Abstract
BACKGROUND
Racial minorities typically have less exposure than non-minorities to antihypertensive medications across an array of cardiovascular conditions in the general population. However, cumulative exposure has not been investigated in dialysis patients.
METHODS
In a longitudinal analysis of 38,381 hypertensive dialysis patients, prescription drug data from Medicaid was linked to Medicare data contained in United States Renal Data System core data, creating a national cohort of dialysis patients dually eligible for Medicare and Medicaid services. The proportion of days covered (PDC) was calculated to determine cumulative exposure to angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), β-blockers, and calcium-channel blockers (CCDs). The factors associated with use of these medications were modeled through weighted linear regression, with derivation of the adjusted odds ratios (AORs) for exposure.
RESULTS
Relative to non-Hispanic Caucasians, African-American, Hispanic, or Other race/ethnicity were significantly associated with less exposure to β-blockers (AOR 0.56−0.69, P < 0.001 in each case) and CCBs (AOR 0.84−0.85, P < 0.001 in each case); African-American race and Hispanic ethnicity had AORs of 0.78 and 0.73 for ACEIs and ARBs, respectively (P < 0.001 in both cases). Collectively, the odds of exposure to each class of medication for minorities was about three-quarters of that for Caucasians.
CONCLUSIONS
Given that dually Medicare-and-Medicaid–eligible dialysis patients have insurance coverage for prescription medications as well as regular contact with health care providers, differences by race in exposure to antihypertensive medications should with time be minimal among patients who are candidates for these drugs. The causes of differences by race in exposure to antihypertensive medications over the course of time should be further examined.
Keywords: ACEIs, β-blockers, calcium-channel blockers, Medicare, Medicaid, hypertension, cardiovascular disease, dialysis, ESRD, blood pressure.
Racial minorities typically have less exposure than non-minorities to antihypertensive medications, as has been noted across an array of cardiovascular conditions in the general population.1–10 Even in environments in which there is “universal” access to care, such as the US Veterans Administration system (a healthcare system for which all United States military veterans are eligible, regardless of socioeconomic status)11,12 or state Medicaid programs in the United States (which provide financial assistance with prescription drug costs for socioeconomically disadvantaged persons),13 minorities have lower rates of treatment for cardiovascular disorders. Although race-based differences in care have been noted in the chronic dialysis environment for the creation of arteriovenous fistulas14–16 and outcomes after renal transplantation,17,18 there is little understanding of whether chronic dialysis patients face the same race-based differences in exposure to antihypertensive medications as does the general population.
Patients undergoing chronic dialysis have high rates of cardiovascular disease19 and are frequently treated with antihypertensive agents that have demonstrated cardioprotective properties in other populations20–32 or in animal models,33,34 such as angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), β-adrenergic antagonists (β-blockers), and calcium-channel blockers (CCBs). Our previous work in the dually Medicaid-and-Medicare–eligible dialysis population showed, perhaps paradoxically, that non-Caucasians are treated with these classes of antihypertensive agents at higher rates than are Caucasians when a cross-sectional approach is used,35 but to date, the cumulative exposure of these groups to these medications over the course of time has not been specifically investigated.
To study this issue in detail, we created a novel linkage of data from the United States Renal Data System (USRDS), which contains Medicare claims data in chronic dialysis patients, with Medicaid prescription claims data,35 to create a large cohort of incident dialysis patients who, by virtue of their geographic diversity, are subject to a wide spectrum of policies, procedures, and cultures of care. Chronic dialysis patients who have dual eligibility for Medicare and Medicaid, and who are therefore indigent, constitute a uniquely informative population in which to study how prescribing patterns vary by race, because: (i) such patients have ready access to care and prescription medications via these two public programs, and (ii) chronic dialysis is a setting in which all patients have similarly frequent contact with health care providers. We determined the percentage of time that patients had exposure to ACEIs or ARBs or both, β-blockers, and CCBs. The term “exposure” was deliberately selected because a filled prescription cannot be used to infer either “use” (which suggests proof of ingestion), or adherence (which implies a volitional action by a patient to take a drug as prescribed). To confine our analysis to individuals who were judged suitable candidates for a drug, we deliberately modeled only individuals who received at least one prescription for these antihypertensive agents. Given the importance of studying the way in which race influences care,36 we reasoned that if race is associated with differential exposure to medication over time, further work would be imperative to understand the factors that undermine or facilitate exposure.
MATERIALS
Data sources for analysis
We conducted an analysis of ACEI–ARB, β-blocker, and CCB prescription drug exposure for an incident cohort of dually eligible (Medicare–Medicaid) chronic dialysis patients for the years 2000–2005. As previously described,35,37 data for these patients were obtained from the USRDS and the Centers for Medicare & Medicaid Services (CMS) Medicaid files; from the CMS we obtained Medicaid Analytic eXtract Personal Summary Files as well as the final action prescription drug claims files. The USRDS performed a deterministic match of these Medicaid beneficiaries against the core USRDS files to identify dually eligible individuals on chronic dialysis.
Study cohort and rationale for analytic approach
We identified unique individuals over the age of 18 years who were receiving chronic dialysis, who survived for at least 90 days after the initiation of dialysis, who were continuously enrolled in Medicare and Medicaid from the initiation of dialysis during the observation period (2000–2005), and who had hypertension designated as a comorbidity or as the cause of end-stage renal disease (ESRD) on the CMS 2728 dialysis intake form.38 After surviving the initial 90-day survival run-in period (in accordance with USRDS guidelines), individuals were removed from the study when they lost either Medicare or Medicaid eligibility, received a kidney transplant, died, or reached the end of the observation window. Patients enrolled in Medicaid managed care plans (e.g., as in Arizona or Tennessee) or in the US Department of Veterans Affairs health system were excluded because medication data were not available for them. (Persons receiving chronic dialysis were generally not enrolled in Medicare managed care plans before 2006.) We excluded residents of Ohio because the number of days of medication supplied, a variable required for computing drug exposure over time in our study, was not recorded in the Medicaid claim form for this state. To assure that patients in our study were actually accessing benefits, we eliminated individuals who did not fill at least one Medicaid prescription during the first 90 days of dialysis. Additionally, because our goal was to study cumulative outpatient medication exposure over time, we eliminated all individuals in our cohort who were institutionalized for ≥ 33% of their respective observation windows, because individuals who are frequently hospitalized have long periods during which their medication exposures are unobservable.
Descriptive variables
Demographic and clinical variables were drawn from the dialysis intake form, also known as the Medical Evidence Form (CMS 2728). Demographic variables included age, sex, and race by ethnicity and employment status. Race–ethnicity consisted of four mutually exclusive groups consisting of non-Hispanic Caucasians, non-Hispanic African-Americans, Hispanics, and Others (which would typically consist of Asian-Americans and Pacific Islanders, Native Americans, and individuals who declined self-identification. Risk behaviors included smoking and substance abuse (i.e., alcohol or illicit drugs), and functional status markers were ability to ambulate and transfer from a bed to a chair. The cause of ESRD was categorized as diabetes, hypertension, glomerulonephritis, or other. Major clinical comorbidities were diabetes (types I and II combined), congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, and cardiac dysrhythmia. Because the CMS 2728 form is structured so that diseases such as diabetes or hypertension may be considered as either a cause or a comorbidity of ESRD, diabetes and hypertension were considered to exist in an individual if they were listed as either the cause or as a comorbidity of ESRD.
We supplemented the medical information provided by the CMS 2728 form with an adapted form of the Liu comorbidity index,39 an overall measure of comorbidity burden developed specifically for the chronic dialysis population. In our adaptation, we used only 90 (rather than 180) days in which to acquire claims, because we found little difference in indices generated by using 90 or 180 days of claims data and because we had required that patients have complete Medicaid and Medicare coverage throughout the first 90 days of dialysis. Hemoglobin was dichotomized at 11g/dl, in accord with the CMS 2728 form. The modality used for dialysis at the time of its initiation was categorized as either in-center hemodialysis or self-care dialysis (home hemodialysis or peritoneal dialysis). The two home-based therapies were grouped together because frequency of contact with healthcare providers might be a determinant of cumulative medication exposure, and patients receiving such therapies typically interact with their physicians on a monthly (as opposed to a thrice-weekly) basis.
Medication exposure
We matched drug name and therapeutic class information for ACEI–ARBs, β-blockers, or CCBs in the Medicaid drug claims at the level of the US Federal Drug Administration National Drug Code (NDC), using Multum Lexicon (Cerner Corporation, www.cerner.com). Details of our specific medication identification strategy have been described.40
We determined the number of days for which each drug was supplied from the Medicaid prescription claims files, to establish the PDC.41,42 If a prescription was filled > 7 days before completion of a previous prescription, treatment was considered to have changed in that the previous prescription had been truncated and then superseded by the ensuing prescription. If a prescription was filled ≤ 7 days before the completion of a previous prescription, the overlap was “carried forward,” in that the exposure to the ensuing prescription was extended by the duration of the overlap. For time spent in the hospital or in a skilled nursing facility (“institutionalized”), we made no assumptions about medication exposure, and those days were entirely excluded from calculation of an individual’s medication exposure. Each individual’s medication exposure was then calculated as the proportion of that individual’s non-institutionalized days with a Medicaid prescription divided by the total number of the individual’s non-institutionalized days in the respective observation window for each patient.
Statistical analysis.
We generated descriptive statistics (means and standard deviations for continuous variables and frequencies for categorical variables) to examine the distributions of our study measures for each individual drug class as well as for the use of at least one class of the drugs included in our study. In bivariate analyses for each individual drug class, the difference between the percentages of users and nonusers was determined for each characteristic. Percentiles (median, 25th, and 75th) were also generated for the medication-exposure measures. To identify independent factors associated with medication use, we generated ordinary least squares (OLS) regression models, with medication status being regressed simultaneously on all a priori selected explanatory variables. Each model was limited to users of that medication (i.e., nonusers were excluded). The response measures ranged from (0, 1), and residual analyses indicated failure to adhere to OLS regression model assumptions (not shown). Thus, we transformed our response measures using the logit transformation. (For individuals with a medication exposure value of 1 (i.e., logit (100%)), we added a single day to the non-institutionalized denominator to produce a value of less than 1, as the logit transformation of 1 does not exist owing to division by 0.) The use of the logit transformation enabled parameter interpretation in the familiar context of AORs, with the antilog of the logit used to mathematically generate the AOR. To account for the differences in subject follow-up times for the response measure, we weighted the OLS regression models by the number of non-institutionalized days for which each subject was a member of the study-subject cohort. Visual inspection of residual plots and their empiric histograms were used for to assess the models.
Because of the large sample size for the study, statistical significance was inferred only when P < 0.01. All statistical analyses were done with SAS 9.2 (SAS Institute, Inc., www.sas.com).
Compliance and research participant protection.
The research protocol for the study was approved by the institutional review board at the University of Kansas Medical Center (KUMC), and the project was undertaken according to the principles of the Declarations of Helsinki. Data Use Agreements were in place between KUMC and the USRDS and CMS.
RESULTS
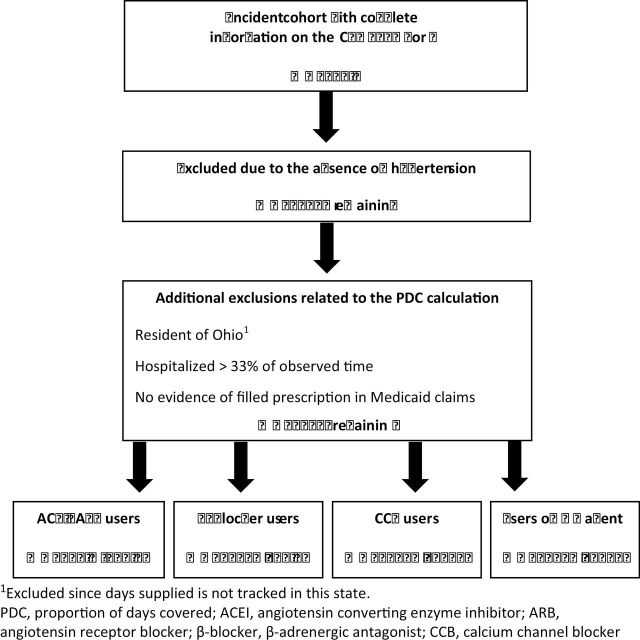
The construction of the study cohort is shown in Figure 1. A total of 70,114 dialysis patients who began having dialysis between January 1, 2000 and December 31, 2005 met the initial inclusion criteria for the study (dual eligibility, age ≥ 18 years, single state of residence, survival > 90 days after initiation of dialysis, and no Department of Veterans Affairs coverage). After limiting the cohort to individuals who had documentation of hypertension as a comorbidity or cause of ESRD on their CMS 2728 form on initiation of dialysis, 59,569 individuals remained. The cohort was then further refined by excluding residents of Ohio (because no record of days of medication supplied is kept by Medicaid in this state), individuals who were hospitalized > 33% of the observation
Figure 1.
Flow diagram for construction of the study cohort.
window, and those who, despite having Medicaid benefits, were not accessing them. This resulted in 38,381 individuals remaining in the study cohort. The percentage of users of any one of these medications ranged from 63.0% for ACEIs–ARBs to 66.6% for CCBs, and 90.6% of the study population used at least one of the agents.
The characteristics of the users of ACEIs–ARBS, β-blockers, CCBs, and a combined measure of any of the agents are shown in Table 1. Each column in the table describes the distribution of characteristics of the users of the different classes of agents. For each individual drug class, the percentage of users (shown) is compared with the percentage of nonusers (not shown) for each characteristic. For example, for ACEIs–ARBs, the percentage of users who were female was significantly different (at a level of P < 0.01, as shown by the asterisk) than the percentage of nonusers who were female. Similarly, the distribution of races differed significantly between users and nonusers of ACEIs–ARBs.
Table 1.
Descriptive statistics for dually-eligible dialysis patients using ACEI/ARBs, β-blockers, and CCBs.
| Characteristic | ACEI/ARBs | β-Blockers | CCBs | ≥ 1 Agent |
|---|---|---|---|---|
| Number of users, n (%) | 24,177 (63.0) | 24,818 (64.7) | 25,551 (66.6) | 34,790 (90.6) |
| Age, years | 60.0 (15.1)* | 60.3 (15.2)* | 60.3 (15.3)* | 60.8 (15.3)* |
| Female gender, % | 58.0* | 57.1 | 59.0* | 57.7* |
| Race/ethnicity, % | −* | −* | −* | −* |
| African-American | 43.0 | 43.0 | 45.2 | 42.4 |
| Caucasian | 29.5 | 31.5 | 27.9 | 31.1 |
| Hispanic | 20.2 | 18.6 | 19.9 | 19.5 |
| Other | 7.4 | 6.8 | 7.0 | 7.0 |
| Unable to ambulate, % | 4.1* | 4.4* | 4.1* | 4.6* |
| Unable to transfer, % | 1.1* | 1.3* | 1.2* | 1.4* |
| Unemployed, % | 97.6 | 97.6 | 97.4 | 97.5 |
| BMI category, % | −* | −* | −* | − |
| < 20kg/m2 | 9.5 | 9.3 | 9.9 | 9.7 |
| 20 − < 25kg/m2 | 30.0 | 29.9 | 30.1 | 29.7 |
| 25 − < 30kg/m2 | 27.4 | 27.6 | 27.4 | 27.2 |
| ≥ 30kg/m2 | 33.2 | 33.2 | 32.6 | 33.3 |
| Smoker, % | 7.1* | 7.0 | 7.0 | 6.8 |
| Substance abuser, % | 2.9 | 2.8* | 2.9* | 2.8 |
| Cause of ESRD, % | −* | −* | −* | −* |
| Diabetes | 55.4 | 52.4 | 52.5 | 52.5 |
| Hypertension | 28.8 | 30.1 | 30.9 | 30.3 |
| Glomerulonephritis | 7.2 | 7.5 | 7.4 | 7.3 |
| Other | 8.6 | 10.0 | 9.3 | 9.9 |
| Liu comorbidity score | 6.2 (3.5) | 6.3 (3.6)* | 5.9 (3.4)* | 6.2 (3.5)* |
| Liu comorbidity distribution, %: | −* | −* | −* | −* |
| 0 | 4.2 | 4.1 | 4.3 | 4.2 |
| 1-5 | 42.8 | 41.7 | 45.5 | 42.8 |
| 6-10 | 41.1 | 41.4 | 39.5 | 40.7 |
| 11+ | 12.0 | 12.7 | 10.7 | 12.3 |
| Major comorbidities, % | ||||
| DM | 65.7* | 63.1* | 62.6* | 62.9* |
| CHF | 34.1 | 34.9* | 31.3* | 33.9 |
| CAD | 24.7 | 27.2* | 22.3* | 24.9 |
| PVD | 14.3 | 14.6 | 13.1* | 14.3 |
| CVA | 10.5 | 10.8 | 10.5 | 10.7 |
| Hb at baseline < 11.0g/dL, % | 74.7 | 74.4 | 75.4* | 74.4 |
| In-center hemodialysis, % | 94.7 | 94.9 | 94.9 | 94.8 |
| Years in the cohort | 2.0 (1.4)* | 2.0 (1.4)* | 2.0 (1.4)* | 1.9 (1.4)* |
Bivariate analyses represent comparisons between percentage of users (shown) and non-users (not shown) for each characteristic, within an individual drug class; * represents a difference at a level of P < 0.01.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; β-blocker, β-adrenergic antagonist; CCB, calcium channel blocker; BMI, body mass index; ESRD, end stage renal disease; HTN, hypertension; DM, diabetes; CHF, congestive heart failure; CAD, coronary artery disease; PVD; peripheral vascular disease; CVA, cerebrovascular accident; HB, hemoglobin.
Across the medication cohorts, the mean age was approximately 60 years, the majority was female, and African-Americans constituted the largest single racial–ethnic group. The primary cause of ESRD was diabetes (present in more than 50% of the study population). The distribution of the adapted Liu comorbidity index demonstrates that as is typical of chronic dialysis patients, the study cohort had a substantial comorbidity burden; fewer than 5% did not have any comorbidity included in the Index. More than 80% had between 1 and 10 concurrent conditions. In examination of individual comorbidities as noted in on the CMS 2728 form, nearly two-thirds of the cohort had diabetes and roughly one-third had CHF. Nearly 95% were using in-center HD. The mean observation time in the study was about 2 years.
Medication exposure statistics describing these distributions were remarkably consistent across drug classes (Table 2), with exposure ranging from 0.52±0.31 to 0.55±0.31 for the individual classes, meaning that on average, an individual member of the study cohort had the drug available for 52%–55% of that individual’s observation time. In considering the potential for serial exposures to different classes of antihypertensive agents, the mean PDC was 0.70±0.29 for use of any of the study medications, indicating a higher level of coverage over time. Medians (50th percentiles) were very similar to means, suggesting that there was minimal skew to the distributions. The 75th percentile for the individual classes of antihypertensive agents was nearly 0.80, the typical threshold or “cut-score” used in studies of medication compliance.
Table 2.
Distribution of proportion of days covered for each class of medication.
| Drug class | Mean ± SD | 25th % | Median | 75th % |
|---|---|---|---|---|
| ACEI/ARBs | 0.52±0.31 | 0.23 | 0.53 | 0.80 |
| β-Blockers | 0.53±0.31 | 0.25 | 0.54 | 0.81 |
| CCBs | 0.55±0.31 | 0.28 | 0.58 | 0.84 |
| ≥ 1 Agent1 | 0.70±0.29 | 0.50 | 0.79 | 0.95 |
1 On at least one of the above 3 classes of medications.
Abbreviations: SD, standard deviation; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; β-Blocker, β-adrenergic antagonist; CCB, calcium channel blocker.
In the adjusted statistical analyses of factors associated with medication exposure, several findings demonstrated substantial consistency across classes of agents (Table 3). Increasing ww(P < 0.0001), while highest BMI strata (> 30kg/m2) was significantly associated with decreased exposure (P < 0.0001) for each drug class. A history of a cerebrovascular event was significantly associated with increased exposure, whereas diabetes mellitus was significantly associated with increased exposure except in the case of β-blockers.
Table 3.
Ordinary least squares regression models for the adjusted odds ratios of factors associated with the medication exposure over time (measured as the proportion of days covered), for each class.
| ACEI/ARBs | B-Blockers | CCBs | ≥ 1 Agent | |||||
|---|---|---|---|---|---|---|---|---|
| AOR | P -value | AOR | P -value | AOR | P -value | AOR | P -value | |
| Age, per 10 yrs | 1.05 | < 0.0001 | 1.05 | < 0.0001 | 1.10 | < 0.0001 | 1.10 | < 0.0001 |
| Male sex | 0.98 | 0.51 | 1.06 | 0.035 | 0.95 | 0.034 | 0.93 | 0.0010 |
| Race/Ethnicity | ||||||||
| Caucasian | 1.0 | − | 1.0 | − | 1.0 | − | 1.0 | − |
| African- American | 0.78 | < 0.0001 | 0.69 | < 0.0001 | 0.85 | < 0.0001 | 0.78 | < 0.0001 |
| Hispanic | 0.73 | < 0.0001 | 0.56 | < 0.0001 | 0.84 | < 0.0001 | 0.68 | < 0.0001 |
| Other | 0.97 | 0.57 | 0.67 | < 0.0001 | 0.84 | 0.0005 | 0.85 | 0.0004 |
| Inability to ambulate | 1.16 | 0.052 | 1.13 | 0.10 | 1.08 | 0.29 | 1.10 | 0.13 |
| Inability to transfer | 0.61 | 0.0006 | 0.75 | 0.038 | 0.89 | 0.39 | 0.61 | < 0.0001 |
| Employed | 1.14 | 0.12 | 1.04 | 0.61 | 1.07 | 0.44 | 1.00 | 0.99 |
| BMI category | ||||||||
| < 20kg/m2 | 0.95 | 0.25 | 0.99 | 0.81 | 0.90 | 0.024 | 0.90 | 0.017 |
| 20-24.9kg/m2 | 1.0 | − | 1.0 | − | 1.0 | − | 1.0 | − |
| 25-29.9kg/m2 | 0.99 | 0.87 | 0.97 | 0.38 | 0.90 | 0.0007 | 0.93 | 0.0085 |
| 30+ kg/m2 | 0.84 | < 0.0001 | 0.85 | < 0.0001 | 0.79 | < 0.0001 | 0.72 | < 0.0001 |
| Smoker | 1.00 | 0.98 | 0.90 | 0.032 | 0.97 | 0.53 | 0.94 | 0.15 |
| Substance abuser | 0.97 | 0.66 | 0.90 | 0.15 | 0.95 | 0.51 | 0.92 | 0.24 |
| Liu Comorbidity Score2 | 1.02 | 0.47 | 1.05 | 0.12 | 0.93 | 0.020 | 0.99 | 0.79 |
| Comorbidities | ||||||||
| DM | 1.28 | < 0.0001 | 0.99 | 0.66 | 1.14 | < 0.0001 | 1.23 | < 0.0001 |
| CHF | 0.99 | 0.68 | 1.00 | 0.94 | 0.93 | 0.023 | 0.98 | 0.48 |
| CAD | 1.00 | 0.96 | 1.31 | < 0.0001 | 0.93 | 0.033 | 1.11 | 0.0003 |
| PVD | 1.02 | 0.57 | 0.91 | 0.014 | 0.88 | 0.0014 | 0.93 | 0.057 |
| CVA | 1.23 | < 0.0001 | 1.29 | < 0.0001 | 1.25 | < 0.0001 | 1.30 | < 0.0001 |
| Hb < 11.0 | 0.93 | 0.012 | 0.90 | 0.0001 | 1.00 | 0.89 | 0.96 | 0.13 |
| Self-care dialysis | 0.94 | 0.27 | 0.99 | 0.84 | 0.73 | < 0.0001 | 0.82 | < 0.0001 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor; β-blocker, β-adrenergic antagonist; CCB, calcium channel blocker; statin, AOR, adjusted odds ratios; BMI, body mass index; HTN, hypertension; DM, diabetes; CHF, congestive heart failure; CAD, coronary artery disease; PVD; peripheral vascular disease; CVA, cerebrovascular accident; Hb, hemoglobin.
Race had a marked effect on medication exposure. Medication exposure was significantly lower for each medication class, as well as for the use of any of the classes, for all minority groups relative to Caucasians, except for ACEIs–ARBs and individuals of “other” race. AORs were 0.78 and 0.73 for ACEI s–ARBs in African-Americans and Hispanics, respectively. Medication AORs ranged from 0.56 to 0.69 among minority groups for β-blockers, and from 0.84 to 0.85 among minorities for CCBs. When use of any of these agents was modeled, the AORs for medication exposure were 0.68 for Hispanics (P < 0.0001), 0.78 for African-Americans (P < 0.0001), and 0.85 for individuals of other races (P = 0.0005).
DISCUSSION
In this study, we applied for the first time to dialysis patients, a measure of cumulative drug exposure, to determine the factors associated with long-term use of antihypertensive medications with putative cardioprotective properties. In a large cohort of dually Medicaid–Medicare-eligible incident dialysis patients, we found several factors to be associated with increased cumulative exposure to each of several classes of these medications, including age, history of a cerebrovascular event, and diabetes; a BMI ≥ 30kg/m2 was associated with decreased exposure to the classes of medications included in the study. The most striking finding, however, was that race/ethnicity was strongly and negatively associated with cumulative exposure. Medication exposure for each of the three classes of medications included in the study, as well as for use of any one of the three classes, was consistently lower in non-Caucasians, with the sole exception of ACEIs–ARBs in individuals of “other” race. The odds of exposure to these medications in minorities were only about three-quarters that for Caucasians.
Little work has been done in examining the association between race–ethnicity and exposure to antihypertensive medication in dialysis patients, with many large-scale studies not having addressed this question systematically.43–47 A more rigorous approach than those previously used, specifically one that accounts for cumulative drug exposure over time, was therefore needed for several reasons. First, among studies that did explicitly examine this issue, conflicting results have emerged. For example, such conflicting results were found for the specific issue of CCB use by African-Americans,45,48,49 with one study showing an association of African-American race with CCB use, another showing no such association, and a yet another showing that African-Americans were more likely to be prescribed dihydropyridine-type CCBs than other types of CCBs. Second, previous work on race–ethnicity and exposure to antihypertensive medication in dialysis patients has been limited by use of a cross-sectional approach at only a single time point; indeed, our own recent work, using such an approach, showed that minorities were actually more likely to receive these medications than were Caucasians.35 Third, older work in a small study of patient–related adherence to antihypertensive medications in hemodialysis patients found that adherence was lower for minorities,50 suggesting the possibility that exposure over time in minorities could be less than for non-minorities. We therefore elected to use the more granular detail provided by the PDC,41,42 which has never been used in the dialysis population. Another key element of our study was that we analyzed only individuals who received at least one prescription for the antihypertensive agents included in the study, thereby restricting the study to persons who were considered suitable candidates for these drugs by their providers.
Why minorities showed have less cumulative exposure to these antihypertensive medications is unclear. The factors contributing to race-based differences in care are complex and encompass both provider-centric factors (e.g., provider behavior or site of care51) and patient-centric factors (e.g., health literacy or healthcare costs52). One intriguing possibility is that minorities might have more discontinuous use of these medications than do Caucasians, (i.e., that prescriptions for the medications might be used through a sequence of lapse and reinitiation at higher rates in non-Caucasians than in Caucasians). Discontinuation followed by later resumption of medications occurs often in dialysis, but why this would occur at higher rates in minorities is uncertain. Another possibility is that differences among providers may exist in beliefs about the relative risks and benefits of antihypertensive agents for different racial groups, even after the initial selection of an agent. It it also possible that adherence to the filling or refilling of prescriptions over time is lower in non-Caucasians than in Caucasians, as suggested by limited earlier work.50 It is important to note that our results should not be misconstrued as measuring medication adherence per se, since it was not possible to determine, in the present analysis, whether either patients or providers were making decisions to stop or modify therapy. We therefore deliberately termed our analysis one of “exposure,” because it did not permit us to ascertain whether a drug was actually ingested or whether cessation of medication was a volitional choice of the patient or the provider. However, all provider- and patient-centric factors, including adherence to prescribed medication use, should be investigated in future work, with detailed attention given to the complex challenge of characterizing variability in prescription-refill patterns over time at the level of the individual patient.
It is important to contextualize our findings in the present understanding of the utility of the antihypertensive medications included in our study. Whether these antihypertensive agents actually provide any cardioprotective benefits to chronic dialysis patients is unclear.53–55 It would therefore be premature to label the differential use of medications by race as a “disparity.” However, agreement about the utility of these medications on the part of providers would not seem to be sufficient reason to explain why minorities would have less cumulative exposure to these medications than do Caucasians, and further efforts to extend this work to other types of medications may provide insights about the scope of this phenomenon.
The present study has several important limitations. First, we studied only patients dually eligible for Medicaid and Medicare. In having Medicaid, the patients we studied probably represent the neediest patients and were more likely to be female and non-Caucasian, to have functional limitations, to engage in risk-creating behaviors, and to be receiving in-center hemodialysis than hemodialysis at home or peritoneal dialysis.37 Although it may not be possible to completely extend our results to the general dialysis population, they do highlight important differences in care for emerging classes of dialysis patients. Second, our use of pharmacy prescription records to infer medication exposure does not equate to proof of medication use. However, it seems implausible that patients would repeatedly fill prescriptions for medications that they are not taking. Third, although some patients may have qualified for participation in Medicaid through a spend-down program (whereby they must meet a certain threshold of out-of-pocket costs before qualifying for full coverage), we attempted to minimize this bias by requiring all patients to demonstrate actual use of Medicaid for acquiring their prescriptions during the initial 90 days of dialysis. Lastly, although we stipulated that all patients were required to have a diagnosis of hypertension at the time of initiation of dialysis, it is conceivable that rates of hypertension decrease disproportionately over time in minorities relative to Caucasians, obviating the disproportionate use of antihypertensive medications in minority populations. However, this seems an unlikely explanation for our findings, in that African-Americans in particular have better survival with dialysis than do Caucasians,19 and that hypertension is associated with improved survival in ESRD.56
In summary, cumulative exposure to ACEIs–ARBs, β-blockers, and CCBs was substantially lower among non-Caucasians than among Caucasians in a cohort of dually Medicaid-and-Medicare–eligible chronic dialysis patients. Because these dialysis patients have insurance coverage for prescription medications as well as regular contact with healthcare providers, differences by race in exposure to these drugs over time should be minimal among patients who are candidates for the drugs. Given this, the factors responsible for this finding must be studied in future detailed work.
DISCLAIMER
The data reported here were supplied by the United States Renal Data System (DUA#2007–10 and 2009–19) and the Centers for Medicare & Medicaid Services (DUA#19707). The interpretation and reporting of these data are the responsibility of the author(s) and should in no way be seen as an official policy or interpretation of the US Government.
CONFLICTS OF INTEREST
The authors have no financial conflicts of interest to declare.
ACKNOWLEDGMENTS
We thank Connie Wang for technical assistance in the preparation of this manuscript. Funding for this study was provided by NIH (NIDDK) grants R01 DK080111-02 (T.I.S.) and K23 DK085378-01 (J.B.W.), a National Kidney Foundation Young Investigator Award (J.B.W.), and a Sandra A. Daugherty Foundation Grant (J.B.W.).
REFERENCES
- 1. Sonel AF, Good CB, Mulgund J, Roe MT, Gibler WB, Smith SC, Jr, Cohen MG, Pollack CV, Jr, Ohman EM, Peterson ED. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation 2005;111:1225–1232 [DOI] [PubMed] [Google Scholar]
- 2. Sheats N, Lin Y, Zhao W, Cheek DE, Lackland DT, Egan BM. Prevalence treatment, and control of hypertension among African Americans and Caucasians at primary care sites for medically under-served patients. Ethnic Dis 2005;15:25–32 [PubMed] [Google Scholar]
- 3. Setoguchi S, Glynn RJ, Avorn J, Levin R, Winkelmayer WC. Ten-year trends of cardiovascular drug use after myocardial infarction among community-dwelling persons > or =65 years of age. Am J Cardiol 2007;100:1061–1067 [DOI] [PubMed] [Google Scholar]
- 4. Riehle JF, Lackland DT, Okonofua EC, Hendrix KH, Egan BM. Ethnic differences in the treatment and control of hypertension in patients with diabetes. J Clin Hypertens (Greenwich) 2005;7:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidem Dr S 2010;19:834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olomu AB, Grzybowski M, Ramanath VS, Rogers AM, Vautaw BM, Chen B, Roychoudhury C, Jackson EA, Eagle KA. Evidence of disparity in the application of quality improvement efforts for the treatment of acute myocardial infarction: the American College of Cardiology’s Guidelines Applied in Practice Initiative in Michigan. Am Heart J 2010;159:377–384 [DOI] [PubMed] [Google Scholar]
- 7. Jha AK, Staiger DO, Lucas FL, Chandra A. Do race-specific models explain disparities in treatments after acute myocardial infarction? Am Heart J 2007;153:785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall SA, Chiu GR, Kaufman DW, Kelly JP, Link CL, Kupelian V, McKinlay JB. General exposures to prescription medications by race/ethnicity in a population-based sample: results from the Boston Area Community Health Survey. Pharmacoepidem Dr S 2010;19:384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vulic D, Lee BT, Dede J, Lopez VA, Wong ND. Extent of control of cardiovascular risk factors and adherence to recommended therapies in US multiethnic adults with coronary heart disease: from a 2005–2006 national survey. Am J Cardiovasc Drugs 2010;10:109–114 [DOI] [PubMed] [Google Scholar]
- 10. Bagchi AD, Stewart K, McLaughlin C, Higgins P, Croghan T. Treatment and outcomes for congestive heart failure by race/ethnicity in TRICARE. Med Care 2011;49:489–495 [DOI] [PubMed] [Google Scholar]
- 11. Mehta JL, Bursac Z, Mehta P, Bansal D, Fink L, Marsh J, Sukhija R, Sachdeva R. Racial disparities in prescriptions for cardioprotective drugs and cardiac outcomes in Veterans Affairs Hospitals. Am J Cardiol 2010;105:1019–1023 [DOI] [PubMed] [Google Scholar]
- 12. Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother 2009;43:185–193 [DOI] [PubMed] [Google Scholar]
- 13. Litaker D, Koroukian S, Frolkis JP, Aron DC. Disparities among the disadvantaged: variation in lipid management in the Ohio Medicaid program. Prev Med 2006;42:313–315 [DOI] [PubMed] [Google Scholar]
- 14. Wasse H, Speckman RA, Frankenfield DL, Rocco MV, McClellan WM. Predictors of delayed transition from central venous catheter use to permanent vascular access among ESRD patients. Am J Kidney Dis 2007;49:276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis 2008;52:753–760 [DOI] [PubMed] [Google Scholar]
- 16. Cavanaugh KL, Wingard RL, Hakim RM, Elasy TA, Ikizler TA. Patient dialysis knowledge is associated with permanent arteriovenous access use in chronic hemodialysis. Clin J Am Soc Nephrol 2009;4:950–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chakkera HA, O’Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, Chertow GM. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol 2005;16:269–277 [DOI] [PubMed] [Google Scholar]
- 18. Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol 2011;22:743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Renal Data System, USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases , Betheseda, MD, 2012. [Google Scholar]
- 20. Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non- insulin-dependent diabetes and hypertension. N Engl J Med 1998;338:645–652 [DOI] [PubMed] [Google Scholar]
- 21. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–2007 [PubMed] [Google Scholar]
- 22. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin- converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145–153 [DOI] [PubMed] [Google Scholar]
- 23. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–1390 [DOI] [PubMed] [Google Scholar]
- 24. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 25. Kjeldsen SE, Dahlof B, Devereux RB, Julius S, Aurup P, Edelman J, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn S, Wedel H. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA 2002;288:1491–1498 [DOI] [PubMed] [Google Scholar]
- 26. Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362:7–13 [DOI] [PubMed] [Google Scholar]
- 27. McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–771 [DOI] [PubMed] [Google Scholar]
- 28. Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893–1906 [DOI] [PubMed] [Google Scholar]
- 29. Wassertheil-Smoller S, Psaty B, Greenland P, Oberman A, Kotchen T, Mouton C, Black H, Aragaki A, Trevisan M. Association between cardiovascular outcomes and antihypertensive drug treatment in older women. JAMA 2004;292:2849–2859 [DOI] [PubMed] [Google Scholar]
- 30. Demers C, McMurray JJ, Swedberg K, Pfeffer MA, Granger CB, Olofsson B, McKelvie RS, Ostergren J, Michelson EL, Johansson PA, Wang D, Yusuf S. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. Jama 2005;294:1794–1798 [DOI] [PubMed] [Google Scholar]
- 31. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895–906 [DOI] [PubMed] [Google Scholar]
- 32. Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–2428 [DOI] [PubMed] [Google Scholar]
- 33. Mohan IK, Khan M, Wisel S, Selvendiran K, Sridhar A, Carnes CA, Bognar B, Kalai T, Hideg K, Kuppusamy P. Cardioprotection by HO-4038, a novel verapamil derivative, targeted against ischemia and reperfusion-mediated acute myocardial infarction. Am J Physiol Heart Circ Physiol 2009;296:H140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed LA, Salem HA, Attia AS, El-Sayed ME. Enhancement of amlodipine cardioprotection by quercetin in ischaemia/reperfusion injury in rats. J Pharm Pharmacol 2009;61:1233–1241 [DOI] [PubMed] [Google Scholar]
- 35. Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, Spertus JA, Shireman TI. Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis 2011;58:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Powe NR. Let’s get serious about racial and ethnic disparities. J Am Soc Nephrol 2008;19:1271–1275 [DOI] [PubMed] [Google Scholar]
- 37. Wetmore JB, Rigler SK, Mahnken JD, Mukhopadhyay P, Shireman TI. Considering health insurance: how do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant 2010;25:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Volkova N, McClellan W, Soucie JM, Schoolwerth A. Racial disparities in the prevalence of cardiovascular disease among incident end-stage renal disease patients. Nephrol Dial Transplant 2006;21:2202–2209 [DOI] [PubMed] [Google Scholar]
- 39. Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 2010;77:141–151 [DOI] [PubMed] [Google Scholar]
- 40. Wetmore JB, Shireman TI. The ABCs of cardioprotection in dialysis patients: a systematic review. Am J Kidney Dis 2009;53:457–466 [DOI] [PubMed] [Google Scholar]
- 41. Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–461 [DOI] [PubMed] [Google Scholar]
- 42. Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int 2002;62:1784–1790 [DOI] [PubMed] [Google Scholar]
- 44. Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant 2004;19:1842– –1848 [DOI] [PubMed] [Google Scholar]
- 45. Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. beta-Blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med 2004;164: 2465–2471 [DOI] [PubMed] [Google Scholar]
- 46. Ishani A, Herzog CA, Collins AJ, Foley RN. Cardiac medications and their association with cardiovascular events in incident dialysis patients: cause or effect? Kidney Int 2004;65:1017–1025 [DOI] [PubMed] [Google Scholar]
- 47. Lopes AA, Bragg-Gresham JL, Ramirez SP, Andreucci VE, Akiba T, Saito A, Jacobson SH, Robinson BM, Port FK, Mason NA, Young EW. Prescription of antihypertensive agents to haemodialysis patients: time trends and associations with patient characteristics, country and survival in the DOPPS. Nephrol Dial Transplant 2009;24:2809–2816 [DOI] [PubMed] [Google Scholar]
- 48. Kestenbaum B, Gillen DL, Sherrard DJ, Seliger S, Ball A, Stehman-Breen C. Calcium channel blocker use and mortality among patients with end-stage renal disease. Kidney Int 2002;61:2157–2164 [DOI] [PubMed] [Google Scholar]
- 49. Griffith TF, Chua BS, Allen AS, Klassen PS, Reddan DN, Szczech LA. Characteristics of treated hypertension in incident hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 2003;42:1260–1269 [DOI] [PubMed] [Google Scholar]
- 50. Curtin RB, Svarstad BL, Keller TH. Hemodialysis patients’ noncompliance with oral medications. ANNA J 1999;26:307–316; discussion 317, 335 [PubMed] [Google Scholar]
- 51. Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes 2011;4:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Charles H, Good CB, Hanusa BH, Chang CC, Whittle J. Racial differences in adherence to cardiac medications. J Natl Med Assoc 2003;95:17–27 [PMC free article] [PubMed] [Google Scholar]
- 53. Sinha AD, Agarwal R. Should all hypertensive dialysis patients receive a blocker of the renin-angiotensin system? Curr Hypertens Rep 2010;12:356–363 [DOI] [PubMed] [Google Scholar]
- 54. Chan KE, Ikizler TA, Gamboa JL, Yu C, Hakim RM, Brown NJ. Combined angiotensin- converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int 2011;80:978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol 2010;5:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Rev Nephrol 2007;3:493–506 [DOI] [PubMed] [Google Scholar]