Abstract
BACKGROUND
The role of folate deficiency and associated hyperhomocysteinemia in the pathogenesis of metabolic syndrome is not fully established. In the current study, we analyzed the role of folate deficiency in pathogenesis of the metabolic syndrome in the spontaneously hypertensive rat (SHR).
METHODS
Metabolic and hemodynamic traits were assessed in SHR/Ola rats fed either folate-deficient or control diet for 4 weeks starting at the age of 3 months.
RESULTS
Compared to SHRs fed a folate-replete diet, SHRs fed a folate-deficient diet showed significantly reduced serum folate (104±5 vs. 11±1 nmol/L, P < 0.0005) and urinary folate excretion (4.3±0.6 vs. 1.2±0.1 nmol/16h, P < 0.0005) together with a near 3-fold increase in plasma total homocysteine concentration (4.5±0.1 vs 13.1±0.7 μmol/L, P < 0.0005), ectopic fat accumulation in liver, and impaired glucose tolerance. Folate deficiency also increased systolic blood pressure by approximately 15mm Hg (P < 0.01). In addition, the low-folate diet was accompanied by significantly reduced activity of antioxidant enzymes and increased concentrations of lipoperoxidation products in liver, renal cortex, and heart.
CONCLUSIONS
These findings demonstrate that the SHR model is susceptible to the adverse metabolic and hemodynamic effects of low dietary intake of folate. The results are consistent with the hypothesis that folate deficiency can promote oxidative stress and multiple features of the metabolic syndrome that are associated with increased risk for diabetes and cardiovascular disease.
Keywords: blood pressure, ectopic fat accumulation, folate deficiency, homocysteine, hypertension, oxidative stress, spontaneously hypertensive rat.
Metabolic syndrome is a cluster of clinical and metabolic disorders that can increase the risk for coronary artery disease and diabetes. Mild hyperhomocysteinemia, a common finding in patients with arteriosclerosis, has been described as another possible component of the metabolic syndrome.1,2 Because folate and B vitamins modulate metabolism of homocysteine and of other sulfur amino acids, mild hyperhomocysteinemia may be a secondary consequence of deficiencies of these vitamins.3 In the current study, we analyzed the possible role of folates and sulfur amino acids in the pathogenesis of metabolic syndrome in the spontaneously hypertensive rat (SHR). The SHR is the most widely studied animal model of essential hypertension and under special environmental conditions, e.g., when fed a high-fructose diet, can be prone to disturbances in lipid and glucose metabolism that are characteristic of the metabolic syndrome.4 Although hyperhomocysteinemia has been reported to affect vascular function in the SHR, the effects of folate deficient diets on blood pressure and features of the metabolic syndrome in this model have not been previously studied.5–7 Here we investigated whether the SHR model is susceptible to the adverse metabolic and hemodynamic effects of a low-folate diet.
MATERIALS AND METHODS
Animals
The SHR/OlaIpcv rats (hereafter referred to as SHRs) were housed in an air-conditioned animal facility and allowed free access to food and water. Baseline biochemical, metabolic, and hemodynamic phenotypes were assessed in nonfasted male rats that were fed either a folate-deficient diet (TD.01505 with 1% succinylsulfathiazole, containing <0.1mg folate/kg diet) (n = 10) or a control diet (TD.08138 with 2mg folate/kg diet, Harlan Teklad, Madison, WI) (n = 10). The rats were fed both the experimental and control diets for 4 weeks starting at the age of 3 months. All experiments were performed in agreement with the Animal Protection Law of the Czech Republic and were approved by the Ethics Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic, Prague.
Biochemical parameters
Folate levels in serum, erythrocytes, and urine were determined by the Folate III Assay Kit (Roche GmbH, Basel, Switzerland) (the coefficient of variation for the assays for folate is <5%).8 Concentrations of total homocysteine and cysteine in plasma were determined by reversed-phase high-performance liquid chromatography (HPLC) with fluorescent detection after derivatization with ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate. The reduction of disulfides and protein-bound homocysteine and cysteine was performed with tris(2-carboxyethyl)phosphine as described previously (the coefficients of variation for the assays for homocysteine and cysteine are <3%).9 Blood glucose levels were measured by the glucose oxidase assay (Pliva-Lachema, Brno, Czech Republic) using tail vein blood drawn into 5% trichloracetic acid and promptly centrifuged. Nonesterified fatty acid levels were determined using an acyl-CoA oxidase-based colorimetric kit (Roche Diagnostics GmbH, Mannheim, Germany). Serum triglyceride concentrations were measured by standard enzymatic methods (Pliva-Lachema). Serum insulin concentrations were determined using a rat insulin enzyme-linked immunosorbent assay kit (Mercodia, Uppsala, Sweden) (the coefficient of variation for the assays for insulin is <3%).
Tissue triglyceride measurements
For determination of triglycerides in liver and soleus muscle, tissues were powdered under liquid N2 and extracted for 16h in chloroform:methanol, after which 2% KH2PO4 was added and the solution was centrifuged. The organic phase was removed and evaporated under N2. The resulting pellet was dissolved in isopropyl alcohol, and triglyceride content was determined by enzymatic assay (Pliva-Lachema).
Oral glucose tolerance testing
Oral glucose tolerance tests were performed using a glucose load of 300mg/100g body weight after overnight fasting. Blood was drawn from the tail without anesthesia before the glucose load (0min time point) and at 30, 60, and 120min thereafter.
Blood pressure measurement
Arterial blood pressures were measured continuously by radiotelemetry in paired experiments between conscious, unrestrained male rats. All rats were allowed to recover for at least 7 days after surgical implantation of radiotelemetry transducers before the start of blood pressure recordings. Pulsatile pressures were recorded in 5-sec bursts every 10min throughout the day and night, and 24-h averages for systolic arterial blood pressure were calculated for each rat. The results from each rat in the same group were then averaged to obtain the group means.
Parameters of oxidative stress
The activity of Cu,Zn-superoxide dismutase (SOD) was analyzed using the reaction of blocking nitrotetrazolium blue reduction and nitroformazan formation. Catalase (CAT) activity measurement was based on the ability of H2O2 to produce with ammonium molybdate a color complex detected spectrophotometrically. The activity of seleno-dependent glutathione peroxidase (GSH-Px) was monitored by oxidation of gluthathione by Ellman reagent (0.01М solution of 5,5’-dithiobis-2 nitrobenzoic acid). The reduced (GSH) and oxidized form of glutathione was determined by HPLC with fluorescent detection (Chromsystems, Germany). Glutathione reductase activity was measured by the decrease of absorbance at 340nm using a molar extinction coefficient of 6220M-1cm-1 for NADPH (using Sigma assay kit). The levels of conjugated dienes were analyzed by extraction in the media (heptane:isopropanol = 2:1) and measured spectrophotometrically in the heptane layer. The levels of thiobarbituric acid reactive substances were determined by the reaction with thiobarbituric acid.10
Statistical analysis
The data are expressed as mean ± SEM. Individual groups were compared by unpaired Student t test. The 24-h mean values of systolic blood pressure were analyzed by repeated-measures analysis of variance with grouping effect of strain and repeated measurements in time. Statistical significance was defined as P < 0.05.
RESULTS
Folate deficiency and hyperhomocysteinemia
As shown in Table 1, SHRs fed a low-folate diet compared to SHRs fed the control diet exhibited significantly lower serum and erythrocyte folate concentrations and urinary excretion of folate. Reduced folate levels were associated with approximately 3-fold greater levels of total plasma homocysteine whereas cysteine concentrations were lower in SHRs with folate deficiency.
Table 1.
Effects of folate deficiency on folate and aminothiol levels in the spontaneously hypertensive rat
| Low-folate diet | Control diet | |
|---|---|---|
| Serum folate (nmol/L) | 11±1** | 104±5 |
| Erythrocyte folate (nmol/L) | 5,123±234** | 10,071±580 |
| Folate excretion in urine (nmol/16h) | 1.2±0.1** | 4.27±0.6 |
| Plasma total cysteine (µmol/L) | 222±4* | 242±5 |
| Plasma total homocysteine (µmol/L) | 13.1±0.7** | 4.5±0.1 |
*P < 0.05, **P < 0.0005.
Metabolic parameters
The results for metabolic measurements are shown in Table 2. There were no obvious group differences in nonfasting insulin, glucose, and triglyceride levels (Table 2). However, reduced folate levels and increased serum concentrations of homocysteine were associated with ectopic fat accumulation in liver as judged by increased hepatic triglyceride levels and increased liver weight (Table 2). Folate deficiency was also associated with a modest increase in the relative weight of epididymal adipose tissue, which contained a reduced percentage of protein, suggesting the presence of larger and metabolically less active adipocytes (Table 2). In addition, SHRs fed the low-folate diet exhibited increased glucose and insulin levels after oral glucose loading, consistent with impaired glucose tolerance and apparent insulin resistance (Figure 1). Areas under the curve for glucose and for insulin were significantly lower in folate-replete versus folate-deficient SHRs (707±10 vs. 753±9 mmol/L/2h, P = 0.005 and 7.1±0.3 vs. 10.5±0.7 nmol/L/2h, P = 0.0003, respectively).
Table 2.
Effects of folate deficiency on parameters of lipid and glucose metabolism in the spontaneously hypertensive rat
| Low-folate diet | Control diet | |
|---|---|---|
| Body weight (g) | 342±6 | 342±7 |
| Relative fat pad weight (g/100g body weight) | 1.2±0.03* | 1.0±0.03 |
| Relative liver weight(g/100g body weight) | 3.35±0.03* | 3.18±0.04 |
| Serum glucose (mmol/L) | 7.4±0.2 | 7.0±0.1 |
| Serum insulin (nmol/L) | 0.578±0.044 | 0.487±0.039 |
| Serum triglycerides (mmol/L) | 0.70±0.06 | 0.70±0.06 |
| Serum nonesterified fatty acid (mmol/L) | 1.07±0.05 | 1.09±0.06 |
| Liver triglycerides (µmol/g) | 25.6±1.5* | 19.5±0.8 |
| Muscle triglycerides (µmol/g) | 2.1±0.2 | 1.9±0.2 |
| Protein content in epididymal fat (%) | 1.21±0.06 | 1.43±0.04* |
*P < 0.05.
Figure 1.
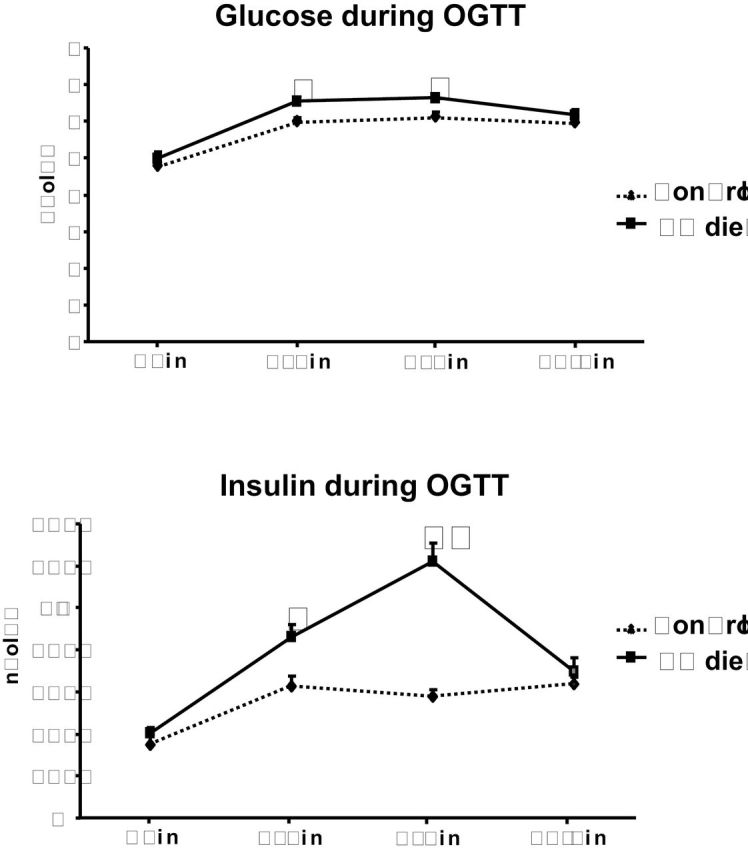
Effects of folate intake on oral glucose tolerance test in spontaneously hypertensive rats (SHR) fed a low-folate (LF) diet (solid lines) and SHR controls (dotted lines). Glucose concentrations after glucose loading were modestly increased in SHRs fed a LF diet compared with control rats at 30 and 60min after glucose loading. Insulin concentrations after glucose loading were prominently increased in SHRs fed a LF diet compared with SHRs fed a control diet. *P < 0.05, **P < 0.005. Abbreviation: OGTT, oral glucose tolerance test.
Blood pressure
Figure 2 shows systolic blood pressures determined by telemetry. Blood pressure was significantly greater in rats fed the low-folate diet than in those fed the folate-replete diet.
Figure 2.
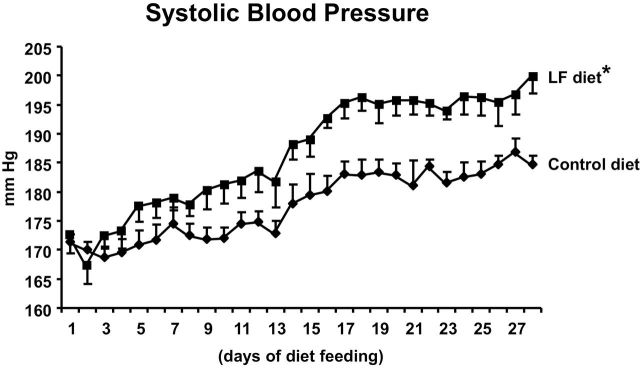
Systolic blood pressures. The daily 24-h average systolic blood pressures measured by radiotelemetry in conscious, unrestrained spontaneously hypertensive rats (SHRs) fed a low-folate (LF) diet were significantly greater than in SHRs fed a control diet (*P < 0.01)
Parameters of oxidative stress
As shown in Table 3, folate deficiency was associated with alterations in antioxidant enzyme activities in liver, kidney, and heart. The antioxidant enzyme activities were generally reduced except for catalase activity, which was increased in liver, reduced in heart, and unchanged in kidney cortex. In addition, rats fed a folate-deficient diet showed decreased levels of reduced glutathione in all 3 tissues. The liver, heart, and kidney all showed evidence of oxidative tissue damage as reflected by increased concentrations of lipoperoxidation products including conjugated dienes and/or thiobarbituric acid reactive substances.
Table 3.
Effects of low-folate vs. control diet on parameters of oxidative stress in the spontaneously hypertensive rat
| SOD(U/mg) | GSH-Px(µM GSH/min/mg) | GSSG(µM NADPH/ min/mg) | CAT(µM H2O2/min/mg) | GSH(mM/g) | CD(nM/mg) | TBARS (nM/mg) | |
|---|---|---|---|---|---|---|---|
| Liver | |||||||
| Control | 0.118±0.009 | 574±30 | 577±26 | 729±13 | 24.3±1.8 | 36.5±1.6 | 0.881±0.048 |
| Low folate | 0.074±0.004** | 427±5** | 402±13** | 861±5* | 16.5±1.8* | 45±2* | 1.747±0.095** |
| Kidney cortex | |||||||
| Control | 0.066±0.006 | 279±28 | 117±24 | 534±46 | 18.7±0.9 | 30±1.4 | 1.214±0.089 |
| Low folate | 0.044±0.005* | 160±38* | 135±25 | 525±39 | 12.6±1.1** | 32.3±2.7 | 1.787±0.205* |
| Heart | |||||||
| Control | 0.062±0.006 | 477±20 | 197±14 | 405±30 | 24.2±2.1 | 28.2±2.4 | 0.991±0.033 |
| Low folate | 0.049±0.005 | 381±32* | 132±17* | 243±32* | 15.5±1.5* | 24.9±1.4 | 2.281±0.155** |
Abbreviations: CAT, catalase; CD, conjugated dienes; GSH, reduced glutathione; GSSH, oxidized form of glutathione; GSH-Px, glutathione peroxidase; SOD, Cu,Zn-superoxide dismutase; TBARS, thiobarbituric acid reactive substances..
*P < 0.05, **P < 0.005.
DISCUSSION
Previous studies in SHRs have provided important evidence that mild hyperhomocysteinemia is associated with target organ damage and hypertension and that folate supplementation can prevent these adverse effects.5–7 In contrast, the relationships between folate deficiency/hyperhomocysteinemia and insulin resistance are controversial both in humans and animal models11-16 and have not been studied in the SHR. In the current studies, we have found that the SHR model is clearly susceptible to the adverse metabolic and hemodynamic effects of a low-folate diet. In this model, we observed that reduced dietary intake of folate causes low serum folate levels and hyperhomocysteinemia together with impaired glucose tolerance, increased ectopic fat accumulation in liver, oxidative tissue damage in liver, heart, and kidneys, and increased blood pressure. The mechanisms connecting folate deficiency and hyperhomocysteinemia with disturbances of lipid and glucose metabolism are not fully understood and may include alterations in AMPK kinase function,17 methylation status,18 and oxidative stress.19 For example, reduced availability of folate for homocysteine remethylation might result in deficiency of S-adenosylmethionine and accumulation of S-adenosylhomocysteine, which together could lead to reduced production of phosphatidylcholine, an essential factor for very low-density lipoprotein assembly and transport of triglycerides out of the liver. The attendant disturbances in hepatic lipid transport could in turn be contributing to ectopic accumulation of fat in liver, a known determinant of insulin resistance.20 The current observations of increased hepatic triglycerides in SHRs fed a low-folate diet are in good agreement with previous reports of hepatic steatosis in rodents elicited by dietary folate deficiency.21
Oxidative stress has been frequently implicated in the pathogenesis of both hypertension and related metabolic disorders.22 In the current study, folate deficiency caused pronounced hyperhomocysteinemia and oxidative damage in liver, kidney, and heart. We also observed that the folate-deficient diet induced decreased activity levels of the antioxidant enzymes CAT, SOD, and GSH-Px, which could be contributing further to the pathogenesis of oxidative stress associated with the low-folate diet. Whereas SOD and GSH-Px activities were decreased in several tissues, CAT activity appeared to be decreased only in the heart. It is possible that folate deficiency may cause different effects on enzyme activity in various tissues/organs owing to inherent differences in organ pathways regulating folate metabolism as well as antioxidant enzyme synthesis, function, or turnover. Mechanisms connecting folate deficiency/hyperhomocysteinemia with oxidative stress are not fully understood. For instance, folate deficiency might induce oxidative stress by increasing homocysteine levels that are associated with oxidation and auto-oxidation of homocysteine and with reduction of antioxidant enzyme activities, such as superoxide dismutase and glutathione peroxidase.23,24 In addition, folate may exert antioxidant effects independent of homocysteine lowering by inhibiting NADPH oxidase-mediated superoxide anion production.25
Folate deficiency accompanied by hyperhomocysteinemia is also thought to be linked to hypertension, at least in part through disturbances in endothelial and vascular function associated with impaired bioavailability of tetrahydrobiopterin, an essential cofactor of eNOS26. Vascular dysfunction has been previously reported in Sprague-Dawley rats fed a high-methionine, low-folate diet, although no effects of folate deficiency on blood pressure were noted in the Sprague-Dawley model.27 However, different strains of rats may vary in suceptibility to hemodynamic effects of dietary-induced alterations in folate and homocysteine levels. For example, dietary administration of homocysteine has been reported to exacerbate hypertension in the SHR and other rats of Wistar origin.28,29 Supplementation with folic acid or with tetrahydrobiopterin has also been reported to reduce blood pressure in SHR models30,31. These observations, together with the current findings, suggest the possibility that the SHR model may be particularly susceptible to the blood pressure effects of folate deficiency.
The relevance of animal models including the SHR for the pathogenesis of metabolic syndrome in humans remains to be established. However, several lines of evidence suggest that folate deficiency can play a role in at least some features of metabolic syndrome in humans similar to those seen in the SHR model. We have shown previously that patients with essential juvenile hypertension and other signs of metabolic syndrome exhibit decreased plasma folate concentrations.32 There is a vast body of additional epidemiological evidence showing that decreased folate levels and/or low folate intake can be associated with increased risk of cardiovascular disease and hypertension in humans.33 More importantly, folic acid or 5-methyltetrahydrofolate administration not only improves endothelial dysfunction and decreases blood pressure, but also ameliorates insulin resistance.34,35 Recent reports suggest that MTHFR c.677C>T homozygous individuals with genetically determined mild decreases in folate levels exhibit features of metabolic syndrome, namely, low birth weight and higher insulin levels.36 Folate supplementation in pregnant women has also been reported to have transgenerational effects of reducing insulin resistance in offspring.37
In summary, our results demonstrate that the SHR model is clearly susceptible to the effects of folate deficiency on multiple features of the metabolic syndrome. Despite mandatory folic acid fortification of the diet beginning in 1998, there remains significant interindividual variation in plasma and red cell folate levels in the population even when taking into account the use of folate supplements.38–40 Thus, it is possible that genetic factors may contribute to variation in individual responsiveness to folate administration. Given that SHR strains are known to harbor variants in multiple genes that can affect glucose and lipid metabolism as well as promote increased blood pressure, it is possible that the SHR model may be unusually predisposed to the effects of a folate-deficient diet on risk for insulin resistance, dyslipidemia, and hypertension. Thus, the current findings should motivate future linkage and correlation studies in segregating populations derived from the SHR to identify genetic factors that might influence susceptibility to the adverse metabolic and hemodynamic effects of low dietary intake of folate. Identification of genes in the SHR model that influence biologic responses to variation in folate intake could help to reveal genetic pathways that influence the metabolic and cardiovascular effects of altered dietary folate intake in humans. Identification of such genetic pathways could ultimately be useful for guiding the design of clinical intervention trials for testing the effects of folate supplementation on risk of hypertension, metabolic syndrome, and cardiovascular disease in specific population subgroups.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We acknowledge the support by grants NS10036-4/2008 and MZO00023001 (Ministry of Health of the Czech Republic) and institutional support provided by the Research Projects RVO:67985823 and by Charles University–First Faculty of Medicine programs PRVOUK P24 and UNCE 204011. This work was also supported by grants ME10019 and 7E10067 from the Ministry of Education of the Czech Republic, P303/10/0505 from the Grant Agency of the Czech Republic, and by the European Commission within the Seventh Framework Programme through the Integrated Project EURATRANS (contract no. HEALTH-F4-2010–241504). We also acknowledge support from National Institutes of Health grants HL35018, HL56028, and HL63709 to T.W.K. We also thank Ms. A. Dutá, Ms. A. Musilová, and Ms. K. Pelinková, MSc, for technical help.
REFERENCES
- 1. Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, D’Agostino RB, Sr, Wilson PW. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care 2001; 24: 1403–1410 [DOI] [PubMed] [Google Scholar]
- 2. Oron-Herman M, Rosenthal T, Sela BA. Hyperhomocysteinemia as a component of syndrome X. Metabolism 2003; 52: 1491–1495 [DOI] [PubMed] [Google Scholar]
- 3. Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res 2007; 4: 143–150 [DOI] [PubMed] [Google Scholar]
- 4. Pravenec M, Kurtz TW. Recent advances in genetics of the spontaneously hypertensive rat. Curr Hypertens Rep 2010; 12: 5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yen CH, Lau YT. Vascular responses in male and female hypertensive rats with hyperhomocysteinemia. Hypertension 2002; 40: 322–328 [DOI] [PubMed] [Google Scholar]
- 6. Miller A, Mujumdar V, Palmer L, Bower JD, Tyagi SC. Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats. Am J Hypertens 2002; 15(2 Pt 1):157–163 [DOI] [PubMed] [Google Scholar]
- 7. Miller A, Mujumdar V, Shek E, Guillot J, Angelo M, Palmer L, Tyagi SC. Hyperhomocyst(e)inemia induces multiorgan damage. Heart Vessels 2000; 15: 135–143 [DOI] [PubMed] [Google Scholar]
- 8. Veselá K, Pavlíková M, Janošíková B, Anděl M, Zvárová J, Hyanek J, Kožich V. Genetic determinants of folate status in central Bohemia. Physiol Res 2005; 54: 295–303 [PubMed] [Google Scholar]
- 9. Krijt J, Vacková M, Kožich V. Measurement of homocysteine and other aminothiols in plasma: advantages of using tris (2-carboxyethyl)phosphine as reductant compared with tri-n-butylphosphine. Clin Chem 2001; 47: 1821–1828 [PubMed] [Google Scholar]
- 10. Malínská H, Oliyarnyk O, Hubová M, Zídek V, Landa V, Šimáková M, Mlejnek P, Kazdová L, Kurtz TW, Pravenec M. Increased liver oxidative stress and altered PUFA metabolism precede development of non-alcoholic steatohepatitis in SREBP-1a transgenic spontaneously hypertensive rats with genetic predisposition to hepatic steatosis. Mol Cell Biochem 2010; 335: 119–125 [DOI] [PubMed] [Google Scholar]
- 11. Rosolova H, Simon J, Mayer O, Jr, Racek J, Dierzé T, Jacobsen DW. Unexpected inverse relationship between insulin resistance and serum homocysteine in healthy subjects. Physiol Res 2002; 51: 93–98 [PubMed] [Google Scholar]
- 12. Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, D’Agostino RB, Sr, Wilson PW. Framingham Offspring Study. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham Offspring Study. Diabetes Care 2001; 24: 1403–1410 [DOI] [PubMed] [Google Scholar]
- 13. Abbasi F, Facchini F, Humphreys MH, Reaven GM. Plasma homocysteine concentrations in healthy volunteers are not related to differences in insulin-mediated glucose disposal. Atherosclerosis 1999; 146: 175–178 [DOI] [PubMed] [Google Scholar]
- 14. Fonseca V, Dicker-Brown A, Ranganathan S, Song W, Barnard RJ, Fink L, Kern PA. Effects of a high-fat-sucrose diet on enzymes in homocysteine metabolism in the rat. Metabolism 2000; 49: 736–741 [DOI] [PubMed] [Google Scholar]
- 15. Wijekoon EP, Hall B, Ratnam S, Brosnan ME, Zeisel SH, Brosnan JT. Homocysteine metabolism in ZDF (type 2) diabetic rats. Diabetes 2005; 54: 3245–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noll C, Lacraz G, Ehses J, Coulaud J, Bailbe D, Paul JL, Portha B, Homo-Delarche F, Janel N. Early reduction of circulating homocysteine levels in Goto-Kakizaki rat, a spontaneous nonobese model of type 2 diabetes. Biochim Biophys Acta 2011; 1812: 699–702 [DOI] [PubMed] [Google Scholar]
- 17. Buettner R, Bettermann I, Hechtl C, Gäbele E, Hellerbrand C, Schölmerich J, Bollheimer L. Dietary folic acid activates AMPK and improves insulin resistance and hepatic inflammation in dietary rodent models of the metabolic syndrome. Horm Metab Res 2010; 42: 769–774 [DOI] [PubMed] [Google Scholar]
- 18. Obeid R, Hermann W. Homocysteine and lipids: A-adenosyl methionine as a key intermediate. FEBS Letters 2009; 583: 1215–1225 [DOI] [PubMed] [Google Scholar]
- 19. Matté C, Stefanello FM, Mackedanz V, Pederzolli CD, Lamers ML, Dutra-Filho CS, Dos Santos MF, Wyse AT. Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci 2009; 27: 337–344 [DOI] [PubMed] [Google Scholar]
- 20. Sironi AM, Sicari R, Folli F, Gastaldelli A. Ectopic fat storage, insulin resistance, and hypertension. Curr Pharm Des 2011; 17: 3074–3080 [DOI] [PubMed] [Google Scholar]
- 21. Christensen KE, Wu Q, Wang X, Deng L, Caudill MA, Rozen R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J Nutr 2010; 140: 1736–1741 [DOI] [PubMed] [Google Scholar]
- 22. Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med 2011; 12: 21–29 [DOI] [PubMed] [Google Scholar]
- 23. Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem 1998; 273: 14085–14089 [DOI] [PubMed] [Google Scholar]
- 24. Handy DE, Zhang Y, Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem 2005; 280: 15518–15525 [DOI] [PubMed] [Google Scholar]
- 25. Hwang SY, Siow YL, Au-Yeung KK, House J, Karmin O. Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney. Am J Physiol Renal Physiol 2011; 300: F189–198 [DOI] [PubMed] [Google Scholar]
- 26. Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 2006; 114: 1193–1201 [DOI] [PubMed] [Google Scholar]
- 27. Hansrani M, Stansby G. The use of an in vivo model to study the effects of hyperhomocysteinaemia on vascular function. J Surg Res 2008; 145: 13–18 [DOI] [PubMed] [Google Scholar]
- 28. Miller A, Mujumdar V, Palmer L, Bower JD, Tyagi SC. Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats. Am J Hypertens 2002; 15: 157–163 [DOI] [PubMed] [Google Scholar]
- 29. Resstel LB, de Andrade CR, Haddad R, Eberlin MN, de Oliveira AM, Correa FM. Hyperhomocysteinaemia-induced cardiovascular changes in rats. Clin Exp Pharmacol Physiol 2008; 35: 949–956 [DOI] [PubMed] [Google Scholar]
- 30. Hong HJ, Hsiao G, Cheng TH, Yen MH. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 2001; 38: 1044–1048 [DOI] [PubMed] [Google Scholar]
- 31. Perez SC, Vianna LM. Favorable effects of pyridoxine and folic acid supplementation of shr-sp. Arch Neurocien (Mex) 2005; 10: 146–149 [Google Scholar]
- 32. Kahleová R, Palyzová D, Zvára K, Zvárová J, Hrach K, Nováková I, Hyánek J, Bendlová B, Kozich V. Essential hypertension in adolescents: association with insulin resistance and with metabolism of homocysteine and vitamins. Am J Hypertens 2002; 15: 857–864 [DOI] [PubMed] [Google Scholar]
- 33. Kalin SR, Rimm EB. Folate and vascular disease. In Bailey LB. (ed), Folate in Health and Disease. 2ndedn. CRC Press: Boca Raton, FL, 2010, pp. 263–290 [Google Scholar]
- 34. Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes (Lond) 2006; 30: 1197–1202 [DOI] [PubMed] [Google Scholar]
- 35. Cagnacci A, Cannoletta M, Volpe A. High-dose short-term folate administration modifies ambulatory blood pressure in postmenopausal women. A placebo-controlled study. Eur J Clin Nutr 2009; 63: 1266–1268 [DOI] [PubMed] [Google Scholar]
- 36. Frelut ML, Nicolas JP, Guilland JC, de Courcy GP. Methyle netetrahydrofolate reductase 677 C->T polymorphism: a link between birth weight and insulin resistance in obese adolescents. Int J Pediatr Obes 2011; 6: e312–317 [DOI] [PubMed] [Google Scholar]
- 37. Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr 2009; 139: 1575–1581 [DOI] [PubMed] [Google Scholar]
- 38. Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 1999; 340: 1449–1454 [DOI] [PubMed] [Google Scholar]
- 39. Choumenkovitch SF, Jacques PF, Nadeau MR, Wilson PW, Rosenberg IH, Selhub J. Folic acid fortification increases red blood cell folate concentrations in the Framingham study. J Nutr 2001; 131: 3277–3280 [DOI] [PubMed] [Google Scholar]
- 40. Yeung L, Yang Q, Berry RJ. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA 2008; 300: 2486–2487 [DOI] [PubMed] [Google Scholar]


