Abstract
BACKGROUND
Renal artery stenosis (RAS) resulting in reduced renal blood flow (RBF) is a common cause of secondary hypertension and deterioration of renal function, which may lead to end-stage renal disease. Recruitment and formation of periarterial collateral vessels may serve to bypass RAS and restore distal blood supply. We hypothesized that development of collaterals around RAS may preserve kidney function.
METHODS
Collateral formation index (CI) was assessed using multidetector computed tomography as fractional vascular volume surrounding the stenosis in 31 pigs with unilateral RAS. Single kidney RBF and glomerular filtration rate (GFR) were also measured.
RESULTS
Of 25 pigs that developed significant stenosis (≥65%), 8 demonstrated minor collateral development (CI < 0.3), and 17 showed major collateral development (CI ≥ 0.3). The degree of RAS was significantly higher in pigs with major collaterals compared with pigs with minor collaterals, and poststenotic kidney cortical volume, perfusion, RBF, and GFR were significantly lower. In a subset of pigs matched for the degree of RAS, RBF and GFR remained lower in pigs with major collaterals.
CONCLUSIONS
We conclude that collaterals develop in animals with significant RAS in proportion to its severity and might be triggered by distal injury, such as decreases in cortical volume and perfusion. However, development of collaterals was unable to confer measurable benefits for stenotic kidney function distal to severe RAS.
Keywords: blood pressure, collateral circulation, computed tomography, hypertension, renal artery stenosis, renal function.
Renal artery stenosis (RAS), characterized by a reduction in renal blood flow (RBF), is a common cause of secondary hypertension and deterioration of renal function,1 which may progress to ischemic nephropathy and end-stage renal disease. Atherosclerosis coexisting with RAS (ARAS) is associated with even poorer outcomes than nonatherosclerotic RAS in terms of renal and cardiovascular disease.2
When a major arterial vessel is occluded, periarterial collateral vessel recruitment and formation may develop in order to bypass the obstruction and restore blood supply to the distal ischemic territory. Myocardial collateral vessels can emerge by increased flow through recruitment of native nonfunctional collaterals (that are already present)3 or by vasculogenesis of new vessels stimulated by ischemia. In the renal artery, a significant occlusion leads to rapid endothelial cell replication within as little as 1 day, with angiographically detectable collaterals becoming evident in 10 days.4 Inflammation, ischemia, or changes in shear stress are likely the dominant mechanisms underlying development of extra-renal peristenotic collateral pathways4 that have been demonstrated in RAS rats.5 In addition to neovascularization outside the kidney, ischemic nephropathy or inflammation of the stenotic kidney may also trigger intra-renal compensatory mechanisms aimed at preserving normal kidney function. We have previously shown that inflammation due to hypercholesterolemia (HC), a surrogate for early atherosclerosis, leads to intra-renal microvascular proliferation, which contributes to sustain renal perfusion.6 Therefore, proliferation of extra-renal conduits may also theoretically contribute to sustaining renal perfusion in HC superimposed on RAS.
However, the renal hemodynamic forces that determine the extent of collateral circulation in RAS and the efficacy of this mechanism have not been fully described. Therefore, this study was designed to test the hypothesis that kidney function is preserved by development of collaterals around a stenosis in the renal artery. We also examined whether collateral formation is affected by coexisting HC. For this purpose we used multidetector computed tomography to measure single-kidney function in a swine model of unilateral RAS with and without superimposed HC and to assess the degree of corresponding collateral circulation growth.
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. Gradual development of unilateral RAS was induced by placement of a local-irritant coil in the main renal artery in 31 domestic pigs (37–60kg), as previously described.7 The pigs were fed either normal pig chow (n = 19) or a high cholesterol diet (n = 12) starting 6 weeks prior to induction of RAS. Following RAS induction, all pigs were observed for an additional 6 weeks, whence in vivo studies were performed.
On the day of the in vivo studies, animals were anesthetized with an intramuscular injection of telazol (5mg/kg) and xylazine (2mg/kg), intubated, and placed on a mechanical ventilator with room air. Anesthesia was maintained with an infusion of ketamine (11mg/kg/hour) and xylazine (1.8mg/kg/hour) in normal saline through an ear vein cannula (3ml/kg/hour). This was followed by placing 9F vascular sheaths into the carotid artery and external jugular vein via a neck cut-down under sterile conditions. Using fluoroscopic guidance, an 8F guide catheter was advanced to the renal artery for an angiogram to determine the angiographic degree of RAS following standard techniques of quantitative angiography.7–9 Mean arterial blood pressure was measured from the side-arm of the arterial vascular sheath. Blood samples were collected from each renal vein and the inferior vena cava for measurement of plasma renin activity (PRA) by radioimmunoassay (Diasorin, Stillwater, MN) and serum creatinine by enzyme-linked immunosorbent assay (ELISA) (Arbor Assays, Ann Arbor, MI). In addition, systemic plasma levels of basic fibroblast growth factor (R&D Systems, Minneapolis, MN) and the soluble vascular endothelial growth factor receptor fms-like tyrosine kinase 1 (NovaTeinBio, Cambridge, MA) were measured by ELISA to assess angiogenesis; transforming growth factor beta (R&D Systems), plasminogen activator inhibitor 1 (Oxford Biomedical Research, Rochester Hills, MI), and endothelin 1 (Cayman Chemical, Ann Arbor, MI), all measured by ELISA, served as markers of fibrosis.
A 5F pigtail catheter was inserted through the venous vascular sheath into the superior vena cava for injection of low-osmolar nonionic contrast medium iopamidol (Isovue-370; Squibb Diagnostics, Princeton, NJ) during subsequent imaging studies. The animals were then transferred to the helical multidetector computed tomography scanner (SOMATOM Definition 64; Siemens, Forcheim, Germany), where in vivo flow studies were performed to assess bilateral single-kidney RBF, glomerular filtration rate (GFR), and regional renal perfusion, as previously described.10,11 Briefly, this consisted of sequential acquisition of 140 consecutive scans after a bolus injection of iopamidol (0.5 cc/kg over 2 seconds). The first 70 scans were collected at 0.68 second intervals during respiratory suspension at end expiration, whereas the following 70 scans were acquired every 2 seconds during suspension of breathing with assisted breathing every 30 seconds. The flow study was followed by a volume study for the measurement of cortical and medullary volumes11 as well as the degree of collateral formation around the stenosis. During the volume study, the kidneys were scanned from pole to pole during infusion of iopamidol (0.5ml/kg over 5 seconds).
Data analysis of renal function.
Regions of interest were manually traced in multidetector computed tomography images of the aorta, renal cortex, and medulla of both kidneys, and their densities sampled over time. Time–density curves were fitted and used to calculate bilateral single-kidney regional perfusion (ml/minute/cc tissue), RBF (ml/minute), and GFR (ml/minute), as shown previously.10 Renal volume was determined by planimetry. Renal vascular resistance was calculated by dividing the mean arterial pressure by RBF.
Data analysis of collateral formation.
Reconstruction of the volume data acquired from the abdominal CT scan produced 512×512 matrix images at a resolution of 0.39×0.39mm and a 0.6-mm slice thickness with 0.3-mm overlap through the planes containing the renal artery. Collateral index (CI) was calculated by assessment of the fractional vascular volume in the immediate zone around the stenosis.
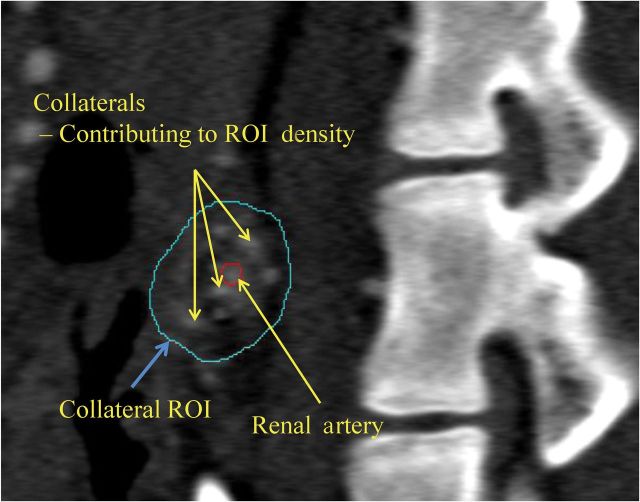
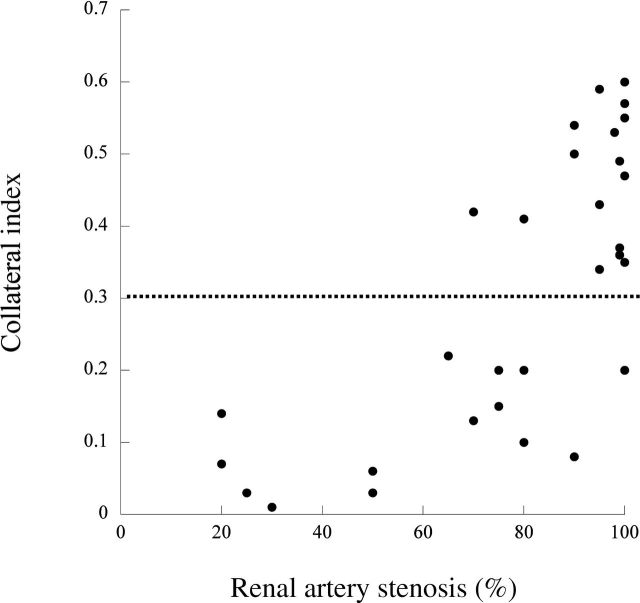
For this purpose, the zone encompassing visually discernible collaterals around the stenotic segment of the renal artery was manually traced. Two regions of interest were then sampled (Figure 1), representing the collateral vessel zone surrounding the stenotic main renal artery and the contrast-filled residual lumen of the renal artery. The fractional vascular area containing blood vessels around the stenosis, representing the collateral vessel growth, was determined as the ratio between the opacity of the two regions of interest, as previously used to calculate vascular volume fraction.10 Significant collateral formation was observed in pigs with degrees of RAS ≥65%, which is also considered hemodynamically significant.8 Because no pig with a degree of RAS <65% showed a CI ≥0.30 (Figure 2), a CI of 0.30 was chosen as the cut-off between minor and major collateral development.
Figure 1.
Computed tomography image demonstrating the regions of interest (ROIs), including the renal artery (red circle) and collateral vessels.
Figure 2.
Distribution of collateral index in comparison with the severity of renal artery stenosis.
Similarly, the extent of perirenal collaterals that enter the kidney via the renal capsule was assessed using a region of interest surrounding the kidney. A perirenal CI was calculated by the same method used for the collaterals surrounding the stenotic renal artery.
Statistical analysis.
Results are expressed as mean ± SEM. Comparisons between groups were performed using unpaired Student’s t test. Least-square regression was used to assess the relationship between degree of stenosis or CI and renal function. Multiple simple linear regression analysis was done to determine differences between groups’ correlations. Statistical significance was accepted for P < 0.05.
RESULTS
Of the 31 pigs, 6 pigs (n = 3 HC diet) had mild, hemodynamically insignificant RAS (≤50%), whereas 25 had hemodynamically significant RAS (≥65%). The CI in pigs fed normal diet (0.37±0.04) did not differ from that in pigs fed an HC diet (0.36±0.05, P = 0.44) and their degrees of stenosis were similar (Table 1). Therefore, data from both groups were subsequently compiled. Of the total 25 pigs with significant RAS, 8 (n = 3 HC diet) demonstrated minor (CI < 0.30) collateral development, and 17 (n = 7 HC diet) had major (CI > 0.30) collateral development (Table 2, Figure 2 and 3). Most of the observed collateral circulation spawned directly from the aorta to the poststenotic segment of renal artery or to the kidney pelvis, and some extended to the renal capsule. A measurement of the perirenal index demonstrated that pigs with major peristenotic collaterals also exhibited significant collateral development around the entire kidney, compared with pigs with mild RAS, although it did not differ from that in pigs with minor collaterals (Figure 4).
Table 1.
Characteristics and renal function Mean ± SEM in pigs with significant renal artery stenosis on normal and high cholesterol diet
| Parameter | Normal diet, n = 15 | High cholesterol diet, n = 10 | ||
|---|---|---|---|---|
| Body weight, kg | 47.2±1.7 | 48.7±2.4 | ||
| Collateral index, % | 0.37±0.04 | 0.36±0.05 | ||
| Degree of stenosis, % | 88.1±3.3 | 92.3±3.2 | ||
| Serum creatinine, mg/dl | 1.66±0.05 | 1.71±0.07 | ||
| Systemic plasma renin activity, ng/ml/h | 6.7±2.9 | 15.9±9.9 | ||
| Systolic blood pressure, mm Hg | 148±7 | 147±5 | ||
| Diastolic blood pressure, mm Hg | 115±6 | 118±4 | ||
| Stenotic kidney | ||||
| Renal cortical volume, cc | 73.8±7.3** | 87.3±7.1** | ||
| Cortical perfusion, ml/min/cc tissue | 3.6±0.4** | 4.0±0.5 | ||
| Renal medullary volume, cc | 12.2±1.0** | 12.5±1.6 | ||
| Medullary perfusion, ml/min/cc tissue | 2.3±0.3 | 2.0±0.3 | ||
| Renal blood flow, ml/min | 314±57** | 382±52** | ||
| Renal vascular resistance, mm Hg/ml*min–1 | 0.63±0.21** | 0.45±0.12** | ||
| Glomerular filtration rate, ml/min | 49±5** | 67±5*,** | ||
| Contralateral kidney | ||||
| Renal cortical volume, cc | 146.7±12.9 | 125.1±8.4 | ||
| Cortical perfusion, ml/min/cc tissue | 4.7±0.3 | 4.7±0.4 | ||
| Renal medullary volume, cc | 22.4±2.6 | 16.6±2.2 | ||
| Medullary perfusion, ml/min/cc tissue | 2.8±0.3 | 2.6±0.4 | ||
| Renal blood flow, ml/min | 756±58 | 616±58 | ||
| Renal vascular resistance, mm Hg/ml*min–1 | 0.18±0.02 | 0.22±0.02 | ||
| Glomerular filtration rate, ml/min | 102±5 | 93±6 | ||
*P < 0.05 vs normal diet; **P < 0.05 vs contralateral kidney
Table 2.
Characteristics, renal function, and collateral formation Mean ± SEM in pigs with mild and significant renal artery stenosis (RAS)
| Significant RAS | |||||
|---|---|---|---|---|---|
| Parameter | Mild RAS, n = 6 | Minor collaterals, n = 8 | Major collaterals, n = 17 | ||
| Body weight, kg | 49.7±4.3 | 50.2±2.2 | 46.6±1.7 | ||
| Collateral index, % | 0.03±0.01 | 0.16±0.02** | 0.46±0.02*, ** | ||
| Degree of stenosis, % | 32.5±5.7 | 79.4±4.0** | 94.7±2.0*, ** | ||
| Serum creatinine, mg/dl | 1.74±0.05 | 1.61±0.08 | 1.73±0.05 | ||
| Systemic plasma renin activity, ng/ml/h | 0.11±0.1 | 3.61±3.3 | 15.41±6.4 | ||
| Systolic blood pressure, mm Hg | 117±5 | 130±4** | 157±5*, ** | ||
| Diastolic blood pressure, mm Hg | 89±4 | 101±4** | 124±4*, ** | ||
| Stenotic kidney | |||||
| Renal cortical volume, cc | 95.3±4.3 | 101.7±7.4*** | 68.6±5.4*, **, *** | ||
| Cortical perfusion, ml/min/cc tissue | 5.5±0.4 | 4.7±0.7 | 3.3±0.3*,**,*** | ||
| Renal medullary volume, cc | 18.3±3.1 | 13.1±1.9 | 11.3±0.9**,*** | ||
| Medullary perfusion, ml/min/cc tissue | 2.7±0.4 | 2.7±0.6 | 1.9±0.2** | ||
| Renal blood flow, ml/min | 562±16*** | 512±75 | 261±33*, **, *** | ||
| Renal vascular resistance, mm Hg/ml*min–1 | 0.18±0.01 | 0.49±0.30 | 0.72±0.15**, *** | ||
| Glomerular filtration rate, ml/min | 76±3 | 70±6*** | 50±5*, **, *** | ||
| Contralateral kidney | |||||
| Renal cortical volume, cc | 100.0.3±4.8 | 124.7±9.9** | 147.0±11.9** | ||
| Cortical perfusion, ml/min/cc tissue | 5.6±0.3 | 5.5±0.4 | 4.3±0.3*, ** | ||
| Renal medullary volume, cc | 18.3±3.3 | 14.4±2.4 | 22.2±2.4* | ||
| Medullary perfusion, ml/min/cc tissue | 3.1±0.5 | 3.4±0.6 | 2.4±0.2 | ||
| Renal blood flow, ml/min | 603±23 | 739±79 | 681±53 | ||
| Renal vascular resistance, mm Hg/ml*min–1 | 0.16±0.00 | 0.16±0.01 | 0.22±0.02*, ** | ||
| Glomerular filtration rate, ml/min | 84±6 | 89±7 | 102±5** | ||
*P < 0.05 vs minor collaterals; **P < 0.05 vs mild RAS; ***P < 0.05 vs contralateral kidney.
Figure 3.
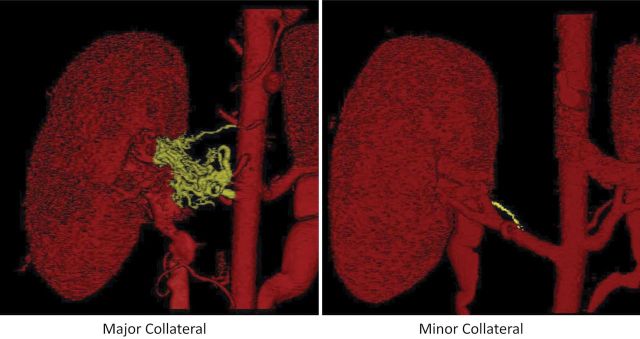
Three-dimensional reconstruction of kidneys imaged using multidetector computed tomography, showing significant renal artery stenosis with minor (right) and major (left) collateral formation.
Figure 4.
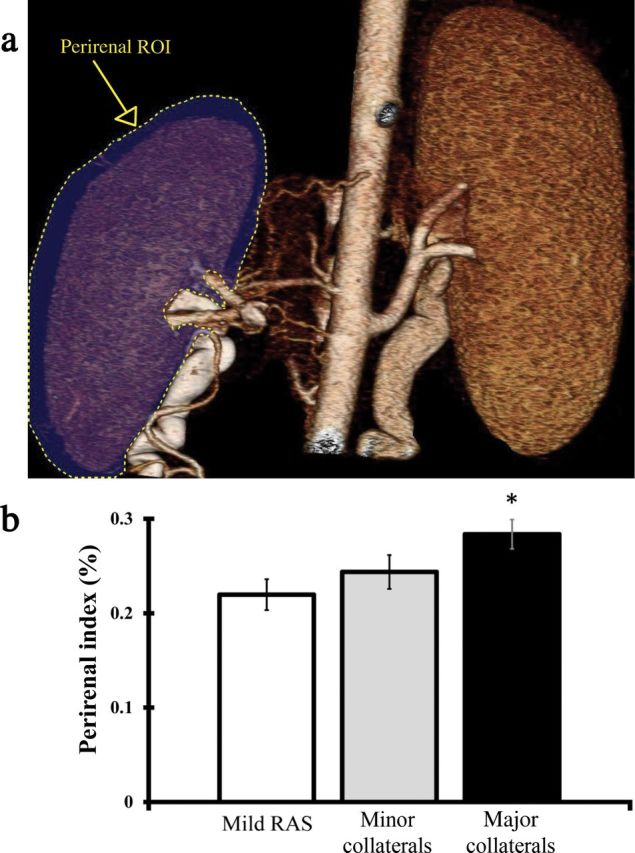
Perirenal collaterals in pig kidneys. (a) Illustration of perirenal region of interest surrounding stenotic kidney. (b) Measure of perirenal index in pigs with mild renal artery stenosis (RAS) or with significant RAS accompanied by major and minor collaterals. *P < 0.01 vs mild RAS.
Pigs with significant RAS and minor collaterals had cortical and medullary volumes and perfusions, RBF, and GFR similar to pigs with mild hemodynamically insignificant RAS, whereas their blood pressures were higher (Table 2).
In animals with significant RAS, the degree of RAS was significantly greater in pigs with major collaterals than in pigs with minor collaterals, their blood pressures were higher, and their serum creatinine levels tended to be higher as well (P = 0.07) (Table 2). Elevation of PRA did not reach statistical significance due to high variability. Stenotic kidney cortical volume and perfusion, as well as RBF and GFR, were significantly lower in pigs with major collaterals than in those with minor collaterals (Table 2). There were no significant differences between these 2 groups in medullary volume or perfusion, although only in major collateral group were these lower than in mild RAS and their contralateral kidneys (CLK).
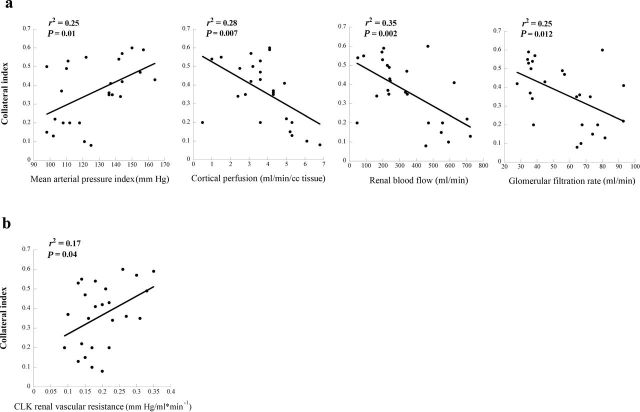
CI showed a significant direct correlation with mean arterial pressure (r 2 = 0.25; P = 0.01) but significant inverse correlations with stenotic kidney perfusion (r 2 = 0.28; P = 0.007), RBF (r 2 = 0.35; P = 0.002), and GFR (r 2 = 0.25; P = 0.01) (Figure 5a), as well as with cortical volume (r 2 = 0.28; P = 0.007).
Figure 5.
Relationships between collateral index and renal function. (a) Correlations of blood pressure, cortical perfusion, renal blood flow, and glomerular filtration rate in the stenotic kidney with collateral index. (b) The contralateral kidney (CLK) demonstrated a significant direct relationship between stenotic kidney collateral index and renal vascular resistance.
Cortical volume and GFR of the CLK were significantly greater in both collateral-forming RAS groups compared with mild RAS pigs, yet cortical perfusion of the CLK was reduced compared with mild RAS only in pigs with major collaterals. CI was directly correlated with CLK renal vascular resistance (r 2 = 0.17; P = 0.04) (Figure 5b). Furthermore, CI tended to correlate inversely with CLK cortical perfusion (r 2 = 0.14; P = 0.06) but not with its volume (r 2 = 0.01; P = 0.62), RBF (r 2 = 0.03; P = 0.45), or GFR (r 2 = 0.01; P = 0.63).
Systemic levels of angiogenic and fibrogenic factors did not differ among the groups (Table 3). CI did not correlate with systemic levels of the angiogenic factors b- FGF (r 2 = 0.007; P = 0.75) and fms-like tyrosine kinase 1 (r 2 = 0.01; P = 0.67) or the fibrogenic factors transforming growth factor beta (r 2 = 0.10; P = 0.21), plasminogen activator inhibitor 1 (r 2 = 0.01; P = 0.68), and endothelin 1 (r 2 = 0.04; P = 0.48).
Table 3.
Systemic levels of angiogenic and fibrogenic factors Mean ± SEM in pigs with mild and significant renal artery stenosis (RAS)
| Significant RAS | |||
|---|---|---|---|
| Circulating Factor | Mild RAS, n = 6 | Minor collaterals, n = 8 | Major collaterals, n = 17 |
| b-FGF (pg/ml) | 36.8±8.9 | 32.1±7.4 | 39.1±6.5 |
| sFlt-1 (ng/ml) | 0.18±0.04 | 0.25±0.07 | 0.18±0.03 |
| TGF-beta (pg/ml) | 1,208.0±192.6 | 1,010.2±102.5 | 1,176.7±82.0 |
| PAI-1 (ng/ml) | 11.1±1.8 | 11.3±1.8 | 11.7±2.0 |
| Endothelin 1 (pg/ml) | 36.3±1.5 | 37.9±1.2 | 38.9±1.9 |
Abbreviations: b-FGF, basic fibroblast growth factor; PAI-1, plasminogen activator inhibitor 1; sFLT-1, soluble vascular endothelial growth factor receptor fms-like tyrosine kinase 1; TGF-beta, transforming growth factor beta.
In order to determine the contribution of the degree of RAS, we also matched subsets of animals with major (n = 8) or minor (n = 6) collateral development for the degrees of stenosis (P = 0.14) (Table 4). Interestingly, despite similar degrees of stenosis, blood pressure continued to be higher, whereas stenotic kidney RBF and GFR remained lower in the animals showing major collateral development compared with matched stenoses inducing minor collateral formation (Table 4). Although cortical and medullary perfusion were seemingly lower in pigs with major collaterals, the difference between groups was not statistically significant. Conversely, cortical and medullary perfusion of the CLK were significantly reduced in the subset of animals with major collaterals, whereas RBF and GFR in those animals were comparable (Table 4).
Table 4.
Characteristics and renal function Mean ± SEM in pigs with minor and major collaterals with similar degrees of renal artery stenosis
| Parameter | Minor collaterals, n = 6 | Major collaterals, n =8 |
|---|---|---|
| Body weight, kg | 45.6±2.9 | 49.8±2.9 |
| Collateral index, % | 0.15±0.02 | 0.47±0.03* |
| Degree of stenosis, % | 83.3±4.0 | 89.1±3.4 |
| Serum creatinine, mg/dl | 1.63±0.10 | 1.75±0.08 |
| Systemic plasma renin activity, ng/ml/h | 4.7±4.4 | 10.5±4.6 |
| Systolic blood pressure, mm Hg | 133±5 | 160±9* |
| Diastolic blood pressure, mm Hg | 104±4 | 126±7* |
| Stenotic kidney | ||
| Renal cortical volume, cc | 93.0±6.4** | 63.3±9.3*, ** |
| Cortical perfusion, ml/min/cc tissue | 4.6±0.9 | 3.2±0.4** |
| Renal medullary volume, cc | 12.0±2.4 | 11.9±1.6** |
| Medullary perfusion, ml/min/cc tissue | 2.5±0.7 | 1.8±0.3 |
| Renal blood flow, ml/min | 445±83 | 244±59*, ** |
| Renal vascular resistance, mm Hg/ml*min–1 | 0.61±0.39 | 0.87±0.29** |
| Glomerular filtration rate, ml/min | 65±6 | 43±7*, ** |
| Contralateral kidney | ||
| Renal cortical volume, cc | 121.6±13.2 | 143.0±12.7 |
| Cortical perfusion, ml/min/cc tissue | 5.6±0.5 | 4.4±0.4* |
| Renal medullary volume, cc | 13.5±3.1 | 24.4±23.8* |
| Medullary perfusion, ml/min/cc tissue | 3.9±0.7 | 2.3±0.4* |
| Renal blood flow, ml/min | 732±107 | 674±52 |
| Renal vascular resistance, mm Hg/ml*min–1 | 0.17±0.02 | 0.21±0.02 |
| Glomerular filtration rate, ml/min | 88±9 | 102±8 |
*P < 0.05 vs minor collaterals; **P < 0.05 vs contralateral kidney.
DISCUSSION
Our study demonstrates that peristenotic collaterals develop in pigs with hemodynamically significant RAS in proportion to its severity. Yet, factors other than the degree of RAS alone contribute to this process because different extents of collateral development were also observed in subgroups of pigs with similar degrees of stenosis. Predominantly, a decrease in stenotic kidney cortical volume and perfusion seems to trigger major collateral formation in those pigs. Interestingly, a fall in CLK cortical perfusion also emerged as an important determinant of collateral formation in the stenotic kidney. Nevertheless, the collaterals succeeded in preserving kidney function only in moderate RAS, as cortical perfusion, RBF, and GFR were not maintained in the stenotic kidney distal to severe RAS.
Development of a collateral blood supply may serve as a mechanism to preserve function distal to an occlusion in the cardiovascular conduit vessels. Preserved cardiac function has been shown in hypertensive patients that have increased number of angiographically apparent coronary collaterals.3 Coronary collateral circulation has a relevant protective effect in patients with coronary artery disease,12 as patients with good collateralization have better survival rates. Levels of coronary collateralization can be variable among individuals.13 We have previously shown that in normal animals an acute obstruction of the left anterior descending coronary artery caused a significant reduction in myocardial perfusion distal to the stenosis, which was preserved in hypertensive or hypercholesterolemic animals due to recruitment or neovascularization of intra-myocardial microvessels.3 Likewise, in a chronic coronary artery stenosis model, myocardial function was impaired, but early hypertension led to microvascular proliferation that partly and temporarily protected the myocardium from ischemic insults.14
In the kidney, in response to renal artery occlusion, collaterals have been described to develop in 3 phases.5 Preexisting vessels fill and perfuse the kidney almost immediately. These collaterals then dilate and elongate within 24 hours, followed by neovascularization in 1–5 days, progressing to a network of tortuous vessels, as we also observed in our animals. Extrarenal collateral circulation can develop from several sources, most commonly from lumbar, inferior phrenic, adrenal, testicular, ovarian, ureteric, inferior mesenteric, and superior capsular arteries.15 Alternative perfusion pathways are therefore achieved by collateral vessels that either develop around the stenosis as bypass conduits or directly penetrate the renal capsule. As demonstrated in our study, when increased peristenotic collaterals fail to preserve kidney function, perirenal collaterals develop.
Microvascular proliferation may also occur within the kidney as a result of HC and inflammatory mechanisms, and it maintains RBF and GFR.6 The importance of this mechanism is underscored by our observation that in animals with RAS, a single injection of endothelial progenitor cells into a stenotic kidney preserves its blood supply, hemodynamics, and function by promoting generation of new vessels.16 However, although HC elicits microvascular proliferation within the kidney, it does not appear to amplify formation of extra-renal collaterals, as in the present study we found no differences in collateral growth in RAS animals with or without superimposed early atherosclerosis. This observation also argues against impairment in collateral formation in our model, as suggested in other HC models.17,18 The reason for the slightly higher GFR in the HC group is unclear and warrants further studies.
The association between renal functional impairment and hypoperfusion has been chronicled in the acute and chronic pig model of RAS.16,19,20 A progressive acute experimental constriction of the renal artery demonstrated that significant hemodynamic effects do not occur until the degree of stenosis reached 50%–70%.19 Similarly, in our chronic model, significant collateral formation did not occur under conditions of mild RAS. Because animals with major collaterals had significantly greater degrees of stenosis than those with minor collateral development, we also compared subgroups of animals with minor and major collaterals matched by degree of stenosis. Interestingly, in spite of the similar degrees of stenosis, animals with major collaterals in this subanalysis were still characterized by significant decreases in cortical volume, RBF, and GFR. This suggests that collateral formation may have been triggered by remodeling of the poststenotic kidney. Possibly, loss of intra-renal microvasculature in these kidneys triggers extra-renal collateral formation as a defense mechanism to sustain blood supply to the kidney. Levels of circulating angiogenic and fibrogenic factors did not show a significant relationship with collateral development, yet may not fully reflect changes within the kidney. Nevertheless, the combination of an increase in blood pressure and extra-renal collaterals was evidently effective in preserving kidney function only in moderate RAS, as the combination failed to sustain RBF and GFR distal to severe RAS.
Furthermore, we cannot rule out a critical contribution of the CLK to collateral development in the stenotic kidney. The CLK renal vascular resistance and GFR were higher, whereas both cortical and medullary perfusion were lower, in pigs with major collaterals, possibly because their blood pressure was also higher. We have previously shown that chronic renovascular hypertension leads to fibrosis20 that may impair renal perfusion. Conceivably, the simultaneously decreased perfusion in both the contralateral and stenotic kidneys might accelerate mechanisms that attempt to restore stenotic kidney blood supply and overall RBF. This is also supported by the increased renal vascular resistance in the CLK of pigs with major collateral development.
Our study was limited by the unavailability of tissue to evaluate the structure and microvasculature of the kidneys. Also, we used young pigs, with short-term exposure to atherosclerosis and renovascular disease relative to humans, and the progression of kidney injury might evolve differently with a longer duration of RAS. Estimation of the degree of stenosis using angiography also suffers from some limitations, yet it is considered the reference standard and has been shown to be reproducible.9 In addition, the spatial resolution of the CT is too low to visually detect collaterals <0.5mm.
In summary, using unique imaging methods, the present study demonstrates that extra-renal periarterial collaterals develop more extensively as the degree of RAS increases and are followed by formation of perirenal collaterals that penetrate the renal capsule. Kidney fibrosis or remodeling might be responsible for triggering the onset of collateral formation in an attempt to preserve cortical perfusion and GFR. A decrease in CLK perfusion might also signal to accelerate collateral formation. Nevertheless, these collaterals are not an effective mechanism for preservation of blood supply or function in the stenotic kidney.
DISCLOSURE
The authors declared no conflicts of interest.
ACKNOWLEDGMENT
This study was partly supported by the National Institutes of Health (grantsDK73608, HL77131, HL085307, and C06 RR018898) and by the American Heart Association.
REFERENCES
- 1. Meyrier A, Hill GS, Simon P. Ischemic renal diseases: new insights into old entities. Kidney Int 1998; 54 2–13 [DOI] [PubMed] [Google Scholar]
- 2. Urbieta-Caceres VH, Lavi R, Zhu XY, Crane JA, Textor SC, Lerman A, Lerman LO. Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol 2010; 299 F135–F140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu XY, Daghini E, Chade AR, Versari D, Krier JD, Textor KB, Lerman A, Lerman LO. Myocardial microvascular function during acute coronary artery stenosis: effect of hypertension and hypercholesterolaemia. Cardiovasc Res 2009; 83 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ilich N, Hollenberg NK, Williams DH, Abrams HL. Time course of increased collateral arterial and venous endothelial cell turnover after renal artery stenosis in the rat. Circ Res 1979; 45 579–582 [DOI] [PubMed] [Google Scholar]
- 5. Donahoe PK, Osmond JD, III, Stewart DR, Hendren WH., III Renal parenchymal tolerance to artery occlusion: a time and damage study in rats developing collateral circulation. Ann Surg 1973; 178 138–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chade AR, Krier JD, Galili O, Lerman A, Lerman LO. Role of renal cortical neovascularization in experimental hypercholesterolemia. Hypertension 2007; 50 729–736 [DOI] [PubMed] [Google Scholar]
- 7. Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 1999; 10 1455–1465 [DOI] [PubMed] [Google Scholar]
- 8. Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 2002; 106 1165–1171 [DOI] [PubMed] [Google Scholar]
- 9. Bell MR, Britson PJ, Chu A, Holmes DR, Jr, Bresnahan JF, Schwartz RS. Validation of a new unix-based quantitative coronary angiographic system for the measurement of coronary artery lesions. Cathet Cardiovasc Diagn 1997; 40 66–74 [DOI] [PubMed] [Google Scholar]
- 10. Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 2001; 281 F630–F638 [DOI] [PubMed] [Google Scholar]
- 11. Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector ct: comparison with electron-beam CT. Radiology 2007; 243 405–412 [DOI] [PubMed] [Google Scholar]
- 12. Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta- analysis. Eur Heart J 2012;33(5):614–621 [DOI] [PubMed] [Google Scholar]
- 13. Zhang S, Zhao L, Shen L, Xu D, Huang B, Wang Q, Lin J, Zou Y, Ge J. Comparison of various niches for endothelial progenitor cell therapy on ischemic myocardial repair: coexistence of host collateralization and akt-mediated angiogenesis produces a superior microenvironment. Arterioscler Thromb Vasc Biol 2012; 32 910–923 [DOI] [PubMed] [Google Scholar]
- 14. Urbieta Caceres VH, Lin J, Zhu XY, Favreau FD, Gibson ME, Crane JA, Lerman A, Lerman LO. Early experimental hypertension preserves the myocardial microvasculature but aggravates cardiac injury distal to chronic coronary artery obstruction. Am J Physiol Heart Circ Physiol 300 H693–H701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hietala SO, Kunz R. Collateral circulation in stenosis or occlusion of the renal artery. Cardiovasc Radiol 1979; 2 249–255 [DOI] [PubMed] [Google Scholar]
- 16. Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 2009; 119 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Weel V, de Vries M, Voshol PJ, Verloop RE, Eilers PH, van Hinsbergh VW, van Bockel JH, Quax PH. Hypercholesterolemia reduces collateral artery growth more dominantly than hyperglycemia or insulin resistance in mice. Arterioscler Thromb Vasc Biol 2006; 26 1383–1390 [DOI] [PubMed] [Google Scholar]
- 18. Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation 2000; 102 III370–III376 [DOI] [PubMed] [Google Scholar]
- 19. Rognant N, Rouviere O, Janier M, Le QH, Barthez P, Laville M, Juillard L. Hemodynamic responses to acute and gradual renal artery stenosis in pigs. Am J Hypertens 2010;23 1216–1219 [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez-Porcel M, Krier JD, Lerman A, Sheedy PF, Jr, Romero JC, Napoli C, Lerman LO. Combination of hypercholesterolemia and hypertension augments renal function abnormalities. Hypertension 2001; 37 774–780 [DOI] [PubMed] [Google Scholar]