Abstract
BACKGROUND
Although dual blockade of the renin–angiotensin–aldosterone system (RAAS) has gained popularity for the treatment of kidney disease, its benefits and potential risks have not been fully elucidated. We conducted a meta-analysis of all randomized controlled trials comparing the efficacy and safety of combined vs. single RAAS blockade therapy in chronic kidney disease (CKD).
METHODS
We performed a literature search using MEDLINE, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, scientific abstracts from meetings, and bibliographies of retrieved articles. We used random-effects models to compute net changes and rate differences in variables.
RESULTS
Fifty-nine (25 crossover and 34 parallel-arm) randomized controlled trials (RCTs) comparing the efficacy and safety of combined vs. single RAAS blockade therapy in CKD were identified (4,975 patients). Combined RAAS blockade therapy was associated with a significant net decrease in glomerular filtration rate (GFR) (–1.8ml/min or ml/min/1.73 m2; P = 0.005), albuminuria (–90mg/g of creatinine; P = 0.001 or –32mg/day; P = 0.03), and proteinuria (–291mg/g; P = 0.003 or –363mg/day; P < 0.001). Combined RAAS blockade therapy was associated with a 9.4% higher rate of regression to normoalbuminuria and a 5% higher rate of achieving the blood pressure (BP) goal (as defined in individual trials). However, combined RAAS blockade therapy was associated with a significant net increase in serum potassium level, a 3.4% higher rate of hyperkalemia, and a 4.6% higher rate of hypotension. There was no effect on doubling of the serum creatinine level, hospitalization, or mortality.
CONCLUSIONS
Although combined RAAS blockade therapy in CKD is associated with a decrease in albuminuria and proteinuria, it is associated with a decrease in GFR and a higher incidence of hyperkalemia and hypotension relative to monotherapy. The potential long-term kidney benefits of combined RAAS blockade therapy require further study.
Keywords: Combined, RAAS blockade, chronic kidney disease, proteinuria, GFR, potassium, hypotension, randomized controlled trial, hypertension, blood pressure.
The prevalence of chronic kidney disease (CKD) is rising throughout the world, partly as the result of an aging population and an increasing prevalence of hypertension, obesity, diabetes, and cardiovascular disease.1,2 Chronic kidney disease is associated with increased morbidity and mortality,3 including significant consumption of resources and healthcare expenditures.4 Hypertension and proteinuria are well-known predictors of the progression of CKD.5 For the same decrease in systemic blood pressure (BP), agents that block the renin–angiotensin–aldosterone system (RAAS) exert a stronger antiproteinuric effect than other antihypertensive drugs such as calcium-channel blockers.6–8 Because of this, current clinical-practice guidelines recommend using blockers of the RAAS as preferred agents for treating kidney disease.9,10 Although prior meta-analyses have demonstrated a beneficial effect of dual RAAS blockade therapy with an angiotensin-converting enzyme inhibitor (ACEI) and an angiotensin-II type-2 receptor blocker (ARB) in reducing proteinuria in patients with kidney disease, no discernible effect of this drug combination was noted on kidney function.11–13 Other combination therapies, including that of an ACEI, ARB, or both with an aldosterone receptor antagonist (ARA) and, most recently, an ACEI or ARB with a direct renin inhibitor (DRI), have also been shown to further reduce urinary protein excretion in kidney disease beyond that achieved with single RAAS blockade,14,15 leading to a more widespread clinical use of combination therapies in treating CKD.
The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET),16 the largest trial of dual vs. single RAAS blockade therapy in patients with coronary, peripheral, or cerebrovascular disease or diabetes with end-organ damage, has called into question the trend in clinical practice toward using combination therapies for RAAS blockade. Indeed, in that trial, the use of an ACEI and ARB was associated with a higher rate of syncope and kidney dysfunction than monotherapy, without benefit on the composite endpoint of fatal and nonfatal cardiovascular outcomes.16 In a subsequent ONTARGET analysis of kidney-related endpoints, doubling of serum creatinine or dialysis was more frequent in the combination-therapy group.17 Several cautionary notes on dual RAAS blockade therapy have since appeared in the literature.18–20 The Canadian Heart and Stroke Foundation clinical guidelines now recommend that combined RAAS blockade therapy be discontinued for the treatment of hypertension.21 In light of scarce data on the potentially deleterious effect of combined RAAS blockade therapy on kidney-related endpoints in patients with CKD, we conducted a meta-analysis of all randomized controlled trials (RCTs) comparing the efficacy and safety of combined vs. single RAAS blockade therapy in patients with CKD.
METHODS
Data sources and searches.
We performed a MEDLINE literature search beginning in August 2011 to identify eligible studies using the Medical Subject Headings (MeSH) database search terms “diabetic nephropathy,” “hypertensive nephropathy,” “glomerular disease,” “proteinuric kidney disease,” “renal insufficiency,” “kidney disease,” “chronic renal failure,” “chronic kidney disease,” “dual therapy,” “dual blockade,” “renin–angiotensin system,” “angiotensin-converting enzyme inhibitor,” “angiotensin-receptor blocker,” “aldosterone blockade,” “selective aldosterone blockade,” “renin inhibitor,” or “direct renin inhibitor.” The search was limited to human studies. We also searched the Cochrane Central Register of Controlled Trials and ClinicalTrials.gov for completed studies using similar search terms, and reviewed the American Society of Nephrology scientific abstracts (2003–2011 meetings), as well as the bibliographies of retrieved articles.
Study selection.
We included randomized, controlled crossover and parallel-arm trials examining the effect of combined vs. single RAAS blockade therapy on kidney-related endpoints, BP parameters, and other outcomes of interest in patients with proteinuria or low GFR (< 60ml/min or ml/min/1.73 m2). There were no restrictions on language, sample size, or study duration. Two authors (PS and KS) independently screened the titles and abstracts of all electronic citations, and full-text articles were retrieved for comprehensive review and independently re-screened.
Data extraction and quality assessment.
The following data were extracted for the RCTs examined in the study: country of origin, year of publication, study design, sample size, duration of intervention, percentage of men, mean age of subjects, serum creatinine, GFR, urine albumin or protein excretion, systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP), and in studies of patients with diabetes, the duration of diabetes and mean concentration of hemoglobin A1C (HbA1C). For each RCT included in the meta-analysis, we also tabulated the exclusion criteria pertaining to the level of kidney function and serum potassium.
For assessment of kidney function, we extracted data on methods of measuring GFR that included measured, estimated, or calculated GFR. We extracted data on the urine albumin and protein specimen collection methods used in each study, including the use of random or timed (24-hour) samples.
When indicated, we used the G3data graph analyzer ( version 1.5.3; GNU General Public License, www.frantz.fi/software/g3data.php) to extract data from graphs. Disagreements were resolved through consensus and arbitration by a third author (BLJ). Study quality was assessed with a modified version of the Jadad scale, which assesses randomization adequacy, blinding, and attrition, with higher scores reflecting better quality.22,23
Data synthesis and analysis.
We used random-effects model meta-analyses to assess absolute and standardized net changes in continuous outcomes. The standardized net change was computed to overcome the use of different units of measurement, and allowed us to include trials that reported only net changes among study groups. The standardized effect size is derived by dividing the mean change in the continuous outcome level of a particular variable by the standard deviation of the mean change in the variable. The variance of the standardized effect size is estimated through the inverse of the sample size. Binary outcomes were examined through random-effects model meta-analyses that assessed rate differences, as well as through Peto fixed-effect model meta-analyses that assessed odds ratios (ORs). The latter approach was used because of the small number of observed events. All pooled estimates are displayed with a 95% confidence interval (CI).
Existence of heterogeneity among effect sizes estimated by individual studies was described with the I2 index and the chi-square test. An I2 index ≥ 50% was used to indicate medium-to-high heterogeneity.24 We investigated sources of heterogeneity for the outcomes of interest by performing random-effects model meta-regression analyses based on a priori selected study characteristics, including trial design (crossover vs. parallel-arm), population setting (diabetic, nondiabetic, or mixed populations), status of hypertension control at enrollment (poorly vs. well-controlled), urine albumin or protein excretion rate (microalbuminuria (30–300mg/day or mg/g of creatinine, macroalbuminuria (> 300mg/day or mg/g of creatinine) vs. overt proteinuria (> 500mg/day or mg/g of creatinine)), baseline GFR (≥ 60ml/min or ml/min/1.73 m2 vs. < 60ml/min or ml/min/1.73 m2), duration of follow up (1–6 months, 7–12 months, or >12 months), type of combination therapy (ACEI and ARB, ACEI or ARB and ARA, ACEI or ARB and DRI vs. ACEI and ARB and ARA), GFR, and albuminuria/proteinuria specimen collection method (random vs. timed), and study quality. Student’s t-test was used to compare subgroups. Publication bias was formally assessed using funnel plots and the Egger test, a test that assesses asymmetry of the funnel plot, whereby a value of P < 0.05 indicates publication bias.25 The meta-analyses were performed with Comprehensive Meta-Analysis version 2.0 (www.meta-analysis.com; Biostat, Englewood, NJ), and OpenMeta (http://tuftscaes.org/open_meta/ download. html). The subgroup analysis figures were generated with the R system software version 2.13.0 (cran.rproject.org/bin/windows/base/old/2.13.0).
RESULTS
Characteristics and quality of the studies.
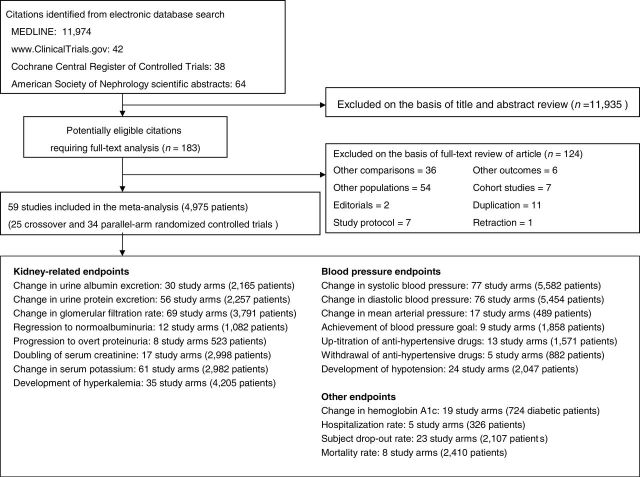
A total of 12,118 potentially relevant citations were identified and screened; 183 articles were retrieved for detailed evaluation, of which 59, consisting of 25 crossover and 34 parallel-arm randomized controlled trials, fulfilled the eligibility criteria for inclusion in the meta-analysis (Fig. 1).14,15,26–82 Twenty-seven trials had two single-therapy groups that included an ACEI or ARB,29,32,34,35,38,40–43,48–51,54,56,58,59,62,66,67,69–71,73,75,80,82 each of which were each compared to the combination-therapy group. Two trials tested different doses of RAAS blockade combination therapies14,65, which was compared with the single-therapy group. In addition, one trial tested different doses of single therapies,33 each of which was compared with the combination-therapy group, and one trial tested double and triple combination therapies,72 each of which was compared with the single-therapy group. In terms of combined RAAS blockade therapy, 74 study arms used an ACEI and ARB, 10 study arms used an ACEI or ARB and an ARA, 5 study arms used an ACEI or ARB and a DRI, and 2 study arms used a combination of an ACEI, ARB, and ARA (Fig. 2).
Figure 1.
Flow diagram for selection of studies of combined vs. single-agent blockade of the renin–angiotensin–aldosterone system (RAAS) included in the meta-analysis.
Figure 2.
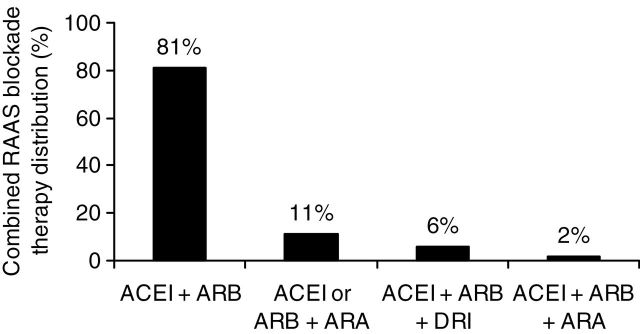
Distribution of combined renin–angiotensin–aldosterone system (RAAS) blockade therapies. Abbreviations: ACEI, Angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor type-2 blocker; ARA, aldosterone receptor antagonist; DRI, direct renin inhibitor.
Characteristics of the individual trials are displayed in Table 1. The trials spanned more than 10 years, varied in sample size (10–599 patients), and involved three types of populations, consisting of diabetics, nondiabetics, or a mixture of the two populations. The mean age of the subjects of the trials ranged from 25 to 66 years, and the duration of follow up ranged from 1–49 months. Thirty-one (52.5%) studies enrolled patients with preserved kidney function (GFR ≥ 60ml/min or ml/min/1.73 m2) and 7 studies enrolled patients with a low GFR (< 60ml/min or ml/min/1.73 m2). Twenty-one studies did not report the subjects’ baseline kidney function. At enrollment, the subjects’ hypertension was well controlled in 13 studies and poorly controlled in 46 studies. The GFR was assessed in a total of 44 studies, in which it was measured in 12 studies, estimated in 14 studies, and calculated in 18 studies. Urine albumin or protein excretion was measured on random samples in 17 studies and on timed samples in 40 studies. At enrollment, the patients in 10 studies had microalbuminuria, those in 9 studies had macroalbuminuria, and those in 38 studies had overt proteinuria. Thirty-four studies were of fair quality (score 1–3) and 25 were of good quality (score 4–5).
Table 1.
Characteristics of randomized controlled trials included in this meta-analysis of trials of single-agent vs. combined therapy for blockade of the renin–angiotensin–aldosterone system
| Renin–angiotensin–aldosterone system blockade | Exclusion criteria | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Study design | Combined therapy | Single therapy | Number of patients | Duration of follow up (months) | Mean age (years) | Men (%) | Mean serum creatinine (mg/dl) | Mean glomerular filtration rate (ml/min or ml/min /1.73 m2) | Mean albuminuria or proteinuria (g/g or g/day) | Mean systolic blood pressure (mm Hg) | Mean diastolic blood pressure (mm Hg) | Population settings | Diabetes duration (years) | Mean hemoglobin A1C (%) | Kidney function | Serum potassium (mEq/l) | Jadad score |
| Ruilope65 | 2000 | Multinational | Parallel-arm | Benazepril + valsartan | Valsartan | 64 | 1.25 | 57 | 71 | NR | NR | 1.8 | NR | NR | Nondiabetic | - | - | Cr clearance < 20ml/min | NR | 3 |
| Parallel-arm | Benazepril + valsartan (varying dosing regimens) | Valsartan | 66 | 1.25 | 58 | 68 | NR | NR | 1.7 | NR | NR | Nondiabetic | - | - | Cr clearance < 20ml/min | NR | 3 | |||
| Agarwal44 | 2001 | USA | Crossover | Lisinopril + losartan | Lisinopril | 16 | 1 | 53 | 88 | 2.0 | NR | 3.6 | 156 | 88 | Mixed | NR | NR | eGFR < 30ml/min | > 5.5 | 3 |
| Russo38 | 2001 | Italy | Crossover | Enalapril + losartan | Enalapril | 19 | 2 | 25 | 40 | NR | 110 | 1.5 | 119 | 76 | Nondiabetic | - | - | eGFR < 90ml/min/1.73 m2 | NR | 3 |
| Crossover | Enalapril + losartan | Losartan | 19 | 2 | 25 | 40 | NR | 110 | 1.5 | 119 | 76 | Nondiabetic | - | - | eGFR < 90ml/min/1.73 m2 | NR | 3 | |||
| Tutuncu51 | 2001 | Turkey | Parallel-arm | Enalapril + losartan | Enalapril | 22 | 12 | 54 | NR | NR | NR | 0.1a | 117 | 75 | Diabetic | 8.1 | 7.6 | Renal impairment | NR | 3 |
| Parallel-arm | Enalapril + losartan | Losartan | 22 | 12 | 58 | NR | NR | NR | 0.1 a | 117 | 78 | Diabetic | 7.6 | 7.6 | Renal impairment | NR | 3 | |||
| Kincaid-Smith45 | 2002 | Australia | Crossover | Any ACEI + candesartan | Any ACEI | 65 | 3 | NR | NR | 2.0 | NR | 2.3 | 139 | 82 | Mixed | NR | NR | sCr > 3.96mg/dl | NR | 3 |
| Berger39 | 2002 | Germany | Crossover | ACEI + candesartan | ACEI + placebo | 12 | 2 | 52 | 50 | 1.1 | NR | 2 | NR | NR | Nondiabetic | - | - | NR | NR | 5 |
| Ferrari40 | 2002 | Switzerland | Crossover | Fosinopril + irbesartan | Fosinopril | 11 | 1.5 | 48 | 64 | 1.5 | 77 | 7.9 | 143 | 91 | Nondiabetic | - | - | eGFR < 30ml/min | NR | 3 |
| Crossover | Fosinopril + irbesartan | Irbesartan | 11 | 1.5 | 48 | 64 | 1.5 | 77 | 7.9 | 143 | 91 | Nondiabetic | - | - | eGFR < 30ml/min | NR | 3 | |||
| Jacobsen26 | 2002 | Denmark | Crossover | Any ACEI + irbesartan | Any ACEI | 21 | 2 | 45 | 81 | NR | NR | 1.9* | 156 | 87 | Diabetic | 29 | NR | eGFR < 20ml/min | > 4.8 | 5 |
| Rossing27 | 2002 | Denmark | Crossover | Any ACEI + candesartan | Any ACEI | 18 | 2 | 58 | 77 | NR | NR | 1.8* | 159 | 85 | Diabetic | 13 | NR | eGFR < 25ml/min | > 4.6 | 5 |
| Kuriyama60 | 2002 | Japan | Parallel-arm | Temocapril + candesartan | Candesartan+ amdopidine | 17 | 3 | 53 | 53 | 2.3 | 29 | 4.2 | 153 | 91 | Diabetic | NR | 7.7 | sCr > 4.0mg/dl | NR | 2 |
| Tylicki66 | 2002 | Poland | Parallel-arm | Enalapril + losartan | Enalapril | 32 | 3 | 41 | 72 | 1.2 | 95 | 2.9 | 137 | 88 | Nondiabetic | - | - | sCr > 2.0mg/dl | NR | 3 |
| Parallel-arm | Enalapril + losartan | Losartan | 32 | 3 | 39 | 56 | 1.1 | 93 | 2.7 | 139 | 89 | Nondiabetic | - | - | sCr > 2.0mg/dl | NR | 3 | |||
| Luno67 | 2002 | Spain | Parallel-arm | Lisinopril + candesartan | Lisinopril | 30 | 6 | 46 | 70 | 1.2 | 90 | 3.7 | 135 | 82 | Non-diabetic | - | - | eGFR < 50ml/min/1.73 m2 | > 5.0 | 3 |
| Parallel-arm | Lisinopril + candesartan | Candesartan | 31 | 6 | 44 | 61 | 1.1 | 100 | 3.9 | 134 | 82 | Nondiabetic | - | - | eGFR < 50ml/min/1.73 m2 | > 5.0 | 3 | |||
| Jacobsen28 | 2003 | Denmark | Crossover | Enalapril + irbesartan | Enalapril | 24 | 2 | 42 | 71 | NR | NR | NR | NR | NR | Diabetic | 31 | NR | eGFR < 30ml/min | > 4.8 | 5 |
| Jacobsen29 | 2003 | Denmark | Crossover | Benazepril + valsartan | Benazepril | 20 | 2 | 43 | 72 | NR | NR | 0.4 a | 141 | 81 | Diabetic | 30 | NR | eGFR < 30ml/min | > 4.8 | 5 |
| Crossover | Benazepril + valsartan | Valsartan | 20 | 2 | 43 | 72 | NR | NR | 0.4 a | 141 | 81 | Diabetic | 30 | NR | eGFR < 30ml/min | > 4.8 | 5 | |||
| Rossing30 | 2003 | Denmark | Crossover | Any ACEI + candesartan | Any ACEI | 20 | 2 | 62 | 85 | NR | NR | NR | NR | NR | Diabetic | 15 | NR | eGFR < 25ml/min | > 4.6 | 5 |
| Kim46 | 2003 | Korea | Crossover | Ramipril + candesartan | Ramipril | 43 | 3 | 34 | 46 | NR | 60 | 3.9 | NR | NR | Mixed | NR | NR | eGFR < 25ml/min/1.73 m2 | NR | 5 |
| Song47 | 2003 | Korea | Crossover | Ramipril + candesartan | Ramipril +placebo | 34 | 4 | 34 | 41 | NR | NR | 4.1 | NR | NR | Mixed | NR | NR | eGFR < 25ml/min/1.73 m2 | NR | 5 |
| Campbell41 | 2003 | Italy | Crossover | Benazepril + valsartan | Benazepril | 24 | 2 | 49 | 96 | 1.7 | 69 | 3.3 | 140 | 91 | Nondiabetic | - | - | eGFR < 20ml/min/1.73 m2 | K > 6 | 3 |
| Crossover | Benazepril + valsartan | Valsartan | 24 | 2 | 49 | 96 | 1.7 | 69 | 3.3 | 140 | 91 | Nondiabetic | - | - | eGFR < 20ml/min/1.73 m2 | K > 6 | 3 | |||
| Shoji68 | 2003 | Japan | Parallel-arm | Enalapril + losartan | Enalapril | 16 | 12 | NR | NR | NR | 79 | 2.0 | NR | NR | Nondiabetic | - | - | NR | NR | 1 |
| Segura69 | 2003 | Spain | Parallel-arm | Benazepril + valsartan | Benazepril | 24 | 6 | 49 | 79 | NR | 70 | 4.0 | 152 | 91 | Nondiabetic | - | - | eGFR < 30ml/min | > 5.0 | 3 |
| Parallel-arm | Benazepril + valsartan | Valsartan | 24 | 6 | 49 | 79 | NR | 71 | 4.4 | 151 | 88 | Nondiabetic | - | - | eGFR < 30ml/min | > 5.0 | 3 | |||
| Rutkowski42 | 2004 | Poland | Crossover | Benazepril + losartan | Benazepril | 30 | 4 | 36 | 50 | 1.2 | 86 | 2.1 | 140 | 91 | Nondiabetic | - | - | sCr > 2.0mg/dl | NR | 3 |
| Crossover | Benazepril + losartan | Losartan | 30 | 4 | 36 | 50 | 1.2 | 86 | 2.1 | 140 | 91 | Nondiabetic | - | - | sCr > 2.0mg/dl | NR | 3 | |||
| Cetinkaya33 | 2004 | Turkey | Crossover | Enalapril + losartan | Enalapril | 10 | 3 | 55 | 55 | NR | 65 | 4.8 | 151 | 93 | Diabetic | NR | 6.9 | NR | NR | 3 |
| Crossover | Enalapril + losartan | Losartan | 10 | 3 | 55 | 55 | NR | 65 | 4.8 | 151 | 93 | Diabetic | NR | 6.9 | NR | NR | 3 | |||
| Crossover | Enalapril + losartan | Double-dose monotherapy | 10 | 3 | 55 | 55 | NR | 65 | 4.8 | 151 | 93 | Diabetic | NR | 6.9 | NR | NR | 3 | |||
| Renke70 | 2004 | Poland | Parallel-arm | Enalapril + losartan | Enalapril | 36 | 9 | 41 | 68 | 1.2 | 94 | 2.9 | 137 | 89 | Nondiabetic | - | - | sCr > 2.0mg/dl | NR | 3 |
| Parallel-arm | Enalapril + losartan | Losartan | 36 | 9 | 39 | 54 | 1.1 | 94 | 2.7 | 139 | 90 | Nondiabetic | - | - | sCr > 2.0mg/dl | NR | 3 | |||
| Scaglione71 | 2005 | Italy | Parallel-arm | Ramipril + losartan | Ramipril | 34 | 6 | 56 | 52 | 1.0 | 72 | 0.5 | 161 | 96 | Non-diabetic | - | - | sCr > 1.3mg/dl (women), sCr > 1.4mg/dl (men) | NR | 5 |
| Parallel-arm | Ramipril + losartan | Losartan | 34 | 6 | 57 | 52 | 1.0 | 70 | 0.4 | 163 | 93 | No-diabetic | - | - | sCr > 1.3mg/dl (women), sCr > 1.4mg/dl (men) | NR | 5 | |||
| Matos34 | 2005 | Brazil | Crossover | Peridopril + irbesartan | Peridopril | 20 | 4 | 54 | 25 | NR | NR | 0.9 | NR | NR | Diabetic | 11 | NR | eGFR < 40ml/min/1.73 m2 | > 5.0 | 3 |
| Crossover | Peridopril + irbesartan | Irbesartan | 20 | 4 | 54 | 25 | NR | NR | 0.9 | NR | NR | Diabetic | 11 | NR | eGFR < 40ml/min/1.73 m2 | > 5.0 | 3 | |||
| Schjoedt31 | 2005 | Denmark | Crossover | ACEI or ARB + spinolactone | ACEI or ARB | 22 | 2 | 45 | 75 | NR | NR | NR | NR | NR | Diabetic | 33 | NR | eGFR < 30ml/min/1.73 m2 | > 4.5 | 5 |
| Esnault48 | 2005 | France | Crossover | Ramipril + valsartan | Ramipril | 18 | 1 | 49 | 67 | 1.7 | NR | 3.7 | 149 | NR | Mixed | NR | NR | sCr > 2.83mg/dl | NR | 3 |
| Crossover | Ramipril + valsartan | Valsartan | 18 | 1 | 49 | 67 | 1.7 | NR | 3.7 | 149 | NR | Mixed | NR | NR | sCr > 2.83mg/dl | NR | 3 | |||
| Andersen52 | 2005 | Denmark | Parallel-arm | Lisinopril + candesartan | Lisinopril | 75 | 12 | 55 | 75 | NR | NR | 0.02 a | 141 | 83 | Diabetic | NR | NR | sCr > 1.47mg/dl | NR | 5 |
| Krimholtz53 | 2005 | UK | Parallel-arm | Lisonopril + candesartan | Lisinpopril + amlodipine | 28 | 6 | 47 | 57 | NR | 92 | 0.3 a | NR | NR | Diabetic | 30.5 | 9.3 | Cr >1.7mg/dl | > 5.5 | 5 |
| Song35 | 2006 | Korea | Crossover | Ramipril + candesartan | Ramipril | 25 | 4 | 49 | 52 | NR | NR | 4.2 | 134 | 80 | Diabetic | 8 | 7.4 | eGFR < 30ml/min/1.73 m2 | > 5.5 | 5 |
| Crossover | Ramipril + candesartan | Candesartan | 25 | 4 | 49 | 52 | NR | NR | 4.2 | 134 | 80 | Diabetic | 8 | 7.4 | eGFR < 30ml/min/1.73 m2 | > 5.5 | 5 | |||
| Atmaca54 | 2006 | Turkey | Parallel-arm | Lisinopril + losartan | Lisinopril | 17 | 12 | 55 | 41 | NR | NR | 0.1 a | 120 | 78 | Diabetic | 7.5 | 6.2 | eGFR < 60ml/min/1.73 m2 | NR | 3 |
| Parallel-arm | Lisinopril + losartan | Losartan | 17 | 12 | 55 | 41 | NR | NR | 0.1 a | 120 | 79 | Diabetic | 7.6 | 6.0 | eGFR < 60ml/min/1.73 m2 | NR | 3 | |||
| Epstein14 | 2006 | USA | Parallel-arm | Enalapril + eplerenone | Enalapril | 182 | 3 | 59 | 61 | 0.9 | 74 | 0.4 a | 140 | 86 | Diabetic | NR | 8.0 | eGFR< 70ml/min | > 5.0 | 5 |
| Parallel-arm | Enalapril + eplerenone (double dose) | Enalapril | 177 | 3 | 59 | 60 | 0.9 | 75 | 0.3 a | 143 | 87 | Diabetic | NR | 8.0 | eGFR< 70ml/min | >5.0 | 5 | |||
| Ogawa55 | 2006 | Japan | Parallel-arm | Imidapril + spironolactone | Imidapril + furosemide | 30 | 12 | 63 | NR | 0.8 | 70 | 0.2 a | 155 | 84 | Diabetic | 11.1 | 6.5 | NR | NR | 3 |
| Sengul56 | 2006 | Turkey | Parallel-arm | Lisinopril + telmisartan | Lisinopril | 95 | 7 | 57 | 37 | 0.9 | 95 | 0.2 a | 140 | 82 | Diabetic | 11.1 | 7.6 | sCr > 1.7mg/dl | > 5.5 | 3 |
| Parallel-arm | Lisinopril + telmisartan | Telmisartan | 97 | 7 | 57 | 39 | 1.0 | 94 | 0.2 a | 140 | 84 | Diabetic | 11.3 | 7.6 | sCr > 1.7mg/dl | >5.5 | 3 | |||
| Van den Meiracker57 | 2006 | Netherland | Parallel-arm | ACEI or ARB + spironolactone | ACEI or ARB + placebo | 59 | 12 | 55 | NR | 1.0 | 75 | 0.9 a | 146 | 81 | Diabetic | NR | 8.3 | sCr > 3.0mg/dl | > 5.0 | 5 |
| Igarashi61 | 2006 | Japan | Parallel-arm | Enalapril + losartan | Double-dose enalapril | 26 | 3 | 64 | 69 | 0.8 | NR | 1.8 | 150 | 81 | Diabetic | 14.5 | 7.2 | NR | NR | 3 |
| Chrysostomou72 | 2006 | Australia | Parallel-arm | Ramipril + irbesartan | Ramipril | 20 | 3 | 58 | 75 | NR | NR | 2.6 | 133 | 78 | Non-diabetic | - | - | sCr > 2.26mg/dl | > 5.0 | 5 |
| Parallel-arm | Ramipril + spironolactone | Ramipril | 20 | 3 | 63 | 70 | NR | NR | 2.4 | 137 | 78 | Nondiabetic | - | - | sCr > 2.26mg/dl | > 5.0 | 5 | |||
| Parallel-arm | Ramipril + irbesartan + spironolactone | Ramipril | 21 | 3 | 58 | 62 | NR | NR | 2.9 | 131 | 77 | Nondiabetic | - | - | sCr > 2.26mg/dl | > 5.0 | 5 | |||
| Horita73 | 2006 | Japan | Parallel-arm | Temocapril + losartan | Temocapril | 27 | 12 | 41 | 56 | 0.8 | 92 | 0.7 | 118 | 73 | Non-diabetic | - | - | Cr clearance < 50ml/min | NR | 3 |
| Parallel-arm | Temocapril + losartan | Losartan | 29 | 12 | 40 | 55 | 0.9 | 91 | 0.8 | 123 | 78 | Non-diabetic | - | - | Cr clearance < 50ml/min | NR | 3 | |||
| Kanno74 | 2006 | Japan | Parallel-arm | ACEI + candesartan | Candesartan | 90 | 36 | 60 | 45 | NR | NR | 1.8 | NR | NR | Nondiabetic | - | - | sCr > 5.0mg/dl | NR | 2 |
| Bakris79 | 2007 | USA | Parallel-arm | Ramipril + irbesartan | Ramipril | 405 | 5 | 66 | 62 | NR | NR | 0.2 a | NR | NR | Mixed | 11.5 | NR | Renal impairment | NR | 5 |
| Ogawa58 | 2007 | Japan | Parallel-arm | Temocapril + candesartan | Temocapril | 80 | 12 | 61 | 48 | 0.8 | NR | 0.2 a | 154 | 91 | Diabetic | 16.8 | 6.8 | sCr > 1.2mg/dl | NR | 3 |
| Parallel-arm | Temocapril + candesartan | Candesartan | 80 | 12 | 62 | 48 | 0.7 | NR | 0.3 a | 151 | 90 | Diabetic | 16.2 | 6.9 | sCr > 1.2mg/dl | NR | 3 | |||
| Uresin62 | 2007 | Multinational | Parallel-arm | Ramipril + aliskiren | Ramipril | 555 | 2 | 60 | 60 | NR | NR | NR | 156 | 98 | Diabetic | NR | 7.3 | NR | NR | 5 |
| Parallel-arm | Ramipril + aliskiren | Aliskiren | 559 | 2 | 60 | 58 | NR | NR | NR | 157 | 98 | Diabetic | NR | 7.3 | NR | NR | 5 | |||
| Nakamura75 | 2007 | Japan | Parallel-arm | Temocapril + olmesartan | Temocapril | 16 | 3 | 31 | 50 | 1.1 | 89 | 2.0 | 117 | 68 | Nondiabetic | - | - | NR | NR | 3 |
| Parallel-arm | Temocapril + olmesartan | Olmesartan | 16 | 3 | 33 | 56 | 1.1 | 89 | 2.0 | 118 | 69 | Nondiabetic | - | - | NR | NR | 3 | |||
| Joffe36 | 2007 | USA | Crossover | Enalapril + eplerenone | Enalapril | 16 | 1.5 | 53 | 69 | 1.1 | 97 | 0.3 | 148 | 88 | Diabetic | 11.8 | 8.1 | sCr > 1.5mg/dl | NR | 4 |
| Menne80 | 2008 | Germany | Parallel-arm | Lisinopril + valsartan | Lisinopril | 90 | 7.5 | 60 | 74 | NR | 113 | 0.1 a | 152 | 90 | Mixed | NR | NR | eGFR < 30ml/min | > 5.5 | 5 |
| Parallel-arm | Lisinopril + valsartan | Valsartan | 86 | 7.5 | 58 | 72 | NR | 120 | 0.1 a | 152 | 91 | Mixed | NR | NR | eGFR < 30ml/min | > 5.5 | 5 | |||
| Mori-Takeyama76 | 2008 | Japan | Parallel-arm | Benazepril + candesartan | Candesartan | 86 | 36 | 37 | 59 | 0.9 | 95 | 1.4 | 134 | 83 | Nondiabetic | - | - | NR | NR | 3 |
| Parving15 | 2008 | Multinational | Parallel-arm | Losartan + aliskiren | Losartan | 599 | 6 | 61 | 71 | 1.2 | 68 | 0.5 a | 136 | 78 | Diabetic | 14.0 | 8.0 | eGFR< 30ml/min/1.73m2 | > 5.1 | 5 |
| Tokunaga77 | 2008 | Japan | Parallel-arm | ARB + spironolactone | ARB | 64 | 17 | NR | NR | NR | NR | NR | NR | NR | Nondiabetic | - | - | eGFR < 15ml/min/1.73 m2 | NR | 1 |
| Swaminathan37 | 2008 | UK | Crossover | ACEI or ARB + spironolactone | ACEI or ARB + placebo | 50 | 1 | 63 | 74 | 1.1 | NR | NR | 163 | 89 | Diabetic | NR | 7.03 | Renal impairment | NR | 5 |
| Persson32 | 2009 | Denmark | Crossover | Irbesartan + aliskiren | Irbesartan | 32 | 2 | 60 | 78 | NR | NR | 0.3 a | 142 | 74 | Diabetic | NR | 8.1 | eGFR < 40ml/min/1.73 m2 | NR | 5 |
| Crossover | Irbesartan + aliskiren | Aliskiren | 32 | 2 | 60 | 78 | NR | NR | 0.3 a | 142 | 74 | Diabetic | NR | 8.1 | eGFR < 40ml/min/1.73 m2 | NR | 5 | |||
| Morales49 | 2009 | Spain | Crossover | Lisinopril + candesartan | Lisinopril | 12 | 1.5 | 57 | 58 | 1.4 | 58 | 2.2 | 139 | 78 | Mixed | NR | NR | eGFR < 15ml/min/1.73 m2 | NR | 3 |
| Crossover | Lisinopril + candesartan | Eplerenone | 12 | 1.5 | 57 | 58 | 1.4 | 58 | 2.2 | 139 | 78 | Mixed | NR | NR | eGFR < 15ml/min/1.73 m2 | NR | 3 | |||
| Krairittichai63 | 2009 | Thailand | Parallel-arm | Enalapril + telmisartan | Enalapril | 80 | 6 | 56 | 50 | 1.8 | 46 | 2.3 | 141 | 76 | Diabetic | 9.2 | 7.6 | eGFR < 15 ml/min/1.73 m2 | > 5.5 | 3 |
| Mehdi59 | 2009 | USA | Parallel-arm | Lisinopril + losartan | Lisinopril + placebo | 53 | 12 | 51 | 47 | 1.6 | NR | 0.9 a | 141 | 75 | Diabetic | 15.7 | 7.9 | sCr > 3.0mg/dl (women), sCr > 4.0mg/dl (men) | > 5.5 | 5 |
| Parallel-arm | Lisinopril + sproinolactone | Lisinopril + placebo | 54 | 12 | 51 | 46 | 1.6 | NR | 1.0 a | 137 | 73 | Diabetic | 15.7 | 7.8 | sCr > 3.0mg/dl (women), sCr > 4.0mg/dl (men) | > 5.5 | 5 | |||
| Bianchi78 | 2010 | USA | Parallel-arm | Ramipril + irbesartan + spironolactone | Ramipril | 128 | 36 | 53 | 64 | NR | 64 | 2.6 | 156 | 94 | Nondiabetic | - | - | eGFR < 30ml/min/1.73 m2 | > 5.0 | 3 |
| Ohishi81 | 2010 | Japan | Parallel-arm | Imidapril + valsartan | Olmesartan | 37 | 4 | 64 | 86.5 | 1.7 | NR | 1.7 | NR | NR | Mixed | NR | NR | sCr > 3.0mg/dl | NR | 3 |
| Titan64 | 2011 | Brazil | Parallel-arm | Enalapril + losartan | Enalapril | 56 | 4 | 58 | 62.5 | 1.7 | 52.9 | 2.9 | 149 | 81 | Diabetic | 17.0 | 8.4 | sCr > 2.5mg/dl | > 5.5 | 3 |
| Luno82 | 2011 | Spain | Parallel-arm | Lisinopril + irbesartan | Lisinopril | 131b | 49 b | 65 b | NR | 1.5 b | 45 b | 2.6 b | 155 b | 81 b | Diabetic | NR | 7.0 a | eGFR < 30ml/min/1.73 m2 | NR | 1 |
| Parallel-arm | Lisinopril + irbesartan | Irbesartan | 1 | |||||||||||||||||
| Meier50 | 2011 | Switzerland | Crossover | Lisinopril + losartan | Losartan | 20 | 2 | 53 | 50 | NR | 60 | 6.6 | NR | NR | Mixed | NR | NR | eGFR < 15ml/min/1.73 m2 | NR | 3 |
| Crossover | Lisinopril + losartan | Losartan (double dose) | 20 | 2 | 53 | 50 | NR | 60 | 6.6 | NR | NR | Mixed | NR | NR | eGFR < 15ml/min/1.73 m2 | NR | 3 | |||
| Slagman43 | 2011 | Netherlands | Crossover | Lisinopril + vasartan + low sodium | Lisinopril + low sodium | 52 | 1.5 | 51 | 83 | NR | 71 | 1.6 | 131 | 76. | Nondiabetic | - | - | eGFR < 30ml/min | NR | 5 |
| Crossover | Lisinopril + vasartan + high sodium | Lisinopril + high sodium | 52 | 1.5 | 51 | 83 | NR | 71 | 1.6 | 131 | 76. | Nondiabetic | - | - | eGFR < 30ml/min | NR | 5 | |||
Abbreviations: SBP,systolic blood pressure; DBP, diastolic blood pressure, eGFR, estimated glomerular filtration rate; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin-II type-2 receptor blockers; CKD, chronic kidney disease; Cr, creatinine; sCr, serum creatinine; NR, not reported.
aValue represents urinary albumin excretion rate; bValue refers to both study arms.
Effect of combined renin–angiotensin–aldosterone system blockade therapy on kidney-related endpoints.
Thirty study arms reported changes in albuminuria (2,165 patients) and 56 study arms reported changes in proteinuria (2,257 patients), with 10 and 20 study arms reporting changes in albuminuria in grams per gram of creatinine (1,347 patients) and grams per day (818 patients), respectively, and 10 and 46 study arms reporting changes in proteinuria in grams per gram of creatinine (697 patients) and grams per day (1,560 patients), respectively. Meta-analysis showed that combined RAAS blockade therapy was associated with an absolute net decrease in urine albumin excretion of 0.09g/g of creatinine (95% CI, –0.15 to –0.04; P = 0.001; I2 = 72%) and 0.03g/day (95% CI, –0.06 to –0.003; P = 0.03; I2 = 72%), and with an absolute net decrease in urine protein excretion of –0.29g/g of creatinine (95% CI, –0.48 to –0.10; P = 0.003; I2 = 50%) and –0.36g/day (95% CI, –0.48 to –0.25; P < 0.001; I2=50%). Similar results were observed with the use of standardized net changes (Table 1). Of note was that in the 19 study arms of diabetic subjects that reported changes in HbA1C (724 patients), there was no significant net change in HbA1C during the study period (0.06%; 95% CI, –0.12 to 0.25%). Combined RAAS blockade therapy was associated with a 9.4% higher rate of return to normoalbuminuria (95% CI, 4.1 to 14.6%; P < 0.001; I2 = 3.6%) in 12 study arms (1,082 patients), but with a nonsignificantly 2.7% lower rate of progression to overt proteinuria (95% CI, –7.0 to 1.7%; P = 0.23) in 8 study arms (523 patients) relative to monotherapy.
Sixty-nine study arms reported changes in GFR (3,801 patients), with 35 reporting changes in GFR in ml/min (1,522 patients) and 36 study arms reporting changes in GFR in ml/min/1.73 m2) (2,275 patients). Meta-analysis showed that as compared with monotherapy for RAAS blockade, combined RAAS blockade therapy was associated with an absolute net decrease in GFR of 1.79ml/min or ml/min/1.73 m2 (95% CI, –3.05 to –0.54; P = 0.005; I2 = 0%). Similar results were observed with the use of standardized net changes (Table 2). No effect of combined RAAS blockade therapy as compared with monotherapy was observed on the doubling of serum creatinine (Table 3).
Table 2.
Summary effect of combined vs. single RAAS blockade therapy on kidney-related endpoints and blood pressure parameters in patients with chronic kidney disease
| Assessment of heterogeneity | Egger test | ||||||
|---|---|---|---|---|---|---|---|
| Outcome variable | No. study arms | No. participants | Net change* (95% CI) | P value | I2 index† | P-value (Chi-square) | P value |
| Urine albumin excretion | |||||||
| Standardized | 30 | 2,165 | –0.435 (–0.717, –0.154) | 0.002 | 88.7 | <0.001 | 0.212 |
| Absolute (g/g of creatinine) | 9 | 1,287 | –0.090 (–0.145,–0.036) | 0.001 | 72.0 | <0.001 | NA |
| Absolute (g/day) | 15 | 618 | –0.032 (–0.061, –0.003) | 0.030 | 72.0 | <0.001 | NA |
| Absolute (g/g or g/day) | 24 | 1,905 | –0.062 (–0.097, –0.028) | <0.001 | 90.0 | <0.001 | 0.898 |
| Urine protein excretion | |||||||
| Standardized | 56 | 2,257 | –0.404 (–0.498, –0.309) | <0.001 | 16.6 | 0.148 | 0.170 |
| Absolute (g/g of creatinine) | 10 | 697 | –0.291 (–0.482, –0.099) | 0.003 | 50.0 | 0.036 | NA |
| Absolute (g/day) | 45 | 1,440 | –0.363 (–0.478, –0.247) | <0.001 | 50.0 | <0.001 | NA |
| Absolute (g/g or g/day) | 55 | 2,137 | –0.339 (–0.434, –0.243) | <0.001 | 49.6 | <0.001 | <0.001 |
| Glomerular filtration rate | |||||||
| Standardized | 69 | 3,791 | –0.094 (–0.171, –0.017) | 0.016 | 19.8 | 0.082 | 0.525 |
| Absolute (mL/min or mL/min/1.73m2) | 58 | 2,734 | –1.794 (–3.045, –0.544) | 0.005 | 0 | 0.790 | 0.04 |
| Serum potassium | |||||||
| Standardized | 61 | 2,982 | 0.278 (0.178, 0.377) | <0.001 | 39.2 | 0.001 | 0.123 |
| Absolute (mEq/L) | 54 | 2,255 | 0.134 (0.089, 0.179) | <0.001 | 36.2 | 0.005 | 0.358 |
| Systolic blood pressure | |||||||
| Standardized | 77 | 5,582 | –0.336 (–0.404, –0.268) | <0.001 | 22.7 | 0.044 | 0.175 |
| Absolute (mmHg) | 65 | 4,365 | –3.755 (–4.579, –2.931) | <0.001 | 12.8 | 0.197 | 0.584 |
| Diastolic blood pressure | |||||||
| Standardized | 76 | 5,454 | –0.279 (–0.363, –0.194) | <0.001 | 47.4 | <0.001 | 0.181 |
| Absolute (mmHg) | 64 | 4,237 | –2.214 (–3.116, –1.313) | <0.001 | 73.2 | <0.001 | 0.777 |
| Mean arterial pressure | |||||||
| Standardized | 17 | 489 | –0.179 (–0.358, –0.001) | 0.049 | 0 | 0.677 | 0.212 |
| Absolute (mmHg) | 17 | 489 | –1.718 (–3.100, –0.335) | 0.015 | 0 | 0.778 | 0.185 |
* By random effects model meta-analysis †A measure of statistical heterogeneity across study results; an I2 index ≥ 50% indicates medium-to-high heterogeneity.
Table 3.
Summary effect of combined vs. single RAAS blockade therapy on binary outcomes.
| Outcome variable | Peto fixed-effect model | Random-effects model | Assessment of heterogeneity | Assessment of publication bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. study arm | No. participants | Odds ratio (95% CI) | P value | No. study arms | No. participants | Summary rate difference (95% CI) | P value | I2 index† | Chi-square P value | Egger test P value | |
| Doubling of serum creatinine | 17 | 2,998 | 0.796 (0.523, 1.211) | 0.287 | 17 | 2,998 | –0.7% (–1.9, 0.5) | 0.271 | 18.3 | 0.240 | 0.43 |
| Development of hyperkalemia | 35 | 4,205 | 2.176 (1.685, 2.810) | <0.001 | 35 | 4,205 | 3.4% (1.7, 5.1) | <0.001 | 29.2 | 0.056 | <0.001 |
| Progression to overt proteinuria | 7 | 491 | 0.975 (0.534, 1.782) | 0.935 | 8 | 523 | –2.7% (–7.0, 1.7) | 0.233 | 0 | 0.604 | 0.06 |
| Regression to normoalbuminuria | 12 | 1,082 | 1.772 (1.320, 2.379) | <0.001 | 12 | 1,082 | 9.4% (4.1, 14.6) | <0.001 | 3.6 | 0.409 | 0.21 |
| Achievement of blood pressure goal | 9 | 1,858 | 1.520 (1.169, 1.977) | 0.002 | 9 | 1,858 | 5.0% (2.0, 8.0) | 0.001 | 0 | 0.994 | 0.97 |
| Addition of other anti-hypertensive drugs | 13 | 1,571 | 0.608 (0.459, 0.806) | 0.001 | 13 | 1,571 | –4.4% (–8.8, –0.1) | 0.045 | 28.1 | 0.161 | 0.09 |
| Withdrawal of anti-hypertensive drugs | 5 | 882 | 0.855 (0.488, 1.496) | 0.582 | 5 | 882 | –1.0% (–4.0, 2.1) | 0.541 | 0 | 0.983 | 0.66 |
| Any adverse effect* | 13 | 2,518 | 0.901 (0.747, 1.086) | 0.275 | 13 | 2,518 | –1.5% (–5.6, 2.6) | 0.477 | 12.6 | 0.318 | 0.38 |
| Subject drop-out | 20 | 1,965 | 1.151 (0.739,1.792) | 0.533 | 23 | 2,107 | 0.6% (–1.3, 2.4) | 0.552 | 0 | 0.498 | 0.52 |
| Development of hypotension | 22 | 1,890 | 3.990 (2.649, 6.009) | <0.001 | 24 | 2,047 | 4.6% (2.3, 6.8) | <0.001 | 33.1 | 0.060 | <0.001 |
| Hospitalization | 4 | 246 | 2.958 (0.893, 9.803) | 0.076 | 5 | 326 | 1.5% (–2.8, 5.9) | 0.481 | 27.0 | 0.242 | 0.24 |
| Mortality | 5 | 1,711 | 0.384 (0.061, 2.396) | 0.305 | 8 | 2,410 | –0.3% (–0.7, 0.2) | 0.279 | 0 | 0.855 | 0.80 |
* As defined in the individual studies
Sixty-one study arms reported changes in serum potassium (2,982 patients). By meta-analysis, combined RAAS blockade therapy was associated with an absolute net increase in serum potassium of 0.13 mEq/l (95% CI, 0.09 to 0.18 mEq/l; P < 0.001; I2 = 36%). Similar results were observed using standardized net changes (Table 2). Combined RAAS blockade therapy was associated with a 3.4% higher rate of hyperkalemia (95% CI, 1.7 to 5.1%; P < 0.001; I2 = 29%) relative to monotherapy (Table 3).
Effect of combined renin–angiotensin–aldosterone system blockade therapy on blood pressure parameters.
Seventy-seven study arms reported on changes in SBP (5,582 patients), 76 study arms on changes in DBP (5,454 patients), and 17 study arms (489 patients) on changes in MAP. By meta- analysis, combined RAAS blockade therapy was associated with absolute net decreases in SBP, DBP, and MAP of 3.8mm Hg (95% CI, –4.6 to –2.9mm Hg; P < 0.001; I2 = 13%), 2.2mm Hg (95% CI, –3.1 to –1.3mm Hg; P < 0.001; I2 = 73%), and 1.7mm Hg (95% CI, –3.1 to –0.3mm Hg; P = 0.015; I2 = 0%), respectively. Similar results were observed with the use of standardized net changes (Table 2).
Nine study arms (1,858 patients) reported on the incidence of achieving a BP goal and 13 study arms (1,571 patients) reported on the requirement for additional antihypertensive medications. By meta-analysis, combination therapy produced a 5.0% higher rate of achievement of a BP goal (95% CI, 2.0 to 8.0%; P = 0.001; I2 = 0%) and a 4.4% lower rate of addition of other antihypertensive medications compared to a single-agent regimen (95% CI, –8.8 to –0.1%; P = 0.045; I2 = 28%).
Effect of combined renin–angiotensin–aldosterone system blockade therapy on other endpoints.
Twenty-four study arms reported on the incidence of hypotension (2,047 patients), 13 study arms on the incidence of any adverse effect as defined in the individual trials (2,518 patients), 5 study arms on the incidence of drug withdrawal (882 patients), 23 study arms on dropout rate (2,107 patients), 5 study arms on hospitalization (326 patients), and 8 study arms on all-cause mortality (2,410 patients). By meta-analysis, combined RAAS blockade therapy was not associated with any of these outcomes, with the exception of a 4.6% higher rate of hypotension (95% CI, 2.3 to 6.8%; P < 0.001, I2 = 33%) relative to single therapy (Table 3).
Investigations of heterogeneity.
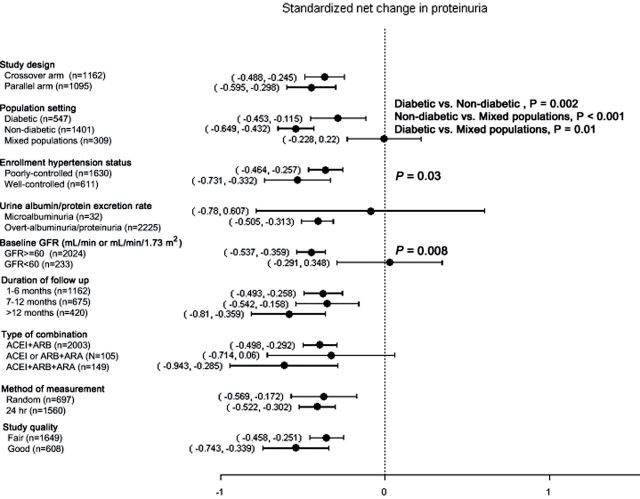
Figures 3 and 4 show the results of subgroup analyses of standardized net changes in albuminuria, proteinuria, and GFR, and of summary differences in the rates of development of hyperkalemia and hypotension, stratified by study design, population type, baseline BP status, albuminuria/proteinuria level, GFR level, type of drug combination, duration of follow up, measurement methods, and study quality. As shown in Figure 3A, larger standardized net decreases in albuminuria were observed in studies of subjects with a low (< 60ml/min or ml/min/1.73 m2) GFR (P = 0.02). Similarly, as shown in Figure 3B, larger standardized net decreases in proteinuria were observed in studies of nondiabetic compared with diabetic subjects (P = 0.002) and mixed populations (P < 0.001), as well as in studies that enrolled patients with well-controlled hypertension (P = 0.03) and preserved (GFR ≥ 60ml/min or ml/min/1.73 m2) kidney function (P = 0.008).
Figure 3.
Subgroup analyses displaying the effect of combined renin–angiotensin–aldosterone system (RAAS) blockade therapy on standardized net change in albuminuria (A) and standardized net change in proteinuria (B). Where shown, P values refer to subgroup comparisons.
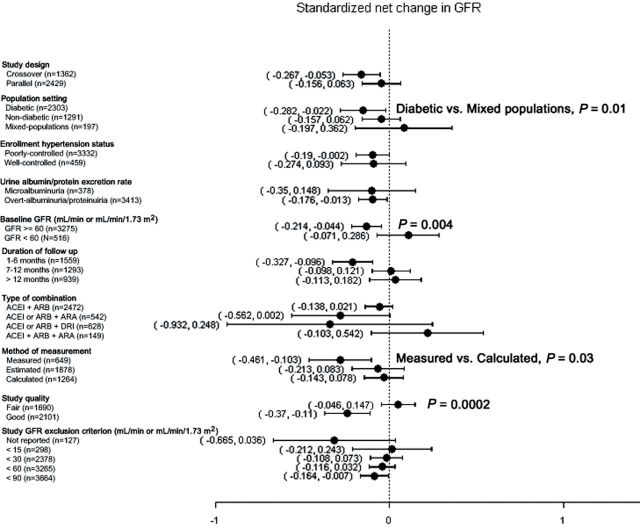
Figure 4.
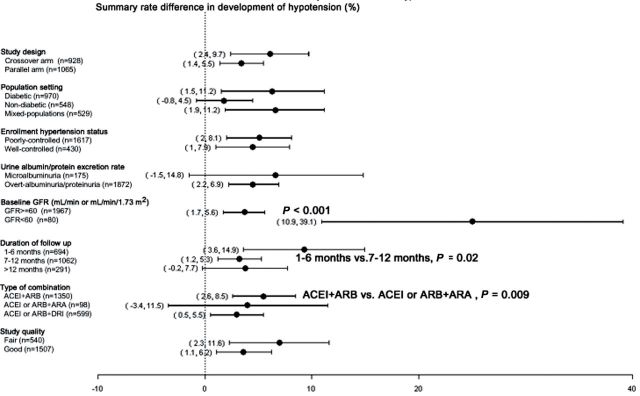
Subgroup analyses displaying the effect of combined renin–angiotensin–aldosterone system (RAAS) blockade therapy on standardized net change in GFR (A), and the summary rate difference in the development of hyperkalemia (B), and hypotension (C). Where shown, P values refer to subgroup comparisons.
As shown in Figure 4A, combined RAAS blockade therapy was associated with larger standardized net decreases in GFR in studies of diabetics as compared with studies of mixed populations (P = 0.01), as well as in studies of subjects with preserved (GFR ≥ 60ml/min or ml/min/1.73 m2) kidney function (P = 0.004), studies that measured rather than calculated GFR (P = 0.03), and studies of good quality (P = 0.0002). As shown in Figure 4B, the highest rates of hyperkalemia were observed in studies that combined an ACEI or ARB with an ARA, although this finding did not reach statistical significance, whereas combination therapy with an ACEI or ARB and a DRI was associated with a higher rate of hyperkalemia than was combination therapy with an ACEI and ARB (P = 0.04). Studies that excluded patients with a baseline serum potassium concentration of > 4.5 and > 5.0 mEq/l were associated with higher rates of hyperkalemia than were studies in which the serum potassium exclusion criterion was not defined (P = 0.02 and P = 0.04, respectively). Furthermore, as shown in Figure 4C, higher rates of hypotension were observed in studies of patients with a low GFR (P < 0.001), studies with a short duration of follow up (1–6 vs. 7–12 months, P = 0.02), and studies combining an ACEI and ARB as compared with studies that combined an ACEI or ARB and ARA (P = 0.009). Regrettably, only one study combining an ACEI or ARB and DRI reported on the development of hypotension, preventing comparison of this with the corresponding effect of other combination therapies.
Funnel plots for the key outcomes of the trials included in the meta-analysis were symmetric and the Egger test was not significant (P > 0.05), suggesting less susceptibility to publication bias (Tables 2 and 3), with the exception of the development of hyperkalemia and hypotension, in which the funnel plots were asymmetric.
DISCUSSION
The present meta-analysis demonstrates that combined RAAS blockade therapy is associated with a significant net improvement in urine albumin/protein excretion and in several BP parameters, including SBP, DBP, and MAP. Combined RAAS blockade therapy is also associated with higher rates of regression to normoalbuminuria and of achievement of BP goals. These beneficial effects were associated with a net decline in GFR, a net increase in serum potassium level, and a higher rate of hyperkalemia and hypotension. Combined RAAS blockade therapy was not associated with higher rates of doubling of serum creatinine, drug withdrawal, development of adverse effects (as defined in the individual studies), patient dropout, hospitalization, or mortality. The overall findings are consistent with results of ONTARGET.17
Chronic kidney disease is a public health problem and an independent risk factor for cardiovascular morbidity and mortality.83,84 A 10-year study of patients with stage 3 CKD demonstrated a cumulative incidence of kidney failure of only 4%, whereas the overall mortality rate rose to 51%.85 Hypertension and proteinuria are well-recognized risk factors for predicting the progression of CKD5 and cardiovascular morbidity and mortality.86,87 Several clinical practice guidelines recommend the use of RAAS blockade therapy for hypertension in patients with CKD, in light of the dual benefit of such therapy on BP and proteinuria.9,10,88,89 Previous meta-analyses of dual RAAS blockade with an ACEI and ARB demonstrated a significant decrease in proteinuria but no clinically meaningful changes in GFR or serum potassium.11–13 These systematic reviews included a smaller number of trials13–39 with total numbers of patients ranging from 425–2,042; these smaller reviews also suffered from potential contamination of the control group in that a variable percentage of study participants were receiving dual RAAS blockade therapy; furthermore, these reviews did not explore more comprehensive measures of efficacy and safety or subgroup analyses.
Our meta-analysis suggests that combined RAAS blockade therapy is associated with a decline in GFR, especially in diabetic patients, patients with preserved kidney function (GFR ≥ 60ml/min or ml/min/1.73 m2), and patients in whom GFR was measured rather than calculated or estimated. We hypothesize that stricter BP goals in studies of diabetic patients might have induced the upward titration of antihypertensive medications. In conjunction with the well-known autonomic sympathetic dysfunction observed in patients with diabetes, this relative lack of strictness increased the likelihood of hypotensive episodes, resulting in acute declines in GFR. By contrast, in studies of patients with impaired kidney function, the use of combined low-dose RAAS blockade therapy and a more cautionary upward titration of these agents might have prevented further declines in GFR. In addition, measured GFR, which is the “gold standard” among measurements of kidney function, is likely to represent a more sensitive marker of hemodynamic changes in response to antihypertensive therapy.
Our meta-analysis demonstrated a clear beneficial effect of combined RAAS blockade therapy in reducing albuminuria and proteinuria. Combined RAAS blockade therapy was also associated with a higher rate of regression to normoalbuminuria. These findings are consistent with the results of prior meta-analyses.11–13 In subgroup analyses, standardized net changes in proteinuria were more pronounced in patients without diabetes, those with well-controlled hypertension, and those with preserved kidney function. We can only speculate as to whether the presence of diabetes, poorly controlled hypertension, and a low GFR are associated with more advanced microvascular endothelial injury, thereby attenuating the benefit of combined RAAS blockade therapy. By contrast, combined RAAS blockade therapy produced a more robust benefit in standardized net changes in abuminuria in patients with a low GFR, a discrepancy that requires further study. Direct comparisons of subgroups within trials would help to address this and other inconsistencies identified in our meta-analysis.
In addition to an improvement in kidney-related endpoints with combined RAAS blockade therapy, we observed a significant improvement in all BP parameters with such therapy, as well as a higher rate of achievement of BP goals (as defined in individual trials) and a lower rate of addition of other antihypertensive medications.
Hypertension is a cause and consequence of CKD, and its treatment is largely inadequate in CKD, especially among patients with diabetes.86 Our subgroup analysis suggests that combined RAAS blockade therapy can help achieve BP goals even in patients with diabetes. Hypotension, however, might have been more clearly recognizable in studies of patients with overt proteinuria and studies with a short duration of follow up (i.e., less than 1 year), which in turn would have impeded the demonstration of a potential benefit of combined RAAS blockade therapy. Importantly, the net increase in serum potassium and higher rate of development of hyperkalemia in patients assigned to combined RAAS blockade therapy are other important safety concerns. This is particularly true for patients with an increased susceptibility to hyperkalemia (e.g., patients with a serum potassium concentration > 4.5 mEq/L or a GFR < 30ml/min, or both, and diabetic patients with hyporeninemic hypoaldosteronism).90
Our data synthesis has several strengths. To our knowledge, this is the largest systematic review of RCTs of patients with CKD to examine the effect of combined vs. single-agent RAAS blockade therapy on kidney-related endpoints, BP parameters, and other clinically important safety endpoints. The results were consistent across a broad range of analyses, including the use of absolute and standardized net changes in the continuous outcomes of interest, as well as the investigations of heterogeneity through the conduct of several informative subgroup analyses. However, several limitations in our analysis should also be noted. We were unable to assess the dosing schedules of combined RAAS blockade therapy, including dosing escalations and maximal dosing schemes, which probably contributed to the heterogeneity of the individual trial-effect estimates in our analysis. Additionally, our observations cannot be generalized to patients with advanced kidney disease (e.g., stage 4 CKD), in which the effect of combined RAAS blockade therapy on both GFR and the development of hyperkalemia remains unknown, as most of the studies in our analysis excluded such patients.
In conclusion, the present meta-analysis of 59 RCTs encompassing 4,975 participants demonstrates that the use of combined RAAS blockade therapy is more effective than monotherapy for RAAS blockade at reducing albuminuria and proteinuria, achieving a higher rate of regression to normoalbuminuria, decreasing BP, and achieving a higher rate of reaching BP goals. However these beneficial effects were compromised by a significant, albeit small, short-term decline in GFR that is of unclear clinical significance, and by higher rates of development of hypotension. The potential long-term benefit of combined RAAS blockade therapy on kidney function in patients with CKD requires further study. In the meantime, combined RAAS blockade therapy should be used judiciously in patients with proteinuric kidney disease, with close monitoring of their BP, kidney function, and serum potassium concentration.
AUTHORS’ CONTRIBUTIONS
Paweena Susantitaphong and Bertrand L. Jaber were responsible for the conception and design of this study, performed the analysis and interpretation of the study data, and wrote the draft of this paper; Paweena Susantitaphong, Ethan M. Balk, S. Eiam-Ong, Nicolas E. Madias, and Bertrand L. Jaber performed critical revision of the paper for important intellectual content; Paweena Susantitaphong, Kamal Sewaralthahab, Ethan M. Balk, Somchai Eiam-Ong, Nicolaos E. Madias, and Bertrand L. Jaber provided final approval of the paper; Bertrand L. Jaber and Ethan M. Balk provided statistical expertise; and Paweena Susantitaphong and Kamal Sewaralthahab collected and assembled the study data.
DISCLOSURE
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was made possible in part through a fellowship to Dr. Susantitaphong funded by the International Society of Nephrology. This work was supported in part by grant UL1 RR025752 from the National Center for Research Resources (NCRR). The content of the work reported in this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the U.S. National Institutes of Health. The authors are grateful to Victor F. Seabra, MD, of the Federal University of Sao Paulo, Brazil, for technical assistance in generating the figures for the subgroup analyses.
REFERENCES
- 1. Suckling R, Gallagher H. Chronic kidney disease, diabetes mellitus and cardiovascular disease: risks and commonalities. J Ren Care 2012; 38 Suppl 1: 4–11 [DOI] [PubMed] [Google Scholar]
- 2. Juutilainen A, Kastarinen H, Antikainen R, Peltonen M, Salomaa V, Tuomilehto J, Jousilahti P, Sundvall J, Laatikainen T, Kastarinen M. Trends in estimated kidney function:the FINRISK surveys. Eur J Epidemiol 2012; 27: 305–313 [DOI] [PubMed] [Google Scholar]
- 3. Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72: 247–259 [DOI] [PubMed] [Google Scholar]
- 4. Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Gustafson S, Li Q, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 2011; 57(1 Suppl 1): A8,e1–526 [DOI] [PubMed] [Google Scholar]
- 5. Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997; 51: 1908–1919 [DOI] [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 7. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 8. Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, Tang AW, Wong TY, Yung CY, Yung JC, Yu AW, Szeto CC. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis 2006; 47: 751–760 [DOI] [PubMed] [Google Scholar]
- 9. Foundation NK. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005; 45(4 Suppl 3): S1–153 [PubMed] [Google Scholar]
- 10. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O’Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. The task force for the management of arterial hypertension of the European Society of H, The task force for the management of arterial hypertension of the European Society of C. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28: 1462–1536 [DOI] [PubMed] [Google Scholar]
- 11. MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48: 8–20 [DOI] [PubMed] [Google Scholar]
- 12. Catapano F, Chiodini P, De Nicola L, Minutolo R, Zamboli P, Gallo C, Conte G. Antiproteinuric response to dual blockade of the renin-angiotensin system in primary glomerulonephritis: meta-analysis and metaregression. Am J Kidney Dis 2008; 52: 475–485 [DOI] [PubMed] [Google Scholar]
- 13. Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148: 30–48 [DOI] [PubMed] [Google Scholar]
- 14. Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006; 1: 940–951 [DOI] [PubMed] [Google Scholar]
- 15. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433–2446 [DOI] [PubMed] [Google Scholar]
- 16. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559 [DOI] [PubMed] [Google Scholar]
- 17. Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372: 547–553 [DOI] [PubMed] [Google Scholar]
- 18. Haynes R, Mason P, Rahimi K, Landray MJ. Dual blockade of the renin-angiotensin system: are two better than one? Nephrol Dial Transplant 2009; 24: 3602–3607 [DOI] [PubMed] [Google Scholar]
- 19. Slagman MC, Navis G, Laverman GD. Dual blockade of the renin-angiotensin- aldosterone system in cardiac and renal disease. Curr Opin Nephrol Hypertens 2010; 19: 140–152 [DOI] [PubMed] [Google Scholar]
- 20. Krause MW, Fonseca VA, Shah SV. Combination inhibition of the renin-angiotensin system: is more better? Kidney Int 2011; 80: 245–255 [DOI] [PubMed] [Google Scholar]
- 21. Mancia G, Grassi G. Impact of new clinical trials on recent guidelines on hypertension management. Ann Med 2011; 43: 124–132 [DOI] [PubMed] [Google Scholar]
- 22. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12 [DOI] [PubMed] [Google Scholar]
- 23. Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5: 1734–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006; 11: 193–206 [DOI] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobsen P, Andersen S, Rossing K, Hansen BV, Parving HH. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial Transplant 2002; 17: 1019–1024 [DOI] [PubMed] [Google Scholar]
- 27. Rossing K, Christensen PK, Jensen BR, Parving HH. Dual blockade of the renin- angiotensin system in diabetic nephropathy: a randomized double-blind crossover study. Diabetes Care 2002; 25: 95–100 [DOI] [PubMed] [Google Scholar]
- 28. Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin- angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int 2003; 63: 1874–1880 [DOI] [PubMed] [Google Scholar]
- 29. Jacobsen P, Andersen S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol 2003; 14: 992–999 [DOI] [PubMed] [Google Scholar]
- 30. Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 2003; 26: 2268–2274 [DOI] [PubMed] [Google Scholar]
- 31. Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Tarnow L, Parving HH. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int 2005; 68: 2829–2836 [DOI] [PubMed] [Google Scholar]
- 32. Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, Danser AH, Boomsma F, Frandsen E, Parving HH. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care 2009; 32: 1873–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cetinkaya R, Odabas AR, Selcuk Y. Anti-proteinuric effects of combination therapy with enalapril and losartan in patients with nephropathy due to type 2 diabetes. Int J Clin Pract 2004; 58: 432–435 [DOI] [PubMed] [Google Scholar]
- 34. Matos JP, de Lourdes Rodrigues M, Ismerim VL, Boasquevisque EM, Genelhu V, Francischetti EA. Effects of dual blockade of the renin angiotensin system in hypertensive type 2 diabetic patients with nephropathy. Clin Nephrol 2005; 64: 180–189 [DOI] [PubMed] [Google Scholar]
- 35. Song JH, Cha SH, Lee HJ, Lee SW, Park GH, Kim MJ. Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant 2006; 21: 683–689 [DOI] [PubMed] [Google Scholar]
- 36. Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab 2007; 92: 2552–2558 [DOI] [PubMed] [Google Scholar]
- 37. Swaminathan K, Davies J, George J, Rajendra NS, Morris AD, Struthers AD. Spironolactone for poorly controlled hypertension in type 2 diabetes: conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia 2008; 51: 762–768 [DOI] [PubMed] [Google Scholar]
- 38. Russo D, Minutolo R, Pisani A, Esposito R, Signoriello G, Andreucci M, Balletta MM. Coadministration of losartan and enalapril exerts additive antiproteinuric effect in IgA nephropathy. Am J Kidney Dis 2001; 38: 18–25 [DOI] [PubMed] [Google Scholar]
- 39. Berger ED, Bader BD, Ebert C, Risler T, Erley CM. Reduction of proteinuria; combined effects of receptor blockade and low dose angiotensin-converting enzyme inhibition. J Hypertens 2002; 20: 739–743 [DOI] [PubMed] [Google Scholar]
- 40. Ferrari P, Marti HP, Pfister M, Frey FJ. Additive antiproteinuric effect of combined ACE inhibition and angiotensin II receptor blockade. J Hypertens 2002; 20: 125–130 [DOI] [PubMed] [Google Scholar]
- 41. Campbell R, Sangalli F, Perticucci E, Aros C, Viscarra C, Perna A, Remuzzi A, Bertocchi F, Fagiani L, Remuzzi G, Ruggenenti P. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int 2003; 63: 1094–1103 [DOI] [PubMed] [Google Scholar]
- 42. Rutkowski P, Tylicki L, Renke M, Korejwo G, Zdrojewski Z, Rutkowski B. Low-dose dual blockade of the renin-angiotensin system in patients with primary glomerulonephritis. Am J Kidney Dis 2004; 43: 260–268 [DOI] [PubMed] [Google Scholar]
- 43. Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 2011; 343: d4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agarwal R. Add-on angiotensin receptor blockade with maximized ACE inhibition. Kidney Int 2001; 59: 2282–2289 [DOI] [PubMed] [Google Scholar]
- 45. Kincaid-Smith P, Fairley K, Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant 2002; 17: 597–601 [DOI] [PubMed] [Google Scholar]
- 46. Kim MJ, Song JH, Suh JH, Lee SW, Kim GA. Additive antiproteinuric effect of combination therapy with ACE inhibitor and angiotensin II receptor antagonist: differential short-term response between IgA nephropathy and diabetic nephropathy. Yonsei Med J 2003; 44: 463–472 [DOI] [PubMed] [Google Scholar]
- 47. Song JH, Lee SW, Suh JH, Kim ES, Hong SB, Kim KA, Kim MJ. The effe ts of dual blockade of the renin-angiotensin system on urinary protein and transforming growth factor-beta excretion in 2 groups of patients with IgA and diabetic nephropathy. Clin Nephrol 2003; 60: 318–326 [DOI] [PubMed] [Google Scholar]
- 48. Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16: 474–481 [DOI] [PubMed] [Google Scholar]
- 49. Morales E, Huerta A, Gutierrez E, Gutierrez Solis E, Segura J, Praga M. [The antiproteinuric effect of the blockage of the renin-angiotensin-aldosterone system (RAAS) in obese patients. Which treatment option is the most effective?]. Nefrologia 2009; 29: 421–429 [DOI] [PubMed] [Google Scholar]
- 50. Meier P, Maillard MP, Meier JR, Tremblay S, Gauthier T, Burnier M. Combining blockers of the renin-angiotensin system or increasing the dose of an angiotensin II receptor antagonist in proteinuric patients: a randomized triple-crossover study. J Hypertens 2011; 29: 1228–1235 [DOI] [PubMed] [Google Scholar]
- 51. Tutuncu NB, Gurlek A, Gedik O. Efficacy of ACE inhibitors and ATII receptor blockers in patients with microalbuminuria: a prospective study. Acta Diabetol 2001; 38: 157–161 [DOI] [PubMed] [Google Scholar]
- 52. Andersen NH, Poulsen PL, Knudsen ST, Poulsen SH, Eiskjaer H, Hansen KW, Helleberg K, Mogensen CE. Long-term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: the CALM II study. Diabetes Care 2005; 28: 273–277 [DOI] [PubMed] [Google Scholar]
- 53. Krimholtz MJ, Karalliedde J, Thomas S, Bilous R, Viberti G. Targeting albumin excretion rate in the treatment of the hypertensive diabetic patient with renal disease. J Am Soc Nephrol 2005; 16 Suppl 1: S42–47 [DOI] [PubMed] [Google Scholar]
- 54. Atmaca A, Gedik O. Effects of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and their combination on microalbuminuria in normotensive patients with type 2 diabetes. Adv Ther 2006; 23: 615–622 [DOI] [PubMed] [Google Scholar]
- 55. Ogawa S, Takeuchi K, Mori T, Nako K, Ito S. Spironolactone further reduces urinary albumin excretion and plasma B-type natriuretic peptide levels in hypertensive type II diabetes treated with angiotensin-converting enzyme inhibitor. Clin Exp Pharmacol Physiol 2006; 33: 477–479 [DOI] [PubMed] [Google Scholar]
- 56. Sengul AM, Altuntas Y, Kurklu A, Aydin L. Beneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res Clin Pract 2006; 71: 210–219 [DOI] [PubMed] [Google Scholar]
- 57. van den Meiracker AH, Baggen RG, Pauli S, Lindemans A, Vulto AG, Poldermans D, Boomsma F. Spironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal function. J Hypertens 2006; 24: 2285–2292 [DOI] [PubMed] [Google Scholar]
- 58. Ogawa S, Takeuchi K, Mori T, Nako K, Tsubono Y, Ito S. Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertens Res 2007; 30: 325–334 [DOI] [PubMed] [Google Scholar]
- 59. Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 2009; 20: 2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kuriyama S, Tomonari H, Tokudome G, Horiguchi M, Hayashi H, Kobayashi H, Ishikawa M, Hosoya T. Antiproteinuric effects of combined antihypertensive therapies in patients with overt type 2 diabetic nephropathy. Hypertens Res 2002; 25: 849–855 [DOI] [PubMed] [Google Scholar]
- 61. Igarashi M, Hirata A, Kadomoto Y, Tominaga M. Dual blockade of angiotensin II with enalapril and losartan reduces proteinuria in hypertensive patients with type 2 diabetes. Endocr J 2006; 53: 493–501 [DOI] [PubMed] [Google Scholar]
- 62. Uresin Y, Taylor AA, Kilo C, Tschope D, Santonastaso M, Ibram G, Fang H, Satlin A. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst 2007; 8: 190–198 [DOI] [PubMed] [Google Scholar]
- 63. Krairittichai U, Chaisuvannarat V. Effects of dual blockade of renin-angiotensin system in type 2 diabetes mellitus patients with diabetic nephropathy. J Med Assoc Thai 2009; 92: 611–617 [PubMed] [Google Scholar]
- 64. Titan SM, M. Vieira J J, Dominguez WV, Barros RT, Zatz R. ACEI and ARB combination therapy in patients with macroalbuminuric diabetic nephropathy and low socioeconomic level: a double-blind randomized clinical trial. Clin Nephrol 2011; 76: 273–283 [DOI] [PubMed] [Google Scholar]
- 65. Ruilope LM, Aldigier JC, Ponticelli C, Oddou-Stock P, Botteri F, Mann JF. Safety of the combination of valsartan and benazepril in patients with chronic renal disease. European Group for the Investigation of Valsartan in Chronic Renal Disease. J Hypertens 2000; 18: 89–95 [PubMed] [Google Scholar]
- 66. Tylicki L, Rutkowski P, Renke M, Rutkowski B. Renoprotective effect of small doses of losartan and enalapril in patients with primary glomerulonephritis. Short-term observation. Am J Nephrol 2002; 22: 356–362 [DOI] [PubMed] [Google Scholar]
- 67. Luno J, Barrio V, Goicoechea MA, Gonzalez C, de Vinuesa SG, Gomez F, Bernis C, Espinosa M, Ahijado F, Gomez J, Escalada P. Effects of dual blockade of the renin-angiotensin system in primary proteinuric nephropathies. Kidney Int Suppl 2002; 82: S47–52 [DOI] [PubMed] [Google Scholar]
- 68. Shoji T, Tomida K, Furumatsu Y, Kaneko T, Togawa M, Okada N, Imai E, Tsubakihara Y. The Long Term Influence of the Combination of Losartan andEnalapril on Erythropoiesis in Japanese Patients with Chronic Glomerulonephritis. J Am Soc Nephrol 2003;; 14:773A Abstract.12595515 [Google Scholar]
- 69. Segura J, Praga M, Campo C, Rodicio JL, Ruilope LM. Combination is better than monotherapy with ACE inhibitor or angiotensin receptor antagonist at recommended doses. J Renin Angiotensin Aldosterone Syst 2003; 4: 43–47 [DOI] [PubMed] [Google Scholar]
- 70. Renke M, Tylicki L, Rutkowski P, Rutkowski B. Low-dose angiotensin II receptor antagonists and angiotensin II-converting enzyme inhibitors alone or in combination for treatment of primary glomerulonephritis. Scand J Urol Nephrol 2004; 38: 427–433 [DOI] [PubMed] [Google Scholar]
- 71. Scaglione R, Argano C, Corrao S, Di Chiara T, Licata A, Licata G. Transforming growth factor beta1 and additional renoprotective effect of combination ACE inhibitor and angiotensin II receptor blocker in hypertensive subjects with minor renal abnormalities: a 24-week randomized controlled trial. J Hypertens 2005; 23: 657–664 [DOI] [PubMed] [Google Scholar]
- 72. Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo- controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1: 256–262 [DOI] [PubMed] [Google Scholar]
- 73. Horita Y, Taura K, Taguchi T, Furusu A, Kohno S. Aldosterone breakthrough during therapy with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in proteinuric patients with immunoglobulin A nephropathy. Nephrology (Carlton) 2006; 11: 462–466 [DOI] [PubMed] [Google Scholar]
- 74. Kanno Y, Takenaka T, Nakamura T, Suzuki H. Add-on angiotensin receptor blocker in patients who have proteinuric chronic kidney diseases and are treated with angiotensin- converting enzyme inhibitors. Clin J Am Soc Nephrol 2006; 1: 730–737 [DOI] [PubMed] [Google Scholar]
- 75. Nakamura T, Inoue T, Sugaya T, Kawagoe Y, Suzuki T, Ueda Y, Koide H, Node K. Beneficial effects of olmesartan and temocapril on urinary liver-type fatty acid-binding protein levels in normotensive patients with immunoglobin A nephropathy. Am J Hypertens 2007; 20: 1195–1201 [DOI] [PubMed] [Google Scholar]
- 76. Mori-Takeyama U, Minatoguchi S, Murata I, Fujiwara H, Ozaki Y, Ohno M, Oda H, Ohashi H. Dual blockade of the rennin-angiotensin system versus maximal recommended dose of angiotensin II receptor blockade in chronic glomerulonephritis. Clin Exp Nephrol 2008; 12: 33–40 [DOI] [PubMed] [Google Scholar]
- 77. Tokunaga M, Tamura M, Kabashima N, Serino R, Shibata T, Matsumoto M, Miyamoto T, Miyazaki M, Furuno Y, Nakamata J, Otsuji Y. Add-On Spironolactone in Patients with Advanced Chronic Kidney Disease Treated with Angiotensin II Receptor Blockers. J Am Soc Nephrol 2008; Abstract. [Google Scholar]
- 78. Bianchi S, Bigazzi R, Campese VM. Intensive versus conventional therapy to slow the progression of idiopathic glomerular diseases. Am J Kidney Dis 2010; 55: 671–681 [DOI] [PubMed] [Google Scholar]
- 79. Bakris GL, Ruilope L, Locatelli F, Ptaszynska A, Pieske B, de Champlain J, Weber MA, Raz I. Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: results of the IMPROVE trial. Kidney Int 2007; 72: 879–885 [DOI] [PubMed] [Google Scholar]
- 80. Menne J, Farsang C, Deak L, Klebs S, Meier M, Handrock R, Sieder C, Haller H. Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: the VALERIA trial. J Hypertens 2008; 2: 1860–1867 [DOI] [PubMed] [Google Scholar]
- 81. Ohishi M, Takeya Y, Tatara Y, Yamamoto K, Onishi M, Maekawa Y, Kamide K, Rakugi H. Strong suppression of the renin-angiotensin system has a renal-protective effect in hypertensive patients: high-dose ARB with ACE inhibitor (Hawaii) study. Hypertens Res 2010; 33: 1150–1154 [DOI] [PubMed] [Google Scholar]
- 82. Luno J, Maria FG, de Vinuesa SG, Praga M. Effect of dual blockade of renin angiotensin system on the progression of type 2 diabetic nephropathy. J Am Soc Nephrol 2011; 22 PMID:17699280 22021708 [Google Scholar]
- 83. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 84. Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ 2010; 341: c4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006; 69: 375–382 [DOI] [PubMed] [Google Scholar]
- 86. De Nicola L, Minutolo R, Chiodini P, Zoccali C, Castellino P, Donadio C, Strippoli M, Casino F, Giannattasio M, Petrarulo F, Virgilio M, Laraia E, Di Iorio BR, Savica V, Conte G. Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention. Kidney Int 2006; 69: 538–545 [DOI] [PubMed] [Google Scholar]
- 87. Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, Kanashiki M, Saito Y, Ota H, Nose T. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 2006; 69: 1264–1271 [DOI] [PubMed] [Google Scholar]
- 88.National Heart Foundation of Australia. Guide to management of hypertension 2010. http://www.heartfoundation.org.au/information-for-professionals/Clinical- Information/Pages/hypertension.aspx. http://www.heartfoundation.org.au/information-for-professionals/Clinical- Information/Pages/hypertension.aspx
- 89. The Canadian Hypertension Education Program Updated standardized recommendations and clinical practice guidelines to detect, treat and control hypertension, 2012. http://www.hypertension.ca/chep-recommendations
- 90. Bakris GL, Kuritzky L. Monitoring and managing urinary albumin excretion: practical advice for primary care clinicians. Postgrad Med 2009; 121: 51–60 [DOI] [PubMed] [Google Scholar]