein was measured as previously described (Kellogg and Pleger, 1989). The maximal amount of facilitation and the EC50 of facilitation were calculated for each experiment using Graph-pad Prism™ software. The experiment was repeated three times for males and five times for females for each of the prenatal exposure groups.
4.5. Statistical analysis
Individual steroids in each brain region were analyzed using a 3-way analysis of variance (ANOVA, Stat View™ 5.0.1) with sex, prenatal treatment (DZ or vehicle), and postnatal age as independent variables. Significant effects were probed using Fisher PLSD or Student’s ‘t’ test. Significant 3-way and 2-way interactions were probed with 2-way or 1-way ANOVA, respectively, or with t tests. Statistical significance was noted when the probability of a Type I error was at least <0.05. Net GABA-mediated chloride uptake (at 10 μM GABA), the maximal facilitation by 3α,5α-THP, and the sensitivity (EC50) to 3α,5α-THP were analyzed using 2-way ANOVA with sex and prenatal treatment as independent variables.
Acknowledgments
This work was supported by funds from the Kilian J. and Caroline F. Schmitt Program (to C.K.K.) and by grants from the National Science Foundation (IBN 03-16083) and from The National Institute of Mental Health (06769801) to C.A.F.
References
- Barker JL, Behar T, Li Y-X, Liu Q-Y, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen X-L, Xian H. Gabaergic cells and signals in CNS development. Perspect Dev Neurobiol. 1998;5:305–322. [PubMed] [Google Scholar]
- Bitran D, Foley M, Audetter D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;15:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat: II. Quantitative analysis. J Neurocytol. 1983;12:697–712. doi: 10.1007/BF01181531. [DOI] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li P-K, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Baulieu E-E, Robel P. Testosterone, dihydrotestosterone and androstanediols in plasma, testes, and prostates of rats during development. Acta Endocrinol. 1981;96:1135–1277. doi: 10.1530/acta.0.0960127. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Morrow AL. 3α-hydroxy-5α-pregnan-20-one levels and GABAA receptor-mediated 36Cl-flux across development in rat cerebral cortex. Dev Brain Res. 2001;131:31–39. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Heenan EJ, Lieberman JA, Morrow AL. Perinatal neurosteroid levels influence GABAergic interneuron localization in adult rat prefrontal cortex. J Neurosci. 2003;23:1832–1839. doi: 10.1523/JNEUROSCI.23-05-01832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg CK. Benzodiazepiness and the developing nervous system: laboratory findings and clinical implications. In: Zagon IS, Slotkin TA, editors. Maternal Substance and the Developing Brain. Academic Press, Inc; 1992. pp. 283–321. [Google Scholar]
- Kellogg CK. Early developmental modulation of GABAA receptor function: influence on adaptive responses. Perspect Dev Neurobiol. 1998;5:219–234. [PubMed] [Google Scholar]
- Kellogg CK. Sex differences in long-term consequences of prenatal diazepam exposure: possible underlying mechanisms. Pharmacol Biochem Behav. 1999;64:673–680. doi: 10.1016/s0091-3057(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Frye CA. Endogenous levels of 5 alpha-reduced progestins and androgens in fetal vs. adult rat brains Dev Brain Res. 1999;115:17–24. doi: 10.1016/s0165-3806(99)00041-3. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Lundin A. Brain androgen-inducible aromatase is critical for adolescent organization of environment-specific social interaction in male rats. Horm Behav. 1999;35:155–162. doi: 10.1006/hbeh.1998.1508. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Pleger GL. GABA-stimulated chloride uptake and enhancement by diazepam in synaptoneurosomes from rat brain during prenatal and postnatal development. Dev Brain Res. 1989;49:87–95. doi: 10.1016/0165-3806(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Primus RJ, Bitran D. Sexually dimorphic influence of prenatal exposure to diazepam on behavioral responses to environmental challenge and on γ-aminobutyric acid (GABA)-stimulated chloride uptake in the brain. J Pharmacol Exp Ther. 1991;256:259–265. [PubMed] [Google Scholar]
- Kellogg CK, Taylor MK, Rodriguez-Zafra M, Pleger GL. Altered stressor-induced changes in GABAA receptor function in the cerebral cortex of adult rats exposed in utero to diazepam. Pharmacol Biochem Behav. 1993;44:267–273. doi: 10.1016/0091-3057(93)90461-2. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced fos immunoreactivity in rat brain. Neuroscience. 1998a;83:681–689. doi: 10.1016/s0306-4522(97)00408-9. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Olson VG, Pleger GL. Neurosteroid action at the GABAA receptor in fetal rat forebrain. Dev Brain Res. 1998b;108:131–137. doi: 10.1016/s0165-3806(98)00042-x. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Yao J, Pleger GL. Sex-specific effects of in utero manipulation of GABAA receptors on pre- and postnatal expression of BDNF in rats. Dev Brain Res. 2000;121:157–167. doi: 10.1016/s0165-3806(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Murray RM. Neruodevelopment and Adult Psychopathology. Cambridge Univ. Press; UK: 1997. pp. 1–282. [Google Scholar]
- Mellon SH, Griddin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Möhler H, Benke D, Fritschy JM, Benson J. The benzodiazepine site of GABAA receptors. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: The View at Fifty Years. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 97–112. [Google Scholar]
- Plassart-Schiess E, Baulieu E-E. Neurosteroids: recent findings. Brain Res Rev. 2001;37:133–140. doi: 10.1016/s0165-0173(01)00113-8. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Experience influences environmental modulation of function at the benzodiazepine (BZD)/GABA receptor chloride channel complex. Brain Res. 1991a;545:257–264. doi: 10.1016/0006-8993(91)91294-b. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal status and pubertal age influence the responsiveness of the benzodiazepine/GABA receptor complex to environmental challenge in male rats. Brain Res. 1991b;561:299–306. doi: 10.1016/0006-8993(91)91608-4. [DOI] [PubMed] [Google Scholar]
- Robard D, Hutt DM. International Atomic energy Agency. Symposium on Radiommunoassay and Related Procedures in Medicine. Uniput; New York: 1974. Statistical analysis of radiommunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. [Google Scholar]
- Roberts AA, Pleger GL, Kellogg CK. Effect of prenatal exposure to diazepam on brain GABAA receptor mRNA levels in rats examined at late fetal or adult ages. Dev Neurosci. 2001;23:135–144. doi: 10.1159/000048705. [DOI] [PubMed] [Google Scholar]
- Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3α-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res Rev. 2001;37:162–171. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JMHM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglegansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, di Michele F, Hermann B, Stroble A, Lancel M, Romeo E, Holsboer F. Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res Rev. 2001;37:59–67. doi: 10.1016/s0165-0173(01)00123-0. [DOI] [PubMed] [Google Scholar]
- Segovia S, Cruz M, del Cerro R, Ortega E, Perez-Laso C, Rodriguez-Zafra M, Angeles M, Isquierdo P, Guillamon A. Role of GABAA receptors in the organization of brain and behavioural sex differences. NeuroReport. 1996;7:2554–2557. doi: 10.1097/00001756-199611040-00030. [DOI] [PubMed] [Google Scholar]
- Smith SS, Woodward DJ, Chapin JK. Sex steroids modulate motor-correlated increases in cerebellar discharge rate. Brain Res. 1989;476:307–316. doi: 10.1016/0006-8993(89)91251-1. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Slotkin TA. Maternal Substance Abuse and the Developing Nervous System. Academic Press, Inc; 1992. pp. 1–378. [Google Scholar]