Abstract
To determine whether serum levels of 25-hydroxyvitamin D (25(OH)D) in young adults are associated with risk of type 1 diabetes mellitus (T1D), we conducted a prospective, nested case-control study among US active-duty military personnel with serum in the US Department of Defense Serum Repository, identifying 310 T1D cases diagnosed between 1997 and 2009 with at least 2 serum samples collected before disease onset and 613 controls matched to cases on age, sex, race/ethnicity, branch of military service, and dates of serum collection. Conditional logistic regression was used to estimate rate ratios and 95% confidence intervals. Among non-Hispanic whites, those with average 25(OH)D levels of ≥100 nmol/L had a 44% lower risk of developing T1D than those with average 25(OH)D levels <75 nmol/L (rate ratio = 0.56, 95% confidence interval: 0.35, 0.90, P for trend = 0.03) over an average follow-up of 5.4 years. In quintile analyses, T1D risk was highest among individuals whose 25(OH)D levels were in the lowest 20% of those measured. There was no association between 25(OH)D levels and risk of T1D among non-Hispanic blacks or Hispanics. Low 25(OH)D levels may predispose healthy, young, non-Hispanic white adults to the development of T1D.
Keywords: nested case-control study, type 1 diabetes, vitamin D
Type 1 diabetes (T1D) is characterized by destruction of the insulin-producing β-cells in the pancreas, with evidence of an autoimmune process in most cases (1). Although there is a strong genetic predisposition to T1D (2, 3), environmental factors are likely to be important, particularly for onset of T1D in adulthood (4). Vitamin D is emerging as an important immunomodulator, and healthy young adults with elevated serum 25-hydroxyvitamin D (25(OH)D) levels have a lower risk of multiple sclerosis (5), an autoimmune disease genetically and epidemiologically related to T1D (6). Evidence for a role of vitamin D in T1D is conflicting and is based on exposure to vitamin D only in utero (7–9) or early childhood (10–12). Although in about 60% of T1D cases onset occurs after the age of 20 years (13), there are no longitudinal studies, to our knowledge, examining the relation between vitamin D levels in adults and T1D risk.
Therefore, we conducted a prospective nested case-control study among healthy US military personnel to examine whether serum 25(OH)D levels in young adults predict their risk of developing T1D.
MATERIALS AND METHODS
Study population
Beginning in the mid-1980s, the US military has tested all military personnel for human immunodeficiency virus antibodies prior to enrollment, before and after overseas deployments, and during regular medical checkups (on average, every 2 years). Sera left over from these tests are documented, stored in freezers at −30°C by the Department of Defense Serum Repository, and made available for medical research (14). The Department of Defense Serum Repository has collected and stored more than 40 million serum samples from more than 8 million personnel from all branches of US military service. The institutional review boards of the Harvard School of Public Health and the Walter Reed Army Institute of Research approved this study and waived the need for informed consent to use archived medical records and stored sera. We were not able to identify individual patients through serum sample labeling, preserving patient anonymity.
Case ascertainment
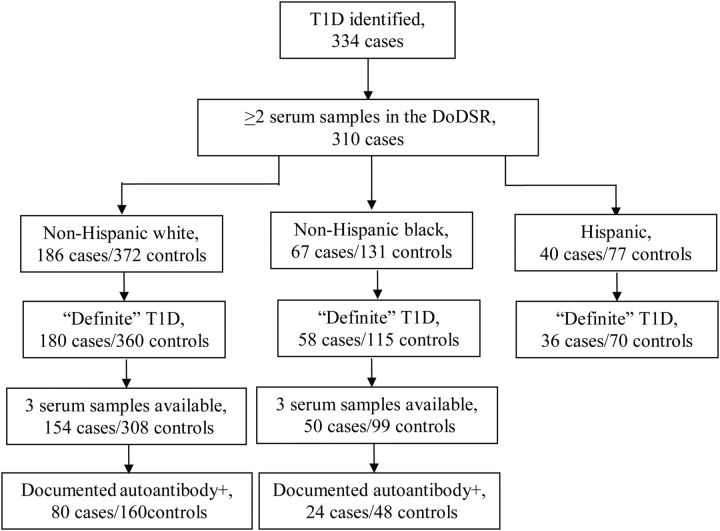
Active-duty personnel diagnosed with any type of diabetes are evaluated for medical discharge from the military. We searched the database of the US Naval Council of Personnel Boards, which is also the physical disability agency for the US Marines, for members who were diagnosed with diabetes between 1997 and 2009. To identify personnel with a specific diagnosis of T1D, we reviewed all medical records and excluded individuals with a diagnosis of type 2 diabetes. Information collected from the medical records included dates of first symptom onset and diagnosis, blood glucose levels (random and/or fasting) at the first medical encounter, signs and symptoms at presentation (polyuria, polydipsia, polyphagia, unintended weight loss, ketoacidosis, coma), and results of autoantibody testing (antiglutamic acid decarboxylase (GAD) immunoglobulin G, anti-islet cell immunoglobulin G). We identified 334 cases of possible T1D; 264 (79%) had a specific diagnosis of T1D in the medical records, 46 (14%) had a diagnosis of insulin-dependent diabetes mellitus, 17 (5.1%) had a diagnosis of “diabetes,” and the remaining 7 (2.1%) had a diagnosis described as “other” or “unknown.” The information available in the medical records for these 24 “uncertain” cases was consistent with T1D. GAD antibody results were reported in the medical records of 179 cases, of whom 140 (78%) were GAD positive, and 26 (15%) were GAD negative. Islet cell antibody testing was reported for 125 cases, including 97 who were also tested for GAD antibodies, and 34 (27%) were reported positive. The main case group included all 334 cases; the “definite” case group excluded the 24 uncertain cases, and the autoantibody-positive subgroup included 156 cases (Figure 1).
Figure 1.
Number of T1D cases and controls by race group, US active-duty military, 1997–2009. Total number with ≥2 sera samples available in the DoDSR (310 cases) includes 17 labeled “other race” that were not further analyzed. Hispanic cases were not further subgrouped because of small sample size. Medical record review was the source of autoantibody documentation. DoDSR, Department of Defense Serum Repository; T1D, type 1 diabetes.
Control and serum sample ascertainment
Two controls were randomly selected from the Department of Defense Serum Repository and matched to each case on age (±1 year), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other (race categories as defined by the US military)), dates of serum collection (±30 days), and branch of military service (navy or marines). All controls were on active duty on the date of symptom onset of the matched case. For 7 cases, only 1 suitable control was identified. Up to 3 serum samples collected before diabetes symptom onset and 1 after diabetes symptom onset were obtained for each case and its matched control.
Laboratory analysis
Serum 25(OH)D levels were measured in all available serum samples by a direct, competitive chemiluminescence immunoassay (CLIA) using the LIAISON 25OH Vitamin D TOTAL assay (DiaSorin, Inc., Stillwater, Minnesota) in the laboratory of 1 of the authors (R.H.). Sera from each matched case-control set were analyzed in the same assay batch. From 369 quality control samples that were dispersed throughout the study samples, we calculated an interassay coefficient of variation of 5.3%, and an intraassay coefficient of variation of 3.1%. Further, the expected seasonal and race variations in 25(OH)D were observed in the controls: The mean 25(OH)D levels were 66.0 nmol/L for samples collected in winter (January through April), 85.5 nmol/L for samples collected in summer (July through October), and 72.3 nmol/L for samples collected in spring and fall (May, June, November, and December). Non-Hispanic whites had higher average 25(OH)D levels (84.6 nmol/L) than non-Hispanic blacks (50.7 nmol/L) (P < 0.0001) and Hispanics (73 nmol/L) (P < 0.0001). Further, 25(OH)D levels appeared to be unaffected by length of storage as mean levels did not vary significantly by year of sample collection (data not shown).
Covariates
Information was available on the state of residence at entry into the navy/marines, and was categorized into tiers of latitude as described in previous studies of this population (5, 15).
Statistical analysis
Because of seasonal fluctuations in 25(OH)D, multiple measures of 25(OH)D are considered a better indicator of an individual's long-term 25(OH)D status than 1 measurement collected at 1 point in time. Thus, our primary hypothesis was that long-term, average 25(OH)D levels are determinant of T1D risk. We reduced random variation in 25(OH)D levels caused by the season during which blood was collected by creating deseasonalized 25(OH)D levels as previously described (5). Briefly, within each race/ethnic group, using linear regression models, we regressed the raw 25(OH)D measurements on the periodic function (−sin(2ΠX/12)−cos(2ΠX/12)), where X is the month of sample collection (16). We also included in the same model indicators for age, sex, and laboratory assay batch, to reduce variations from these sources. The residuals from this model were added to the sex-specific 25(OH)D means derived from the model, as men in general tend to have higher levels of 25(OH)D than women, to obtain adjusted 25(OH)D measurements specific to each race/sex group. For each individual, adjusted preonset 25(OH)D levels from multiple (i.e., 2 or 3) samples were averaged together and used as an estimate of their long-term 25(OH)D exposure status. Cases with only 1 preonset sample (n = 24 cases), as well as their matched controls, were excluded from further examination as a single 25(OH)D measurement at 1 point in time is less likely to reflect longer-term 25(OH)D nutrition, leaving 310 cases in the main case group (Figure 1). The “other” race group had only 17 T1D cases and was not further analyzed in this study. Analyses were also conducted excluding the 24 cases with an uncertain T1D diagnosis. We further restricted the analyses to 1) cases and controls with 3 averaged serum samples to further reduce random variation in average 25(OH)D levels and 2) cases that were GAD positive and/or islet cell antibody positive by medical record report, as they were laboratory-confirmed cases of autoimmune T1D.
Within each race/ethnic group, we used conditional logistic regression to estimate the relative risk of T1D. Multivariate analyses further included latitude of residence at entry into the military. Average 25(OH)D levels were modeled as a continuous variable to assess linearity. In categorical analyses, we used 2 approaches: a priori defined categories and quintiles of 25(OH)D (tertiles for non-Hispanic blacks and Hispanics because of smaller sample sizes); the latter approach was used to avoid making assumptions regarding the shape of the dose-response curve. A priori categories were chosen on the basis of generally accepted levels of vitamin D insufficiency, deficiency, and sufficiency, as used in our prior study of 25(OH)D and multiple sclerosis (5). The categories were defined as <25 nmol/L, 25–<50 nmol/L, 50–<75 nmol/L, 75–<100 nmol/L, and ≥100 nmol/L; however, because of small sample sizes within some categories, in analyses among non-Hispanic whites and Hispanics, the lowest 3 categories were collapsed to <75 nmol/L, and among non-Hispanic blacks, the categories were collapsed to <50 nmol/L, 50–<75 nmol/L, and ≥75 nmol/L. Because of variations between assays and laboratories in measuring 25(OH)D (17, 18), the 25(OH)D levels measured in this study may not be equivalent to those assessed by using other methods (see Appendix for conversion equation, on the basis of analyses of 139 samples using both methods). The quantiles were created on the basis of the distribution of average adjusted 25(OH)D among the controls, and the median of each quantile was modeled as a continuous variable to assess linearity of the rate ratios across the quantiles. For all analyses, a 2-tailed P value of <0.05 was considered significant. All analyses were conducted in SAS, version 9.1, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
General characteristics of the T1D cases and controls with at least 2 available serum samples are shown in Table 1.
Table 1.
Characteristics of T1D Cases and Their Matched Controls With 2 or More Preonset Serum Samples, US Active-Duty Military, 1997–2009
| Cases (n = 310) |
Controls (n = 613) |
|||||
|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Race | ||||||
| Non-Hispanic white | 186 | 60 | 372 | 60.7 | ||
| Non-Hispanic black | 67 | 21.6 | 131 | 21 | ||
| Hispanic | 40 | 12.9 | 77 | 12.6 | ||
| Other | 17 | 5.5 | 33 | 5.4 | ||
| Sex | ||||||
| Male | 294 | 94.8 | 583 | 95.1 | ||
| Female | 16 | 5.2 | 30 | 4.9 | ||
| Latitude of residencea | ||||||
| North | 69 | 22 | 112 | 18.3 | ||
| Middle | 104 | 34 | 188 | 30.7 | ||
| South | 122 | 39 | 266 | 43.4 | ||
| Outside the continental United States | 5 | 1.6 | 5 | 0.8 | ||
| Missing data | 10 | 3.2 | 42 | 6.9 | ||
| No. of preonset serum samples | ||||||
| 2 | 45 | 14.5 | 90 | 14.7 | ||
| 3 | 265 | 85.5 | 523 | 85.3 | ||
| Age at first serum sample, years | 20.6 (4.1) | 20.6 (4.0) | ||||
| Age at symptom onset, years | 26 (5.4) | |||||
| First random glucose level at diagnosis, mg/dL | 262 | 484 (199) | ||||
| First fasting glucose level at diagnosis, mg/dL | 97 | 289 (154) | ||||
| Weight at onset, poundsb | 191 | 173 (28.7) | ||||
| GAD IgG seropositivity | 132 | 78 | ||||
| Islet cell IgG seropositivity | 32 | 28 | ||||
| Signs/symptoms at onset | ||||||
| Polyuria | 253 | 82 | ||||
| Polydipsia | 249 | 80 | ||||
| Polyphagia | 75 | 24 | ||||
| Unintended weight loss | 177 | 57 | ||||
| Ketoacidosis | 112 | 36 | ||||
| Blurred vision | 111 | 36 | ||||
| Coma | 2 | 0.7 | ||||
Abbreviations: GAD, glutamic acid decarboxylase; IgG, immunoglobulin G; SD, standard deviation; T1D, type I diabetes.
a Latitude of residence at entry into the military was categorized as follows: North, states at latitudes higher than 41°–42°; Middle, states at latitudes between 37° and 41°–42°; South, states at latitudes below ∼37°; Outside the continental United States, Alaska, Hawaii, and Puerto Rico.
b One pound = 0.45 kg.
Non-Hispanic whites
The average adjusted 25(OH)D level in the preclinical onset samples was 93.2 nmol/L (range, 42.1–172 nmol/L) in the cases and 97.0 nmol/L (range, 31–211 nmol/L) in the controls.
In a priori categorical analyses adjusted for latitude of residence at entry to the military, among all cases with ≥2 samples, compared with individuals with 25(OH)D levels <75 nmol/L, those with levels 75–<100 nmol/L had a 40% lower risk of T1D, and those with levels ≥100 nmol/L had a 44% lower risk of T1D (P for trend = 0.03) (Table 2). Excluding the 6 uncertain cases and their matched controls strengthened these results (Table 2). Further restricting the analysis to definite cases with 3 samples yielded a 54% lower risk among those with 25(OH)D between 75 and 100 nmol/L and a 63% lower risk among those with levels ≥100 nmol/L when compared with those with levels <75 nmol/L (P for trend = 0.001) (Table 2). These rate ratios were somewhat attenuated in the autoantibody-positive subgroup: (75–<100 nmol/L vs. <75 nmol/L, rate ratio (RR) = 0.62, 95% confidence interval (CI): 0.31, 1.28; ≥100 nmol/L, RR = 0.41, 95% CI: 0.20, 0.84, P for trend = 0.02).
Table 2.
Rate Ratioa of T1D by A Priori Defined Categories of Preonset 25(OH)D Serum Levels—Non-Hispanic Whites, US Active-Duty Military, 1997–2009
| All Cases With ≥2 Samples (n = 186) |
Cases With ≥2 Samples, Excluding Uncertain Diagnosis of T1Db (n = 180) |
Cases With 3 Samples, Excluding Uncertain Diagnosis of T1Db (n = 154) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | No. of Controls | RR | 95% CI | No. of Cases | No. of Controls | RR | 95% CI | No. of Cases | No. of Controls | RR | 95% CI | |
| Levels of 25(OH)D, nmol/L | ||||||||||||
| <75 | 45 | 57 | 1.0 | Referent | 45 | 54 | 1.0 | Referent | 42 | 41 | 1.0 | Referent |
| 75–<100 | 76 | 160 | 0.60 | 0.38, 0.97 | 75 | 155 | 0.58 | 0.36, 0.95 | 63 | 133 | 0.46 | 0.27, 0.79 |
| ≥100 | 65 | 155 | 0.56 | 0.35, 0.90 | 60 | 151 | 0.50 | 0.31, 0.81 | 49 | 134 | 0.37 | 0.21, 0.63 |
| P for trend | 0.03 | 0.01 | 0.001 | |||||||||
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; RR, rate ratio; T1D, type 1 diabetes.
a Adjusted for matching factors (age, race, date of blood collection, sex, and branch of military service) and latitude of residence at entry into the military.
b Excludes 6 cases that did not have a clear diagnosis of T1D in the medical record and their 12 matched controls.
In quintile analyses adjusting for latitude of residence at entry into the military, the highest risk of T1D was among individuals whose 25(OH)D levels were in the lowest 20% (i.e., quintile 1) of those measured. Compared with this quintile, cases in quintiles 2–5 had similar rate ratios, with a 30% lower risk of T1D in the top versus bottom quintile among individuals with ≥2 serum samples, and a 35% lower risk when excluding uncertain T1D cases. Among those with 3 samples, the rate ratios of cases in the top versus bottom quintiles were similar both when excluding uncertain cases (P for trend = 0.008) and when further restricting to antibody-positive cases (P for trend = 0.04) (Table 3).
Table 3.
Rate Ratioa of T1D by Quintile of Preonset 25(OH)D Serum Level—Non-Hispanic Whites, US Active-Duty Military, 1997–2009
| Range of 25(OH)D levels, nmol/L | Median 25(OH)D level, nmol/L | All Cases With ≥2 Samples (n = 186) |
Cases With ≥2 Samples, Excluding Uncertain Diagnosis of T1Db (n = 180) |
Cases With 3 Samples, Excluding Uncertain Diagnosis of T1Db (n = 154) |
Cases With 3 Antibody-Positivec Samples, Excluding Uncertain Diagnosis of T1Db (n = 83) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | No. of Controls | RR | 95% CI | No. of Cases | No. of Controls | RR | 95% CI | No. of Cases | No. of Controls | RR | 95% CI | No. of Cases | No. of Controls | RR | 95% CI | |||
| Quintile | ||||||||||||||||||
| 1 | 31–<77 | 67.6 | 52 | 67 | 1 | Referent | 52 | 63 | 1 | Referent | 47 | 50 | 1 | Referent | 25 | 28 | 1 | Referent |
| 2 | 77–<88.7 | 82.8 | 36 | 77 | 0.56 | 0.32, 0.99 | 35 | 75 | 0.52 | 0.29, 0.93 | 30 | 62 | 0.47 | 0.25, 0.88 | 15 | 28 | 0.63 | 0.26, 1.49 |
| 3 | 88.7–<100.7 | 95.1 | 34 | 78 | 0.55 | 0.32, 0.96 | 34 | 76 | 0.53 | 0.30, 0.92 | 29 | 66 | 0.45 | 0.24, 0.82 | 15 | 34 | 0.5 | 0.22, 1.15 |
| 4 | 100.7–<114.1 | 106.5 | 25 | 78 | 0.42 | 0.24, 0.75 | 21 | 76 | 0.34 | 0.18, 0.62 | 19 | 66 | 0.3 | 0.16, 0.58 | 9 | 30 | 0.33 | 0.13, 0.83 |
| 5 | 114.1–210.7 | 126.6 | 39 | 72 | 0.7 | 0.42, 1.17 | 38 | 70 | 0.65 | 0.38, 1.10 | 29 | 64 | 0.47 | 0.26, 0.86 | 16 | 40 | 0.48 | 0.22, 1.05 |
| P for trend | 0.14 | 0.07 | 0.008 | 0.04 | ||||||||||||||
Abbreviations: CI, confidence interval; GAD, glutamic acid decarboxylase; IC, islet cell; 25(OH)D, 25-hydroxyvitamin D; RR, rate ratio; T1D, type 1 diabetes.
a Adjusted for matching factors (age, race, date of blood collection, sex, and branch of military service) and latitude of residence at entry into the military.
b Excludes 6 cases that did not have a clear diagnosis of T1D in the medical record and their 12 matched controls.
c GAD or IC positive by medical record report.
Non-Hispanic blacks
Among non-Hispanic blacks, there were 67 cases and 131 controls with at least 2 serum samples; 57 cases and 111 controls had 3 serum samples. Among those with 2 samples, average adjusted 25(OH)D levels were 49.1 nmol/L (range, 19.2–92 nmol/L) in the cases and 49.8 nmol/L (range, 12.7–113 nmol/L) in the controls. Overall, there were no significant associations between 25(OH)D levels and risk of T1D among blacks. After adjusting for latitude of residence at entry to the military, we found that a 50-nmol/L increase in 25(OH)D serum levels resulted in a rate ratio of developing T1D of 0.97 (95% CI: 0.45, 2.12, P = 0.94) among individuals with ≥2 samples. In a priori categorical analyses, using <50 nmol/L 25(OH)D as the reference (39 cases and 75 controls), the rate ratio was 0.99 (95% CI: 0.52, 1.88) for 25(OH)D levels 50–<75 nmol/L (24 cases and 47 controls) and 0.77 (95% CI: 0.22, 2.66) for 25(OH)D) levels ≥75 nmol/L (4 cases and 9 controls). The rate ratio for the top tertile (median 25(OH)D: 65.2 nmol/L) versus the bottom tertile (median 25(OH)D: 34.3 nmol/L) was 1.06 (95% CI: 0.53, 2.09, P for trend = 0.85) among those with ≥2 samples. Excluding the uncertain cases and restricting to those with 3 serum samples or GAD/islet cell antibodies did not materially change these results (data not shown).
Hispanics
The sample size of this group was small with only 39 cases and 75 controls (32 cases and 61 controls with 3 samples). The mean 25(OH)D level was 84.4 nmol/L (range: 35.4–142 nmol/L) in the cases and 82.2 nmol/L (range: 47.0–152 nmol/L) in the controls. No significant associations were observed between 25(OH)D levels and risk of T1D.
DISCUSSION
In this large, prospective study based on repeated measures of 25(OH)D in serum samples collected prior to the onset of T1D, we found that among non-Hispanic whites the risk of developing T1D was highest among those with levels of 25(OH)D in the bottom 20% (<77 nmol/L) of those measured, with little change in risk at levels of 25(OH)D ≥75 nmol/L, suggesting that increasingly higher levels of 25(OH)D do not confer additional protection. There was no association between 25(OH)D levels and T1D among non-Hispanic blacks or among Hispanics.
This is the first study, to our knowledge, to assess 25(OH)D serum levels in otherwise healthy adults and follow them for a clinical onset/diagnosis of T1D. Previous investigations of vitamin D and T1D have primarily examined vitamin D exposure during pregnancy (7–9) or childhood (10–12) and risk of a T1D diagnosis before adulthood and have had mixed results. The finding of an inverse association in the current study is consistent with previous work in this military cohort demonstrating that high 25(OH)D levels are associated with a lower risk of multiple sclerosis (5), suggesting inadequate vitamin D in adulthood may be an important risk factor for autoimmune disease in general. Studies of serum 25(OH)D among individuals with T1D are limited by their retrospective design, as all have been conducted at or after the time of onset/diagnosis of T1D (19–23), when 25(OH)D levels may be influenced by the disease itself or individual behavior changes in response to disease. In the current study, the preonset samples were collected on average 5.4 years before clinical onset of T1D; thus, it is unlikely that 25(OH)D levels would be influenced by overt symptoms of diabetes per se. Although we cannot rule out the possibility that the subclinical autoimmune process in T1D, which begins years prior to complete loss of insulin production (24), affects vitamin D metabolism resulting in lower circulating 25(OH)D levels, our results suggest that low 25(OH)D levels may contribute to the autoimmune process, or through another mechanism, lead to an increased risk of T1D.
Although our study has several strengths, including a prospective design with a representative control group and repeated measures of 25(OH)D, there are some limitations to consider. First is the potential misclassification of type of diabetes. All cases of “diabetes” were identified and then further reviewed for type. Individuals with a clear diagnosis of type 2 diabetes in the medical records were excluded from the study. The majority of cases included here (93%) were diagnosed with T1D or insulin-dependent diabetes mellitus by a physician. The remaining 7% did not have a specific diagnosis of T1D, but there was not enough information in the medical records to rule it out. Thus, we performed secondary analyses excluding these cases. However, certain ethnic groups, including blacks and Hispanics, are more likely to develop “idiopathic T1D,” which is characterized by an acute onset with no evidence of autoimmunity and commonly includes other hallmarks of a type-2 diabetic process (25); some have suggested that idiopathic T1D may be more properly described as “ketosis-prone type 2 diabetes” (26). Thus, because there may be more misclassification of true T1D in these ethnic groups, we restricted analyses to GAD-positive and/or islet cell antibody–positive cases, and results were similar to those for the entire study population. Second, as in all observational studies, possible confounding by other environmental or genetic risk factors cannot be ruled out. Sun exposure is an important determinant of circulating 25(OH)D levels (27). Because ultraviolet B radiation may have other immunosuppressive actions outside of the vitamin D pathway (28), we cannot exclude the possibility that the low T1D risk among individuals with elevated 25(OH)D is caused by a direct protective effect of ultraviolet B radiation rather than a vitamin D effect. This interpretation, however, would leave the previously reported inverse association between dietary vitamin D supplementation and T1D (10) unexplained. Confounding by a gene that both increases T1D risk and is associated with decreased 25(OH)D levels should also be considered. The cytochrome P450, family 2, subfamily R, polypeptide 1 gene (CYP2R1) encoding the 25-hydroxylase that converts vitamin D to 25(OH)D, has been associated with both circulating levels of 25(OH)D (29) and risk of T1D (30), though the latter associations are weak and cannot completely explain the inverse association seen here. Moreover, none of the other genes (31) that have been widely associated with T1D were associated with 25(OH)D in a large genome-wide association study (29). Finally, because of the demographic makeup of the US military, all T1D cases were adult-onset (i.e., age 18 years or later) and 95% of the cases were male; thus, these findings may not be generalizable to childhood- and adolescent-onset T1D or to women, and similar studies in these groups are needed.
The lack of any association between 25(OH)D levels among non-Hispanic blacks and Hispanics appears in contradiction to the hypothesis that low 25(OH)D levels increase risk of T1D, as both groups tend to have lower levels of 25(OH)D compared with whites. While some cases of T1D are likely idiopathic (see above), restricting our analysis to T1D cases with antibodies against GAD and/or islet cells did not change the results. One possible explanation is lack of power to detect an association because of the small sample sizes in these groups, compounded by the small number of non-Hispanic blacks with 25(OH)D levels in the range inversely associated with T1D in non-Hispanic whites (25(OH)D ∼>75 nmol/L). Continued follow-up in this population, thus increasing the sample size in these groups, will help to elucidate any associations.
The biological mechanism by which vitamin D may protect against T1D is not known but may involve a complex interplay of immunomodulation and β-cell protection (32). In nonobese diabetic mice, 1,25-dihydroxyvitamin D or its analogs can inhibit the maturation of dendritic cells, decrease interleukin-12 production, reduce the helper T cell 1TH populations driving the autoimmune response, and increase the 2TH and T regulatory cell populations (33–35). Further, 1,25-dihydroxyvitamin D may protect β-cells against cytokine-induced death (36–38). Vitamin D has also been shown to reduce the incidence and delay onset in other autoimmune disease models (39–41).
In summary, in this large prospective study based on repeated measures of serum 25(OH)D, we found that risk of developing T1D among young, apparently healthy, non-Hispanic white adults was highest among individuals whose levels of 25(OH)D were in the lowest 20% of those measured. Although an approximate 2-fold difference in risk of T1D was observed when comparing those with 25(OH)D levels above 75 nmol/L with those with levels below 75 nmol/L, this absolute level should be interpreted cautiously because of variations among 25(OH)D assays and laboratories performing those assays. If the association reported here is causal, a substantial proportion of T1D cases could be prevented by vitamin D supplementation in a dose largely considered to be safe. Whereas it would be premature to recommend widespread use of vitamin D supplements for prevention of T1D, the feasibility of a primary prevention trial should be considered.
Note added in proof: While our manuscript was in press, there was a publication by Gorham et al.43 based on the same source population of our study reporting an inverse association between serum 25(OH)D levels and diabetes risk. Gorham et al., however, included in their analyses all cases of “insulin dependent diabetes” identified in an electronic database without differentiating type 1 from type 2 diabetes, while, in contrast, we restricted our investigation to cases of type 1 diabetes confirmed by a review of the individual medical records and evidence of the presence of autoantibodies against glutamic acid decarboxylase or islet cells. Further, our review of the medical records also permitted the identification of the date of diabetes symptom onset, and thus the selection of multiple serum samples collected before that date.
ACKNOWLEDGEMENTS
Author affiliations: Department of Nutrition, Harvard School of Public Health, Harvard University, Boston, Massachusetts (Kassandra L. Munger, Jennifer Massa, Alberto Ascherio); Department of Epidemiology, Division of Preventive Medicine, Walter Reed Army Institute of Research, Silver Spring, Maryland (Lynn I. Levin); Department of Epidemiology, Harvard School of Public Health, Harvard University, Boston, Massachusetts (Jennifer Massa, Alberto Ascherio); Heartland Assays, Ames, Iowa (Ronald Horst); Orban Biotech, LLC, Boston, Massachusetts (Tihamer Orban); and Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, and Harvard Medical School, Harvard University, Boston, Massachusetts (Alberto Ascherio).
This study was funded by the National Institute of Neurological Disorders and Stroke (grant NS046635).
We thank Dr. Mark Rubertone and Dr. Angelia Eick, Armed Forces Health Surveillance Center, Silver Spring, Maryland, for control and serum sample identification and retrieval, Dr. Noel Howard, Naval Council of Personnel Boards, Washington Navy Yard, Washington, DC, for T1D case identification in the US Navy and Marines, and Leslie Unger, Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts, for technical assistance.
This study was presented as a poster at the 71st Annual Meeting of the American Diabetes Association in San Diego, California, June 24–28, 2011.
The views expressed are those of the authors and should not be construed to represent the positions of the US Department of the Army, the Department of the Navy, the Department of Defense, the National Institute of Neurological Disorders and Stroke, or the National Institutes of Health. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflicts of interest: K.L.M., L.I.L., J.M., and A.A. have no conflicts of interest to declare. T.O. is founder, Chief Executive Officer, and Chief Scientific Officer of Orban Biotech, LLC, and R.H. is the President and Chief Executive Officer of Heartland Assays, Inc.
APPENDIX
Test results indicating levels of 25(OH)D are known to vary by the analytical methods used (17, 18) and across laboratories (42). Because the actual 25(OH)D levels are important for comparison with those of other studies and for the generalization of our findings, we calibrated the 25(OH)D levels obtained in the current study with those obtained in our previous investigation of multiple sclerosis in the same population to facilitate comparisons. In the multiple sclerosis study, we used a radioimmunoassay (RIA) to measure 25(OH)D. We then measured 25(OH)D in 139 of the multiple sclerosis control serum samples with the CLIA. In these samples, the mean 25(OH)D levels were 8.1 nmol/L higher using the current CLIA (73.2 nmol/L) compared with the RIA (65.1 nmol/L). We derived a conversion equation by regressing the original RIA measurements on the new CLIA measurements:
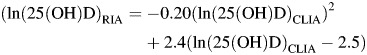 |
For reference of comparison with our previous study on multiple sclerosis, CLIA 50 nmol/L = RIA 45.1 nmol/L; CLIA 75 nmol/L = RIA 61.2 nmol/L; CLIA 100 nmol/L = RIA 73.0 nmol/L. The difference between the CLIA and the RIA results explains why the cutoff levels for 25(OH)D quintiles in Figure 1 are not the same for the 2 studies.
REFERENCES
- 1.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.Varney MD, Valdes AM, Carlson JA, et al. HLA DPA1, DPB1 alleles and haplotypes contribute to the risk associated with type 1 diabetes: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2010;59(8):2055–2062. doi: 10.2337/db09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32(4):457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Emery LM, Babu S, Bugawan TL, et al. Newborn HLA-DR DQ genotype screening: age- and ethnicity-specific type 1 diabetes risk estimates. Pediatr Diabetes. 2005;6(3):136–144. doi: 10.1111/j.1399-543X.2005.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 6.Handel AE, Handunnetthi L, Ebers GC, et al. Type 1 diabetes mellitus and multiple sclerosis: common etiological features. Nat Rev Endocrinol. 2009;5(12):655–664. doi: 10.1038/nrendo.2009.216. [DOI] [PubMed] [Google Scholar]
- 7.Stene LC, Ulriksen J, Magnus P, et al. Use of cod liver oil during pregnancy associated with lower risk of type I diabetes in the offspring. Diabetologia. 2000;43(9):1093–1098. doi: 10.1007/s001250051499. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen ME, Reinert L, Kinnunen L, et al. Serum 25-hydroxyvitamin D level during early pregnancy and type 1 diabetes risk in the offspring. Diabetologia. 2012;55(5):1291–1294. doi: 10.1007/s00125-012-2458-8. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen IM, Joner G, Jenum PA, et al. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61(1):175–178. doi: 10.2337/db11-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 11.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case–control study. Am J Clin Nutr. 2003;78(6):1128–1134. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 12.Simpson M, Brady H, Yin X, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 2011;54(11):2779–2788. doi: 10.1007/s00125-011-2278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rewers M, Norris J, Dabelea D. Epidemiology of type 1 diabetes mellitus. Adv Exp Med Biol. 2004;552:219–246. [PubMed] [Google Scholar]
- 14.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92(12):1900–1904. doi: 10.2105/ajph.92.12.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293(20):2496–2500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 16.Bliss C. Periodic Regression in Biology and Climatology. New Haven: The Connecticut Agricultural Experimental Station; 1958. pp. 3–55. [Google Scholar]
- 17.Carter GD, Jones JC, Berry JL. The anomalous behaviour of exogenous 25-hydroxyvitamin D in competitive binding assays. J Steroid Biochem Mol Biol. 2007;103(3-5):480–482. doi: 10.1016/j.jsbmb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–1556. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Pozzilli P, Manfrini S, Crino A, et al. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005;37(11):680–683. doi: 10.1055/s-2005-870578. [DOI] [PubMed] [Google Scholar]
- 20.Littorin B, Blom P, Scholin A, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49(12):2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 21.Bierschenk L, Alexander J, Wasserfall C, et al. Vitamin D levels in subjects with and without type 1 diabetes residing in a solar rich environment. Diabetes Care. 2009;32(11):1977–1979. doi: 10.2337/dc09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greer RM, Rogers MA, Bowling FG, et al. Australian children and adolescents with type 1 diabetes have low vitamin D levels. Med J Aust. 2007;187(1):59–60. doi: 10.5694/j.1326-5377.2007.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 23.Svoren BM, Volkening LK, Wood JR, et al. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154(1):132–134. doi: 10.1016/j.jpeds.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserfall CH, Atkinson MA. Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmun Rev. 2006;5(6):424–428. doi: 10.1016/j.autrev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350–357. doi: 10.7326/0003-4819-144-5-200603070-00011. [DOI] [PubMed] [Google Scholar]
- 26.Umpierrez GE. Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care. 2006;29(12):2755–2757. doi: 10.2337/dc06-1870. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 28.Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006;92(1):140–149. doi: 10.1016/j.pbiomolbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin d genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–1631. doi: 10.2337/db10-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57(2):176–185. doi: 10.1373/clinchem.2010.148221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takiishi T, Gysemans C, Bouillon R, et al. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39(2):419–446. doi: 10.1016/j.ecl.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Mathieu C, Waer M, Laureys J, et al. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37(6):552–558. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 34.Gregori S, Giarratana N, Smiroldo S, et al. A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 35.Adorini L, Penna G, Giarratana N, et al. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88(2):227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 36.Sandler S, Buschard K, Bendtzen K. Effects of 1,25-dihydroxyvitamin D3 and the analogues MC903 and KH1060 on interleukin-1 beta-induced inhibition of rat pancreatic islet beta-cell function in vitro. Immunol Lett. 1994;41(1):73–77. doi: 10.1016/0165-2478(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 37.Riachy R, Vandewalle B, Moerman E, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11(2):151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 38.Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146(4):1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 39.Branisteanu DD, Waer M, Sobis H, et al. Prevention of murine experimental allergic encephalomyelitis: cooperative effects of cyclosporine and 1 alpha, 25-(OH)2D3. J Neuroimmunol. 1995;61(2):151–160. doi: 10.1016/0165-5728(95)00076-e. [DOI] [PubMed] [Google Scholar]
- 40.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128(1):68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 41.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(15):7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binkley N, Krueger DC, Morgan S, et al. Current status of clinical 25-hydroxyvitamin D measurement: an assessment of between-laboratory agreement. Clin Chim Acta. 2010;411(23-24):1976–1982. doi: 10.1016/j.cca.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorham ED, Garland CF, Burgi AA, et al. Lower prediagnostic serum 25-hydroxyvitamin D concentration is associated with higher risk of insulin-requiring diabetes: a nested case-control study. Diabetologia. 2012;55(12):3224–3227. doi: 10.1007/s00125-012-2709-8. [DOI] [PubMed] [Google Scholar]