Abstract
Background
Thrombolysis remains the only approved therapy for acute ischaemic stroke (AIS); however, its utilisation is reported to be low.
Aims
This study aimed to determine the reasons for the low utilisation of thrombolysis in clinical practice.
Method
Five metropolitan hospitals comprising two tertiary referral centres and three district hospitals conducted a retrospective, cross-sectional study. Researchers identified patients discharged with a principal diagnosis of AIS over a 12-month time period (July 2009–July 2010), and reviewed the medical record of systematically chosen samples.
Results
The research team reviewed a total of 521 records (48.8% females, mean age 74.4 ± 14 years, age range 5-102 years) from the 1261 AIS patients. Sixty-nine per cent of AIS patients failed to meet eligibility criteria to receive thrombolysis because individuals arrived at the hospital later than 4.5 hours after the onset of symptoms. The factors found to be positively associated with late arrival included confusion at onset, absence of a witness at onset and waiting for improvement of symptoms. However, factors negatively associated with late arrival encompassed facial droop, slurred speech and immediately calling an ambulance. Only 14.7% of the patients arriving within 4.5 hours received thrombolysis. The main reasons for exclusion included such factors as rapidly improving symptoms (28.2%), minor symptoms (17.2%), patient receiving therapeutic anticoagulation (6.7%) and severe stroke (5.5%).
Conclusion
A late patient presentation represents the most significant barrier to utilising thrombolysis in the acute stroke setting. Thrombolysis continues to be currently underutilised in potentially eligible patients, and additional research is needed to identify more precise criteria for selecting patients for thrombolysis.
Keywords: Tissue Plasminogen Activator (tPA), Alteplase, Thrombolysis, Stroke, Cerebrovascular accident
What this study adds:
Thrombolysis constitutes the only approved therapy for acute ischaemic stroke. However, reports indicate its utilisation remains consistently low. This study identified the factors associated with the underutilisation of thrombolysis. Late patient presentation creates the most significant barrier to utilising thrombolysis in the acute stroke setting. Thrombolysis continues to be currently underutilised in potentially eligible patients, and additional research needs to identify more precise criteria for selecting patients for thrombolysis.
Background
In Australia, acute ischaemic stroke (AIS) treatment used thrombolysis with recombinant tissue plasminogen activator (rPA) starting about a decade ago.1 The national and international stroke management guidelines2-4 recommend thrombolysis in the treatment of AIS based on the superior level of evidence for its safety and effectiveness 5-6 The recently reported International Stroke Trial 3 (IST-3) affirms the benefit for tPA delivered within three hours from stroke onset, also supports the use of tPA in patients over age 80 and in patients with more severe stroke.7 However, the current utilisation of thrombolysis in everyday clinical practice remains low.8 The literature points out several factors associated with the low frequency of utilisation. The presentation of stroke patients at hospitals beyond the narrow time window for safe and effective administration (4.5 hours) represents the most common reason for non-use of thrombolysis.2,9 Even among patients arriving within the time window, rates of thrombolysis utilisation continue to be low suggesting other significant barriers to thrombolysis exist.8,10 Several interventions may overcome these barriers such as public awareness campaigns,11,12 pre-hospital triage by paramedics, hospital bypass protocols coupled with pre-notification systems13 and urgent neuroimaging protocols.14 These published interventions demonstrated success, however their impact on the national and global thrombolysis utilisation rates shows limited utilisation, revealed by the fact that only a small number of stroke treating centres use the published interventions.
This study primarily aims to further explore reasons for the low rates of utilisation of thrombolysis in clinical practice in the Australian setting. The specific objectives plan to identify and quantify the reasons for patients presenting within the time window and not receiving thrombolysis, and to document factors associated with patients’ presenting outside the time window.
Method
Study design
The study design encompassed a retrospective, crosssectional study of AIS patients presenting over a one-year period from July 2009 to July 2010 to any of the study hospitals. The study included five metropolitan hospitals in total, two tertiary referral centres (TRC) and three district hospitals (DH) with varying characteristics as outlined in Table 1. This sampling strategy aimed to capture a representative sample of ischaemic stroke patients presenting to public hospitals at different geographic locations across NSW. The International Classification of Diseases (tenth revision) codes for AIS (I63 x, I64)15 assisted in identification of records for detailed review. To cover the entire study period, the researchers used a systematic sampling technique (i.e., starting with the first record and then selecting every nth record from a patient list ordered by admission date). Due to varying admission numbers at each hospital every second record was reviewed at TRC-1 and DH-2, every third record was reviewed at TRC-2, every fourth record was reviewed at DH-3 while all the records were reviewed at DH-1.
Table 1. Hospital characteristics.
| Characteristics | Hospitals (N=5) | ||||
|---|---|---|---|---|---|
| TRC-1 | TRC-2 | DH-1 | DH-2 | DH-3 | |
| Size (beds) | 740 | 650 | 200 | 400 | 485 |
| Acute Stroke Unit | present | present | present | present | present |
| Demographics29 | Urban population, Australian-born and multicultural population | Urban and rural populations, mainly Australian-born population | Urban population, mainly Australian-born population | Urban population, multicultural population | Urban population, mainly Australianborn population |
| Thrombolysis protocol | Both I.V and I.A thrombolysis are utilised: - I.V >3 h from onset of stroke - I.A >6 h from stroke onset - mechanical clot retrieval is also utilised for selected patients | PAST (Prehospital Acute Stroke Triage) protocol,13 consisting of a pre-hospital stroke assessment tool, an ambulance protocol for hospital bypass for potentially thrombolysiseligible patients, and a prehospital notification system of an acute stroke team. | Transfer protocol: to transfer eligible patients presenting to the hospital within two hours of stroke onset to the nearby TRC-2 for administration of thrombolysis. Note: DH-1 is located in the Catchment area covered by the PAST protocol.13 | Recently commenced a protocol for thrombolysis administration for eligible patients on March 2010, i.e. during the study period. | FASTER protocol 30 (Face, Arm, Speech, Time, Emergency Response), consisting of a pre-hospital assessment tool followed by contacting an acute stroke team which allows hospital bypass, alerts triage and CT radiology, and rapidly assess the patient on arrival to the emergency department. |
TRC, tertiary referral centre; DH, district hospital; h, hour; IV, intravenous; IA, intra-arterial
Data collection
A thorough assessment of medical records, ambulance transfer sheets and electronic health information databases encompassed the review process. The extracted patient information went into a specifically designed data form that included demographics, medical history, medication history, stroke severity, blood pressure, initial laboratory and neuroimaging results, the time of stroke onset, time of arrival, time of neuro-imaging, time of thrombolysis administration, in-hospital complications, mortality and discharge information. The definition of the time of stroke onset comprised the time when the patient or a bystander first noticed any neurological symptoms. For patients found with symptoms, or where symptoms became first noticed upon awaking, the stroke onset time definition encompassed the time at which the patient was last known to be symptomfree.
Data analysis
The statistical analyses incorporated the use of the Statistical Package for the Social Sciences (SPSS) (version 19). The Student’s t-test and the Mann–Whitney U test compared parametric and non-parametric continuous variables, respectively, while the independent samples chisquare test compared categorical variables. A multivariate analysis, using backward stepwise logistic regression, assessed the impact of a number of factors on the likelihood of the patients presenting within the treatment window (≤4.5 hours) versus late (>4.5 hours). Combining variables with a univariate P <0.10 into a multivariate backward stepwise logistic regression model assessed their predictive ability while controlling for the effects of other predictors in the model. The following independent variables initially tested in the univariate analyses included: patient age, gender, co-morbidities, previous history of stroke or TIA, the absence of witnesses at the stroke onset, whether an ambulance was immediately called at the onset, weekend admission, mode of hospital arrival, stroke severity (using the National Institutes of Health Stroke Scale and the Glasgow Coma Scale),3 and stroke symptoms at onset. A P value of <0.05 comprised the alpha level of significance in all of the analyses.
Relevant human research ethics committees at each hospital approved the study protocol prior to commencement of the study.
Results
Patient characteristics
Researchers completed a review of a total of 521 patients’ medical records; these represented 41.3% of all the AIS admissions to the five hospitals during the 12-month study period. Table 2 outlines the patient demographics. The results showed a mean age of 74 ±14 years, and a similar proportion of males and females. The age group over 80 years characterised a substantial proportion (43.6%) of the patients who presented within the thrombolysis time window (≤4.5 h). Since the national guidelines considers the cut-off age at 80 years,2 the study currently considers this age as a relative contraindication to thrombolysis.
Table 2. The demographic characteristics of sampled acute ischemic stroke patients, n=521.
| Characteristic | All patients No. (%) | Patients arrival interval | |
|---|---|---|---|
| ≤4.5 h (%) | >4.5 h (%) | ||
| Age (years) mean ±SD range | 74.4 ±14 (5-102) | 75.8 ±13 (28-94) | 73.8 ±15 (5-102) |
| Age ≥65 (years) | 414 (79.5) | 82.8 | 77.9 |
| Age >80 (years) | 207 (39.7) | 43.6 | 38.0 |
| Female | 254 (48.8) | 50.3 | 48.0 |
| Pre-morbid living status † | |||
| Patient lives alone | 154 (29.9) | 25.2 | 31.6 |
| Patient lives in nursing home | 53 (10.3) | 10.4 | 10.1 |
| Patient lives with family | 301 (58.4) | 62.6 | 55.6 |
| Admitted via ambulance† | 404 (79.8) | 86.8 | 76.7 |
| Presenting hospital | |||
| TRC-1 | 133 (25.5) | 28.2 | 24.3 |
| TRC-2 | 117 (22.5) | 25.8 | 20.9 |
| DH-1 | 94 (18.0) | 13.5 | 20.1 |
| DH-2 | 85 (16.3) | 14.1 | 17.3 |
| DH-3 | 92 (17.7) | 18.4 | 17.3 |
| Medical history | |||
| Hypertension | 343 (65.8) | 62.6 | 67.3 |
| Hypercholesterolemia | 239 (45.9) | 47.2 | 45.3 |
| Previous stroke/TIA | 173 (33.2) | 34.4 | 32.7 |
| Atrial fibrillation | 159 (30.5) | 33.7 | 29.1 |
| Tobacco use (current/past) | 151 (29.0) | 28.2 | 29.3 |
| Ischemic heart disease | 132 (25.3) | 25.2 | 25.4 |
| Diabetes | 124 (23.8) | 19.0 | 26.0 |
| Cancer (current/past) | 84 (16.1) | 15.3 | 16.5 |
| Dementia | 51 (9.8) | 12.9 | 8.4 |
| High Alcohol use (current/past) | 36 (6.9) | 8.0 | 6.4 |
| PVD | 31 (6.0) | 6.1 | 5.9 |
TRC, tertiary referral centres; DH, district hospitals; SD, standard deviation; TIA, transient ischaemic attack;
PVD, peripheral vascular disease.
† Data available for 506 patients
Stroke Presentation
• Patients arriving in time (≤4.5 h)
Almost one-third of the AIS patients arrived within the required time frame (within the thrombolysis time window, ≤4.5 h), and the remaining two thirds arrived passed the recommended time frame (beyond the thrombolysis time window, >4.5 h) (Figure 1).
Figure 1. Acute ischaemic stroke patients who were admitted and excluded from thrombolysis.
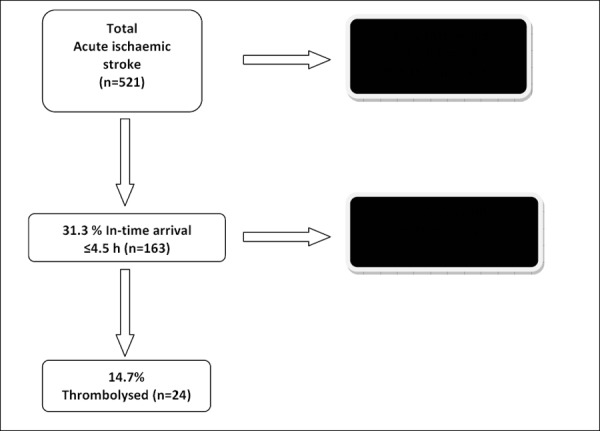
The majority of the patients admitted within the thrombolysis time window did not receive thrombolysis. The most commonly documented reasons for not using thrombolysis related to rapidly improving symptoms and minor symptoms (Table 3).
Table 3. The reasons for not using thrombolysis in the patients presenting in time, n=163.
| Reason | No. (%) |
|---|---|
| Rapidly improving symptoms | 46 (28.2) |
| Minor symptoms | 28 (17.2) |
| Receiving an anticoagulant with an INR >1.5 | 11 (6.7) |
| Severe stroke | 9 (5.5) |
| No salvageable penumbra | 6 (3.7) |
| Severe co-morbidity | 4 (2.5) |
| Major Surgery (within 14 days) | 2 (1.2) |
| Gastrointestinal haemorrhage (within 30 days) | 2 (1.2) |
| History of ICH | 1 (0.6) |
| Seizure | 1 (0.6) |
| Other | 5 (3.1) |
| No documented reason | 24 (14.7) |
INR, International normalised ratio; ICH, Intracerebral haemorrhage
The medical record of a considerable proportion of the patients (14.7%, similar to the proportion who actually received thrombolysis) contained no documented reason for exclusion. Five patients presented with other reasons for exclusion, including such factors as: clot not found upon neuro-imaging (two patients), enoxaparin therapy being used (low molecular weight heparin anticoagulant), relevant information missing from the medical history and inadequate thrombolysis work-up time. No patients were excluded for the following reasons: symptoms suggestive of subarachnoid haemorrhage, neuro-imaging showing haemorrhage, uncontrolled hypertension, recent myocardial infarction within the previous 30 days, head trauma or prior stroke within the previous three months or patient or family refusal of thrombolysis.
• Late arrival of patients (>4.5h)
Approximately half (51.4%) of the patients’ medical records contained a documented time of stroke onset. The median onset-to-arrival time for the patients arriving within 4.5 hours showed significantly shorter times than those arriving late (88 min vs. 585 min, respectively, P <0.001). The median time from ambulance contact to arrival did not differ significantly between the patients arriving in time and those arriving late (55 min vs. 58 min, respectively, P >0.05). However the onset to ambulance contact time demonstrated significantly longer times in the patients arriving late (19 min vs. 386 min, P <0.001), suggesting that this factor may validate one reason for the delay. In a key side note, the data revealed that 81 (23%) of the patients in this group arrived via private vehicle and did not use the ambulance.
Forty-four per cent (159/358) of the patients exhibited an unknown onset time; of these patients, 17% experienced stroke onset during their sleep and discovered the symptoms after awaking. One-fifth of the patients arrived late due to being discovered by another person finding the patient in a state that rendered them unable to seek help, and an additional 12% were found while unaware of their symptoms. Forty-two per cent of patients presenting late reported being alone at stroke-onset and 14% of these cases demonstrated altered consciousness at onset.
The AIS patients (n=521) took the following actions when discovering their first symptoms: 50% called an ambulance, 8.2% contacted their (general practitioner) GP, 6.2% went to the hospital, 5% contacted a relative, friend, neighbour or stranger, and 30.5% sought no help at all.
Sixty-one per cent of the patients presenting late sought no medical attention immediately after symptom onset. Of those, 41% (146) of patients waited for their symptoms to improve, and 21 patients tried to ‘sleep off the symptoms’. Sixty-two (17%) of the patients contacted their GP after symptom onset rather than an ambulance.
The medical record contained the stroke severity score on admission in 41.5% of AIS patients. However, this study failed to find a significant difference in the degree of stroke severity between the patients arriving in time versus those arriving late (P =0.45).
The majority (70%) of the strokes occurred at a private home or a nursing home. Compared to the patients whose stroke occurred at a private home, patients with stroke onset at a nursing home exhibited a significantly shorter median onset-to-arrival time (227 minutes vs. 112 minutes, respectively, P =0.017).
Factors associated with patients arriving late (>4.5 h)
The univariate analyses identified nine independent variables as potential predictors of late arrival (Table 4). These variables provided the data for the multivariate analysis. The final model contained five independent variables, ‘patient alone at onset’, ‘patient waiting for symptoms to improve’, ‘confusion (accompanying symptom onset)’, ‘presence of facial droop/slurred speech at the onset of stroke’ and ‘immediately calling an ambulance at the onset of stroke’(Table 5).
Table 4. Univariate analyses, showing the associations between variables and in-time/late arrival.
| Variables | In time arrival (.4.5 h) N=163 (%) | Late arrival (>4.5 h) N=358 (%) | OR (95% CI) | Univariate P |
|---|---|---|---|---|
| Age, median (years) | 78.6 | 77.0 | 1.01 (1.00-1.02) | 0.155 |
| Age ≥65 | 82.8 | 77.9 | 0.73 (0.45-1.18) | 0.200 |
| Age >80 | 43.6 | 38.0 | 0.79 (0.54-1.15) | 0.228 |
| Female Gender | 50.3 | 48 | 1.10 (0.76-1.58) | 0.632 |
| Australian born | 71.4 | 74.6 | 1.18 (0.75-1.85) | 0.487 |
| English speaking | 92.4 | 90.4 | 0.77 (0.39-1.54) | 0.457 |
| Hypertension | 62.6 | 67.3 | 1.23 (0.83-1.82) | 0.290 |
| Ischemic heart disease | 25.2 | 25.2 | 1.01 (0.66- 1.56) | 0.948 |
| Diabetes | 19.0 | 26.0 | 1.49 (0.94-2.38) | 0.084 |
| Atrial fibrillation | 33.7 | 29.1 | 0.81 (0.54-1.19) | 0.281 |
| Hypercholesterolemia | 47.2 | 45.3 | 0.93 (0.64-1.33) | 0.93 (0.64-1.33) |
| PVD | 6.1 | 5.9 | 0.95 (0.44-2.08) | 0.904 |
| Cancer | 15.3 | 16.5 | 1.09 (0.65-1.82) | 0.742 |
| Dementia | 12.9 | 8.4 | 0.62 (0.34-1.11) | 0.109 |
| Previous stroke/ TIA | 34.4 | 32.7 | 0.93 (0.63-1.37) | 0.707 |
| Tobacco use (current/past) | 28.2 | 29.3 | 1.05 (0.70-1.59) | 0.796 |
| High Alcohol use | 8.0 | 6.4 | 0.79 (0.39-1.61) | 0.518 |
| Pre-morbid mRS ≤1 | 89.8 | 89.9 | 1.01 (0.30-3.33) | 0.992 |
| Pre-morbid independence in ADLs | 80.3 | 86.3 | 1.56 (0.93-2.56) | 0.085 |
| Alone at onset | 34.8 | 58.8 | 2.63 (1.72-4.17) | 0.000* |
| Action taken when symptoms were discovered: immediate call for ambulance | 66.9 | 34.9 | 0.27 (0.18-0.39) | 0.000* |
| Action taken when symptoms were discovered: went to hospital | 7.4 | 4.7 | 0.63 (0.29-1.35) | 0.228 |
| Patient waited for symptoms to improve | 16.7 | 62.9 | 8.33 (5.00-14.29) | 0.000* |
| Pre-hospital consultation | 3.7 | 18.4 | 5.88 (2.5-14.29) | 0.000* |
| Weekend admission | 30.1 | 25.7 | 1.25 (0.79-1.96) | 0.341 |
| Mode of arrival by Ambulance | 86.8 | 76.7 | 0.5 (0.30-0.84) | 0.008* |
| Admission: NIHSS ≤22† | 95.2 | 100 | 0.78 (0.68-0.91) | 0.461 |
| Admission: GCS ≤9‡ | 5 | 3.25 | 0.62 (0.25-1.59) | 0.314 |
| Facial droop, drooling, slurred speech | 73.6 | 52.5 | 0.40 (0.26-0.60) | 0.000* |
| Limb weakness | 63.2 | 52.8 | 0.65 (0.44-0.95) | 0.027* |
| Confusion | 1.7 | 13.6 | 4.17 (2.04-8.33) | 0.000* |
| Unsteady gait | 8 | 23.5 | 3.57 (1.92-6.67) | 0.000* |
| Fall/ collapse at onset | 34.4 | 29.6 | 0.81 (0.54-1.19) | 0.278 |
PVD, peripheral vascular disease; TIA, transient ischaemic attack; mRS, modified Rankin scale; ADL, activities of daily living; NIHSS, National Institutes of Health Stroke Scale; GSC, Glasgow Coma Scale.
† data available for only 53 patients
‡ data available for 494 patients
* Statistical significance at P<0.05
Table 5. Multivariate analysis, showing the factors independently predicting late patient arrival (>4.5 h).
| Variable | B | Odds ratio | 95% Confidence interval |
|---|---|---|---|
| Patient alone at onset | 0.91 | 2.50 | 1.35-4.55 |
| Patient waited for symptoms to improve | 1.52 | 4.55 | 2.22-9.09 |
| Confusion at onset | 1.80 | 5.88 | 2.17-16.67 |
| Facial droop/ slurred speech at onset | -0.99 | 0.37 | 0.20-0.70 |
| Ambulance immediately called at onset | -1.34 | 0.26 | 0.13-0.51 |
| Constant | 2.62 | 13.74 |
Among the three factors positively associated with late arrival to hospital (>4.5 h), ‘confusion (accompanying symptom onset)’ and ‘patient waiting for symptoms to improve’ proved to be stronger predictors of late arrival than ‘patient being alone at onset’. Regarding the factors negatively associated with late arrival (>4.5 h), ‘immediately calling an ambulance at the onset of stroke’ established itself as a stronger predictor, than the ‘presence of facial droop/slurred speech at the onset of stroke’.
The model as a whole explained between 31.2% (Cox and Snell square) and 42.2% (Nagelkerke R squared) of the variance in patient arrival time and correctly classified 76.4% of the cases (X2=109.3, P >0.001).
Discussion
In an attempt to identify and prioritise the key areas needing potential interventions and to increase thrombolysis utilisation and consequently decrease stroke disability, this study identified and quantified reasons as to why patients arriving at the hospital in time do not receive thrombolysis. Furthermore, this study identified the factors associated with late arrival of patients. A strength of the study encompasses including real-life stroke cases and recording which actions the patients and/or bystanders actually took at the onset of symptoms or the time of symptom discovery. This strength contrasts with earlier studies which focused on assessing hypothetical actions that patients may take at stroke onset. The study found that in addition to the patient being alone or confused at onset, failure of the patient to take appropriate action represented a significant factor leading to late patient arrival.
For the patients arriving to hospital in time, minor and rapidly improving symptoms remain the primary reasons for not using thrombolysis. The guidelines2-4 recommend excluding patients with minor or rapidly improving symptoms because the risks outweigh the benefits of treatment. A study in Canada confirmed that the main reason for excluding patients who arrive within the time frame involved minor and rapidly improving symptoms.16 However, the Canadian study also found that 32% of these patients either died or remained dependent at hospital discharge, and it questioned the appropriateness of excluding these patients from thrombolysis. Currently, no clear definition of ‘rapidly improving symptoms’ in any of the recognised guidelines (American, Australian or European)2-4 exists, and many cases are excluded from thrombolysis because of an improvement in the motor symptoms after the initial onset and despite incomplete resolution on admission. Future research could identify those patients with improving symptoms who are likely to benefit from thrombolysis.16
A considerable proportion of the patients arriving in time represented the age group of 80 and above. According to the Australian guidelines,2 age greater than 80 years remains a relative contraindication for thrombolysis. American guidelines, places an absolute contraindication on patients presenting between 3-4.5 hours.9 The European guidelines,4 recommend thrombolysis in certain patients over the age of 80, although the labeling of tPA (AlteplaseR) fails to include this age group in the current European marketing of the product.17 This exclusion represents the previous limited evidence from randomised controlled trials (RCT) to show benefits from thrombolysis administration in this group of patients rather than related to a higher demonstrated risk of haemorrhagic complications.18 The recently reported IST-3 trial now provides evidence that thrombolysis is both safe and effective in patients over 80 and most likely the international guidelines will undergo modification to reflect this new evidence. 7
In our study, only a minority (14.7%) of the patients arriving in time actually received thrombolysis. This study describes the proportion to be lower than the proportions reported in other studies in which 19.4%19 and 26.7%16 of patients arriving in time received thrombolysis. Also, an additional 14.7% of the patients in our study who presented in time and with no documented contraindications failed to receive thrombolysis, which highlights the underutilisation of thrombolysis in potentially eligible patients. This deficit could be overcome by the development of decision-making tools to assist clinicians in the timely assessment of the risks and benefits of thrombolysis.
The largest barrier to thrombolysis continues to be late patient presentation. Exclusion comprised approximately 69% of the patients in the study for this reason; other studies reported exclusion rates of 73%16 and 85%.19 However, it must be noted that the recent extension of the thrombolysis time window from 3 to 4.5 hours occurred prior to these studies and the majority of studies reported in the literature. All of the studies conducted to date, including the current one found that shortening the time from stroke onset to hospital arrival produced the single greatest impact on thrombolysis utilisation rates.19
Patient reluctance to seek treatment still exists as the main factor leading to this delay.20 Our study found that ‘patient waiting for symptoms to improve’ stood out as an important predictor of late arrival and that ‘immediately contacting the ambulance’ predicted in-time arrival. Another discovery indicated that transportation to the hospital by an ambulance service caused only a small portion of the delay and no difference existed in the ambulance-contact-to-arrival times of the patients presenting in time and those presenting late. The data confirms the findings of other studies20 in which the ambulance service contributed minimally to the prehospital delay.
The presence of facial droop and/or slurred speech at stroke-onset significantly predicted arrival in time. This association may stem from these symptoms being somewhat alarming in contrast to other stroke symptoms, such as numbness or minor limb weakness. Similarly, another Australian study found that speech problems showed an association with earlier ambulance contact. 21 The study also found that stroke severity failed to be associated with either in-time or late arrival, which may be explained by some patients with severe, unattended strokes having long delays before reaching a hospital.21 No significant association exists between any of the stroke risk factors and the time of presentation. Previous studies reported similar findings,20 although not for previous stroke/TIA. The studies found these factors associated with decreased pre-hospital delay, presumably because the patients had previous experience and education on how to respond appropriately. These findings provide evidence that targeted patient education reduces pre-hospital delay.
Our study found that ‘the patient being alone at onset’ signifies a critical factor leading to delay. This finding appears in other studies.20 The majority of stroke attacks occurred while the patients resided at home, and approximately 30% of the patients reported living alone. Given that stroke symptoms can affect a patient’s ability to seek help, a significant number of patients can be expected to present late because of this factor. Moreover, the presence of confusion at onset of stroke symptoms indicates the most salient predictor of late arrival. This delay could be avoided by utilising currently available personal emergency communication services. These services provide patients with a water-proof pendant that can be attached to a necklace or a wristband and that can be easily activated by the patient at the onset of initial symptoms (even in the presence of mild confusion) to alert a dispatcher to alert an ambulance service promptly.
A significant proportion of stroke patients presented with an unknown onset time for reasons that included onset during sleep, being unaware of symptoms, and an unattended onset of a stroke that significantly impacted the patient’s level of consciousness. In these cases, the onset time reverts back to the last time at which the patient was known to be well, and this assumption consequently leads to exclusion from thrombolysis because of late patient presentation. In some of our study patients, an advanced neuroimaging technique detected the presence of a salvageable penumbra (i.e., viable brain tissue that is under an ischaemic attack),22 and some of the patients presenting in time became excluded from thrombolysis due to failure to detect a salvageable penumbra. This technique is currently being investigated in some centres to confirm the presence of a salvageable penumbra in thrombolysiseligible patients prior to administering thrombolytics and clinical trials study select patients presenting beyond 4.5 hours or with sleep-onset stroke using advanced imaging.23,24
Public awareness campaigns that educate patients and their families on the appropriate actions continue to be needed. In this study, we found that an ambulance was contacted immediately after first discovery of symptoms in only 50% of the cases and that ‘immediately contacting an ambulance’ predicted arrival in-time for treatment. Several public awareness campaigns appear worldwide, the Brain Attack campaign in the US and the F.A.S.T campaign in Australia, for example.11,12 These campaigns aim to increase community knowledge of stroke, stroke risk factors, stroke warning signs and symptoms, and the importance of promptly contacting an emergency service.25 Further research needs to identify the reasons for the limited effect of these campaigns. It has been suggested that the message being delivered needs to be modified to include such items as “do not call a GP”, “seek help even if you think symptoms are minor”, “do not wait for symptoms to improve” and “drug treatment is only available for patients who seek help early”.26 However, these campaigns may fail to reach their target population, which consists of patients with elevated stroke risk and their families. Community pharmacists play an important role because they are in a position to identify patients at risk for stroke through specifically developed screening programs,27 reinforce key messages regarding the early recognition of stroke symptoms and the appropriate actions to take at the onset of a stroke and encourage adherence to medication.28
The limitations of this study include its retrospective design and our reliance on ICD-10 codes to identify the AIS patients. In addition, the data remains limited to the information documented in the medical records and ambulance transfer forms. In some cases, the research team relied on the physician’s interpretation of whether the symptoms rated minor or rapidly improving rather than on a validated stroke-specific tool due to the score failing to be documented (a significant finding in and of itself). Finally, only five metropolitan hospitals in a single state comprise the study population, necessitating caution when interpreting the findings of this study. Despite these limitations, this study provided important insights into why patients were excluded from thrombolysis in clinical practice and identified areas for possible interventions that may increase thrombolysis utilisation and consequently decrease patient disability from stroke.
Conclusion
In conclusion, minor and rapidly improving symptoms remain the major reasons for exclusion in the patients presenting within the thrombolysis time window, and a considerable proportion of the patients being older than 80 years failed to meet inclusion criteria. Further research should consider identifying patients with improving symptoms who might benefit from thrombolysis. Among the patients who arrive late, however, a delay in seeking treatment exists as the major factor leading to late arrival, which emphasized the need for a more tailored and targeted public education campaign.
ACKNOWLEDGEMENTS
We thank the casemix, statistics, and medical records departments at each hospital for providing support for this study.
Footnotes
ETHICS COMMITTEE APPROVAL
Northern Sydney Local Health District
Western Sydney Local Health District
Hunter New England Health District
PPlease cite this paper as: Eissa A, Krass I, Levi C, Sturm J, Ibrahim R, Bajorek B. Understanding the reasons behind the low utilisation of thrombolysis in stroke. AMJ 2013, 6, 3, 152-167. http//dx.doi.org/10.4066/AMJ.2013.1607
References
- 1.Australian Public Assessment Report for Alteplase 2011. Therapeutic Goods Administration [website]. http://www.tga.gov.au/(accessed February 2012). [Google Scholar]
- 2.Melbourne: National Stroke Foundation.; Clinical Guidelines for Stroke Management 2010. [Google Scholar]
- 3.Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines for Management of Ischaemic Stroke update 2009. The European Stroke Organization (ESO) [website] http://www.eso-stroke.org(accessed Dec 2012). [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995 Dec 14;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.IST-3 collaborative group; Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, Ricci S, Murray V, Berge E, Slot KB, Hankey GJ, Correia M, Peeters A, Matz K, Lyrer P, Gubitz G, Phillips SJ, Arauz A. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012 Jun 23;379(9834):2352–63. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Stroke Audit - Acute Services Clinical Audit Report 2011. Melbourne: National Stroke Foundation; [Google Scholar]
- 9.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009 Aug;40(8)(8):2945. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudd AG, Hoffman A, Grant R, Campbell JT, Lowe D. Intercollegiate Working Party for Stroke. Stroke thrombolysis in England, Wales and Northern Ireland: how much do we do and how much do we need? J Neurol Neurosurg Psychiatry. 2011 Jan;82(1):14–9. doi: 10.1136/jnnp.2009.203174. [DOI] [PubMed] [Google Scholar]
- 11.F.A.S.T campaign. National Stroke Foundation, Australia [website] http://www.strokefoundation.com.au (accessed Mar 2012). [Google Scholar]
- 12.Wang MY, Lavine SD, Soukiasian H, Tabrizi R, Levy ML, Giannotta SL. Treating stroke as a medical emergency: a survey of resident physicians' attitudes toward “brain attack" and carotid endarterectomy. Neurosurgery. 2001 May;48(5):1109–15. doi: 10.1097/00006123-200105000-00028. discussion 1115-7. [DOI] [PubMed] [Google Scholar]
- 13.Quain DA, Parsons MW, Loudfoot AR, Spratt NJ, Evans MK, Russell ML, Royan AT, Moore AG, Miteff F, Hullick CJ, Attia J, McElduff P, Levi CR. Improving access to acute stroke therapies: a controlled trial of organised pre-hospital and emergency care. Med J Aust. 2008 Oct;20189(8):429–33. doi: 10.5694/j.1326-5377.2008.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindsberg PJ, Häppölä O, Kallela M, Valanne L, Kuisma M, Kaste M. Door to thrombolysis: ER reorganization and reduced delays to acute stroke treatment. Neurology. 2006 Jul 25;67(2):334–6. doi: 10.1212/01.wnl.0000224759.44743.7d. [DOI] [PubMed] [Google Scholar]
- 15.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005 Aug;36(8):1776–81. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 16.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001 Apr 24;56(8):1015–20. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 17.Actilyse. European Agency for the Evaluation of Medicinal products. UK Medicines and Healthcare products Regulatory Agency (MHRA) and the European Medicines Agency (EMEA). [website]. http://emc.medicines.org.uk (accessed Dec 2012) [Google Scholar]
- 18.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients of > or = 80 versus < 80 years of age--a systematic review across cohort studies. Age Ageing. 2006 Nov;35(6):572–80. doi: 10.1093/ageing/afl104. [DOI] [PubMed] [Google Scholar]
- 19.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Cleveland Clinic Health System Stroke Quality Improvement Team. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004 Mar;61(3):346–50. doi: 10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 20.Moser DK, Kimble LP, Alberts MJ, Alonzo A, Croft JB, Dracup K, Evenson KR, Go AS, Hand MM, Kothari RU, Mensah GA, Morris DL, Pancioli AM, Riegel B, Zerwic JJ. Reducing delay in seeking treatment by patients with acute coronary syndrome and stroke: a scientific statement from the American Heart Association Council on cardiovascular nursing and stroke council. Circulation. 2006 Jul 11;114(2):168–82. doi: 10.1161/CIRCULATIONAHA.106.176040. [DOI] [PubMed] [Google Scholar]
- 21.Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Department of Infrastructure T, Regional Development and Local Government. Road deaths Australia: 2008 Statistical summary. Stroke. Canberra: Department of Infrastructure, Transport, Regional Development. 2007 2009 Feb;38(2):361–6. doi: 10.1161/01.STR.0000254528.17405.cc. [DOI] [PubMed] [Google Scholar]
- 22.Heiss WD. The concept of the penumbra: can it be translated to stroke management? Int J Stroke. 2010 Aug;5(4):290–5. doi: 10.1111/j.1747-4949.2010.00444.x. [DOI] [PubMed] [Google Scholar]
- 23.Davis SM, Donnan GA. MR mismatch and thrombolysis: appealing but validation required. Stroke. 2009 Aug;40(8):2910. doi: 10.1161/STROKEAHA.109.552893. [DOI] [PubMed] [Google Scholar]
- 24.Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND), Clinical trials [website]. http://clinicaltrials.gov/ct2/show/NCT00887328(accessed Mar 2012) [Google Scholar]
- 25.Kwan J, Hand P, Sandercock P. Improving the efficiency of delivery of thrombolysis for acute stroke: a systematic review. QJM. 2004 May;97(5):273–9. doi: 10.1093/qjmed/hch054. [DOI] [PubMed] [Google Scholar]
- 26.Kleindorfer D, Khoury J, Broderick JP, Rademacher E, Woo D, Flaherty ML, Alwell K, Moomaw CJ, Schneider A, Pancioli A, Miller R, Kissela BM. Temporal trends in public awareness of stroke: warning signs, risk factors, and treatment. Stroke. 2009 Jul;40(7):2502–6. doi: 10.1161/STROKEAHA.109.551861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangum SA, Kraenow KR, Narducci WA. Identifying at-risk patients through community pharmacybased hypertension and stroke prevention screening projects. J Am Pharm Assoc. 2003;43:50–55. [PubMed] [Google Scholar]
- 28.Fagan SC, Zarowitz BJ, Robert S. "Brain attack": an indication for thrombolysis? Ann Pharmacother. 1992 Jan;26(1):73–80. doi: 10.1177/106002809202600114. [DOI] [PubMed] [Google Scholar]
- 29.National Regional Profile. Australian Bureau of Statistics [website]. http://www.abs.gov.au. [Google Scholar]
- 30.O'Brien W, Crimmins D, Donaldson W, Risti R, Clarke TA, Whyte S, Sturm J. FASTER (Face, Arm, Speech, Time, Emergency Response): experience of Central Coast Stroke Services implementation of a pre-hospital notification system for expedient management of acute stroke. J Clin Neurosci. 2012 Feb;19(2):241–5. doi: 10.1016/j.jocn.2011.06.009. [DOI] [PubMed] [Google Scholar]


