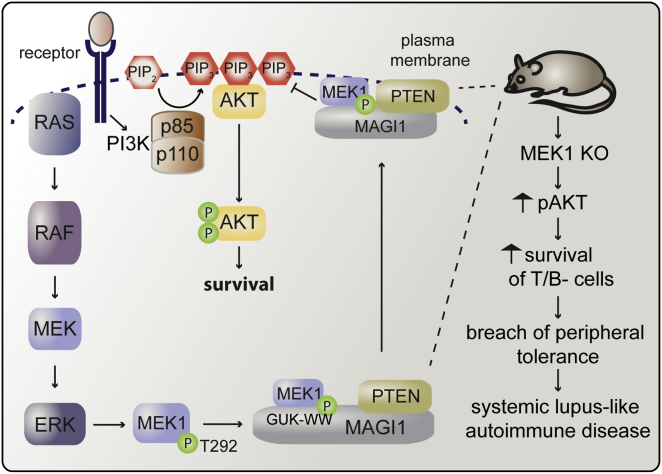
Summary
The Raf/MEK/ERK and PI3K/Akt pathways are prominent effectors of oncogenic Ras. These pathways negatively regulate each other, but the mechanism involved is incompletely understood. We now identify MEK1 as an essential regulator of lipid/protein phosphatase PTEN, through which it controls phosphatidylinositol-3-phosphate accumulation and AKT signaling. MEK1 ablation stabilizes AKT activation and, in vivo, causes a lupus-like autoimmune disease and myeloproliferation. Mechanistically, MEK1 is necessary for PTEN membrane recruitment as part of a ternary complex containing the multidomain adaptor MAGI1. Complex formation is independent of MEK1 kinase activity but requires phosphorylation of T292 on MEK1 by activated ERK. Thus, inhibiting the ERK pathway reduces PTEN membrane recruitment, increasing phosphatidylinositol-3-phosphate accumulation and AKT activation. Our data offer a conceptual framework for the observation that activation of the PI3K pathway frequently mediate resistance to MEK inhibitors and for the promising results obtained by combined MEK/PI3K inhibition in preclinical cancer models.
Graphical Abstract
Highlights
► A complex of MEK1, MAGI1, and PTEN regulates PIP3 turnover and AKT signaling ► Ablation/inhibition of MEK1 blocks complex formation and PTEN membrane recruitment ► ERK-mediated MEK1 phosphorylation coordinates the timing of ERK and AKT signaling ► In vivo, MEK1 ablation induces AKT activation and a breach in peripheral tolerance
Introduction
The Raf/MEK/ERK pathway is among the most thoroughly studied downstream effectors of activated Ras (Wimmer and Baccarini, 2010). Deregulation of the pathway is implicated in both developmental disorders and cancer (Maurer et al., 2011; Schubbert et al., 2007). Consequently, numerous RAF and MEK inhibitors aimed at blocking ERK activation have been designed (Chapman and Miner, 2011; Poulikakos and Solit, 2011).
The dual specificity kinases MEK1 and MEK2 are activated by RAF and mediate phosphorylation of ERK1 and ERK2 (Roskoski, 2012). MEK1 and MEK2 are very similar but differ structurally in a proline-rich domain in the C-terminal half of the catalytic core, which in MEK1 contains the negative regulatory phosphorylation sites T286, targeted by Cdk5 mainly in postmitotic neurons, and T292, essential for the negative feedback regulation of MEK by ERK1 and ERK2 (Roskoski, 2012).
MEK1 and MEK2 also bind differentially to scaffolds such as MP1, which plays a role in ERK1 activation at late endosomes (Teis et al., 2002), and IQGAP1, which regulates adhesion/migration, promotes signaling from MEK1 to ERK, and attenuates MEK2/ERK signaling (Roy et al., 2005).
Finally, disruption of the mek1 gene in vivo causes abnormal placenta development and lethality around embryonic day 9.5 (Bissonauth et al., 2006; Catalanotti et al., 2009; Giroux et al., 1999), while mek2−/− mice are normal (Bélanger et al., 2003).
We have recently reported that MEK1 is essential for the regulation of the timing and strength of ERK signaling. By phosphorylating the T292 site in the proline-rich region of MEK1, ERK exerts negative feedback control on MEK1/MEK2 dimers. If MEK1 is absent, this control is disabled, leading to increased ERK signaling (Catalanotti et al., 2009).
Besides this negative feedback within the pathway, ERK can interfere with phosphatidylinositol-3 kinase (PI3K)/AKT signaling, another major protumorigenic Ras effector (Liu et al., 2009; Song et al., 2012). In response to growth factors, PI3K converts phosphatidylinositol 4,5 diphosphate (PIP2) in the second messenger phosphatidylinositol 3,4,5 triphosphate (PIP3). AKT binds to PIP3 via its pleckstrin homology (PH) domain and translocates to the membrane, where it is activated by phosphorylation of T308 and S473. Tumor suppressor phosphatase and tensin homolog deleted on chromosome ten (PTEN) dephosphorylates PIP3, counteracting PI3K activity and restraining AKT activation (Liu et al., 2009; Song et al., 2012). Inhibition or silencing of MEK increases growth factor-stimulated AKT activation (Hoeflich et al., 2009; Ussar and Voss, 2004; Yu et al., 2002), a finding which was linked to ERK-mediated inhibition of PI3K recruitment to the epidermal growth factor (EGF) receptor via phosphorylation of the adaptor GAB1 (Lehr et al., 2004; Zhang et al., 2002).
We show that MEK1 is required for the control of PIP3 accumulation at the cell membrane, which it regulates by ensuring the proper localization of PTEN in the context of a ternary complex with a multiprotein adaptor, MAGI1. MEK1 ablation deregulates the AKT pathway which, in vivo, induces a breach of peripheral self tolerance and myeloproliferation.
Results
MEK1 Is Essential for the Negative Regulation of AKT Signaling
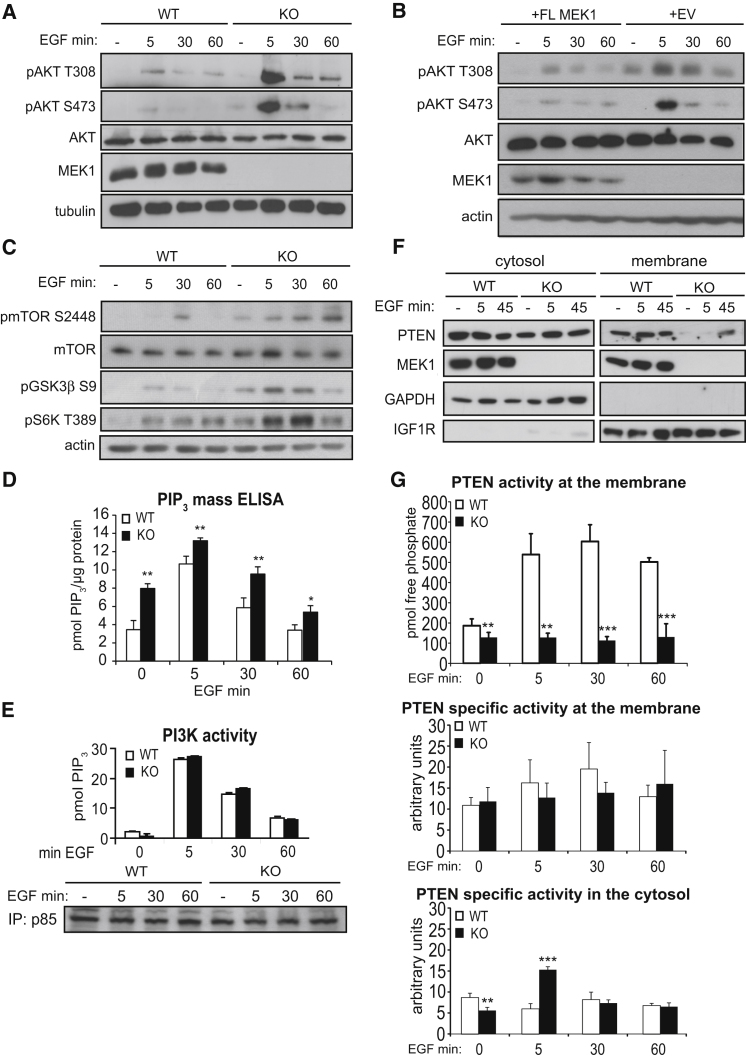
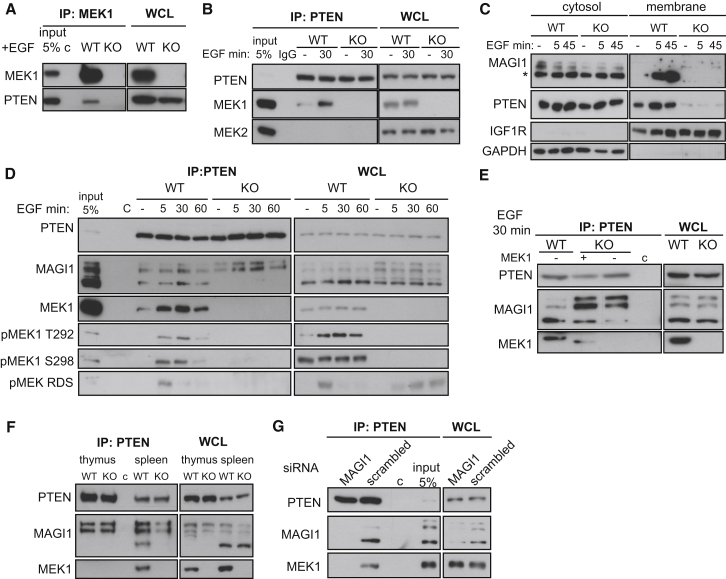
We monitored the impact of MEK1 on AKT signaling in wild-type (WT) and MEK1 knockout (KO) mouse embryonic fibroblasts (MEFs) (Catalanotti et al., 2009) stimulated with epidermal growth factor (EGF). Phosphorylation of AKT and its downstream effectors mTOR, GSK3β, and S6K was reproducibly enhanced in MEK1-deficient cells (Figures 1A and 1C). The increase in AKT phosphorylation was rescued by the re-expression of full-length MEK1 (Figure 1B). The levels of the membrane lipid PIP3, necessary for AKT activation (Liu et al., 2009), were significantly higher in KO MEFs than in control cells (Figure 1D and Figures S1A and S1B available online). In contrast, PI3K expression and activity (Figures 1E and S1C) were comparable in WT and KO cells, as were PTEN expression and the EGF-induced dephosphorylation of the C-terminal residues which negatively regulate PTEN activity, its association with PDZ-containing proteins (Rahdar et al., 2009; Vazquez et al., 2001) and with PIP2 (Figure S1D). However, PTEN was hardly detectable at the membrane of KO MEFs, even after EGF stimulation (Figures 1F, S1E, and S1F), and membrane-associated PTEN activity was greatly reduced (Figure 1G). Consistently, focal adhesion kinase (FAK), a membrane-associated PTEN protein substrate (Tamura et al., 1998; Yamada and Araki, 2001), was also hyperphosphorylated in KO cells (Figure S1G). MEK1 ablation did not impact the specific activity of membrane PTEN; in the cytosol, activity transiently increased at 5 min of EGF treatment (Figure 1G). PTEN can be regulated by a variety of posttranslational modifications and by the interaction with other proteins (Song et al., 2012); which of these mechanisms is responsible for this transient increase in cytosolic PTEN activity is currently unknown. Thus, MEK1 is required for correct membrane localization of PTEN and for limiting PIP3 accumulation and AKT activation.
Figure 1.
MEK1 Ablation Promotes PIP3 Signaling by Preventing PTEN Membrane Translocation
(A–C) WT and KO MEFs (A and C), and KO MEFs reconstituted with full-length MEK1 (+FL MEK1) or empty vector (+EV) (B) were stimulated with EGF and lysed at the indicated time points. Proteins and phosphorylated proteins were detected by immunoblotting as indicated.
(D) ELISA measurement of cellular PIP3 in MEFs upon EGF stimulation.
(E) PI3K activity in p85 IPs from untreated or EGF-stimulated MEFs was assessed by ELISA. The amount of PIP3 was normalized to the amount of immunoprecipitated p85.
(F) Extracts of starved or EGF-stimulated MEFs separated into cytosolic and membrane fractions. The presence of PTEN, MEK1, GAPDH (cytosolic marker), and IGF1R (plasma membrane marker) was assessed by immunoblotting.
(G) PTEN activity in membrane and cytosol fractions of EGF-stimulated MEFs. In the middle and bottom panels, arbitrary units were calculated by division of picomoles of released phosphate by the amount of immunoprecipitated PTEN determined by the densitometry of western blots.
The values in (D), (E), and (G) represent the mean of three experiments ±SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S1.
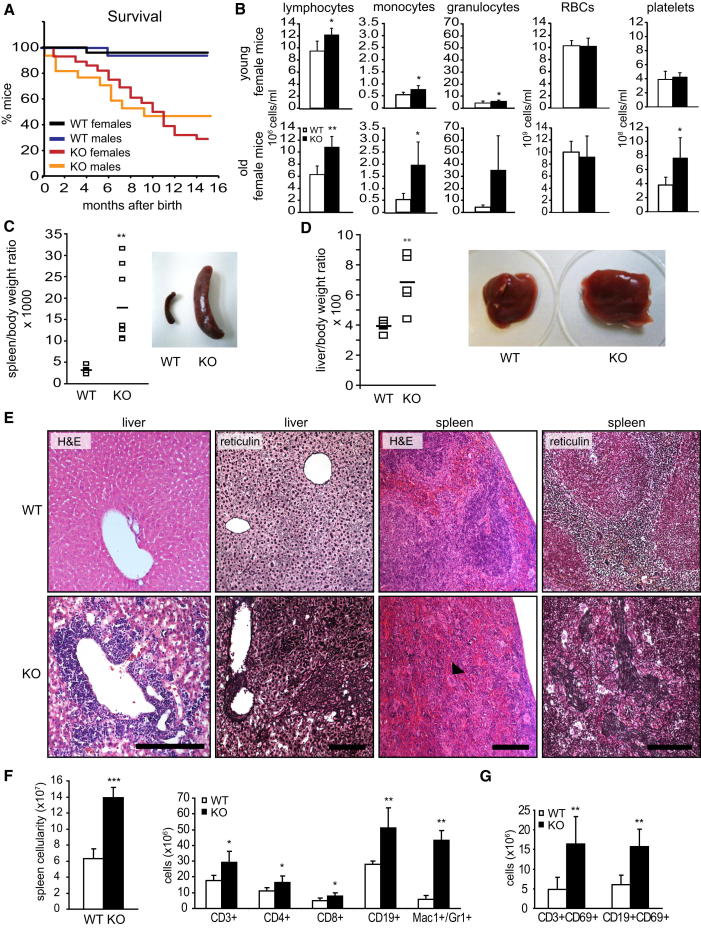
MEK1 KO Mice Develop Myeloproliferation and Autoimmune Disease
To investigate the function of MEK1 in vivo, we used mice with epiblast-restricted MEK1 ablation (mek1f/f; Sox2Cre mice [Catalanotti et al., 2009], referred to as KO mice). These animals, in particular the females, had a significantly decreased survival rate (Figure 2A). Increased numbers of circulating lymphocytes could be detected in the blood of 1- to 3-month-old mice; this was exacerbated in old MEK1 KO animals, in which it was accompanied by granulocytosis and thrombocytosis (Figure 2B). By 8–10 months of age, 83% MEK1-deficient females had developed severe splenomegaly (Figure 2C), hepatomegaly with lesser frequency (Figure 2D; observed in 45% of the animals), and, occasionally, lymphadenopathy. Liver and spleen showed effacement of architecture, extramedullary hematopoiesis, accumulation of atypical megakaryocytes, and fibrosis (Figures 2E and S2A). Splenomegaly correlated with a massive increase in immature Mac1+Gr1+ myeloid cells (Figure 2F), a population observed in pathological conditions such as cancer and autoimmunity (Gabrilovich and Nagaraj, 2009). KO bone marrow and splenocytes isolated from young, unaffected animals gave rise to a significant higher number of colony forming units in semisolid media, indicating a cell-autonomous phenotype (Figure S2B). In addition, KO spleens contained significantly increased numbers of T (CD3+) and B (CD19+) cells (Figure 2F). The CD4+/CD8+ ratio was normal, but more T and B cells were activated, as shown by the coexpression of the activation marker CD69 with CD3 and CD19 (Figure 2G). Despite the autoimmune disease, splenic Tregs were not decreased (data not shown). A slight increase in activated T cells was the only phenotype detected in the spleen of young KO mice (Figure S2C). In contrast to the marked phenotypic alterations in spleen and liver, KO thymi were normal in terms of cellularity and subset distribution (Figure S2D).
Figure 2.
Myeloproliferation and Lymphocyte Activation in MEK1 KO Mice
(A) Survival of female (n = 28) and male (n = 17) KO mice monitored over a 15 month period.
(B) Peripheral blood cell counts of young (1–3 months) and old (8–12 months) female KO mice and sex-matched WT littermates. Values represent mean ±SD (n = 5).
(C and D) Spleno- and hepatomegaly in MEK1 KO mice. The plots show the weight of spleens (C; n = 6) and livers (D; n = 5) isolated from affected mice.
(E) Effacement of tissue architecture, extramedullary hematopoiesis (hematoxylin and eosin staining; arrowhead, giant megakaryocyte) and fibrosis in KO livers and spleens. Scale bars represent 200 μm.
(F and G) Increased Mac1+/Gr1+ cells and activated lymphocytes in spleens of affected KO mice (age 5–12 months, n = 5), detected by FACS analysis of lineage-specific and activation-induced markers (G; CD69).
Values represent mean ±SD (n = 5). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S2.
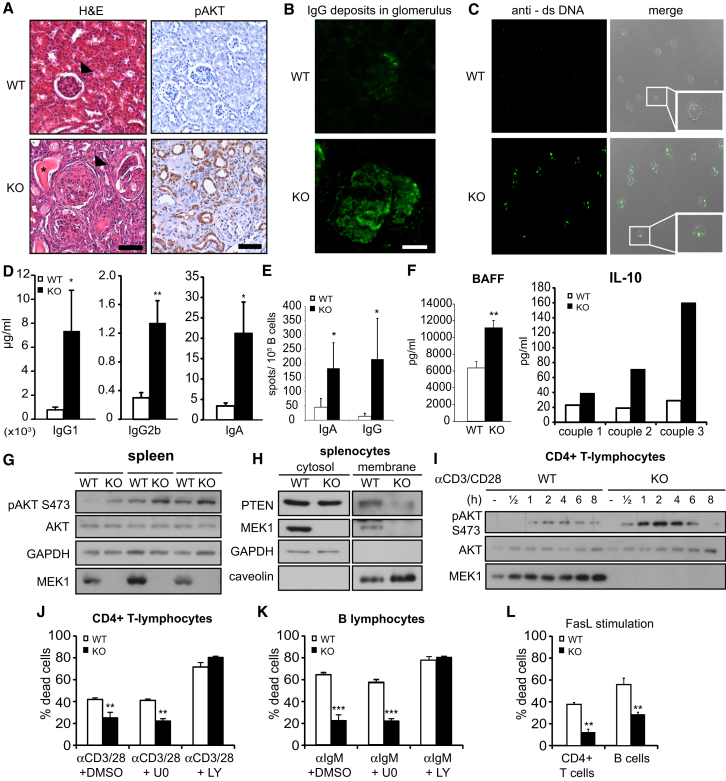
Among the nonhematopoietic organs, KO lungs showed thickening of the interstitial alveolar spaces with vascular congestion (Figure S2E); however, the kidneys were the organs most severely affected. The tubules were dilated and filled with proteinaceous material, and the glomeruli displayed signs of focal proliferation and sclerosis reminiscent of glomerulonephritis, accompanied by the deposition of immunocomplexes in the glomeruli (Figures 3A and 3B). In addition, antibodies against double-stranded DNA (dsDNA), a hallmark of lupus-like autoimmune diseases, were detected in the sera of seven out of seven KO 5- to 10-month-old females (Figure 3C; titer of 1:100 in five mice; 1:1000 in two mice). This correlated with a marked increase in the concentrations of serum immunoglobulins (IgG1, IgG2b, IgA) and in the frequency of IgA and IgG-producing B cells affected mice (Figures 3D and 3E). Circulating BAFF and interleukin-10 (IL-10) (Figure 3F), crucially implicated in the pathogenesis of lupus-like disease (Su et al., 2012), were also elevated, and the frequency of cells producing IL-10, GM-CSF, transforming growth factor β, and interferon γ (IFNγ) was elevated to varying degrees (Figure S3A). In all cases, the increase was most pronounced in CD4+ lymphocytes. IFNα, IL-3, IL-6, IL-17, and IL-23 were tested but proved similar in WT and KO (data not shown).
Figure 3.
MEK1 Ablation Leads to Systemic Autoimmune Disease
(A) Enlarged glomeruli (arrows), tubules filled with proteinaceous material (asterisk), and increased pAKT in KO kidneys. Scale bars represent 100 μm.
(B) Immune complex deposition in the glomerulus of a 10-month-old KO female visualized by mouse IgG antibodies (green). The scale bar represents 30 μm.
(C) dsDNA autoantibodies detected in the serum of KO mice (5–10 months, n = 7) by Crithidia luciliae kinetoplast staining.
(D) IgG1, IgG2b, and IgA levels in the sera of 8- to 10-month-old mice (n = 3) assessed by ELISA.
(E) Frequency of B cells secreting IgA and IgG in 8- to 10-month-old mice determined by ELISpot (n = 4). Error bars represent the SD.
(F) BAFF and IL-10 serum levels determined as in (D).
(G) AKT phosphorylation and expression of AKT, MEK1, and GAPDH (loading control) were determined in lysates of 8-week-old WT and KO spleens.
(H) PTEN, MEK1, GAPDH (cytosolic marker), and caveolin (membrane marker) detected by immunoblotting in subcellular fractions of freshly isolated WT and KO splenocytes.
(I) Splenic CD4+ T cells stimulated with anti-CD3 and anti-CD28 (3 μg/ml each). The indicated proteins were visualized by immunoblotting.
(J–L) Survival of CD4+ T cells and B cells in response to AICD and FasL-induced cell death.
Values represent the mean of three experiments ±SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S3.
Thus, the KO mice are prone to develop both myeloproliferation and a lupus-like autoimmune disorder reminiscent of those reported for pten+/− (Di Cristofano et al., 1999), hematopoetic (Guo et al., 2008; Yilmaz et al., 2006) or T cell-restricted PTEN KO mice (Liu et al., 2010; Suzuki et al., 2001), and constitutively active AKT transgenic mice (Kharas et al., 2010; Parsons et al., 2001).
MEK1 Ablation Deregulates AKT Activation and Impairs Activation-Induced Cell Death
The AKT deregulation caused by PTEN mislocalization (Figures 1 and S1) could contribute to the phenotypes of MEK1 KO mice. Indeed, AKT phosphorylation was detected to variable extents in KO kidneys, livers, lungs, spleens, and lymphnodes (Figures 3A, S2F, and S2G). In contrast, ERK phosphorylation, deregulated in cultured fibroblasts and in some organs in vivo (Catalanotti et al., 2009), was not affected (Figure S2H). Given the poor conditions of these animals, however, it is impossible to distinguish whether this phosphorylation is cause or consequence of the phenotypes observed. We therefore investigated in more detail the spleens of young MEK1 KO mice, which were normal but for a slight increase in the number of activated T cells (Figure S2C). pAKT (Figure 3G) was increased and membrane PTEN was decreased (Figure 3H) in the KO samples. Increased pAKT was also observed in KO CD4+ T cells stimulated with anti-CD3/CD28 (Figure 3I). KO CD4+ T lymphocytes and B cells were less responsive to activation-induced cell death (AICD), a phenotype abrogated by a PI3K, but not by a MEK inhibitor (Figures 3J and 3K), and to Fas-induced apoptosis (Figure 3L). In contrast, other functions of CD4+ cells and B cells, such as their ability to proliferate and express the activation marker CD69 in response to T or B cell receptor stimulation, were unaffected (Figures S3B and S3C), indicating that the resistance to AICD is the major cell-autonomous phenotype. KO MEFs were also resistant to various proapoptotic stimuli (Figures S3D and S3E). This is reminiscent of the impaired apoptosis observed in PTEN-deficient lymphocytes (Buckler et al., 2008; Di Cristofano et al., 1999; Liu et al., 2010; Suzuki et al., 2003) and fibroblasts (Stambolic et al., 1998).
A MEK1/MAGI-1/PTEN Complex Regulates PTEN Membrane Recruitment
To gain insight in PTEN regulation by MEK1, we tested whether MEK1 and PTEN interacted in cells. PTEN was present in MEK1 immunoprecipitates (IPs) from growth-factor stimulated fibroblasts (Figure 4A). Conversely, MEK1 but not MEK2 could be detected in PTEN IPs from WT cells, and interaction was enhanced upon growth factor stimulation (Figures 4B and 4D). Recombinant PTEN and MEK1 did not interact in vitro (Figure S4A), indicating a requirement for a scaffold protein that, since MEK1 impacts membrane localization of PTEN, should be involved in PTEN membrane recruitment. Membrane-associated guanylate kinase (MAGUK) family member with inverted domain structure-1 (MAGI1) is a PTEN binding partner which localizes to the plasma membrane, specifically in domains such as adherens junctions, which act as focal points for the regulation of PIP3 pools and therefore control the recruitment and activation of AKT by assembling signaling complexes involving E-cadherin, β-catenin, and PTEN (Bonifant et al., 2007; Kotelevets et al., 2005). MAGI1 contains six PDZ domains, a guanylate kinase (GUK) domain, and two WW domains flanked by two PDZ domains (Dobrosotskaya et al., 1997); PTEN binds selectively to the second PDZ domain of MAGI1 (Kotelevets et al., 2005). Several isoforms and splice variants of MAGI1 are expressed in different cell types (Dobrosotskaya et al., 1997; Emtage et al., 2009; Kotelevets et al., 2005; Laura et al., 2002; Xu et al., 2008). MAGI1 in MEFs appeared as multiple bands, with a predominant band of approximately 100 kD (MAGI1100, marked by an asterisk) and two further bands of about 130 and 150 kD. The 100 kD isoform was selectively recruited to the membrane upon growth factor stimulation of WT cells, but MEK1 ablation prevented MAGI1 membrane translocation (Figure 4C). In addition, MAGI1100 was detected in PTEN IPs from WT but not MEK1 KO MEF whole-cell lysates and membrane fractions (Figures 4D and S4B), and the interaction between PTEN and MAGI1 could be restored by addition of recombinant MEK1 to the KO lysates prior to PTEN immunoprecipitation (Figure 4E). MAGI1 was detectable in MEK1, but not in MEK2 IPs (Figures S4C and S4D). Thus, MEK1 is essential for the formation of a complex containing MAGI1 and PTEN and for their membrane translocation upon growth factor stimulation. The kinetics of EGF-induced complex formation was similar to that of MEK1 phosphorylation on the ERK-dependent negative regulatory residue T292, unique to MEK1. pT292 MEK1 was enriched in PTEN immunoprecipitates, as assessed by densitometry (10% of total pT292 MEK1 versus 0.7% of total MEK1 in PTEN IPs from EGF-stimulated cells), while neither the activating RAF-dependent (S218 and S222) nor the PAK-dependent (S298) sites correlated well with binding (Figure 4D). Thus, T292 might work as a temporal switch, ensuring that ternary complex formation and membrane translocation occur after ERK activation.
Figure 4.
A MEK1/MAGI1/PTEN Ternary Complex Regulates MAGI1 and PTEN Membrane Recruitment
(A and B) MEK1 (A) or PTEN (B) IPs prepared from EGF-stimulated WT and KO MEFs. MEK1, MEK2, and PTEN were detected by immunoblotting. WCL, whole-cell lysates; c, unspecific binding to beads or isotype control antibody (IgG).
(C) Translocation of MAGI1100 (∗) and PTEN to the membrane. MAGI1, PTEN, IGF1R (membrane marker), and GAPDH (cytosolic marker) detected by immunoblotting of cytosol and membrane fractions of EGF-stimulated MEFs.
(D) PTEN, MAGI1, MEK1, and pMEK1 (pS298, pT292, and Raf-dependent sites, pRDS) detected by immunoblotting in WCL and PTEN IPs from EGF-stimulated MEFs.
(E) PTEN was immunoprecipitated from EGF-stimulated (30 min) WT and KO MEFs. Recombinant MEK1 (rMEK1; 1 μg) was added to the KO lysate as indicated. rMEK1 lacks 34 N-terminal amino acids and runs faster than endogenous MEK1.
(F) WCL and PTEN IPs from WT and KO thymocytes and splenocytes immunoblotted with PTEN, MAGI1, and MEK1 antibodies.
(G) WT MEFs transfected with small interfering RNA (siRNA) against MAGI1 or control siRNA (scrambled). PTEN, MAGI1, and MEK1 were detected by immunoblotting of WCL and PTEN IPs from EGF-stimulated cells (30 min) MEFs.
See also Figure S4.
MEK1 ablation decreases PTEN membrane localization and increases AKT phosphorylation in splenocytes (Figures 3G and 3H). MAGI1100 is expressed in spleen, and it is detectable, together with MEK1, in PTEN immunoprecipitates from WT but not KO organs (Figure 4F). Therefore, the mechanism described for the MEFs operates also in the spleen. In line with the lack of phenotype and AKT activation in KO thymi (Figures S2D and S2G), MAGI1100 was not expressed in thymus, and MEK1 was absent in PTEN immunoprecipitates from this organ (Figure 4F).
In MEFs, MAGI1 is the most efficiently expressed MAGI protein (Figure S4E). Consistently, MAGI1 knockdown efficiently abrogated PTEN binding to MEK1 (Figure 4G), reduced PTEN membrane localization, and increased AKT activation (Figures S4F and S4G). Thus, MAGI1 is the scaffold mediating the interaction between MEK1 and PTEN. The ternary complex was also found in human, rat, and chicken cells (Figure S4H) and in monkey cells (Figure 5H), indicating that it represents an important conserved regulatory principle.
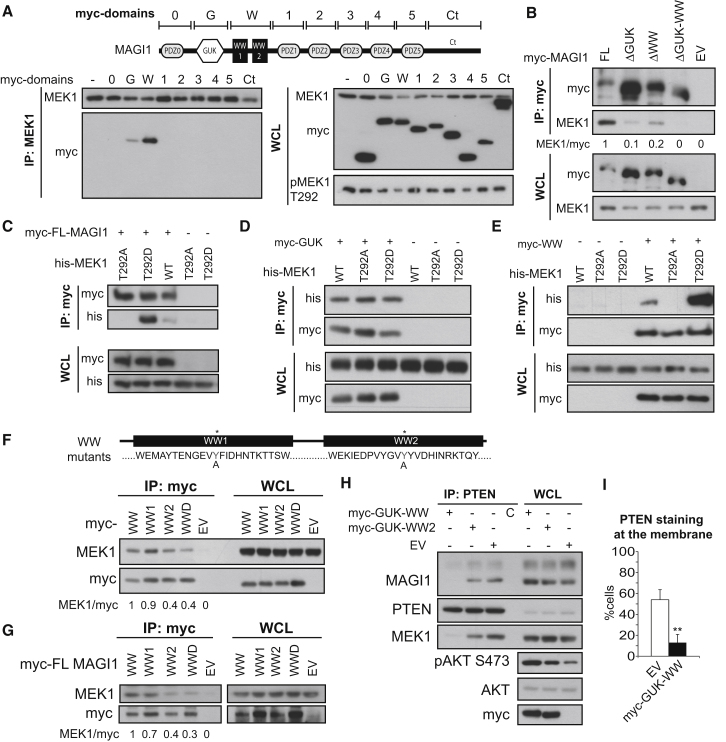
Figure 5.
Structure-Function Analysis of MEK1/MAGI1 Complexes
COS7 cells continuously growing in the presence of serum were transiently transfected with the indicated constructs.
(A) myc-tagged MAGI1 domains (schematically depicted in the upper panel) in endogenous MEK1 IPs and WCL were detected by immunoblot.
(B) myc-tagged MAGI1 and endogenous MEK1 were detected in myc-tag IPs and WCL of cells transfected with myc-tagged MAGI1 deletion mutants.
(C–E) myc-tagged MAGI1 proteins and His-tagged MEK1 were detected in myc-tag IP and WCL of cells transfected with His-tagged WT or mutant MEK1 and myc-tagged full-length MAGI1 (C), GUK (D), or WW (E) domains. Lysates were subjected to immunoblotting with His and myc antibodies.
(F and G) myc-tagged MAGI1 proteins and endogenous MEK1 were detected by immunoblotting in myc-tag IPs and in lysates of cells transfected with WT or mutant WW domains of MAGI1 (1, 2, and D, double mutant; F) or full-length MAGI1 bearing the same mutations (G). EV, empty vector.
(H and I) COS7 cells transfected with a plasmid encoding the WT or mutated GUK-WW domains of MAGI1 or EV were subjected to PTEN IP (H). The indicated antigens were detected by immunoblotting. PTEN membrane localization was assessed by immunofluorescence (I; n = 3). Values represent mean ± SD. ∗∗p < 0.01.
The numbers in (B), (F), and (G) indicate the ratio between MEK1:myc-MAGI1 proteins, measured by densitometry. The MEK1:WT myc-MAGI1 ratio is set as 1.
We next examined the interaction of MEK1 with selected MAGI1 domains expressed as myc-fusion proteins (Xu et al., 2008). The GUK and, to a greater extent, the two WW domains were detectable in MEK1 IPs, whereas the PTEN-binding PDZ domains were not (Figure 5A). Consistently, deletion of either the GUK or the WW domains reduced MAGI1 binding to MEK1, and deletion of both domains abolished it (Figure 5B). MEK1 was detectable in full-length MAGI1, GUK, and WW domain IPs. GUK domains are phosphopeptide-binding modules (Zhu et al., 2011), and WW motifs recognize proline-rich ligands, also in conjunction with S/T phosphorylation (Gao et al., 2009; Ingham et al., 2005; Macias et al., 2002). Mutation of T292 in the proline-rich region of MEK1 to a nonphosphorylatable A (T292A) decreased, and a phosphomimetic (T292D) mutation increased, binding to MAGI1 and WW, but not GUK (Figures 5C–5E). Thus, if the GUK domain is phosphosensitive, binding must depend on a different phosphorylation site. Mutation of the WW domains of MAGI1, in particular of WW2, strongly reduced MEK1 binding by a WW MAGI1 fragment or by full-length MAGI1 (Figures 5F and 5G). Remarkably, a MAGI1 GUK-WW fragment, which binds to MEK1 but not to PTEN, strongly reduced the binding of endogenous MEK1 and MAGI1 to PTEN, reduced PTEN membrane recruitment, and triggered AKT hyperactivation (Figures 5H and 5I), mimicking the MEK1 KO. A MAGI1 GUK-WW2 domain mutant was less efficient in preventing MEK1/MAGI1 binding to PTEN and causing AKT activation (Figure 5H). The residual MEK1 binding/dominant negative activity of the MAGI1 GUK-WW2 domain mutant is likely due to the interaction of the MAGI1 GUK domain with other MEK1 binding sites (Figures 5A, 5B, and 5D). Together, the data support the hypothesis that perturbations of the ternary complex PTEN/MAGI/MEK1 result in AKT activation.
MEK1 T292 Phosphorylation Controls Complex Formation
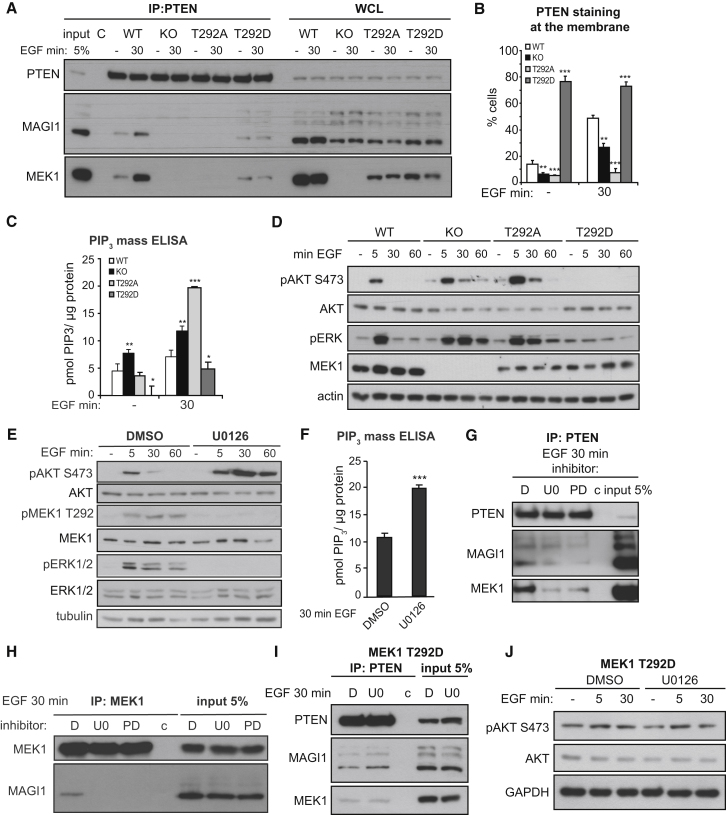
To further investigate the role of T292, we monitored complex formation in KO MEFs reconstituted with nonphosphorylatable T292A or phosphomimetic T292D MEK1 mutants. The mutants expressed at comparable levels, slightly lower than WT MEK1. MAGI1100 formed a complex with PTEN in WT and T292D cells, but not in KO or T292A cells; in addition, complex formation between MAGI1, PTEN, and the phosphomimetic MEK1 T292D mutant was not regulated by growth factor treatment (Figure 6A).
Figure 6.
T292 Phosphorylation, but Not MEK1 Kinase Activity, Promotes MEK1/MAGI1/PTEN Binding and Decreases PIP3 Pathway Activation
(A) WT, KO, and MEK1 mutant fibroblasts were stimulated with EGF. PTEN, MAGI1, and MEK1 in endogenous PTEN IPs and WCL were detected by immunoblotting.
(B–D) MEK1 WT, KO, and mutant fibroblasts were stimulated with EGF. PTEN membrane recruitment was determined by immunofluorescence. The plot in (B) depicts the percentage of cells showing membrane PTEN. The plot in (C) shows PIP3 levels, determined by ELISA. pAKT, pERK, AKT, MEK, and tubulin (loading control) were detected by immunoblotting (D).
(E and F) WT MEFs were pretreated with MEK inhibitors or DMSO and stimulated with EGF. Phosphorylation and expression of AKT, ERK, MEK1, and tubulin (loading control) were determined by immunoblotting (E). Intracellular PIP3 levels in WT MEFs pretreated with U0126 or DMSO and stimulated with EGF were measured by ELISA (F).
(G and H) WT fibroblasts pretreated with DMSO or MEK inhibitors (U0126, PD0325901) and stimulated with EGF were subjected to PTEN (G) or MEK1 IP (H). PTEN, MAGI1, and MEK1 were detected by immunoblotting. c, nonspecific binding to the beads.
(I and J) T292D MEK1 mutant MEFs were treated with U0126 or DMSO and stimulated with EGF. PTEN, MEK1, and MAGI1 in PTEN IPs (I) and pAKT, AKT, and GAPDH (loading control) in WCL (J) were visualized by immunoblotting. In (J), c represents nonspecific binding to the beads.
The values in (B), (C), and (F) show the mean of three experiments ±SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S5.
Like KO MEFs, MEK1 T292A cells showed defective PTEN membrane recruitment, and PIP3 accumulation and AKT phosphorylation were increased above the levels observed in KO cells. The exact reasons for these differences between KO and reconstituted cells are unknown. In contrast, MEK1 T292D mutant cells displayed constitutive PTEN membrane recruitment, as well as low levels of PIP3 and AKT phosphorylation (Figures 6B–6D). Consistent with this biochemical behavior, MEK1 T292A cells were resistant to TNFα-induced apoptosis (Figure S5A). Thus, T292 phosphorylation is a prerequisite for proper complex formation and for the regulation of the PIP3 pathway.
T292 is phosphorylated by ERK, which might thus regulate the timing of PTEN membrane recruitment and AKT signaling. Indeed, MEK inhibitors blocked both ERK and T292 phosphorylation, concomitantly increasing AKT phosphorylation (Figures 6E and S5B) and PIP3 accumulation (Figure 6F) and, most importantly, reducing the binding of MEK1 and MAGI1100 to PTEN and of MAGI1100 to MEK1 (Figures 6G and 6H). In MEK1 T292D MEFs, MEK inhibition did not perturb the association of PTEN with MEK1 or MAGI1100 or AKT phosphorylation (Figures 6I and 6J); thus, phosphorylation of T292 is required, and MEK1 kinase activity is dispensable, for the interaction of MEK1 with MAGI100 and PTEN.
Two other MAGI family proteins, MAGI2 and MAGI3, were also able to form complexes with MEK1. Consistent with the data obtained for MAGI1, chemical inhibition of T292 phosphorylation decreased the recruitment of MAGI2 and 3 to the membrane (Figures S5C and S5D).
Discussion
A Kinase-Independent Role for MEK1 in the Regulation of PTEN Function
We report the existence of a ternary complex between MEK1, MAGI1, and PTEN, mediating the translocation of PTEN to the membrane and therefore regulating the concentration of PIP3 and AKT activation. Both MEK1 and MAGI1 are necessary for complex formation, and PTEN will not bind to one component if the other is missing. The complex is held together by MAGI1, which contacts PTEN via its PDZ domain (Kotelevets et al., 2005) and MEK1. MAGI1 binds to MEK1 via its GUK and WW2 domains, but only binding to the WW domains is sensitive to the ERK-mediated phosphorylation of T292 in MEK1. The MAGI1 WW domains are very similar to the group Ib WW2 and WW3 domains of the ubiquitin ligase Nedd4L, whose binding to SMAD2 and SMAD3 depends on the phosphorylation of a T in the linker region (Gao et al., 2009); the NEDD4 WW2 domain has been found to bind to proteins containing PR sequences in an unbiased proteomic screen (Ingham et al., 2005). T292 of MEK1 is embedded in such a PR containing sequence (PPRPRTPGRP in MEK1, SPRPRPPGRP in MEK2); in addition, the MEK1 sequence does not contain any other predicted WW binding sites. Since the proline-rich loop of MEK1 is disordered in all available crystal structures, its interaction with the MAGI1 WW domains cannot be modeled, and at this point it is not possible to distinguish whether phosphorylation creates a reversible MAGI1 binding site on MEK1 or induces exposure of another MAGI1 binding site through an allosteric mechanism.
Besides being essential for the assembly of the MEK1/MAGI/PTEN complex, pT292 is also required for the downregulation of MEK2-dependent ERK signaling, mediated by MEK1 in the context of a MEK1/MEK2 heterodimer (Catalanotti et al., 2009). Thus, phosphorylation of MEK1 T292 relays a negative feedback within the ERK pathway and initiates the deactivation of the PIP3/AKT pathway through the membrane localization of MAGI/PTEN, acting as a temporal switch for both cascades.
MEK1 has been reported to bind to another WW domain containing protein, namely the proapoptotic tumor suppressor WOX1, associated with the death of activated T cell leukemia. In this case, however, the WOX1/MEK1 complex dissociates upon ERK activation (Lin et al., 2011). Thus, ERK differentially regulates binding of MEK1 to WW domain containing proteins and may negatively affect survival by promoting the translocation of WOX1 to the mitochondria (Lin et al., 2011) and the membrane recruitment of PTEN in the context of the MEK1/MAGI1/PTEN complex.
Our data show that MEK1 binding to the GUK and WW domains of MAGI1 promotes both complex formation between MAGI1 and PTEN and their membrane translocation. We do not know in detail how this is accomplished. One hypothesis is that binding to MEK1 will cause a conformational change in MAGI1, exposing the PDZ domains necessary for PTEN binding (PDZ2 [Kotelevets et al., 2005]) and for the binding to membrane proteins such as cadherins (PDZ4 and PDZ5 [Kotelevets et al., 2005; Mizuhara et al., 2005; Xu et al., 2008]). Intramolecular interactions between the SH3 and the GUK domain of other MAGUK proteins have been described, postulated to keep the proteins in an inactive, “closed” conformation (Montgomery et al., 2004). In MAGI, the WW domain replaces the SH3. It is conceivable that the WW domain may also be involved in intramolecular interactions that can be interrupted by MEK1 binding. Whatever the mechanism involved, MEK1 kinase activity is not required for complex formation and MEK1 binds to MAGI1 as a monomer or as a MEK1 dimer, but not as a heterodimer.
It is noteworthy that MEK1, but not PTEN, binds selectively to MAGI100, the only isoform translocated to the membrane in response to growth factors. Binding of PTEN to other MAGI1 isoforms may localize the phosphatase to other subcellular compartments, such as the nucleus (Dobrosotskaya et al., 1997; Laura et al., 2002), but it does not rescue membrane recruitment in the absence of MEK1. In addition, MAGI1100 is selectively expressed in organs and cells that are affected by MEK1 ablation (compare thymus and spleen in Figure 4F), where it correlates with the lack of PTEN membrane recruitment.
The possibility also exists that it is MEK1, rather than MAGI1, that mediates the membrane recruitment of the ternary complex. In this context, MEK reportedly binds to paxillin, and growth factor treatment induces the recruitment of Raf and ERK to this complex. Activated ERK phosphorylates paxillin on S83, promoting its association with activated FAK and PI3K and increasing cell spreading (Ishibe et al., 2004). The study did not differentiate between MEK1 and MEK2; however, our data would predict that in the absence of MEK1 at least a certain number of focal adhesions would fail to recruit MAGI/PTEN, resulting in the increased FAK phosphorylation, growth factor-induced migration, and cell spreading (Catalanotti et al., 2009) observed in the MEK1 KO fibroblasts.
Finally, some PTEN membrane binding, unaffected by EGF stimulation, can be observed in MEK1 KO fibroblasts (on average, approximately 20% of the WT). It is conceivable that this binding is mediated by the N-terminal PIP2-binding motif of PTEN, which has been shown to be necessary for membrane association (Rahdar et al., 2009). Alternative binding mechanisms likely underlie the rather modest EGF-mediated membrane recruitment of PTEN and MEK1, compared with the robust increase of MAGI1; consistent with this, a strong increase in the amount of both MEK1 and MAGI1100 is observed in membrane PTEN IPs from stimulated cells, indicating that EGF causes the preferential recruitment of the ternary complex to the membrane.
Together, these data define a unique kinase-independent function of MEK1 outside the canonical pathway and provide insight into PTEN regulation.
Biological Implications of a Disabled Feedback
MEK1 KO mice succumb early to an autoimmune disease accompanied by extramedullary hematopoiesis. The latter phenotype has been observed in PTEN cell-type-restricted KO or AKT transgenic mice (Guo et al., 2008; Kharas et al., 2010; Yilmaz et al., 2006), and we could show that KO bone marrow and spleen from unaffected animals contain significantly higher numbers of colony forming units, indicating a cell-autonomous phenotype. At this point, however, the contribution of the MEK1 KO environment, with its lymphoid dysregulation and autoimmune disease, is unclear. Cell-type-restricted MEK1 ablation and transplantation experiments are planned to clarify this matter.
The autoimmune disease clearly correlates with a reduction in T cell activation-induced cell death and with AKT activation in T cells and kidney. A similar phenotype can be achieved by genetic manipulations increasing PIP3/AKT signaling (Borlado et al., 2000; Di Cristofano et al., 1999; Liu et al., 2010; Parsons et al., 2001; Suzuki et al., 2001), but also by adoptive transfer of mouse T lymphocytes treated ex vivo with MEK inhibitor (Deng et al., 2003) or T cell restricted expression of a dominant negative mek transgene (Sawalha et al., 2008). The latter treatments would mimic the MEK1 KO by reducing phosphorylation of the critical T292 residue in MEK1 and therefore PTEN membrane recruitment. Importantly, both reduced ERK activation (Gorelik and Richardson, 2010) and increased AKT signaling (Tang et al., 2009) have been observed in T cells from lupus patients. Together with our data, these results suggest that the activation of ERK and its crosstalk with the PI3K pathway are crucial players in the development of experimental and possibly clinical lupus-like autoimmune diseases.
The MEK1 KO does not phenocopy T cell-restricted PTEN deletion in two aspects: the breach of central tolerance (Suzuki et al., 2001) and the development of T cell lymphomas (Liu et al., 2010). In both cases, the cells responsible for the phenotype originate in the thymus, which is not affected in the MEK1 KO mice. This lack of phenotype correlates with the lack of expression of MAGI100, and underscores the specific effect of MEK1 on this particular mechanism of PTEN regulation. In this context, it is important to consider that the impaired membrane localization of MAGI-1 in KO cells and organs might cause deregulation/mislocalization of one or more of its many interaction partners, thereby playing a role in the phenotype of the MEK1 KO mice.
Is the regulation of PTEN by MEK1/MAGI relevant in the context of cancer? MAGI has been found mutated in human cancer genomes (Berger et al., 2011; Pleasance et al., 2010), and it can suppress the growth of tumor xenografts (Zaric et al., 2012). Based on this, it is in principle possible that MEK1 may act as a tumor suppressor under certain conditions, as observed in a model of Myc-induced B cell lymphoma (Bric et al., 2009). More broadly, inhibition of the Raf/MEK/ERK pathway often causes activation of the AKT pathway in cancer (Hoeflich et al., 2009; Mirzoeva et al., 2009; Wee et al., 2009), due to the release of yet incompletely identified negative feedback loops. The use of Raf or MEK inhibitors might, by preventing the phosphorylation of T292 in MEK1, decrease PTEN membrane recruitment, increasing PIP3 concentrations and favoring the emergence of resistance mechanisms relying on the PI3K/AKT pathway. This would provide a mechanistic framework for the combined inhibition of these pathways, which is being increasingly advocated as the treatment of choice (Hoeflich et al., 2012; Sos et al., 2009; Villanueva et al., 2010).
Experimental Procedures
Mice
mek1f/f; Sox2Cre mice (Catalanotti et al., 2009) were maintained on a mixed Sv129/Bl6 background. Animal experiments were performed in accordance with a protocol authorized by the Austrian Ministry of Science and Communications.
Cell Culture and Transfections
MEFs and stable KO clones re-expressing WT and mutant MEK1 (Catalanotti et al., 2009) were grown in Dulbecco’s modified Eagle’s medium plus 10% (v/v) fetal calf serum (FCS) (PAA) and starved in 0.5% (v/v) FCS overnight prior to treatment with EGF (2 ng/ml, Biomedical Technologies). Inhibitors (10 nM PD0325901, Sigma-Aldrich; 10 μM U0126 and 50 μM LY294002, Cell Signaling) were added 1 hr before stimulation. MEFs were transfected with 30 nM MAGI siRNA duplexes (Sakurai et al., 2006) or nontargeting pool (D-001810-10-05, Dharmacon) with Lipofectamine RNAiMAX (Invitrogen). COS-7 cells were transfected with TurboFect (Fermentas).
Splenic CD4+ T and B cells were purified with MACS negative selection kits (MiltenyiBiotec; purity approximately 95% by fluorescence-activated cell sorting [FACS]) and cultured in RPMI 1640 (PAA) plus 10% FCS, 1 mM sodium pyruvate (Sigma-Aldrich), 1% nonessential amino acids (Sigma-Aldrich), 50 μM β-ME, and 10 mM N-acetylcysteine. In T cells, AICD was induced with anti-CD3/CD28 (1 μg/ml and 0.5 μg/ml, both BD PharMingen) for 72 hr, followed by 16 hr stimulation with αCD3 (10 μg/ml). B cells were treated with soluble anti-IgM Fab2 fragment (0.1 μg/ml, Jackson ImmunoResearch) and analyzed 24 hr later. FasL (100 ng/ml, Adipogen) induced apoptosis was monitored 6 hr after the treatment. Cell death was measured by annexinV/propidium iodide staining (eBiosciences).
Immunoprecipitation, Pull-Down, Cell Fractionation, Immunoblotting, ELISA, and ELISpot
Cells were lysed in a buffer containing 20 mM TrisHCl (pH 7.4), 137 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1% NP-40, 1 mM Na3VO4, 50 mM NaF, 2 mM PMSF, and protein inhibitor cocktail (Roche). IPs were prepared by addition of the appropriate antibodies prior to incubation with protein A-sepharose (GE Healthcare) or protein G-agarose (Thermo Scientific). Phosphatase activity in PTEN IPs was monitored as described (Sanchez et al., 2005). Pull-down experiments were carried out in IP buffer plus 0.1% BSA with purified recombinant PTEN (Calbiochem) and MEK1 (gift of I. Moarefi, Crelux). The Thermo Scientific or ProteoJet membrane protein extraction kit (Fermentas) or hypotonic lysis/differential centrifugation were used to produce cytoplasmic and membrane (plasma, mitochondria, and ER/Golgi) fractions. Immunoblotting was performed with the antibodies detailed in the Supplemental Experimental Procedures. Echelon kits were used to determine PIP3 levels (mass ELISA) and PI3K activity (ELISA). Serum antibodies, BAFF, and IL-10 were detected by ELISA (Life Diagnostics and R&D Systems). ELISpot assays were performed with IgA and IgG ELISpot kits (Mabtech).
Immunofluorescence
Anti-dsDNA antibodies were detected by Crithidia luciliae (Orgentec) kinetoplast staining with FITC-conjugated goat anti-mouse Ig antibody (Invitrogen). Confocal images were acquired with LSM Meta (Zeiss). For details, see the Supplemental Experimental Procedures.
Blood Analysis and FACS
Peripheral blood cell counts were acquired with V-Sight (Menarini Diagnostics). Cells were stained with antibodies against CD19, CD3e, CD4, CD8a, Mac1, Gr-1, and CD69 (all BD PharMingen) and analyzed by FACSCalibur and FACSAria (BectonDickinson) and FlowJo software.
Histology and Immunohistochemistry
Hematoxylin and eosin staining and immunohistochemistry were carried out as described (Catalanotti et al., 2009) with pAKT (S473) and pERK (T202/Y204; both Cell Signaling). Reticular fibers were visualized by Gomori staining. Frozen kidney sections were examined as described in (Di Cristofano et al., 1999).
Statistical Analysis
Values are expressed as mean (±SD) of at least three independent experiments. p values were calculated with the two-tailed Student’s t test. A p value ≤0.05 is considered statistically significant.
Acknowledgments
We thank I. Moarefi, E. Ogris, and Z. Xu for providing material, N. Kozakowski for helpful discussions, and N. Seiter and the animal facility for technical assistance. This work was supported by FWF grants T 196-B12 to V.J. and W1220-B09 to M.B.
Published: February 28, 2013
Footnotes
Supplemental Information includes five figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2013.01.037.
Supplemental Information
References
- Bélanger L.F., Roy S., Tremblay M., Brott B., Steff A.M., Mourad W., Hugo P., Erikson R., Charron J. Mek2 is dispensable for mouse growth and development. Mol. Cell. Biol. 2003;23:4778–4787. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M.F., Lawrence M.S., Demichelis F., Drier Y., Cibulskis K., Sivachenko A.Y., Sboner A., Esgueva R., Pflueger D., Sougnez C. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonauth V., Roy S., Gravel M., Guillemette S., Charron J. Requirement for Map2k1 (Mek1) in extra-embryonic ectoderm during placentogenesis. Development. 2006;133:3429–3440. doi: 10.1242/dev.02526. [DOI] [PubMed] [Google Scholar]
- Bonifant C.L., Kim J.S., Waldman T. NHERFs, NEP, MAGUKs, and more: interactions that regulate PTEN. J. Cell. Biochem. 2007;102:878–885. doi: 10.1002/jcb.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlado L.R., Redondo C., Alvarez B., Jimenez C., Criado L.M., Flores J., Marcos M.A., Martinez-A C., Balomenos D., Carrera A.C. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. FASEB J. 2000;14:895–903. doi: 10.1096/fasebj.14.7.895. [DOI] [PubMed] [Google Scholar]
- Bric A., Miething C., Bialucha C.U., Scuoppo C., Zender L., Krasnitz A., Xuan Z., Zuber J., Wigler M., Hicks J. Functional identification of tumor-suppressor genes through an in vivo RNA interference screen in a mouse lymphoma model. Cancer Cell. 2009;16:324–335. doi: 10.1016/j.ccr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler J.L., Liu X., Turka L.A. Regulation of T-cell responses by PTEN. Immunol. Rev. 2008;224:239–248. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalanotti F., Reyes G., Jesenberger V., Galabova-Kovacs G., de Matos Simoes R., Carugo O., Baccarini M. A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat. Struct. Mol. Biol. 2009;16:294–303. doi: 10.1038/nsmb.1564. [DOI] [PubMed] [Google Scholar]
- Chapman M.S., Miner J.N. Novel mitogen-activated protein kinase kinase inhibitors. Expert Opin. Investig. Drugs. 2011;20:209–220. doi: 10.1517/13543784.2011.548803. [DOI] [PubMed] [Google Scholar]
- Deng C., Lu Q., Zhang Z., Rao T., Attwood J., Yung R., Richardson B. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum. 2003;48:746–756. doi: 10.1002/art.10833. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A., Kotsi P., Peng Y.F., Cordon-Cardo C., Elkon K.B., Pandolfi P.P. Impaired Fas response and autoimmunity in Pten+/- mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya I., Guy R.K., James G.L. MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J. Biol. Chem. 1997;272:31589–31597. doi: 10.1074/jbc.272.50.31589. [DOI] [PubMed] [Google Scholar]
- Emtage L., Chang H., Tiver R., Rongo C. MAGI-1 modulates AMPA receptor synaptic localization and behavioral plasticity in response to prior experience. PLoS ONE. 2009;4:e4613. doi: 10.1371/journal.pone.0004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Alarcón C., Sapkota G., Rahman S., Chen P.-Y., Goerner N., Macias M.J., Erdjument-Bromage H., Tempst P., Massagué J. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-β signaling. Mol. Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S., Tremblay M., Bernard D., Cardin-Girard J.F., Aubry S., Larouche L., Rousseau S., Huot J., Landry J., Jeannotte L., Charron J. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- Gorelik G., Richardson B. Key role of ERK pathway signaling in lupus. Autoimmunity. 2010;43:17–22. doi: 10.3109/08916930903374832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Lasky J.L., Chang C.J., Mosessian S., Lewis X., Xiao Y., Yeh J.E., Chen J.Y., Iruela-Arispe M.L., Varella-Garcia M., Wu H. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich K.P., O’Brien C., Boyd Z., Cavet G., Guerrero S., Jung K., Januario T., Savage H., Punnoose E., Truong T. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin. Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- Hoeflich K.P., Merchant M., Orr C., Chan J., Den Otter D., Berry L., Kasman I., Koeppen H., Rice K., Yang N.-Y. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72:210–219. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- Ingham R.J., Colwill K., Howard C., Dettwiler S., Lim C.S.H., Yu J., Hersi K., Raaijmakers J., Gish G., Mbamalu G. WW domains provide a platform for the assembly of multiprotein networks. Mol. Cell. Biol. 2005;25:7092–7106. doi: 10.1128/MCB.25.16.7092-7106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibe S., Joly D., Liu Z.X., Cantley L.G. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell. 2004;16:257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kharas M.G., Okabe R., Ganis J.J., Gozo M., Khandan T., Paktinat M., Gilliland D.G., Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevets L., van Hengel J., Bruyneel E., Mareel M., van Roy F., Chastre E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 2005;19:115–117. doi: 10.1096/fj.04-1942fje. [DOI] [PubMed] [Google Scholar]
- Laura R.P., Ross S., Koeppen H., Lasky L.A. MAGI-1: a widely expressed, alternatively spliced tight junction protein. Exp. Cell Res. 2002;275:155–170. doi: 10.1006/excr.2002.5475. [DOI] [PubMed] [Google Scholar]
- Lehr S., Kotzka J., Avci H., Sickmann A., Meyer H.E., Herkner A., Muller-Wieland D. Identification of major ERK-related phosphorylation sites in Gab1. Biochemistry. 2004;43:12133–12140. doi: 10.1021/bi049753e. [DOI] [PubMed] [Google Scholar]
- Lin H.P., Chang J.Y., Lin S.R., Lee M.H., Huang S.S., Hsu L.J., Chang N.S. Identification of an in vivo MEK/WOX1 complex as a master switch for apoptosis in T cell leukemia. Genes Cancer. 2011;2:550–562. doi: 10.1177/1947601911418498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Karnell J.L., Yin B., Zhang R., Zhang J., Li P., Choi Y., Maltzman J.S., Pear W.S., Bassing C.H., Turka L.A. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J. Clin. Invest. 2010;120:2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M.J., Wiesner S., Sudol M. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett. 2002;513:30–37. doi: 10.1016/s0014-5793(01)03290-2. [DOI] [PubMed] [Google Scholar]
- Maurer G., Tarkowski B., Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- Mirzoeva O.K., Das D., Heiser L.M., Bhattacharya S., Siwak D., Gendelman R., Bayani N., Wang N.J., Neve R.M., Guan Y. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuhara E., Nakatani T., Minaki Y., Sakamoto Y., Ono Y., Takai Y. MAGI1 recruits Dll1 to cadherin-based adherens junctions and stabilizes it on the cell surface. J. Biol. Chem. 2005;280:26499–26507. doi: 10.1074/jbc.M500375200. [DOI] [PubMed] [Google Scholar]
- Montgomery J.M., Zamorano P.L., Garner C.C. MAGUKs in synapse assembly and function: an emerging view. Cell. Mol. Life Sci. 2004;61:911–929. doi: 10.1007/s00018-003-3364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M.J., Jones R.G., Tsao M.S., Odermatt B., Ohashi P.S., Woodgett J.R. Expression of active protein kinase B in T cells perturbs both T and B cell homeostasis and promotes inflammation. J. Immunol. 2001;167:42–48. doi: 10.4049/jimmunol.167.1.42. [DOI] [PubMed] [Google Scholar]
- Pleasance E.D., Cheetham R.K., Stephens P.J., McBride D.J., Humphray S.J., Greenman C.D., Varela I., Lin M.L., Ordóñez G.R., Bignell G.R. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos P.I., Solit D.B. Resistance to MEK inhibitors: should we co-target upstream? Sci. Signal. 2011;4:pe16. doi: 10.1126/scisignal.2001948. [DOI] [PubMed] [Google Scholar]
- Rahdar M., Inoue T., Meyer T., Zhang J., Vazquez F., Devreotes P.N. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc. Natl. Acad. Sci. USA. 2009;106:480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr. MEK1/2 dual-specificity protein kinases: structure and regulation. Biochem. Biophys. Res. Commun. 2012;417:5–10. doi: 10.1016/j.bbrc.2011.11.145. [DOI] [PubMed] [Google Scholar]
- Roy M., Li Z., Sacks D.B. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol. Cell. Biol. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A., Fukuhara S., Yamagishi A., Sako K., Kamioka Y., Masuda M., Nakaoka Y., Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol. Biol. Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T., Thangada S., Wu M.-T., Kontos C.D., Wu D., Wu H., Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2005;102:4312–4317. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha A.H., Jeffries M., Webb R., Lu Q., Gorelik G., Ray D., Osban J., Knowlton N., Johnson K., Richardson B. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 2008;9:368–378. doi: 10.1038/gene.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S., Shannon K., Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Song M.S., Salmena L., Pandolfi P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Sos M.L., Fischer S., Ullrich R., Peifer M., Heuckmann J.M., Koker M., Heynck S., Stückrath I., Weiss J., Fischer F. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc. Natl. Acad. Sci. USA. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V., Suzuki A., de la Pompa J.L., Brothers G.M., Mirtsos C., Sasaki T., Ruland J., Penninger J.M., Siderovski D.P., Mak T.W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Su D.L., Lu Z.M., Shen M.N., Li X., Sun L.Y. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J. Biomed. Biotechnol. 2012;2012:347141. doi: 10.1155/2012/347141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Yamaguchi M.T., Ohteki T., Sasaki T., Kaisho T., Kimura Y., Yoshida R., Wakeham A., Higuchi T., Fukumoto M. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Kaisho T., Ohishi M., Tsukio-Yamaguchi M., Tsubata T., Koni P.A., Sasaki T., Mak T.W., Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Gu J., Matsumoto K., Aota S., Parsons R., Yamada K.M. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Tang H., Tan G., Guo Q., Pang R., Zeng F. Abnormal activation of the Akt-GSK3β signaling pathway in peripheral blood T cells from patients with systemic lupus erythematosus. Cell Cycle. 2009;8:2789–2793. doi: 10.4161/cc.8.17.9446. [DOI] [PubMed] [Google Scholar]
- Teis D., Wunderlich W., Huber L.A. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- Ussar S., Voss T. MEK1 and MEK2, different regulators of the G1/S transition. J. Biol. Chem. 2004;279:43861–43869. doi: 10.1074/jbc.M406240200. [DOI] [PubMed] [Google Scholar]
- Vazquez F., Grossman S.R., Takahashi Y., Rokas M.V., Nakamura N., Sellers W.R. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Villanueva J., Vultur A., Lee J.T., Somasundaram R., Fukunaga-Kalabis M., Cipolla A.K., Wubbenhorst B., Xu X., Gimotty P.A., Kee D. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S., Jagani Z., Xiang K.X., Loo A., Dorsch M., Yao Y.M., Sellers W.R., Lengauer C., Stegmeier F. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- Wimmer R., Baccarini M. Partner exchange: protein-protein interactions in the Raf pathway. Trends Biochem. Sci. 2010;35:660–668. doi: 10.1016/j.tibs.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Xu Z., Peng A.W., Oshima K., Heller S. MAGI-1, a candidate stereociliary scaffolding protein, associates with the tip-link component cadherin 23. J. Neurosci. 2008;28:11269–11276. doi: 10.1523/JNEUROSCI.3833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K.M., Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 2001;114:2375–2382. doi: 10.1242/jcs.114.13.2375. [DOI] [PubMed] [Google Scholar]
- Yilmaz O.H., Valdez R., Theisen B.K., Guo W., Ferguson D.O., Wu H., Morrison S.J. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yu C.F., Liu Z.X., Cantley L.G. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J. Biol. Chem. 2002;277:19382–19388. doi: 10.1074/jbc.M200732200. [DOI] [PubMed] [Google Scholar]
- Zaric J., Joseph J.M., Tercier S., Sengstag T., Ponsonnet L., Delorenzi M., Rüegg C. Identification of MAGI1 as a tumor-suppressor protein induced by cyclooxygenase-2 inhibitors in colorectal cancer cells. Oncogene. 2012;31:48–59. doi: 10.1038/onc.2011.218. [DOI] [PubMed] [Google Scholar]
- Zhang S.Q., Tsiaras W.G., Araki T., Wen G., Minichiello L., Klein R., Neel B.G. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Shang Y., Xia C., Wang W., Wen W., Zhang M. Guanylate kinase domains of the MAGUK family scaffold proteins as specific phospho-protein-binding modules. EMBO J. 2011;30:4986–4997. doi: 10.1038/emboj.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.