Abstract
A prospective, randomized double-blind study comparing the effects of irradiated and unirradiated white blood cells was conducted in 108 acute leukemia patients with life-threatening infections, refractory to antibiotics. The study demonstrated no significant improvement in 30-day survival or overall survival. Transfusion of unirradiated white cells did not compromise the patient's opportunity to undergo allogeneic stem cell transplant, nor the success rate or overall survival after allogeneic transplant. The important positive finding in this study was that the unirradiated white cells produced a significantly higher increment in circulating granulocytes and in a higher proportion of patients granulocyte count exceeded 1000 per microliter, approaching normal concentrations. The increase in the number and the improved survival of the unirradiated granulocytes suggest that this procedure might potentially be a method to improve the utility of granulocyte transfusions and merits further investigation. The study demonstrated non-inferiority for unirradiated white cells. There were no harmful effects such as graft-versus-host disease, indicating that such studies would be safe to conduct in the future.
Keywords: white blood cell transfusions, effects of irradiation, post transfusion increment
Introduction
In the 1950s chemotherapy for leukemia or other malignancies had limited effectiveness because of the occurrence of hemorrhage and infection, which limited the ability to tolerate chemotherapy. The development of allogeneic platelet transfusions for thrombocytopenic recipients greatly reduced the impact of hemorrhage on patient survival.1
The consequence was that infection became and still is the leading cause of morbidity and mortality for patients who receive myelosuppressive therapy and develop leukopenia. In a classic paper,2 Gerald Bodey demonstrated that there was a clear relationship between the circulating granulocyte levels in patients and the incidence of infection. Both the degree of granulocytopenia and its duration were important factors in determining the occurrence of infection. This observation led to the idea that transfusions of allogeneic granulocytes collected from volunteer donors might be as effective in controlling infection as platelets were in controlling hemorrhage.
Unfortunately, the physiology of granulocytes in man made this simple approach unsuccessful. Under normal circumstances 80–90% of the patients' total granulocyte content is in the marrow granulocyte reserve and only 5–10% of the existing differentiated granulocytes are in the circulation. Thus, when one collects granulocytes from peripheral circulation of a donor and injects them into a recipient, only 5% remain in the circulation, and their half-life in the circulation is only 6 h; the remainder move into the bone marrow and tissues. Thus, it was evident that a new strategy had to be devised for transfusing allogeneic granulocytes. The development of the continuous-flow blood cell separator, the addition of high-molecular-weight hydroxy-ethyl starch to improve leukocyte separation from the packed red cells, and the use of granulocyte colony-stimulating factor to mobilize leukocytes from the donor's marrow reserve made it possible to collect a sufficient number of leukocytes to make leukocyte replacement transfusion successful.3
Unfortunately during the development of granulocyte transfusion therapy a number of anecdotal case reports appeared in the literature suggesting that the transfusion of granulocytes could result in a clinical syndrome similar to that observed in patients who receive bone marrow stem cells following immunoablative therapy, so-called transfusion-associated graft-versus-host disease (TAGVHD). These cases occurred in various clinical circumstances, in a small number of patients; usually the reports consisted of single-case reports of patients with advanced immunosuppression from Hodgkin's disease, children with altered immune response and so on. But these granulocyte transfusions were administered to patients with far-advanced disease, most of whom suffered, as a result of their infections, clinical signs and symptoms that resembled graft-versus-host disease, that is, hepatic failure, GI toxicity, bone marrow failure and death. But a careful review of these case reports revealed no common thread, and the evidence that the reports in fact represented graft-versus-host disease was inconclusive.4 Nonetheless, as granulocyte transfusions became more widespread, such reports continued to appear,5 and by 1992 or 1993 it became a recommendation that to prevent the putative TAGVHD, all blood products be irradiated to eliminate the lymphocytes, which were thought to be responsible for this syndrome.
Unfortunately, it has been clearly demonstrated in vitro that radiation of the white cell blood products significantly diminishes neutrophil function.6 More importantly, it literally eradicated functional mononuclear monocytic cell function.7 The effect was to potentially greatly reduce the effectiveness of white cell transfusions. Moreover, transfusion of unirradiated leukocytes had an in-vivo, half-life between 12 and 24 h, whereas irradiated products had half-lives in the circulation of 3–9 h. Thus, we decided to initiate a study comparing irradiated and unirradiated cells with two goals: (1) to determine whether the unirradiated product was more effective in controlling infection and (2) to determine whether this product was safe. Because of the equivocal nature of the evidence to TAGVHD, it is in an unbiased comparison of irradiated and unirradiated cells that we could determine if there truly is an increased likelihood of signs and symptoms of TAGVHD.
Materials and methods
In preparation for this study we conducted a retrospective review of 16 records of acute leukemia patients who had life-threatening infections refractory to antibiotics who had been given irradiated WBC transfusions. We found that these patients had a 50% mortality within 30 days of the start of WBC transfusion (Table 1). Therefore, we initiated a prospective study in which granulocyte transfusions were administered to the patients as frequently as possible during the first 30 days after diagnosis. Our goals were to evaluate the impact on survival and physiological effects of the transfusion to determine whether the persistence of granulocytes in the circulation was significantly different for transfusates that were irradiated in vitro and those that were not. The study was conducted in a prospective randomized double-blind way using an adaptive design (see the Supplemenatry appendix).
Table 1. WBC Transfusion—retrospective review 6/1/2007–8/22/2007.
|
74 Transfusions to 16 patients. 1–17 Transfusions per patient, median 4, average 4.6 | ||
|---|---|---|
|
Mortality 50% |
||
| Success | Fail | |
| No. of patients | 8 patients | 8 patients |
| No. of transfusions | 3–10 | 1–17 |
| Median | 4 | 3.5 |
| Eval. | 4+ | <7 |
| FU (d's) | 4–60 | 1–30 |
| Median | 15 | 8 |
| Age | ||
| Median | 51.51 | 59 |
| Range | 19–7 | 38–80 |
| <40 | 3 | 0 |
The assignment to receive irradiated or unirradiated granulocytes was determined by a computerized program and the assignments were known only to two physicians in the blood bank, who supervised the preparation of the granulocytes and determined whether they were irradiated or not. All products were delivered to the ward for administration to the patient, with the same label, that is, they were labeled ‘irradiated,' so that no one outside the blood bank could distinguish between the two products. None of the staff on the nursing unit, the research nurses, the floor nurses, the physician assistants and the faculty had any way of telling which product was which, and the patients signed an informed consent explaining the potential of TAGVHD and the possible benefit of unirradiated granulocytes, and executed a signed informed consent that indicated that neither the patient, patient's family members who donated the white cells, nor the staff would know the difference between irradiated and unirradiated product.
Donors were recruited from relatives, friends of leukemia patients and community volunteers. All were screened for infectious diseases (according to current regulatory requirements for allogeneic donors). Other criteria for eligibility included a biochemical profile that included alanine aminotransferase, alkaline phosphatase, total bilirubin, hemoglobin S levels and a urine pregnancy test for all female donors. Informed written consent was obtained from all eligible donors before granulocyte collection. The eligible donors first underwent platelet apheresis donation to evaluate their vascular access and to determine whether they could withstand a 2-h collection via two-arm continuous-flow apheresis. GCs were collected via donor stimulation with a single subcutaneous injection of G-CSF (600 μg; Amgen, Thousand Oaks, CA, USA), and first-time donors also received in addition to G-CSF an oral dose of dexamethasone (8 mg) 12 h before the granulocyte collection. GCs were harvested via Hespan-sodium citrate solution (6% hetastarch in 0.9% sodium chloride; BBraun, Inc., Irvine, CA, USA) containing 30 ml of triCitrasol anticoagulant sodium citrate concentrate (46.7% trisodium; Citra Labs, Braintree, MA, USA) 12 h after stimulation. Approximately 1.5 x the whole-blood volume of each donor, or ∼7000–8000 ml, was processed via two-arm peripheral venous access procedure with a COBE Spectra continuous-flow cell separator (CaridianBCT, Lakewood, CO, USA) and COBE Spectra WBC tubing set (CaridianBCT).
The bag with the GC was immediately transferred to the Transfusion Service where it was cross-matched with a sample of the patient's plasma for ABO compatibility. Those units that were ABO incompatible were subject to a drain of the incompatible RBC's by allowing for a sedimentation with the bag upside down, which allowed for the removal of all but 2–3 ml of contaminating RBC's.8 At this time, one of the persons authorized to decide the irradiation/unirradiation status of the unit applied the appropriate label to the unit of GC. If the unit was to be irradiated, a ‘NONIRRADIATED' label was applied and the unit was taken to the Irradiator (name, brand, city) and the standard 25 cGy applied. Once these steps were completed, the unit was released for transfusion to the specific patient, following the standard transfusion service procedures.
Results
After the study of 30 patients the data safety monitoring board recommended that the study be closed because the inferiority boundary had been crossed. However, the investigators requested that the study remain open, not to determine effectiveness but to determine safety and to evaluate the effect of the transfusions on the physiology of the granulocytes. That was agreed to and the study was continued until 108 consecutive patients were registered and randomized. At this point the investigators and the monitoring board believed that we had accumulated sufficient evidence on safety to close the study and to prepare a final evaluation (Table 2).
Table 2. Summary of Study Groups.
| Unirradiated | |
|---|---|
| No. of patients | 48 |
| Age | |
| Median | 57.5 |
| Average | 55.2 |
| Range | 20–84 |
| REL. REF. | 25/48=0.52 |
| No. of transfusions | |
| Median | 5 |
| Average | 6.0 |
| Range | 1–18 |
| Increment | |
| Median | 1.4 |
| Aerage | 2.0 |
| Range | 0.8 |
| >1.0 | 30/48=0.62 |
| Irradiated | |
| No. of patients | 60 |
| Age | |
| Median | 60 |
| Average | 57.1 |
| Range | 19–79 |
| REL. REF. | 32/60=0.53 |
| No. of transfusions | |
| Median | 7 |
| Average | 7.4 |
| Range | 1–32 |
| Increment | |
| Median | 0.5 |
| Average | 1.26 |
| Range | 0–4.3 |
| >1.0 | 22/60=0.36 |
The groups were comparable in age, although the irradiated group was slightly older. They were comparable in the proportion who had advanced relapsed leukemia, were refractory to all known therapy, and were on investigational therapy, that is phase 1 drugs. The number of transfusions received was comparable, although the irradiated group had slightly more transfusions than the unirradiated group. However, the post-transfusion increments (the difference between the pre- and post-transfusion WBC counts) were significantly higher in the unirradiated group, and the proportion that were over a thousand were also substantially higher.
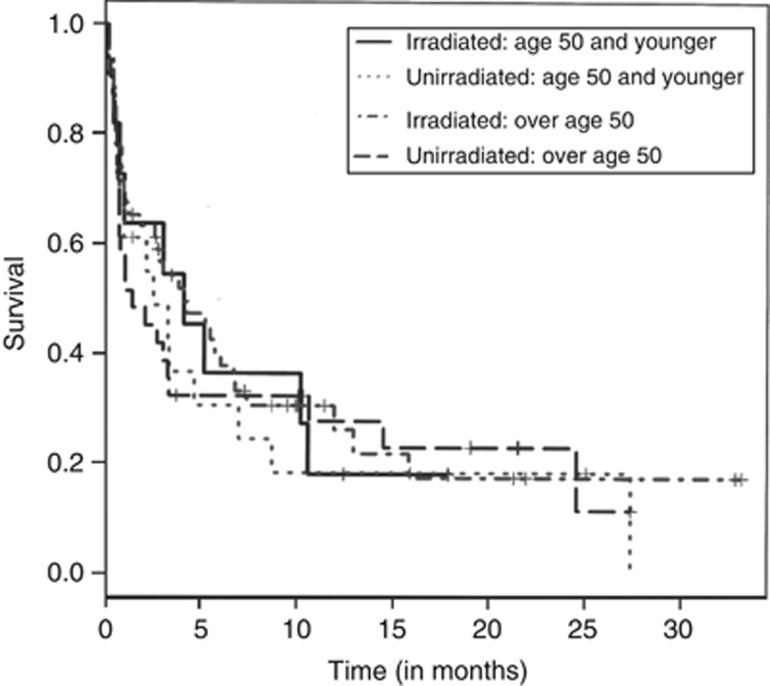
The patients were stratified by age above and below 50 years, because the frequency of response in patients under age 50 was expected to be higher; however, the 30-day mortality was not significantly different for patients either above 50 P-value=0.48 or below 50 P-value=0.67.
Survival was also not significantly different between patients over 50 or under 50 whether irradiated or unirradiated. Therefore, combining the age groups, there was no significant difference in median survival for all patients irradiated 4.1 (2.7, 6.8), unirradiated 2.2 (0.8, 4.7). These data are shown graphically in Figure 1.
Figure 1.
Overall survival of patients following onset of WBC transfusions.
As there was no significant difference based on age or treatment (Table 3); we concluded that unirradiated cells do not significantly change survival over those that are irradiated, but more importantly, the non-inferiority test had a P-value of 0.39, indicating that the unirradiated leukocyte transfusions did not produce any harmful effects that could be detected by 30 days survival or overall survival.
Table 3. Survival analysis.
| Model including age and treatment |
|---|
| Age: P-value=0.48 |
| Treatment: P-value=0.78 |
| As age was not significant, it was dropped and the model refit with just treatment |
| Treatment: P-value=0.433 |
| Conclusion: unirradiated does not significantly change survival |
| Conclusion: the non-inferiority test had a P-value=0.39 |
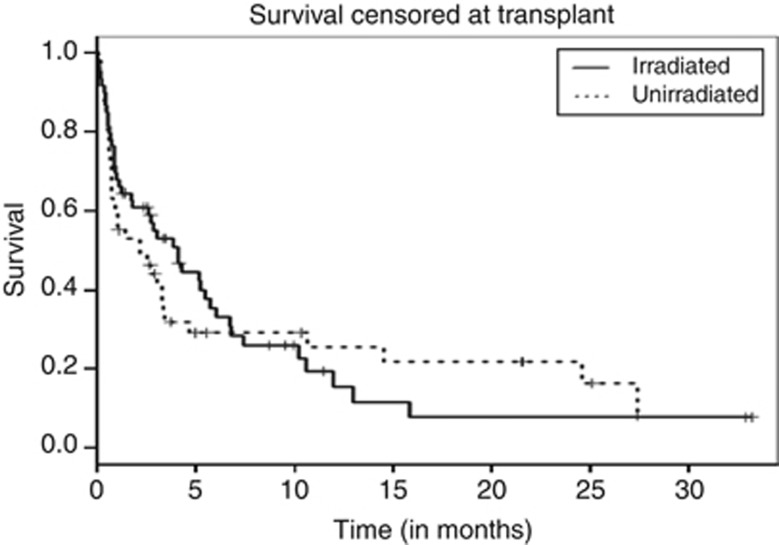
The next major concern was the possibility that there was a difference in the proportion of patients who underwent an allogeneic transplant. Therefore, survival curves were analyzed censored at the time of transplant, and again there were no significant differences between those who received irradiated transfusions and those who received unirradiated transfusions (Figure 2). Moreover, the fraction of patients who underwent transplantation was not different for the two groups, and the overall survival was not significantly different P-value=0.673 (Table 4).
Figure 2.
Survival of patients following onset of WBC transfusions, censored at time of transplant.
Table 4. Transplant patients.
| Unirradiated | Irradiated | Total | |
|---|---|---|---|
| Number (%) | 5/48 (0.10) | 9/60 (0.15) | 14/108 (0.13) |
| Surving (%) | 3/5 (0.60) | 6/9 (0.67) | 9/14 (0.60) |
| Time (months) | 1–16+ | 1.5–18+ | 1–18+ |
| Time (months) | 13+ | 11+ | 11+ |
The survival of patients post-transplant did not differ (P=0.43)
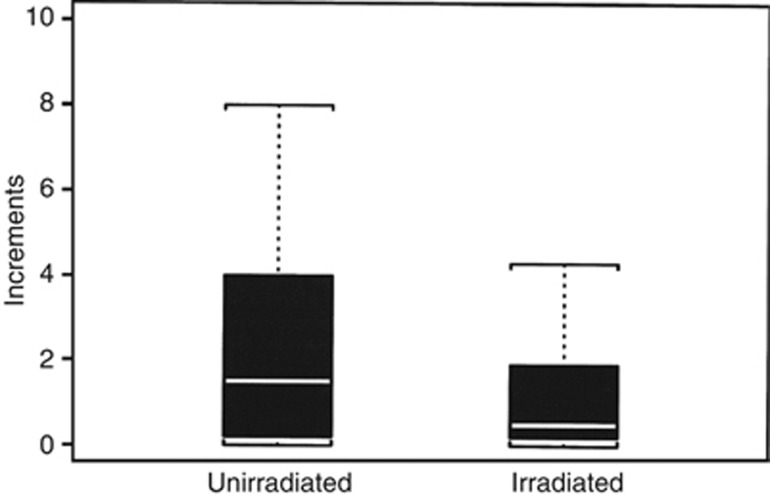
The one difference that was significant was the difference between the measured increments (difference between pre- and post-transfusion values) in granulocytes after transfusion (Figure 3). In Table 5 we show that the unirradiated white cells had a substantially higher mean and medium increment and that the number of patients who had increments that were in the normal range, that is over 1000, was significantly higher in the unirradiated group. Furthermore, the fraction of patients who had increments that were greater than a thousand was significantly higher for unirradiated patients (P-value 0.006).
Figure 3.
Post-transfusion increments in circulating granulocytes (1000s/microliter).
Table 5. Post-transfusion increments in granulocyte concentrations.
| Irradiated: mean 1.35, median 0.5, range (0, 10), 22 patients had increments ⩾1, and 38 had increments <1.0 |
|---|
| Unirradiated: mean 2.023, median 1.5, range (0, 8), 31 patients had increments ⩾1 and 17 had increments <1.0 |
| Test of increments (unirradiated vs irradiated): P-value=0.062 |
| Test of percent ‘Normal' for unirradiated vs irradiated): P-value 0.06 (Fisher's exact test) |
Discussion
This study has demonstrated that irradiation does prevent the differentiation of undifferentiated myeloid cells to differentiated granulocytes. Thus, unirradiated leukocyte transfusions have the potential to improve infection control over transfusions using irradiated leukocytes. However, the impact of this difference on 30-day survival was not significant. The obvious reason for this is that patients received an average of only 6 or 7 transfusions over a period of ∼7–10 days. Even though the unirradiated cells might transiently control infection, infection recurred in nearly all the patients because they had advanced refractory disease and did not achieve hematological remission. Therefore, any difference in infection control or survival was obscured. To demonstrate any improvement in infection control of the unirradiated white cells would likely require patients to be treated earlier, while they had a better potential for achieving remission. Recovery from life-threatening infection depends on either achieving relatively normal granulocyte counts or receiving granulocytes regularly for up to 6 weeks. So if patients were treated earlier in the course of their infection, the duration of granulocyte transfusion could be extended, thus potentially exploiting the improvement in granulocyte recovery from unirradiated transfusions.
Administration of allogeneic granulocytes, whether irradiated or unirradiated, did not compromise eligibility for allogeneic stem cell transplant (13% of the patients). More important is that the effectiveness of transplant in these patients was comparable for both groups, approximately two-thirds of the patients surviving for a year after successful transplant.
Perhaps the most encouraging result from this study is that the outcomes were not inferior for the patients receiving unirradiated granulocytes, and we did not observe a single case of TAGVHD. This indicates that at least for the 108 TAGVHD transfusions administered to the 48 patients in this study there were no harmful effects observed.
Recently, a study published by Guo et al9 reported on the systematic administration of unirradiated granulocytes immediately after intensive chemotherapy for leukemia. The granulocytes were transfused after each course of chemotherapy. They reported a significant increase in long-term survival after 10 months in comparison with the randomly assigned control group. Our group reported similar findings in 1978, when we demonstrated significant improvement after 6 months in overall survival in patients who received unirradiated white cell transfusions compared with that of patients who did not receive transfusion. Thus, the current study adds the information on 48 patients to the 38 reported by McCredie10 and the 29 reported by Guo making a total of 115 patients who received unirradiated white cell transfusions during periods of myelosupression (In the Guo study, there were no reported instances of TAGVHD). Rather, both studies reported a possible antileukemia effect of the unirradiated white blood cell transfusions.
Thus, while it is possible that TAGVHD may occur, the estimated frequency is well below 1%. When you compare the very high mortality associated with life-threatening infections in these myelosuppressed patients, it would seem that the benefit risk ratios would favor continued investigation of unirradiated granulocyte transfusions in a setting where they are more likely to be effective.
Acknowledgments
The authors acknowledge the support of Dr Hagop M Kantarjian, Chair, Department of Leukemia and the physicians of the Department of Leukemia, for making this study possible.
The data in this manuscript was presented orally at: ‘Leukemia and Lymphoma, East and West are Together,' Dubrovnik, Croatia, 9/17/2011-9/21/2011. Published in Abstract Book, P 17: Glasilio Hrvatskoga, Lijecnickdg Zbora, Coden Livjas, issn 1330–4917, Supplement 4, Zagreb, 2011.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Authors Contributions
Emil J Freireich, Director of the Special Medical Education Programs and Director of the Adult Leukemia Research Program, was the principle investigator and responsible for the design, evaluation and preparing the manuscript; Benjamin Lichtiger, Director of the Transfusion Medicine Service and Blood Donor Service, collected and prepared the leukocyte product for transfusion; Gloria Mattiuzzi interviewed patients, obtained informed consent and followed the patients clinically; Fernando Martinez, Co-Director of the Transfusion Medicine Service and Blood Donor Service, assisted in preparing the transfusion product; Virgil Reddy was responsible for recruiting and evaluating donors; J Kyle Wathen was the research statistician responsible for the design and evaluation of the study, and he is currently the Director of Quantitative Decision Strategies at Johnson and Johnson.
Supplementary Material
References
- Freireich EJ. Origins of platelet transfusion therapy. Transfus Med Rev. 2011;25:252–256. doi: 10.1016/j.tmrv.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- Freireich EJ. Leukocyte transfusion and the development of the continuous-flow blood cell separator. Transfus Med Rev. 2011;25:344–350. doi: 10.1016/j.tmrv.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Anderson KC, Weinstein HJ. Transfusion-associated graft-versus-host disease. N Engl J Med. 1990;323:315–321. doi: 10.1056/NEJM199008023230506. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Haluska FG, Dock NL, Dover JS, Kineke EJ, Anderson KC. Brief report: graft-versus-host disease associated with transfusion of blood from unrelated HLA-homozygous donors. N Engl J Med. 1993;328:766–770. doi: 10.1056/NEJM199303183281105. [DOI] [PubMed] [Google Scholar]
- Buescher ES, Gallin JI. Effects of storage and radiation on human neutrophil function in vitro. Inflammation. 1987;11:401–416. doi: 10.1007/BF00915984. [DOI] [PubMed] [Google Scholar]
- Buescher ES, Gallin JI. Radiation effects on cultured human monocytes and on monocyte-derived macrophages. Blood. 1984;63:1402–1407. [PubMed] [Google Scholar]
- Narvios AB, Reddy V, Lichtiger B. Method of removing incompatible red blood cells from granulocyte components. Transfus Apher Sci. 2006;35:179–180. doi: 10.1016/j.transci.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Guo M, Hu KX, Yu CL, Sun QY, Qiao JH, Wang DH, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117:936–941. doi: 10.1182/blood-2010-06-288506. [DOI] [PubMed] [Google Scholar]
- McCredie KB, Hester JP, Dicke KA, Freireich EJ. Blood components in the care of the cancer patient. Year Book Medical Publisher, Inc.: Chicago—London; Current Problems in Cancer. 1978;Vol.III (No. 1:4–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.