Abstract
There is a well-established relationship between increased arterial stiffness and cardiovascular mortality. We examined whether a long-term aerobic exercise intervention (6 months) would increase arterial compliance in older adults with hypertension complicated by Type 2 diabetes (T2DM) and hyperlipidemia. A total of 52 older adults (mean age 69.3±0.6 years, 30 males and 22 females) with diet/oral hypoglycemic-controlled T2DM, hypertension and hypercholesterolemia were recruited. Subjects were randomly assigned to one of two groups: an aerobic group (6 months vigorous aerobic exercise, AT group) and a non-aerobic group (6 months of no aerobic exercise, NA group). Arterial stiffness was measured as pulse-wave velocity (PWV) using the Complior device. Aerobic training decreased arterial stiffness as measured by both radial (P=0.001, 2-way analysis of variance with repeated measures) and femoral (P=0.002) PWV. This was due to a decrease in arterial stiffness in the AT group after 3 months of training, which was not maintained after 6-month training for either radial (P=0.707) or femoral (P=0.680) PWV. Our findings indicate that in older adults with multiple cardiovascular risk factors, short-term improvements in arterial stiffness became attenuated over the long term.
Keywords: gerontology, aerobic exercise, arterial stiffness, Type 2 diabetes
Introduction
Studies of short-duration aerobic training have demonstrated an ability to reduce arterial stiffness in subjects with single cardiometabolic risk factors such as Type 2 diabetes (T2DM),1 advanced age,2, 3 and hypertension,3, 4, 5 but this has not been prospectively examined for multiple cardiometabolic risk factors in the longer term. In fact, a recent systematic review of the state of knowledge regarding exercise and older adults identified a strong need for further research on the impact of physical activity in subjects with multiple risk factors.6
In the current study, we examine whether aerobic exercise can reverse arterial stiffness in adults at very high cardiovascular risk (long-standing diabetes, geriatric age group, hypercholesterolemia and hypertension). Congruent with previous work examining three7 and six4, 5 month aerobic interventions in subjects with a single risk factor only (hypertension), we hypothesized that aerobic exercise would be an effective non-pharmacological therapy for increased arterial stiffness in the short term (3 months) but these benefits would not persist over the longer term (6 months).
Materials and methods
Subjects
Older adults were recruited from the local community through advertisement in local publications (Table 1). All subjects had to be over 65 years of age and were excluded if they had any history of angina, myocardial infarction, stroke, chronic pulmonary disease, smoking in the last 5 years or exercise-limiting orthopedic impairment. All subjects were required to have T2DM, hypertension and hyperlipidemia for at least 5 years. Diabetes and hyperlipidemia were defined by current American Diabetes Association guidelines.8 Hypertension was defined as taking anti-hypertensive agents or having an average blood pressure (based on the mean of three measurements) with a systolic blood pressure (SBP) of >140 mm Hg or a diastolic blood pressure (DBP) of >90 mm Hg as per current Joint National Commitee9 and American Diabetes Association10 guidelines. Entry requirements included a normal resting electrocardiogram, a normal Bruce protocol treadmill maximal exercise stress test, a normal hematocrit and a normal creatinine. Subjects had to be sedentary at the start of the study (as defined as no strength training and <30 min brisk walking/moderate exercise per week and no vigorous exercise in the preceding 6 months).
Table 1. Subject characteristics.
| Measure | All subjects | AT subjects | NA subjects | P-value |
|---|---|---|---|---|
| Age (years) | 69.3±0.6 | 68.5±0.9 | 70.0±0.8 | 0.226 |
| Body mass index (kg m−2) | 29.7±0.6 | 30.9±1.0 | 28.6±0.8 | 0.070 |
| SBP (mm Hg) | 144±3 | 148±4 | 140±3 | 0.161 |
| DBP (mm Hg) | 82±2 | 82±3 | 82±2 | 0.942 |
| Heart rate (beats per minute) | 74±1 | 73±2 | 75±2 | 0.703 |
| Fasting blood glucose (mmol l−1) | 7.8±0.3 | 7.7±0.4 | 7.8±0.4 | 0.985 |
| Glycosylated hemoglobin (%) | 6.7±0.2 | 6.8±0.3 | 6.6±0.2 | 0.923 |
| Years since diabetes diagnosis | 7.9±0.6 | 7.8±1.1 | 7.9±0.7 | 0.520 |
| Total cholesterol (mmol l−1) | 5.0±0.2 | 4.9±0.2 | 5.0±0.3 | 0.888 |
| LDL cholesterol (mmol l−1) | 2.6±0.1 | 2.7±0.2 | 2.6±0.2 | 0.841 |
| HDL cholesterol (mmol l−1) | 1.5±0.1 | 1.5±0.1 | 1.6±0.2 | 0.671 |
| Radial PWV (m s−1) | 10.78±0.32 | 11.17±0.42 | 10.34±0.47 | 0.197 |
| Femoral PWV (m s−1) | 11.97±0.44 | 13.40±0.69 | 12.03±0.63 | 0.162 |
| VO2max (ml kg−1 per minute) | 22.5±0.7 | 22.2±0.9 | 23.4±1.0 | 0.382 |
| Medications (number, percent) | ||||
| Metformin | 34 (66%) | 17 (70%) | 17 (63%) | 0.772 |
| Sulfonylureas | 21 (40%) | 13 (52%) | 8 (30%) | 0.157 |
| Glitazones | 8 (17%) | 3 (13%) | 5 (20%) | 0.715 |
| Statin | 24 (45%) | 14 (57%) | 10 (37%) | 0.175 |
| ACE inhibitors | 25 (47%) | 12 (48%) | 13 (47%) | 0.989 |
| ARB | 12 (21%) | 7 (26%) | 5 (17%) | 0.501 |
| Beta-blockers | 9 (15%) | 4 (17%) | 5 (13%) | 0.715 |
| Calcium channel blocker | 4 (8%) | 3 (13%) | 1 (3%) | 0.305 |
| Hydrochlorothiazide | 16 (30%) | 10 (39%) | 6 (23%) | 0.242 |
| Other diuretics | 3 (6%) | 1 (4%) | 2 (7%) | 0.989 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PWV, pulse-wave velocity; SBP, systolic blood pressure; VO2max, maximal uptake of oxygen.
Demographic data for aerobically trained (AT), untrained (NA) and all subjects are shown as mean±s.e. A P-value of <0.05 was considered as significant.
Subjects were randomized to each of two groups: an aerobic group (AT), and a non-aerobic (NA) group. A simple randomization was performed using a random number generator, equivalent to the flipping of a coin. Allocation concealment was maintained through the use of an off-site randomized list, managed by an individual that had no contact with subjects before completion of recruitment and screening. This study was approved by the Human Subjects Committee of the University of British Columbia, and all subjects gave written informed consent.
Study design
Each subject underwent three evaluation sessions: a baseline session (t0), an assessment after 3 months of training (t3) and an assessment after 6 months of training (t6). Post-intervention vascular studies could be delayed up to 7 days to accommodate each subject's schedule; training was continued if the session was delayed. All study sessions were performed with the subject supine and took place between 0700 h and noon for all subjects to avoid bias due to circadian rhythms. The technician responsible for performing all measures was blinded to subject group.
Training program
The endurance training (AT group) intervention was designed to improve aerobic fitness according to current guidelines,11 and consisted of moderate to vigorous intensity exercise on a treadmill and a cycle ergometer. Training sessions were three times per week, and subjects had to attend 90% of all training sessions to remain enrolled in the study. The aerobic training sessions were 60 min in duration and consisted of 10 min warm-up, 40 min aerobic training and 10 min of cool down/stretching. A clinical exercise physiologist supervised each class, and verified compliance with the exercise regime. Moderate to vigorous intensity exercise was attained via continuous monitoring of heart rates (Polar Vantage Heart Watches; Adelaide, Australia). Based on the resting heart rate (HR) and maximal HR determined during maximal exercise treadmill testing (see below) we set training HR to 60–75% of HR reserve based on the Karvonen formula.12
To avoid the Hawthorne Effect (the phenomenon where subjects improve an aspect of their behavior being experimentally measured simply in response to the fact that they know they are being studied),13 we chose to have an active as opposed to a sedentary control group. Subjects in the NA group also attended sessions three times per week. The NA group sessions were specifically designed to have a minimal aerobic component, and consisted of non-strenuous core (exercise ball) and non-strenuous strength training (very light dumbbells with no increase in weight) exercises. We confirmed minimal aerobic training in the NA group with a test of maximal oxygen consumption in each subject (VO2max, see below). The trainer also contacted each subject weekly to ensure that they were not undertaking any additional exercise. For ethical reasons, each subject in the NA group was given the option of joining the aerobic exercise group after the 6-month intervention period was complete, to encourage them to increase their level of physical fitness.
Exercise classes were held in a hospital-based facility (Healthy Heart Program, St Paul's Hospital, Vancouver, Canada) under supervision of clinical exercise physiologists with cardiology and emergency services available. Cardiovascular measures were taken before, during and after exercise, and included blood pressure, HR and blood glucose. Electrocardiogram telemetry was also available if necessary if unusual HR or rhythms were noted.
Data collection and processing
Subject conditions were standardized according to established guidelines: all measures were performed after 30 min supine rest; the environment was quiet and temperature controlled (25±1 °C); all subjects were fasting; and all subjects had refrained from the consumption of alcohol or caffeine for the preceding 24 h. All patients held all their medications from midnight onwards to avoid confounding the arterial stiffness measures. To avoid the confounding acute effects of exercise, the aerobic training program was also halted for the preceding 24 h.14 Arterial stiffness was measured using the Complior device (Artech Medical, Pantin, France), a semi-automated device that uses two pressure transducers.14 The Complior device is commonly used in previous studies that measure pulse-wave velocity (PWV)15, 16 and it provides measures of arterial compliance that correlate well with other devices17 and are highly reproducible.18 The pressure transducers are held in place by velcro straps that allow them to be fixed over the skin. Each pressure transducer measures the pulse waveform at each site, allowing one to measure transit time of the pulse wave between the two locations. A higher PWV represents greater arterial stiffness. The transducers are placed over the carotid and femoral arteries for a measure of femoral arterial stiffness and over the carotid and radial arteries for a measure of peripheral arterial stiffness.14 PWV was calculated from these transducer measures that are digitally recorded (sampling rate 500 Hz), resulting in measures of radial and femoral PWV. PWV was chosen as our measure of arterial stiffness due to the fact that it is the most commonly used measure both in the literature and in consensus statements.19 Femoral PWV is also one of the only indices of arterial stiffness directly linked with cardiovascular mortality and morbidity.20
HR, SBP, DBP and mean blood pressure were measured using an automated blood pressure cuff that met current hypertension guidelines21 (BpTRU Medical Devices, Coquitlam, Canada). Each subject's weight was measured using a physician's balance scale. Body mass index, waist circumference, hip circumference and waist-to-hip ratio were measured and calculated as per established guidelines.22 VO2max was determined using a maximal Bruce treadmill protocol exercise test and used traditional methods. VO2max was reached by a gradual progression to maximal exertion, and the test was halted when the subject reached a plateau in VO2 uptake, reached volitional exhaustion and demonstrated a respiratory exchange ratio of >1.10.23 The change in VO2max was examined in all groups, including the untrained and strength trained subjects.
Statistical analysis
All data analysis was done in a blinded manner. Results are expressed as the mean±s.e. Our sample size calculations for our three primary outcome measures (radial PWV, femoral PWV and VO2max) assumed a power of 90% and a 5% level of significance. The effects of training on all measures were calculated by a two-way analysis of variance for repeated measures (time × group). For all outcomes, a value of P<0.05 was considered as significant.24 Dropouts were handled on an intention-to-treat basis. Between-group comparisons for proportions (such as medications) were done with Fisher's exact test.
Results
Subject characteristics
Sixty-five subjects responded to the advertisement in local publications and were screened. Thirteen subjects did not meet inclusion and exclusion criteria (including four subjects excluded on the basis of a positive stress test). In all, 52 subjects (30 males and 22 females) were randomized to the AT and NA groups. Randomization resulted in 25 subjects in the AT group (13 males and 12 females) and 27 in the NA group (17 males and 10 females). There were no drop-outs from the study, and all the 52 subjects attended at least 90% of the training sessions. As shown in Table 1, at the time of entry into the study, there was no significant difference between AT and NA subjects with respect to demographic data, resting HR, resting blood pressure, fasting blood sugar, glycosylated hemoglobin or lipid profile. There was no difference between the two groups with respect to the prescription of various types of medications. Only one patient (in the NA group) changed their medication during the intervention (discontinued their statin agent).
Short- and long-term effects of training on measures of arterial stiffness
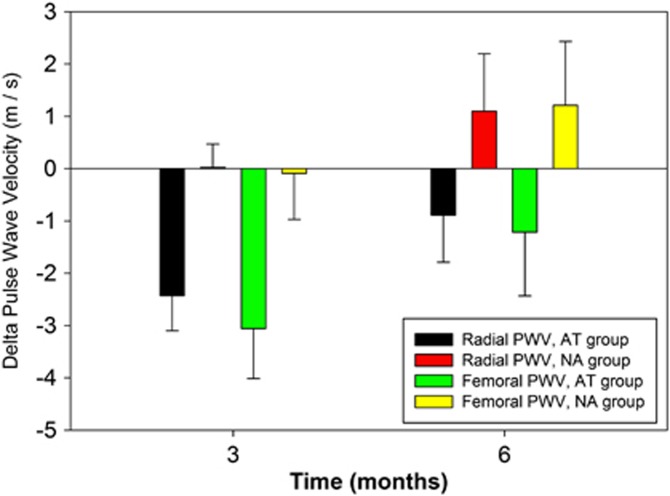
As shown in Figure 1, there was a significant difference in the response to training (group × time) between the AT and NA groups for both radial (P=0.001) and femoral (P=0.002) PWV. The difference in response was primarily due to a decrease in both radial and femoral PWV in the AT group after 3 months of training that was not maintained at the 6-month mark for either radial or femoral PWV (Figure 1). An unpaired t-test between the two groups at 6 months demonstrates no difference between the AT and NA groups for either radial (P=0.707) or femoral (P=0.680) PWV.
Figure 1.
The PWV response to training (group × time) between the AT and NA groups. Black=AT group, radial PWV; Red=NA group, radial PWV; Green=AT group, femoral PWV; Yellow=NA group, femoral PWV.
The relative impact of aerobic training resulted in an ∼21.7±6.7% decrease in radial PWV and 22.8±7.2% decrease in femoral PWV over 3 months. By comparison, the NA group demonstrated a 0.2±4.2% increase in radial PMV and a 0.7±7.3% decrease in femoral PWV at the 3-month mark. None of these reductions in PWV observed in the AT group were maintained throughout the course of the intervention (Figure 1).
Effects of training on measures of fitness
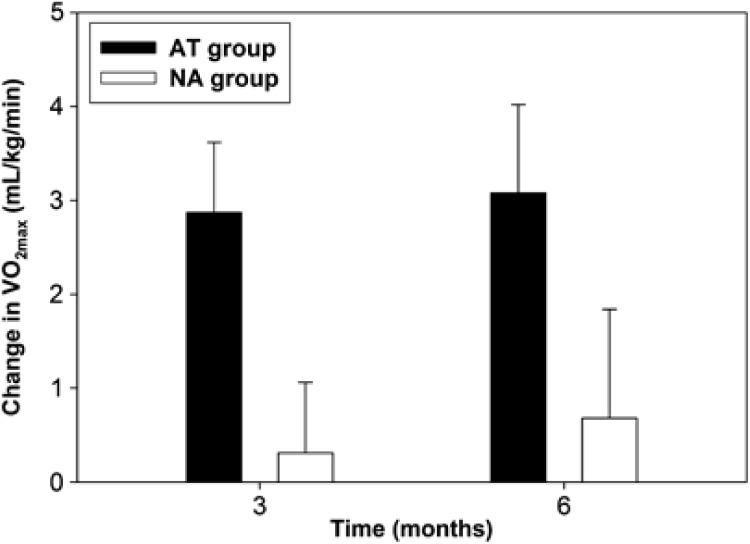
The training response VO2max over the 6-month program was significantly different between the AT and NA groups (P=0.015), with an increase in maximal oxygen uptake only seen in the AT group, as demonstrated in Figure 2. The respiratory exchange ratio was >1.10 for both AT and NA groups at all measurement times. As shown in Table 2, there was a non-significant trend in the AT group for a decrease in weight, SBP and fasting blood glucose as compared with the NA group but no significant difference between the NA and AT groups with respect to changes in body mass index, waist-to-hip ratio, DBP or resting HR.
Figure 2.
The training response of VO2max (maximal uptake of oxygen) over 6 months for both the AT and NA groups. Black=AT group; White=NA group.
Table 2. Change in arterial stiffness and fitness measures after 6 months intervention.
| Measure |
3 months |
6 months |
P-value (time × group) | ||
|---|---|---|---|---|---|
| AT group | NA group | AT group | NA group | ||
| Femoral PWV | 10.35±0.74 | 10.40±0.83 | 12.19±0.71 | 11.76±0.75 | 0.002 |
| Radial PWV | 8.74±0.57 | 9.46±0.70 | 10.28±0.46 | 10.53±0.47 | 0.001 |
| Weight (kg) | 88.5±3.4 | 81.4±2.9 | 87.4±3.6 | 82.1±3.2 | 0.038 |
| BMI (kg m−2) | 31.6±1.1 | 28.3±0.8 | 30.9±1.1 | 28.7±0.9 | 0.101 |
| WHR | 0.98±0.01 | 0.92±0.01 | 0.98±0.01 | 0.95±0.02 | 0.518 |
| SBP (mm Hg) | 138±3 | 138±4 | 134±4 | 138±4 | 0.022 |
| DBP (mm Hg) | 82±2 | 86±2 | 82±2 | 83±2 | 0.078 |
| HR (b.p.m.) | 68±3 | 75±2 | 71±4 | 75±2 | 0.773 |
| FBG (mEq) | 7.19±0.23 | 7.08±0.23 | 7.00±0.41 | 7.54±0.41 | 0.049 |
| HgA1C (%) | 5.9±0.3 | 5.9±0.2 | 6.1±0.3 | 6.4±0.2 | 0.589 |
| Total cholesterol (mmol l−1) | 4.8±0.2 | 5.0±0.3 | 4.8±0.2 | 4.7±0.3 | 0.761 |
| LDL cholesterol (mmol l−1) | 2.5±0.2 | 2.3±0.2 | 2.5±0.2 | 2.6±0.2 | 0.537 |
| HDL cholesterol (mmol l−1) | 1.5±0.1 | 1.4±0.2 | 1.5±0.1 | 1.4±0.2 | 0.614 |
| VO2max (ml kg−1 per minute) | 25.1±0.9 | 23.7±1.0 | 25.3±0.9 | 24.1±1.0 | 0.015* |
Abbreviations: b.p.m., beats per minute; BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; PWV, pulse-wave velocity; SBP, systolic blood pressure; WHR, waist-to-hip ratio; VO2max, maximal uptake of oxygen.
Changes in measures of fitness for aerobically trained (AT) and untrained (NA) are shown as mean±s.e. The effects of training were calculated by a two-way analysis of variance for repeated measures (time × group), with a P-value of <0.05 being significant. *P<0.05, 2-way analysis of variance with repeated measures.
Discussion
Aerobic training temporarily reversed multifactorial (geriatric age, T2DM, hypertension and hypercholesterolemia) arterial stiffness, as shown by significant decreases in both radial and femoral PWV. Although these improvements after 3 months of aerobic training were quite large in magnitude, they became attenuated over the course of the training intervention. In fact there was no statistical difference between the two groups at the 6-month mark, suggesting that the underlying mechanisms behind arterial stiffening in a high cardiometabolic risk population have some constituents that are both progressive and irreversible. The attenuation of the aerobic training effect on arterial stiffness occurred despite a maintenance of improved aerobic fitness (as measured by VO2max) throughout the course of the intervention.
The present study is novel in that the subjects were at high cardiometabolic risk (geriatric age, T2DM, hypertension and hypercholesterolemia), and that the intervention was longer in duration than most previous studies (6 months). Most previous studies have examined short-term interventions, middle-aged subjects and populations with a single risk factor. The benefits of aerobic exercise on arterial stiffness have been demonstrated previously through cross-sectional data on middle-aged normal subjects,2 prospective interventions in young athletes1 and prospective interventions in middle-aged healthy persons. Long-term aerobic training has been shown to improve arterial stiffness in normal older adults3 and short-term aerobic training has successfully reduced arterial stiffness in middle-aged subjects with T2DM.1, 25 The literature examining the effects of aerobic training in hypertensive older subjects has produced mixed results, mainly due to the duration of the training intervention. A cross-sectional study of hypertensive adults has previously demonstrated that there is no relationship between aerobic fitness and arterial stiffness.26 Small studies of older adults with hypertension as a single risk factor have shown that long-duration interventions similar in length to the present study (6 months) have no impact on arterial stiffness,4, 5 while short-term training (3 months or less) can improve arterial compliance27 congruent with the results of the present study. Overall, this suggests that in older adult population with multiple cardiovascular risk factors, there is a component of arterial stiffness that is unresponsive to aerobic training interventions.
Potential mechanisms
Age and disease-associated arterial stiffening are due to a combination of altered vascular wall structure and altered vascular function due to modified autonomic and local vasodilator/vasoconstrictor activity.28 The main structural contributors to increased arterial stiffness are increased collagen concentration (normal aging),29 non-enzymatic glycation resulting in the formation of collagen crosslinks30 (normal aging and diabetes) and vascular smooth muscle hypertrophy31 (hypertension). Of these structural changes, it has been theorized that non-enzymatic glycation would be the most responsive to aerobic exercise, through pulsatile stretching and breaking of collagen crosslinks.30 Breaking down of the diabetes-associated collagen crosslinks, as well as short-term aerobic training-induced improvements in sympathetic tone32 and increase in nitric-oxide induced vasodilatory activity33 could all potentially explain the short-term reductions in arterial stiffness seen in this study. The fact that we were unable to demonstrate any training-induced improvements in fasting blood glucose, blood pressure and lipid levels might be one explanation for the training-resistant nature of arterial stiffness in our high-risk subjects over the longer term.
Clinical implications
In persons with diabetes, previous research has strongly established the relationship between vascular stiffness and cardiovascular mortality.34 Our study population consisted of older subjects with T2DM complicated by co-morbid hypertension, and hyperlipidemia, putting them at very high risk for arterial stiffness and the consequent cardiovascular risks associated with this condition. Although aerobic exercise has numerous well-established benefits in older adults35 our findings suggest that in the longer term, arterial stiffness in a high-risk population is resistant to aerobic training. This suggests that with respect to vascular compliance, the timely prescription of aerobic training to prevent hypertension, diabetes and high cholesterol might impact vascular health more than starting aerobic training once patients are already at high cardiometabolic risk.
Limitations
Since our exercise intervention was only 6 months in duration, it is possible that an even longer intervention might have successfully reduced arterial stiffness. There were trends for a reduction in weight, SBP and fasting blood glucose that might have eventually resulted in a decline in arterial stiffness if the intervention had been continued for longer than 6 months.
Further research is needed to determine the pathophysiological mechanism for the short-term reduction in radial and femoral PWV with aerobic exercise in our population. Our study was unable to detect any significant training effect on weight, body mass index, waist circumference or fasting blood glucose but this is consistent with the other aerobic training literature in older adults with cardiovascular risk factors.36, 37 Since our exercise intervention was completed by a relatively small sample size, the benefits of aerobic training on arterial stiffness need to be confirmed by larger studies. It is also possible that the active control group (NA group) had some degree of aerobic training since it is impossible to completely separate strength from aerobic training, although there was no significant difference in the response of VO2max to training between the aerobic and non-aerobic groups. It is also possible that a mild strength training effect in the NA group might have skewed the results, but this is unlikely given that the intervention only involved an exercise ball and very light weights that were not progressed. Although all subjects had to be non-smokers for at least 5 years, we do not have data on lifetime smoking habits. There might have been some selection bias toward healthier subjects, since all of our subjects were recruited from newspaper advertisements.
Summary
The present study demonstrated that aerobic exercise has no long-term impact on arterial stiffness in older adults with multiple cardiometabolic risk factors.

Acknowledgments
This research was supported by Canadian Institutes of Health Research, as well as the Academic Enhancement Fund (Department of Medicine, University of British Columbia).
The authors declare no conflict of interest.
References
- Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Koyama H, et al. Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Res Clin Pract. 2004;65 (2:85–93. doi: 10.1016/j.diabres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging habitual exercise, and dynamic arterial compliance. Circulation. 2000;102 (11:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tabara Y, Yuasa T, Oshiumi A, Kobayashi T, Miyawaki Y, Miki T, et al. Effect of acute and long-term aerobic exercise on arterial stiffness in the elderly. Hypertens Res. 2007;30 (10:895–902. doi: 10.1291/hypres.30.895. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Petrella RJ. Acute and chronic impact of dynamic exercise on arterial stiffness in older hypertensives. Open Cardiovasc Med J. 2008;2:3–8. doi: 10.2174/1874192400802010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapiro EP, et al. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. 2005;165 (7:756–762. doi: 10.1001/archinte.165.7.756. [DOI] [PubMed] [Google Scholar]
- Hughes SL, Leith KH, Marquez DX, Moni G, Nguyen HQ, Desai P, et al. Physical activity and older adults: expert consensus for a new research agenda. Gerontologist. 2011;51 (6:822–832. doi: 10.1093/geront/gnr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes GV, Ciolac EG, Carvalho VO, D'Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33 (6:627–632. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Introduction: The American Diabetes Association's (ADA) evidence-based practice guidelines, standards, and related recommendations and documents for diabetes care. Diabetes Care. 2012;35 (Suppl 1:S1–S2. doi: 10.2337/dc12-s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43 (1:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Clinical Practice Recommendations 2005. Diabetes Care. 2005;28 (Suppl 1:S1–79. doi: 10.2337/diacare.28.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- Christmas C, Andersen RA. Exercise and older patients: guidelines for the clinician. J Am Geriatr Soc. 2000;48 (3:318–324. doi: 10.1111/j.1532-5415.2000.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Medicine ACoS ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription4th edn.Lippincott Williams and Wilkins: Philadelphia; 2001 [Google Scholar]
- McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Kingwell B, Bank A, Weber M, Struijker-Boudier H. Clinical applications of arterial stiffness: therapeutics and pharmacology. Am J Hypertens. 2002;15 (5:453–458. doi: 10.1016/s0895-7061(01)02329-9. [DOI] [PubMed] [Google Scholar]
- Adragao T, Pires A, Birne R, Curto JD, Lucas C, Goncalves M, et al. A plain X-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol Dial Transplant. 2009;24 (3:997–1002. doi: 10.1093/ndt/gfn584. [DOI] [PubMed] [Google Scholar]
- Asmar R, Topouchian J, Pannier B, Benetos A, Safar M. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens. 2001;19 (4:813–818. doi: 10.1097/00004872-200104000-00019. [DOI] [PubMed] [Google Scholar]
- Rajzer MW, Wojciechowska W, Klocek M, Palka I, Brzozowska-Kiszka M, Kawecka-Jaszcz K. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens. 2008;26 (10:2001–2007. doi: 10.1097/HJH.0b013e32830a4a25. [DOI] [PubMed] [Google Scholar]
- Pereira T, Maldonado J. Comparative study of two generations of the Complior device for aortic pulse wave velocity measurements. Blood Press Monit. 2010;15 (6:316–321. doi: 10.1097/MBP.0b013e32833f5685. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27 (21:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34 (5:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- Mikhail N, Cope D. The JNC-7 guidelines and the optimal target for systolic blood pressure. Hypertension. 2003;42 (6:1206–1252. doi: 10.1161/01.HYP.000012525.15835.f1. [DOI] [PubMed] [Google Scholar]
- Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2006;2007;176 (8:S1–S13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger AR, Sculthorpe N. A new VOmax protocol allowing self-pacing in maximal incremental exercise. Br J Sports Med. 2012;46 (1:59–63. doi: 10.1136/bjsports-2011-090006. [DOI] [PubMed] [Google Scholar]
- Dawson-Saunders BTR. Basic and Clinical Biostatistics. Prentice Hall of Canada: Toronto,; 1994. [Google Scholar]
- Yamamoto A, Katayama Y, Tomiyama K, Hosoai H, Hirata F, Yasuda H. A short-term admission improved brachial-ankle pulse wave velocity in type 2 diabetic patients. Diabetes Res Clin Pract. 2005;70 (3:248–252. doi: 10.1016/j.diabres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kraft KA, Arena R, Arrowood JA, Fei DY. High aerobic capacity does not attenuate aortic stiffness in hypertensive subjects. Am Heart J. 2007;154 (5:976–982. doi: 10.1016/j.ahj.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34 (2:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol. 2006;41 (5:501–507. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588 (Pt 20:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencetti S, Lagi A, Cipriani M, Fattorini L, Bandinelli G, Bernardi L. Autonomic control of the cerebral circulation during normal and impaired peripheral circulatory control. Heart. 1999;82 (3:365–372. doi: 10.1136/hrt.82.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor. Hypertension. 2005;46 (3:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, et al. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009;135 (3:346–352. doi: 10.1016/j.ijcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, et al. Systemic alpha-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol. 2007;293 (3:H1466–H1472. doi: 10.1152/ajpheart.00273.2007. [DOI] [PubMed] [Google Scholar]
- Benetos A. Pulse pressure and arterial stiffness in type 1 diabetic patients. J Hypertens. 2003;21 (11:2005–2007. doi: 10.1097/00004872-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am J Prev Med. 2003;25 (3 Suppl 2:129–136. doi: 10.1016/s0749-3797(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Collier SR, Kanaley JA, Carhart R, Frechette V, Tobin MM, Hall AK, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22 (10:678–686. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32 (8:1531–1535. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]