Sir,
The aim of this study is to report the 6-month anatomic and Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) response after the combination of intravitreal adalimumab and bevacizumab (IVBA) in patients with macular edema of various etiologies.
Case report
We reviewed the clinical records of consecutive patients with macular edema of various etiologies, which were treated with at least one off-label combined intravitreal injection of 1.25 mg/0.05 ml of bevacizumab and 2 mg/0.08 ml of adalimumab (Figure 1). Five consecutive patients (7 eyes), and at least 6 months of follow-up were identified and included for this analysis.
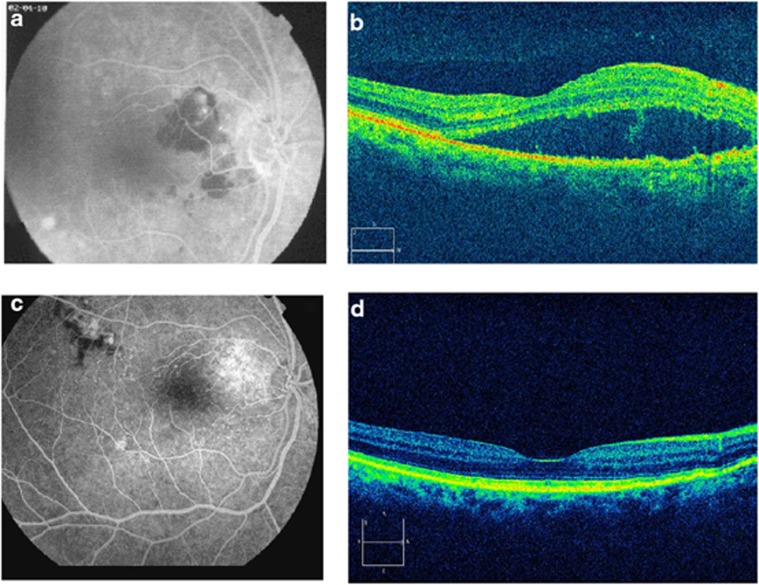
Figure 1.
(a) Late phase fluorescein angiogram demonstrates an extrafoveal choroidal neovascular membrane associated with age-related macular generation with subretinal fluid, with a best-corrected visual acuity (BCVA) of 20/80. (b) Spectral domain optical coherence tomography (SD-OCT) of the lesion shows a hyporreflective image confirming subretinal fluid at baseline. (c) Late phase fluorescein angiogram, and (d) SD-OCT demonstrate a complete resolution of subretinal fluid, with a BCVA of 20/25 at 6 months of follow up, after doses of intravitreal adalimumab and bevacizumab. There is a new area of hypo- and hyperfluorecence supero-temporal to the fovea without leakage (c).
Our results are depicted in Table 1. Eyes 1 and 7 were previously treated with intravitreal bevacizumab (3 doses with 6-week intervals), with no anatomical or functional response ≥12 weeks from last injection. Four (57.1%) of seven eyes gained ≥3 ETDRS lines of BCVA. None of the patients developed systemic complications such as thromboembolic events or a cerebral vascular accident, and none developed any ocular complication.
Table 1. Patient's demographics, etiology, and clinical findings before and after IVBA.
| Patients/Eye # | Lat | Age (years) | Gender | Duration of ME (months) | Etiology |
ETDRS
BCVA |
OCT
CMT |
# of Injections | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | 6 months | Baseline (μ) | 1 month (μ) | 3 months (μ) | 6 months (μ) | |||||||
| 1/1 | OD | 71 | F | 08 | PMCE | 20/125 | 20/63 | 20/63 | 20/63 | 239 | 245 | 280 | 283 | 1 |
| 1/2 | OS | 71 | F | 10 | PMCE | 20/63 | 20/63 | 20/80 | 20/80 | 233 | 250 | 272 | 273 | 1 |
| 2/3 | OD | 51 | M | 06 | DME | 20/80 | 20/40 | 20/40 | 20/40 | 505 | 308 | 320 | 321 | 2 |
| 2/4 | OS | 51 | M | 06 | DME | CF | 20/100 | 20/80 | 20/80 | 664 | 310 | 335 | 332 | 2 |
| 3/5 | OS | 69 | M | 02 | CRVO | 20/200 | CF | CF | CF | 502 | 510 | 516 | 520 | 4 |
| 4/6 | OD | 67 | F | 01 | AMD | 20/80 | 20/25 | 20/25 | 20/25 | 385 | 208 | 224 | 220 | 4 |
| 5/7 | OD | 82 | M | 07 | AMD/BRVO | 20/200 | 20/200 | 20/200 | 20/200 | 289 | 300 | 310 | 310 | 2 |
Abbreviations: AMD, exudative age-related macular degeneration; BCVA, best corrected visual acuity; BRVO, branch retinal vein occlusion; CMT, central macular thickness; CRVO, central retinal vein occlusion; DME, diabetic macular edema: ETDRS, Early Treatment Diabetic Retinopathy Study; F, female; IVBA, intravitreal bevacizumab and adalimumab; lat, laterality; M, male; ME, macular edema; OCT, optical coherence tomography; OD, right eye; #, number; OS, left eye; PMC, pseudophakic cystoid macular edema.
Comment
Sfikakis et al1 reported an improvement in visual acuity and macular edema in four patients with refractory DME, following two intravenous infusions of infliximab (5 mg/kg) given 1 month apart. Wu et al2 did not find any visual benefit with either 1 or 2 mg of infliximab or 2 mg of adalimumab in eyes with refractory DME. In addition, a high rate of intraocular inflammation was seen in eyes injected with infliximab.
Androudi et al3 reported five patients with refractory cystoid macular edema secondary to noninfectious uveitis that were treated with intravitreal adalimumab for 3 months with no apparent benefit. There were no important adverse events reported in this small group of patients.
No important adverse events, including inflammation, were reported in our current series.
The limitations of our study include its small sample size, and retrospective nature. In addition, although our study suggests there is a benefit from combined VEGF and TNF-α inhibition, it is unclear if the same benefit could have been obtained with either agent or only with both. Furthermore, several of the etiologies have a documented natural history to suggest that spontaneous resolution is possible. However, two eyes did not respond to prior treatment with bevacizumab alone and did respond to combined VEGF and TNF-α inhibition. Our results warrant further study.
Acknowledgments
This study was supported in part by the Arevalo-Coutinho Foundation for Research in Ophthalmology, Caracas, Venezuela.
The authors declare no conflict of interest.
Footnotes
Presented in part at the 44th Retina Society Annual Scientific Meeting, Rome, Italy, September 2011.
References
- Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005;28:445–447. doi: 10.2337/diacare.28.2.445. [DOI] [PubMed] [Google Scholar]
- Wu L, Hernandez-Bogantes E, Roca JA, Arevalo JF, Barraza K, Lasave AF. Intravitreal tumor necrosis factor inhibitors in the treatment of refractory diabetic macular edema: a pilot study from the Pan-American Collaborative Retina Study Group. Retina. 2011;31:298–303. doi: 10.1097/IAE.0b013e3181eac7a6. [DOI] [PubMed] [Google Scholar]
- Androudi S, Tsironi E, Kalogeropoulos C, Theodoridou A, Brazitikos P. Intravitreal adalimumab for refractory uveitis-related macular edema. Ophthalmology. 2010;117:1612–1616. doi: 10.1016/j.ophtha.2009.12.011. [DOI] [PubMed] [Google Scholar]