Abstract
Respiratory syncytial virus (RSV) infection of the lower respiratory tract is the leading cause of respiratory failure among infants in the United States of America and annually results in >300,000 deaths worldwide. Despite the importance of RSV, there is no licensed vaccine, and no specific form of therapy. This is largely due to the absence of an appropriate animal model for the evaluation of vaccines and therapeutic agents. We inoculated anesthetized infant (4 wk) baboons (Papio anubis) with a human strain of RSV intranasally or intratracheally. Baboons were monitored daily for clinical changes. Anesthetized baboons were intubated at various intervals, and bronchoalveolar lavage (BAL) was performed for viral culture and determination of leukocyte counts. Sham-infected baboons served as controls. Necropsies were performed on infected baboons on days 1, 3, 5, 8, or 13 after inoculation, with pathological analysis and immunohistochemical staining of lung tissues to detect RSV antigen. Infected baboons developed tachypnea and reduced oxygenation peaking from 4 to 8 days after infection and persisting for ≥14 days. Virus was recoverable in BAL fluid up to 8 days following infection. Necropsy revealed intense interstitial pneumonia, sloughing of the bronchiolar epithelium, and obstruction of the bronchiolar lumen with inflammatory cells and sloughed epithelial cells. RSV antigen was identified in bronchiolar and alveolar epithelium. We conclude that RSV-infected infant baboons develop clinical and pathological changes that parallel those observed in human infants with RSV infection. The infant baboon represents a much-needed model for studying the pathogenesis of RSV infection and evaluating antivirals and vaccines.
Keywords: interstitial pneumonia, bronchiolitis, baboon model, animal models, respiratory syncytial virus vaccine
respiratory syncytial virus (RSV) is the most important respiratory pathogen of early life. RSV infection, in the form of interstitial pneumonia and bronchiolitis, annually results in >120,000 hospitalizations in the United States of America and >300,000 deaths worldwide (21, 24). RSV lower respiratory tract infection (LRTI) in infancy also may predispose to the development of childhood asthma (33). Despite the considerable impact of RSV infection, there is no approved vaccine and no effective specific form of therapy. The lack of an appropriate animal model has resulted in an incomplete understanding of the pathophysiology of the disease and an inability to evaluate the safety and efficacy of vaccines and pharmacological agents. An animal model developing clinical and pathological manifestations similar to those of human infants with RSV infection would provide a significant step toward overcoming these problems. To reflect human disease, an appropriate animal model should develop tachypnea, reduced oxygenation, and interstitial and peribronchiolar inflammation with obstruction of the bronchiolar lumen by invading leukocytes and exfoliated epithelial cells following infection. These manifestations should be most prominent in an infant of the species tested, since RSV infection in humans is most severe in infants.
Existing animal models of RSV infection fall short of these desired characteristics in various respects. In mouse models, RSV disease is mediated by aggressive lymphocyte responses, that is, removal of CD4 or CD8 lymphocytes before infection leads to milder illness despite permitting greater virus replication (14). This is in marked contrast to the situation in humans, where severe RSV bronchiolitis is characterized by an absence of CD8 cytotoxic T lymphocytes in the lung (37). This substantial difference in pathogenesis makes it unlikely that results from testing of vaccines and pharmacological agents in murine models will apply to RSV infection in humans. Lambs infected with RSV develop histological evidence of LRTI, but actual symptoms of LRTI (sustained tachypnea and hypoxia) are minimal or absent (20, 25, 31). Infection of cattle with bovine RSV strains may cause significant LRTI, but human RSV strains induce lesser changes (2). Moreover, in calves raised on farms, RSV-induced lung disease is characterized by intense neutrophilic inflammation, mucopurulent exudate, and growth of a variety of bacterial pathogens, suggesting the presence of bacterial superinfection (2, 35). Such secondary infection is extremely uncommon in human infants with RSV infection (28). When calves are raised in germ-free environments, bovine RSV challenge can result in pneumonia, but the disease seems to be lymphocyte-mediated, as it is in rodents (1). It is therefore uncertain whether findings in calves can be generalized to humans.
Numerous species of monkeys and apes have been experimentally infected with human RSV. Although RSV infection in chimpanzees causes profuse rhinorrhea, it has not resulted in LRTI (3). RSV infection in owl monkeys (27), cebus monkeys (9) (30), bonnet monkeys (26), cynomolgus macaques (8), and African Green monkeys (17) results in histological evidence of mild interstitial pneumonia, but these animals have not developed tachypnea or hypoxia. None of these studies evaluated the effect of RSV on infant animals. The nature of inflammatory responses in infants may differ from those in older children and adults, which could affect the clinical response to RSV infection.
The olive baboon, Papio cynocephalus anubis, has often been used in biomedical research for transplantation, infectious disease, and vaccine studies. Here we report on the use of the infant olive baboon in the development of a novel model of RSV disease that closely mimics the findings observed in human infants.
METHODS
Baboons.
Olive baboons obtained from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center (OUHSC) were infected at 4 wk of age. After infection, study baboons were monitored in a sequestered nursery. Animals surviving the study were returned to the baboon colony. Baboons were maintained and treated in accordance with guidelines and approved protocols of the Institutional Animal Care and Use Committees and the Institutional Biosafety Committees of OUHSC. Study protocols were submitted to and approved by the same committees.
Obtaining of clinical data and specimen collection.
Baboons were monitored daily for respiratory rate, level of activity, and food intake. On study day 0 and at various intervals up to 21 days thereafter, baboons were lightly anesthetized with ketamine (10 mg/kg) and acepromazine (1 mg/kg). On these occasions, measurements of body temperature, respiratory rate, heart rate, and oxygen saturation (O2sat) were obtained, and auscultation of the chest was performed. Venous blood was drawn for complete blood counts and antibody determinations. Animals were then intubated with a 3.0 endotracheal tube (ETT), and bronchoalveolar lavage (BAL) was performed by inserting tygon tubing through the ETT and gently wedging it into the terminal airway. After instilling 0.5-ml aliquots of sterile PBS in the lung, BAL fluid was recovered by suctioning. Recovered fluid (generally 50–60% of the volume instilled) was centrifuged to sediment cells and then frozen for further analysis.
RSV infection of baboons.
After obtaining BAL fluids on day 0, baboons were infected with the A2 strain of human RSV. The RSV inoculum contained ∼2 × 107 plaque-forming units (pfu)/ml of RSV grown in HEp-2 tissue culture cells. Intranasal infection (n = 2 baboons) was performed by inserting a 22-gauge catheter in the nares and instilling 0.5 ml of inoculum in the nasal cavity. This was repeated in each naris, for a total inoculum of 2 ml. Intratracheal infection (n = 13) was performed by placing 4 × 1 ml aliquots of inoculum in the lung via tubing inserted through a cuffed ETT and wedged in the distal lung. For each of the four doses, the baboons were repositioned so upper and lower quadrants of each lung were targeted. Sham infection (n = 3) was carried out by inoculating 4 × 1 ml aliquots of uninfected HEp-2 cells processed in a similar fashion as for the preparation of the RSV inoculum. This preparation was also administered via a cannula passed through the ETT and wedged in the lung.
BAL cell counts and virology.
The number of leukocytes recovered from BAL fluid was counted in a hemocytometer. Leukocyte differentials were determined by a pathologist, who generally counted 200 cells. Supernatant fluid was then frozen at −80°C. Virus titration was completed using published methods (7).
Serology.
RSV-specific antibodies were measured by two methods. First, total RSV-specific IgG was determined using an immune-fluorescent bead assay. Ultraviolet-inactivated RSV in 0.1% Tween 20 was coupled to Bio-Plex Pro magnetic carboxyl beads (Bio-Rad) using the Bio-Plex Amine Coupling Kit (Bio-Rad). Sera were incubated with ∼2,000 coupled beads for 1 h at room temperature in the dark, with shaking at 300 revolutions/min (rpm) in a flat-bottom 96-well plate. Beads were then washed with the Bio-Plex Pro wash station (Bio-Rad) and incubated with biotinylated goat anti-human IgG (Vector Laboratories) for 45 min at room temperature in the dark, with shaking at 300 rpm. The beads were washed again and incubated with streptavidin-PE for 10 min. Beads were washed again and resuspended in 125 μl of PBS by shaking at 1,100 rpm. Data were collected using the Bio-Plex 200 multiplex bead array system (Bio-Rad).
We also determined titers of RSV-neutralizing antibody (NA) in baboon sera using published methods (10). An RSV stock was diluted to contain 500 pfu/ml, and 0.5 ml of this stock was incubated with 0.5 ml of twofold dilutions of baboon sera in growth medium. The neutralization titer was determined to be the highest serum dilution resulting in a 90% reduction in the number of plaques.
Pathology and immunohistochemistry.
Baboons infected intratracheally with RSV were humanely killed on days 1 (n = 2), 3 (n = 3), 5 (n = 2), 8 (n = 1), and 13 (n = 1) after infection; four other baboons survived beyond day 21. One intranasally infected baboon was humanely killed 13 days after infection, and another survived the protocol. Lung tissues from the baboons undergoing necropsy were fixed in formalin, and lung tissue slices were processed for routine histology [hematoxylin and eosin (H&E) staining]. Similar tissues from each of these baboons were processed using immunohistochemical (IHC) techniques for detection of RSV antigen and for enumeration of CD4 and CD8 cells (37). To determine the number of CD4 and CD8 cells present in lung tissues, a pathologist counted the number of cells in 10 high-power microscopic fields (HPF) and averaged the results. Lung tissue from two control baboons with neither RSV nor sham infection was analyzed similarly.
Statistical analysis.
Statistical analysis was carried out with the guidance of the institutional biostatistician. Differences in respiratory rates and O2sats between RSV and sham-infected baboons were determined by combining all observations obtained over the indicated intervals. Because values were not normally distributed, medians were compared using the Mann-Whitney nonparametric test. Differences in other group mean outcomes listed below were also assessed for normality and, since the values had a normal distribution, means were evaluated using t-tests. Correlations of BAL leukocyte numbers with disease outcomes were analyzed by calculating coefficients of correlation. The numbers of CD4 and CD8 cells accumulating in the lung over the course of infection were compared by ANOVA. Data are expressed as means ± 1 SE.
RESULTS
Clinical observations.
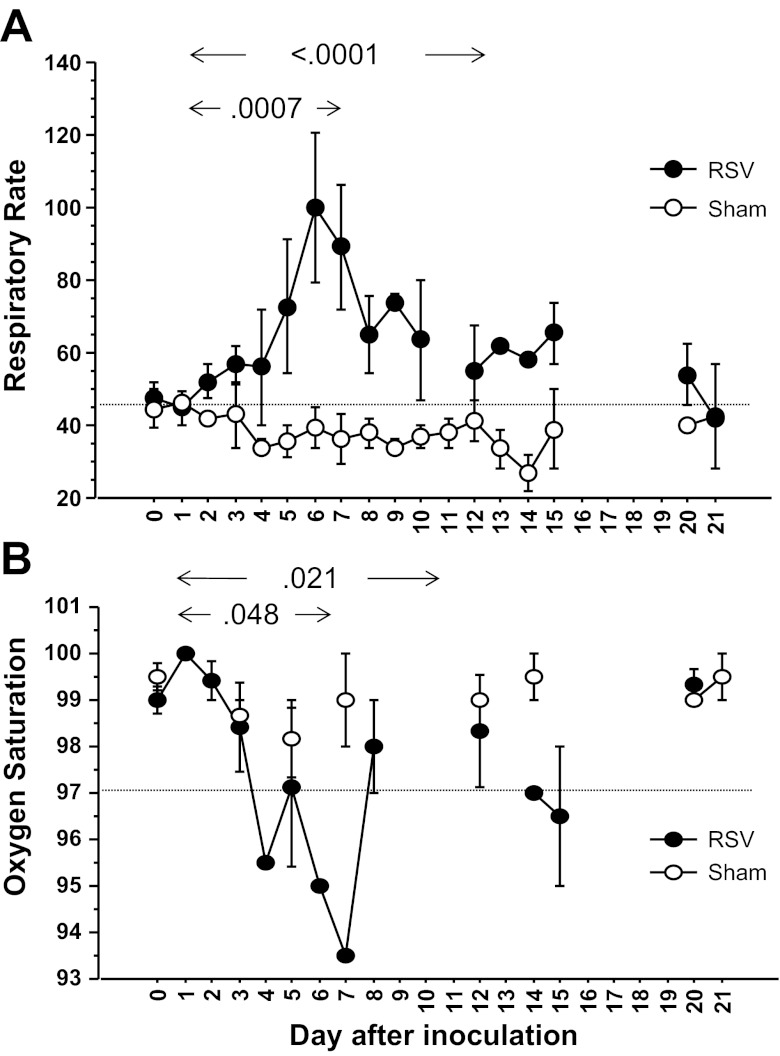
The mean respiratory rate in sedated baboons on day 0, before any procedures were performed, was 44.8 ± 2.5 breaths/min (Fig. 1A). All eight baboons infected intratracheally and allowed to survive for adequate intervals developed marked tachypnea, with respiratory rates 1.5–2.5 times above baseline rates. Peak respiratory rates were observed from days 5 to 8 following infection (Fig. 1A) with daily mean respiratory rates ranging from 64 to 108/min over this interval. Respiratory rates in these baboons remained elevated above preinfection rates for up to 15 days following infection, before returning to baseline values on days 21–22. In sham-infected baboons, mean daily respiratory rates did not exceed 45/min at any point. Respiratory rates in baboons infected intratracheally were higher over the interval from days 1 to 8 (P = 0.0007) and days 1 to 15 (P < 0.0001) than those in sham-infected baboons over the same intervals. Animals infected intratracheally often exhibited labored abdominal breathing with mild-moderate chest wall retractions. Auscultation of the chest revealed unequivocal wheezing in one baboon and slight harshness of breath sounds on expiration in another.
Fig. 1.
Respiratory syncytial virus (RSV) infection causes increased respiratory rates and reduced oxygenation in infant baboons. Values illustrated were obtained from 13 baboons infected it and 3 sham infected (uninfected tissue culture cell preparations). For respiratory rate values in RSV-infected baboons (A), n = 12 on day 0, n = 3–6 from days 1 to 8 and days 2 to 3 thereafter. For sham-infected baboons, n = 2–3 at all points. For oxygen saturation (O2sat) values in RSV-infected baboons (B), n = 12 on day 0 and n = 3–6 at all other points. For sham-infected baboons, n = 2–3 at all points. Each point represents the mean ± SE. The horizontal axis represents days after inoculation of baboons. Respiratory rates determined in it-infected baboons from days 1 to 8 and from days 1 to 15 differed significantly from those in sham-infected baboons over the same intervals (P = 0.0007 and P < 0.0001, respectively). O2sats (B) measured from days 1 to 8 and from days 1 to 15 also differed between it-infected and sham-infected baboons (P < 0.048 and P = 0.025, respectively).
Three of 7 baboons infected intratracheally and tested for oxygenation developed reduced oxygenation, with O2sats of <97% from days 4 to 7 (Fig. 1B). Daily mean O2sats (all 7 baboons included) ranged from 93.5 to 95.5% from days 4 to 7 after infection and did not return to preinfection values of 99–100% until beyond 15 days after infection. Sham-infected baboons maintained mean daily O2sats of >97.5% throughout the entire study period (Fig. 1B). Mean O2sats in baboons infected intratracheally were lower over the interval from days 1 to 8 (P = 0.048) and days 1 to 15 (P = 0.026) than those in sham-infected baboons over the same intervals. Because baboons received the same degree of sedation at each of the time intervals illustrated, the reduced oxygenation could not be attributed to sedation alone.
Baboons infected intranasally experienced only intermittent tachypnea during the 1 wk after RSV infection, and O2sats did not fall below 96% over this interval (data not shown).
Viral replication.
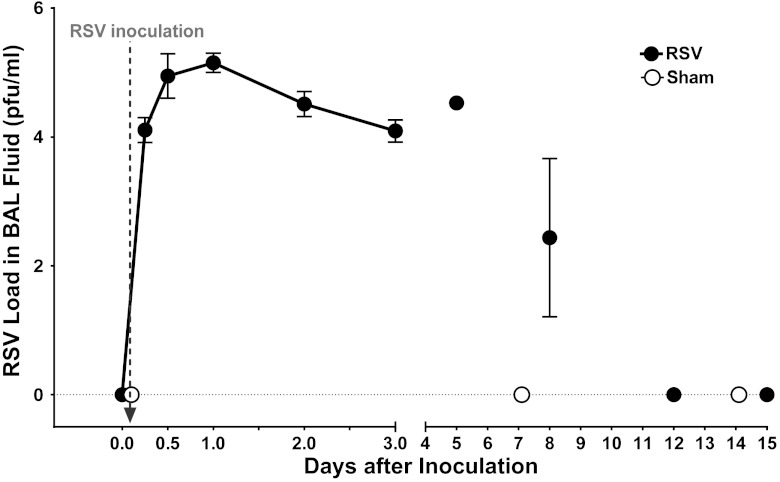
We performed serial BAL on 11 baboons (8 infected intratracheally, 3 sham infected) to determine the pattern of viral replication after infection (Fig. 2). In two intratracheally infected baboons, BAL was performed at 6, 12, 24, 48, and 72 h after infection to determine the pattern of early virus replication. Sham-infected animals were negative for RSV on all occasions. Baboons receiving RSV were culture-negative at time 0 (preinfection). By 6 h after inoculation, >104 pfu/ml of RSV was recoverable in BAL fluid samples. The titer of recoverable virus increased to a peak of >105 pfu/ml of BAL fluid at 12 and 24 h after inoculation and then remained fairly constant at >104 pfu/ml from 2 to 5 days after inoculation before declining on day 8 and being undetectable on day 12 following infection. All baboons inoculated with RSV intratracheally had virus recoverable in BAL fluid on at least one occasion. Five baboons infected either intranasally or intratracheally were studied before we had developed our technique for obtaining BAL fluids and thus did not have results of viral titrations available.
Fig. 2.
Recovery of RSV in bronchoalveolar lavage (BAL) fluid from RSV-infected baboons but not sham-infected baboons. The vertical axis represents the titer of RSV recovered in BAL fluids after inoculation of baboons with 8 × 107 plaque-forming units (pfu) of RSV or an equal volume of control preparation. The horizontal axis represents days after inoculation of baboons. The vertical arrow indicates the time of inoculation (immediately after time 0). RSV was recovered from all 10 baboons that were inoculated it with RSV and subsequently cultured but was not detected in BAL fluids from three sham-infected baboons. BAL fluids for culture were not available from baboons infected in. For RSV-infected baboons, n = 2–6 baboons at each time point, whereas n = 2–3 at each point for sham-infected baboons.
Antibody responses.
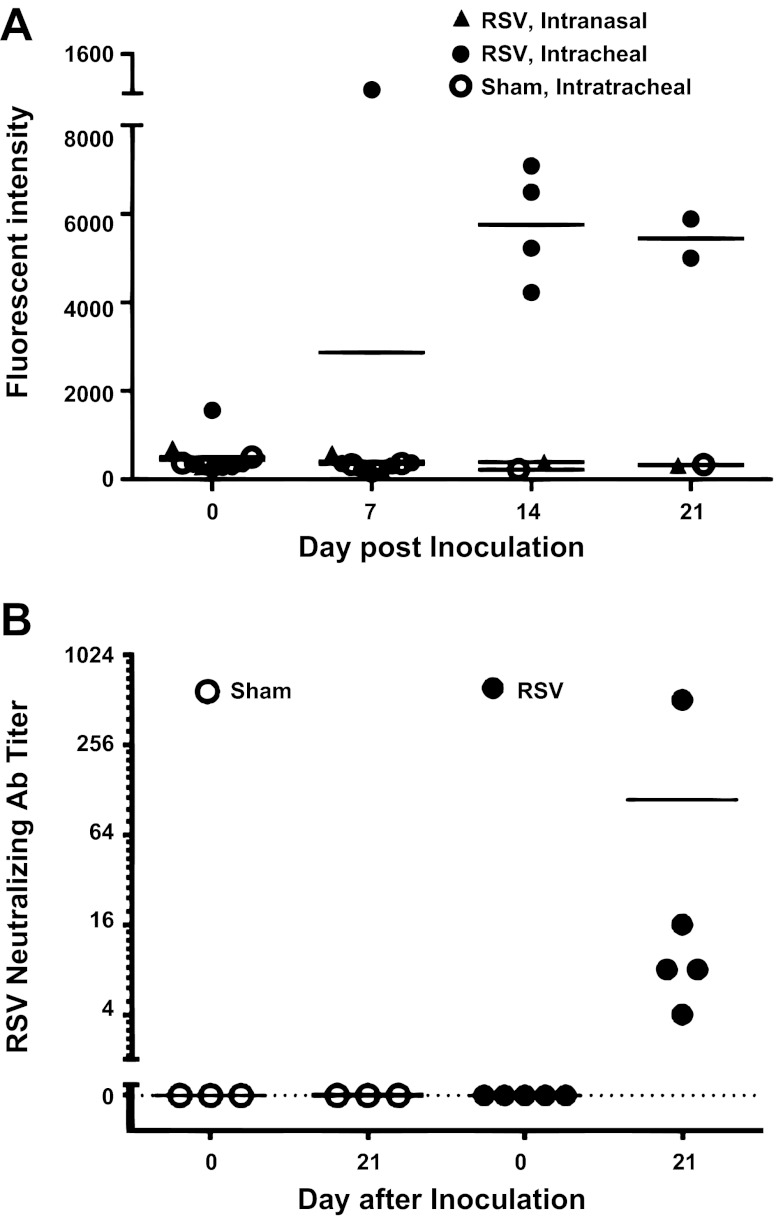
The serum antibody response following inoculation with RSV was determined by measuring the titers of total anti-RSV IgG and of RSV NA. Before inoculation, anti-RSV IgG was generally not present, but total RSV-IgG responses were detectable in RSV-infected baboons on days 14 and 21 (Fig. 3A). No anti-RSV IgG was found throughout the study period in sham-inoculated controls.
Fig. 3.
RSV infection induces RSV-specific IgG and neutralizing antibody (NA) responses in infant baboons. Sera from baboons infected with RSV (in route) or sham infected were tested for total RSV-IgG antibody (A) and for NA to RSV (B). In the vertical axis of A, fluorescent intensity refers to signal strength of RSV-coated beads incubated with baboon sera and stained with fluorescein-conjugated anti-IgG detector antibodies. In B, the vertical axis represents the serum dilution resulting in a 90% neutralization of virus growth. The horizontal axis represents days after inoculation of baboons. The horizontal bars represent the means of values. All baboons were seronegative for total anti-RSV IgG and for NA on day 0 (preinfection). Quantities of RSV-IgG rose progressively from day 0 through day 21 in RSV-infected baboons but not in baboons challenged in or in sham-infected animals (A). All five baboons infected with RSV it demonstrated NA responses (B). All three sham-infected baboons remained seronegative for RSV NA (B).
All baboons were seronegative for NA to RSV before infection. Each of four baboons inoculated intratracheally with RSV developed NA in serum by day 21 after infection, with titers ranging from 4 to 256 (mean = 70, Fig. 3B). Sham-infected baboons remained seronegative for NA to RSV throughout the 21-day period.
Leukocytes in BAL fluid.
The total number of leukocytes in BAL fluid was 84,293 ± 4,740/ml at baseline and remained fairly constant in sham-infected baboons (Fig. 4A). In intratracheally infected baboons, the number of leukocytes in BAL fluid rose ∼10-fold by 24 h after infection and then declined on days 2 and 3 after infection. Thereafter, total leukocyte counts gradually increased again in BAL from day 4 through day 12 before declining by day 15. Neutrophils were the predominant cell type in BAL fluids during the first 3 days following infection, comprising ∼85% of cells present. Macrophages became the predominant cell type from days 4 to 8 following infection and were still present in high numbers through day 12. Lymphocytes began to increase slightly in BAL populations on day 8 and were slightly more numerous than macrophages on day 12 after infection (Fig. 4, B–D). Neither the total number of leukocytes nor the number of any specific cell type correlated in a statistically significant fashion with respiratory rates or O2sats (each probability >0.27).
Fig. 4.
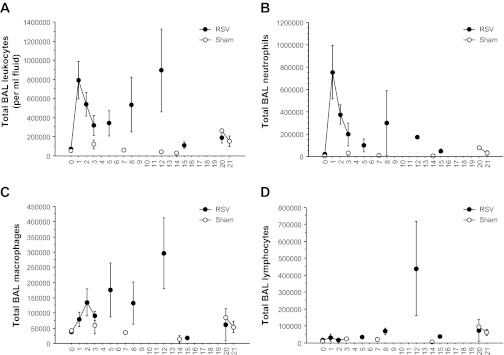
RSV infection causes leukocytosis in BAL fluid. BAL fluids were tested for numbers of leukocytes at various intervals following inoculation of baboons. Data from 11 baboons infected it and 3 sham-infected animals are included, with 2–5 animals represented at each point on the graph. The vertical axis indicates the mean ± SE number of cells present in BAL fluid. The horizontal axis represents days after inoculation. Total white blood cell numbers (A) were counted in a hemocytometer. The numbers of neutrophils (B), macrophages (C), lymphocytes (D), and eosinophils (data not shown) present in BAL were counted by a pathologist. The total number of leukocytes present in BAL fluids from days 1 to 21 after challenge was greater in RSV-infected baboons than in sham-infected baboons (P = 0.012).
Routine pathological analysis.
Groups of baboons were humanely killed on various days following infection (see methods), and lung tissues were processed for routine H&E staining. Lung tissues from central and peripheral portions of the lung were analyzed in all cases. There was approximately equal involvement grossly of central and peripheral areas of the lung. Pathological findings at necropsy are illustrated in Fig. 5. The external appearance of the lung on days 1, 3, and 5 following infection is illustrated in Fig. 5, A–C. On day 1 (Fig. 5A), the external appearance of the lung showed evidence of mild vascular congestion, and the cut surface was essentially normal. On day 3 (Fig. 5B), the external surface showed marked vascular congestion in nearly all areas of the lung, and the cut surface confirmed the presence of marked vascular congestion without internal hemorrhage in the lung. The lungs were grossly edematous. On day 5 (Fig. 5C), vascular congestion and edema were still prominent on the external lung surface, and there was consolidation of part of one lobe. The two cut areas revealed marked congestion internally without hemorrhage. Vascular congestion and edema were still prominent on gross examination of the lungs of animals undergoing necropsy on day 13 (data not shown).
Fig. 5.
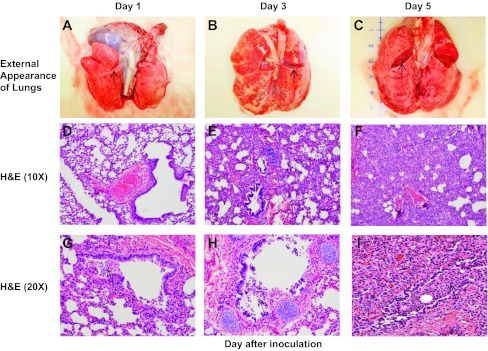
RSV infection induces pathological changes in infant baboons that resemble those in human infants with RSV infection. The external appearance of intact, fresh lung blocks recovered from RSV-infected animals on days 1, 3, and 5 following infection is shown in A–C. The cut surface (arrows) reveals the state of the lung parenchyma in each figure. Lungs demonstrate incrementally greater vascular congestion and edema over time. D–F demonstrate interstitial pneumonia with worsening inflammation, epithelial damage, and consolidation over time. The inflammatory infiltrate is more subtle on day 1 and found primarily in a peribronchiolar distribution with multifocal infiltration in the bronchiolar epithelium. Progression to day 5 reveals severe thickening of the interstitium and alveolar collapse as a result of the worsening inflammatory infiltration. H&E, hematoxylin and eosin. In G–I, there is progressive bronchiolar epithelial damage with sloughing, leading to partial to complete obstruction of some bronchioles with fragmented epithelium mixed with mononuclear inflammatory cells (arrows).
Lung slices were also processed for routine H&E staining. Figure 5, D–I, illustrates the pathological findings observed in lung tissue obtained on days 1, 3, and 5 after RSV infection. On day 1 after infection (Fig. 5D), mild degrees of interstitial inflammation were noted, predominantly in peribronchiolar areas. On days 3 and 5, the degree of inflammation increased, with more intense inflammation of the interstitium (Fig. 5, E and F). Figure 5, G–I, demonstrates progressive bronchiolar epithelial damage and sloughing over the course of infection, leading to partial to complete obstruction of some bronchioles by a mixture of fragmented epithelium and mononuclear inflammatory cells. Loss of the bronchiolar epithelium was noted in several areas of all specimens obtained on each day following infection, and proliferative changes were often noted in the bronchiolar epithelium that had otherwise remained intact. Inflammatory changes remained quite prominent in necropsy tissues obtained on days 8 and 13 after infection (data not shown).
The extent of lung involvement varied from mild to severe among individual animals. The distribution of pathology was patchy in some animals and more diffuse in others. Changes were observed in both central and peripheral areas of the lung. Figure 5 illustrates the close similarity of pathological findings in baboons to those occurring in humans with RSV infection.
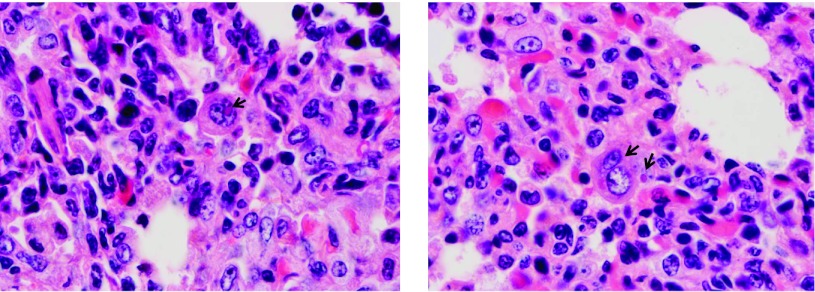
Figure 6 demonstrates areas of the baboon lung in which RSV infection caused the fusion of epithelial cells into characteristic syncytia. Pale pink viral inclusion bodies were also observed in some areas.
Fig. 6.
Formation of syncytial cells and appearance of inclusion bodies in lung tissue of RSV-infected baboons. RSV infection of humans results in fusion of epithelial cells into multinucleate syncytia, and in the formation of intracellular inclusion bodies. Arrows indicate areas where these characteristic findings are also present in the lungs of RSV-infected infant baboons.
IHC staining for RSV antigen.
Figure 7 illustrates the histological findings following IHC staining of the lung for RSV antigen. Figure 7, A–C, demonstrates that RSV antigen is present in ciliated epithelial cells early in the course of infection (Fig. 7A), with staining of primarily basal epithelial cells later (Fig. 7C), presumably after apical ciliated epithelial cells have sloughed as a result of infection. RSV antigen was detected in bronchiolar epithelium in tissues from all animals tested.
Fig. 7.
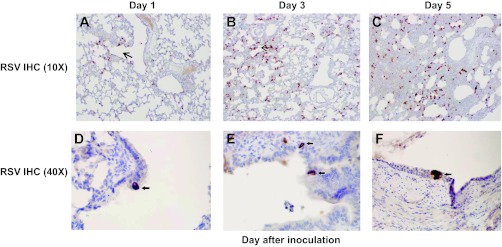
Immunohistochemical (IHC) staining of lung for RSV antigen. Brown peroxidase stain indicates the presence of RSV antigen. A: cluster of positive cells in the bronchiolar epithelium (arrow). B: scattered positive cells found primarily in a peribronchiolar distribution (area surrounding arrow). C: diffuse, patchy distribution of positive cells now involving lung parenchyma. Diffuse increase in positive cells seen in a background of worsening interstitial expansion and inflammation. D–F: bronchioles demonstrating RSV IHC positivity (black arrows) within the single cells of the columnar epithelial lining. Ciliated epithelial cells are infected early, with progression to infection of columnar basal cells as ciliated cells are sloughed.
Figure 7, D–F, illustrates the progression of RSV infection over the first 5 days following infection. In Fig. 7D, infected cells are observed in the bronchiolar epithelium and in the interstitium of alveoli that are adjacent to the bronchioles. In Fig. 7E, RSV antigen is detected in alveolar interstitium in areas more distant from the bronchioles. Finally, in Fig. 7F, the distribution of RSV antigen is more diffuse, and there is a marked increase in the degree of leukocyte infiltration of the interstitium, with greater thickening of the interstitial walls. RSV infection therefore apparently originates in bronchiolar epithelial cells and then spreads to the interstitium over the first few days of infection.
RSV antigen could not be detected by IHC in tissues obtained on day 8 or day 13 from RSV-challenged baboons.
IHC staining for CD4 and CD8 lymphocytes.
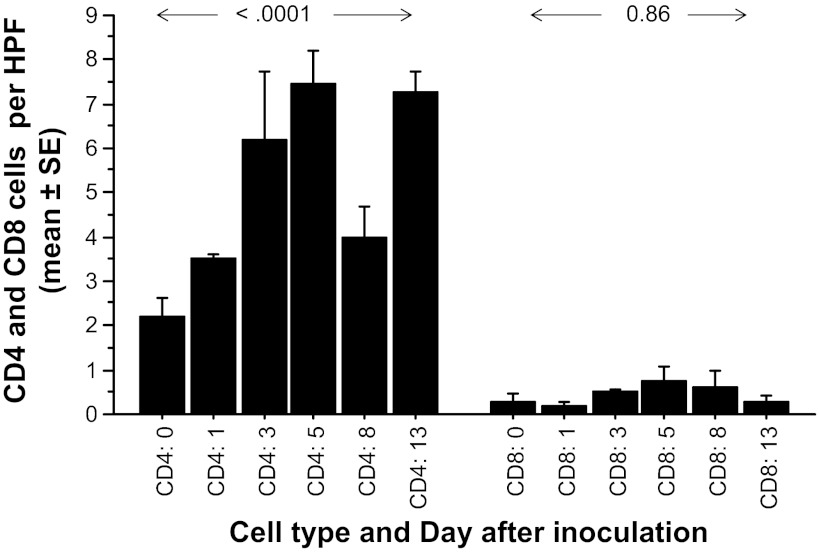
Severe RSV infection in human infants is characterized by replication of RSV in bronchiolar and alveolar epithelium with an absence of cytotoxic CD8 lymphocyte responses in the lung (37). To determine if severe RSV infection in infant baboons is also characterized by reduced CD8 responses, we performed IHC for CD4 and CD8 lymphocyte antigens on lung tissues from baboons infected intratracheally at 1, 3, 5, 8, and 13 days after infection, comparing results with those in two uninfected control baboons (Fig. 8). The number of CD4-positive cells was 2.2 ± 0.4/HPF at baseline and rose to ∼7/HPF over the course of infection (P < 0.0001). The number of CD8-positive cells was 0.3 ± 0.2/HPF at baseline and did not increase in a statistically significant fashion (P = 0.86) during the course of infection. The number of CD8 lymphocytes never exceeded a mean of 1 cell/HPF at any point. CD8+ cells could be detected in the lungs of all baboons tested, indicating that the assay was functional. This suggests that RSV LRTI in infant baboons is associated with a lack of strong CD8 cytotoxic lymphocyte responses, as is the case in human infants with severe disease (37).
Fig. 8.
CD4 and CD8 lymphocytes in lungs of RSV-infected and uninfected baboons. RSV-infected baboons were humanely killed at various intervals after infection (2 at day 0, 3 on day 1, 2 on day 5, 1 on day 8, and 2 on day 13). Lung tissues were then stained for CD4 and CD8 cells using immunohistochemical techniques. The numbers of CD4- and CD8-positive cells in each of 10 high-power fields (HPF) of tissue from each baboon (vertical axis) were counted by a pathologist. The number of CD4 cells in the lungs of infected baboons increased significantly from control values (day 0) over the course of infection (P < 0.0001), but the number of CD8 cells did not vary in a statistically significant fashion (P = 0.86), and never surpassed 1 cell/HPF.
DISCUSSION
One of us (Welliver) previously participated in the only study known to us involving RSV infection of infant baboons (23). In that earlier study, groups of pregnant baboons were immunized with an RSV vaccine or placebo, after which infants born to each group were inoculated with 4 × 107 pfu of RSV. Infants of vaccinated mothers were protected against infection, but the manifestations of illness in infants of unvaccinated mothers were not described in detail. In the present study, we extend these previous results by demonstrating that infant baboons infected with a human strain of RSV develop tachypnea and reduced oxygenation. The degree of tachypnea (daily mean respiratory rates of 64–108/min) and reduced oxygenation (daily mean O2sats of 93.5–95.5%) observed in infant baboons are meaningful in that they are similar to criteria used by clinicians to hospitalize human infants with RSV infection. The most profound abnormalities of respiratory rate and O2sat in infant baboons occurred between days 4 and 7 after infection. Differences in these measures from baseline values (and from sham-infected animals) persisted for at least 15 days after infection. This is similar to the case in human infants where signs of LRTI may persist for weeks following some initial improvement (4). The prolonged duration of signs of lower respiratory tract disease in the infant baboon provides an excellent endpoint to assess the effects of antivirals and vaccines in reducing or preventing RSV-related illness. No other nonhuman primate model has been reported to demonstrate tachypnea or reduced oxygenation following infection with RSV (5, 11, 17, 26, 27, 30).
We cultured serial samples of BAL fluid from infected animals to determine the kinetics of replication of RSV in infant baboons. RSV was recovered in culture of BAL fluid from all baboons inoculated by the intratracheal route but was not tested in baboons inoculated intranasally. RSV was present in BAL fluid in low titers at 6 h after inoculation, reached a maximum at 24 h after inoculation, and then remained at fairly constant levels through the next 5 days. These findings clearly demonstrate that RSV was replicating in the lung. RSV was still detectable in BAL fluid samples obtained 8 days after infection but was undetectable in samples obtained 12 days following infection. This pattern of persistent replication is similar to that reported in human infants (22). The degree of replication of RSV in baboons provides another endpoint for evaluation of the efficacy of vaccines and antivirals.
All baboons were seronegative for RSV NA at baseline, and all sham-infected baboons had undetectable NA titers at intervals up to 21 days after inoculation. In contrast, each of five baboons inoculated intratracheally with RSV developed NA responses by day 21 after infection (arithmetic mean titer = 108), suggesting that true infection occurred. NA responses were generally of low magnitude, similar to NA titers in human infants undergoing primary RSV infection (35a). This may be because of an immaturity in the ability to develop antibody responses in infants of each species.
We also evaluated leukocyte responses in BAL fluids obtained from RSV-infected animals. We found that infection was followed by a biphasic inflammatory response. Neutrophils were the predominant cell type in BAL fluid for the first 3 days following infection, before declining sharply in number thereafter. Macrophages became the predominant cell type present from days 4 to 8, a time when illness was most severe. This may suggest that macrophages are the most important cell responsible for eradicating virus and clearing debris from the airway, as has been demonstrated in mouse models of RSV infection (29). Alternatively, it may mean that exaggerated macrophage responses somehow contribute to illness. We plan to study the role of macrophages in modifying RSV infection, hopefully identifying successful therapeutic approaches to the management of RSV infection.
Total BAL leukocyte counts reached a second peak on day 12 following infection, coincident with the appearance of lymphocytes (along with macrophages) in BAL fluids. Studies in human infants similarly demonstrate that RSV-specific cytotoxic lymphocytes do not appear in the airway of infants with bronchiolitis until 10–12 days following infection, when illness has largely resolved (15). A role for lymphocytes in eradicating RSV infection is suggested by their appearance in BAL fluid at the time when the titer of replicating virus begins to decline. However, the low numbers of lymphocytes present during the time of maximum illness do not support a role for these cells in the pathogenesis of RSV infection in baboons. This appears to be the case in RSV infection of human infants as well (37).
In this study, total BAL leukocyte counts did not correlate with the severity of illness in infant baboons. Total leukocyte counts have not been demonstrated to correlate with clinical indicators of illness in human infants (13). It is possible that the severity of RSV infection is not the result of the number or activity of a single cell type or cytokine, but the end effects of either airway obstruction, or else sloughing of the bronchiolar epithelium, with the loss of homeostatic factors normally produced by airway epithelial cells (12, 32). These abnormalities may increase the work of breathing necessary for the animal to maintain adequate ventilation.
The histopathological findings in RSV-infected baboons included severe vascular congestion, marked interstitial pneumonitis, extensive sloughing of bronchiolar epithelium, and areas of obstruction of the airway lumen with mononuclear cells and sloughed epithelial cells. These are the characteristic findings in RSV LRTI in human infants (16, 37), again emphasizing the similarity of disease induced in the two species. Serial IHC studies in our baboons revealed that RSV first infected ciliated epithelial cells in the bronchiolar airways followed by infection of basal epithelial cells, since the more apical ciliated cells were sloughed. Infection then appeared to spread first to the pulmonary interstitium in peribronchiolar areas before disseminating to areas of the interstitium located further from the airways. The severity of interstitial involvement seemed to reach a maximum at day 5 following infection, simultaneous with the onset of tachypnea and reduced oxygenation in the animal. This suggests that the degree of pneumonitis may underlie the degree of limitation of gas exchange in the lung.
IHC staining also revealed that CD4 lymphocytes increased approximately threefold in lung tissues over the first 5 days of infection, whereas increases in CD8 cells were minimal in lung tissues through day 13 after infection, another finding that is similar to that of human infants with severe RSV infection (37). A lack of cytotoxic CD8 lymphocyte responses might lead to more extensive viral replication and enhanced disease severity in both species. The significance of the increase in CD4 lymphocytes in the lung during RSV infection is less clear. The fact that CD4 lymphocytes, but not CD8 lymphocytes, are present in the lung might suggest that, during RSV infection, there is some interference with the ability of CD4 helper lymphocytes to promote the development of cytotoxic CD8 lymphocytes.
The infant baboon model presents the opportunity to examine the comparative roles of viral replication and inflammatory responses in determining the outcome of RSV infection. A variety of antiviral compounds against RSV are in development (36), but there is an understandable reluctance to test new drugs in infants. Immune modulating drugs may conceivably improve the outcome of RSV infection by regulating the inflammatory response, but the safety of this approach (especially in infants) is in question. Baboons are ∼95% identical genetically to humans (5, 6). Therefore, establishing the safety and efficacy of such compounds in an infant baboon would suggest that these compounds could also be used safely in human infants.
Regarding RSV vaccines, severe illness at the time of natural RSV (not vaccine-induced) infection appears to be related to a limited release of antiviral Th1 cytokines (1, 7, 13) and a failure to generate CD8 cytotoxic lymphocyte responses in the lung (37), that is, the Th1 cytokine interferon-γ (IFNγ) is present in infants with milder forms of RSV infection, but is virtually absent in severe forms of disease (1, 7, 13), whereas CD8 lymphocytes are essentially undetectable in the lungs of human infants with fatal RSV infection (37) but were present in an infant with very mild RSV infection who died in an automobile accident (16). We have not yet studied IFNγ responses in infant baboons, and IFNγ could be produced by the CD4 cells present in the lung. This will be a point of further study. Nevertheless, the number of CD8 lymphocytes in the lung was quite low in our infant baboons with RSV infection. We believe that the induction of antiviral CD8 lymphocyte responses by an RSV vaccine would protect human infants against severe forms of infection. The infant baboon appears to be an ideal model to study whether RSV vaccines can induce protective antibody and CD8 lymphocyte responses safely and effectively.
In summary, the infant baboon model of RSV infection provides an opportunity to study the relative contribution of virus replicating in the lung vs. the resulting inflammatory response in determining the outcome of RSV infection. Additionally, the model permits the evaluation of the safety and effectiveness of vaccines, antivirals, and immune-modulating agents before progressing to studies in humans. Various theories of pathogenesis could be studied in this model, hopefully providing guidance in the development of new therapeutic agents for use in severe forms of RSV LRTI.
GRANTS
This work was supported by the National Institute of Research Resources Grant 5P40RR-012317-14 (G. L. White, R. F. Wolf, and J. F. Papin) as well as departmental funding (R. C. Welliver, Sr.).
DISCLOSURES
The authors of this manuscript do not have a commercial or financial conflict of interest in regard to the publication of this study.
AUTHOR CONTRIBUTIONS
Author contributions: J.F.P., R.F.W., and R.C.W. conception and design of research; J.F.P., R.F.W., S.D.K., J.D.J., S.N.M., and R.C.W. performed experiments; J.F.P., R.F.W., M.P.A., and R.C.W. analyzed data; J.F.P., R.F.W., and R.C.W. interpreted results of experiments; J.F.P., R.F.W., and R.C.W. prepared figures; J.F.P., R.F.W., and R.C.W. drafted manuscript; J.F.P., R.F.W., and R.C.W. edited and revised manuscript; J.F.P., R.F.W., S.D.K., J.D.J., S.N.M., M.P.A., and R.C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Parts of this project were presented at the 49th annual meeting of the Infectious Disease Society of America, October 20–23, 2011 in Boston, MA.
REFERENCES
- 1. Aberle JH, Aberle SW, Rebhandl W, Pracher E, Kundi M, Popow-Kraupp T. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin Exp Immunol 137: 146–150, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angen Ø, Thomsen J, Larsen LE, Larsen J, Kokotovic B, Heegaard PMH, Enemark JMD. Respiratory disease in calves: microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol 137: 165–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antonis AFG, de Jong MC, van der Poel WHM, van der Most RG, Stockhofe-Zurwieden N, Kimman T, Schrijver RS. Age-dependent differences in the pathogenesis of bovine respiratory syncytial virus infections related to the development of natural immunocompetence. J Gen Virol 91: 2497–2506, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Belknap EB, Ciszewski DK, Baker JC. Experimental respiratory syncytial virus infection in calves and lambs. J Vet Diagnostic Invest 7: 285–298, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Belshe RB, Richardson LS, London WT, Sly DL, Lorfeld JH, Camargo E, Prevar DA, Chanock RM. Experimental respiratory syncytial virus infection of four species of primates. J Med Virol 1: 157–162, 1977 [DOI] [PubMed] [Google Scholar]
- 6. Bisgaard H, Flores-Nunez A, Goh A, Azimi P, Halkas A, Malice MP, Marchal JL, Dass SB, Reiss TF, Knorr BA. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Resp Crit Care 178: 854–860, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bont L, Heijnen CJ, Kavelaars A, Aalderen WMCv, Brus F, Draaisma JMT, Pekelharing-Berghuis M, Diemen-Steenvoorde RAAMv, Kimpen JLL. Local interferon-γ levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 184: 355–358, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Chen FC, Li WH. Genomic divergences between humans and other hominoids and the effective population size of the common ancestor of humans and chimpanzees. Am J Hum Genet 68: 444–456, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper GM, Brudno M, Program NCS, Green ED, Batzoglou S, Sidow A. Quantitative estimates of sequence divergence for comparative analyses of mammalian genomes. Genome Res 13: 813–820, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crowe JE, Jr, Murphy BR, Chanock RM, Williamson RA, Barbas CF, 3rd, Burton DR. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci USA 91: 1386–1390, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Swart RL, Kuiken T, Timmerman HH, Amerongen Gv, van den Hoogen BG, Vos HW, Neijens HJ, Andeweg AC, Osterhaus ADME. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol 76: 11561–11569, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farah OR, Li D, McIntyre BAS, Pan J, Belik J. Airway epithelial-derived facror relaxes pulmonary vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol 296: L115–L120, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1α (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis 184: 393–399, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 88: 1026–1033, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heidema J, Lukens MV, van Maren WWC, van Dijk MEA, Otten HG, van Aught AJ, van der Werff DBM, van Gestel SJP, Semple MG, Smyth RL, Kimpen van Bleek GM JLL. CD8T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol 179: 8410–8417, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 20: 108–119, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kakuk TJ, Soike K, Brideau RJ, Zaya RM, Cole SL, Zhang JY, Roberts ED, Wells PA, Wathen MW. A human respiratory syncytial virus (RSV) primate model of enhanced pulmonary pathology induced with a formalin-inactivated rsv vaccine but not a recombinant FG subunit vaccine. J Infect Dis 167: 553–561, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89: 422–434, 1969 [DOI] [PubMed] [Google Scholar]
- 20. Lapin CD, Hiatt PW, Langston C, Mason E, Piedra PT. A lamb model for human respiratory syncytial virus infection. Pediatr Pulmonol 15: 151–156, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 21: 629–632, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, Buckingham S, Vernacchio L, Ambrosino DM. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis 178: 1555–1561, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Murthy KSM, Welliver R, Rice KS, Carey KD. Maternal immunization with respiratory syncytial virus vaccine protects infants from viral infection. In: RSV After 45 Years. Segovia, Spain: 2001, p. 69. [Google Scholar]
- 24. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375: 1545–1555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olivier A, Gallup J, de Macedo MM, Varga SM, Ackermann M. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int J Exp Pathol 90: 431–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ponnuraj EM, Hayward AR, Raj A, Wilson H, Simoes EAF. Increased replication of respiratory syncytial virus (RSV) in pulmonary infiltrates is associated with enhanced histopathological disease in bonnet monkeys (Macaca radiata) pre-immunized with a formalin-inactivated RSV vaccine. J Gen Virol 82: 2663–2674, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Prince GA, Suffin SC, Prevar DA, Camargo E, Sly DL, London WT, Chanock RM. Respiratory syncytial virus infection in owl monkeys: viral shedding, immunological response, and associated illness caused by wild-type virus and two temperature-sensitive mutants. Infect Immun 26: 1009–1013, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Randolph AG, Reder L, Englund JA. Risk of bacterial infection in previously healthy respiratory syncytial virus-infected young children admitted to the intensive care unit. Pediatr Infect Dis J 23: 990–994, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed JL, Brewah YA, Delaney T, Welliver TP, Burwell T, Benjamin E, Kuta E, Kozhich A, McKinney L, Suzich J, Kiener PA, Avendano L, Velozo L, Humbles A, Welliver RC, Sr, Coyle AJ. Macrophage impairment underlies airways occlusion in primary respiratory syncytial virus bronchiolitis. J Infect Dis 198: 1783–1793, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Richardson LS, Belshe RB, Sly DL, London WT, Prevar DA, Camargo E, Chanock RM. Experimental respiratory syncytial virus pneumonia in cebus monkeys. J Med Virol 2: 45–59, 1978 [DOI] [PubMed] [Google Scholar]
- 31. Sow FB, Gallup JM, Olivier A, Krishnan S, Patera AC, Suzich J, Ackermann MR. Respiratory syncytial virus is associated with an inflammatory response in lungs and architectural remodeling of lung-draining lymph nodes of newborn lambs. Am J Physiol Lung Cell Mol Physiol 300: L12–L24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spina D. Epithelium smooth muscle regulation and interactions. Am J Respir Crit Care Med 158: S141–S145, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354: 541–545, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Tristram DA, Welliver RC, Hogerman DA, Hildreth SW, Paradiso P. Second-year surveillance of recipients of a respiratory syncytial virus (RSV) F protein subunit vaccine, PFP-1: evaluation of antibody persistence and possible disease enhancement. Vaccine 12: 551–556, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Viuff B, Uttenthal Å, Tegtmeier C, Alexandersen S. Sites of replication of bovine respiratory syncytial virus in naturally infected calves as determined by in situ hybridization. Vet Pathol Online 33: 383–390, 1996 [DOI] [PubMed] [Google Scholar]
- 35a. Welliver RC, Kaul TN, Putnam PI, Sun M, Riddlesberger K, Ogra PL. The antibody response to primary and secondary infection with respiratory syncytial virus infection; kinetics of class-specific responses. J Pediatr 96: 808–813, 1980 [DOI] [PubMed] [Google Scholar]
- 36. Welliver RC. Pharmacotherapy of respiratory syncytial virus infection. Curr Opin Pharmacol 10: 289–293, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 195: 1126–1136, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]