Abstract
Obesity and alcohol interact to increase the risk of death from liver failure in men. In the present study, we aimed to examine whether obesity and alcohol were multiplicative or additive in increasing the risk of hepatocellular carcinoma (HCC) in both men and women. We conducted a prospective, population-based study of 23,712 Taiwanese residents (50.3% men) from 7 townships who underwent an evaluation for liver disease and were followed for 11.6 years for incident HCC. The mean age was 47 (standard deviation, 10) years and the mean body mass index (weight (kg)/height (m)2) was 24 (standard deviation, 3). Overall, 305 cases of HCC were identified over 275,126 person-years of follow-up. Age, male sex, alcohol drinking, cigarette smoking, elevated alanine aminotransferase, serum hepatitis B surface antigen, anti-hepatitis C virus positivity, and diabetes mellitus were each statistically significant predictors of incident HCC in univariate analyses (P < 0.05). Alcohol use and obesity (body mass index ≥30) showed a synergistic association with the risk of incident HCC in both unadjusted analyses (hazard ratio = 7.19, 95% confidence interval: 3.69, 14.00; P < 0.01) and multivariable-adjusted analyses (age, sex, smoking, serum alanine aminotransferase, serum hepatitis B surface antigen, anti-hepatitis C virus antibody, and diabetes mellitus) (hazard ratio = 3.82, 95% confidence interval: 1.94, 7.52; P < 0.01). Relative excess risks due to interaction, attributable proportion, and synergy index were 4.83, 0.67, and 4.53, respectively, suggesting a multiplicative interaction between alcohol use and obesity. Obesity and alcohol synergistically increase the risk of incident HCC.
Keywords: alcoholic liver disease, community-dwelling, fatty liver, liver cancer, nonalcoholic fatty liver, prospective cohort
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer mortality worldwide (1). It is estimated that by the year 2030, HCC will become the third most fatal cancer (1). This rising incidence of HCC is preceded by the obesity epidemic worldwide (2, 3). Several studies have shown that obesity increases the risk of HCC (2, 4). Another modifiable risk factor that is strongly associated with liver disease and HCC is excessive alcohol use (5–8). Both alcohol use and obesity are associated with fatty liver disease. Epidemiologic studies have suggested that fatty liver disease, both alcoholic and nonalcoholic in etiology, is associated with increased risk of HCC (9–15).
Previous studies have shown that alcohol and obesity synergistically increase the risk of elevated serum alanine aminotransferase and aspartate aminotransferase levels (16). It is plausible that obesity and alcohol both lead to increased cytokine release by hepatocytes and Kupffer cells that may lead to hepatic stellate cell activation (17). These mechanistic pathways, along with other hitherto unknown pathways, increase the risk of progressive steatohepatitis. Understanding the impact of this synergism between obesity and alcohol use at the population level is important because these are modifiable risk factors. Previous epidemiologic investigations have shown that seeing a synergistic interaction strengthens the expectation that there is biologic plausibility for a relationship between a primary risk factor and disease, for example, cigarette smoking and alcohol use with oral cancer (18–20). Given that incidence and mortality rates due to HCC mirror each other, prevention is the preferred strategy in reducing the worldwide burden of HCC (3, 21).
In a recent study, Hart et al. (22) followed 9,559 Scottish men for up to 42 years and found that obesity and alcohol use had a supra-additive interaction in their association with the risk of liver disease morbidity and mortality. Because of the small number of events, the effect of obesity and alcohol on HCC was not examined. That study included only men. We recently conducted a pilot study in a high-risk population and found that obesity and alcohol use raised the risk of HCC in Taiwanese men with hepatitis B (23). However, the study was limited, as the confidence intervals for synergy index overlapped 1 because of the smaller sample size.
In the present study, we aimed to examine whether obesity and alcohol were either multiplicative or additive in increasing the risk of incident HCC in both men and women in a larger, well-characterized, population-based cohort that was representative of the general population of Taiwan.
MATERIALS AND METHODS
Study participants
The present prospective cohort study included participants from a Taiwanese cohort that has been previously described (9). A detailed description of the recruitment and blood sample collection and assay procedures have been previously published (9, 24, 25). A total of 47,079 male and 42,214 female residents (total = 89,293) from 7 townships in Taiwan were invited to participate in a cancer screening program between 1991 and 1992. Of those residents, a total of 11,973 men (25%) and 11,847 women (28%) agreed to participate and provided written informed consent. Information on demographic characteristics and lifestyle factors was collected during a structured personal interview administered by well-trained public health nurses. Overnight fasting blood samples were collected at baseline, and measurements of serum hepatitis B surface antigen, anti-hepatitis C antibody, and alanine aminotransferase levels were performed using commercial kits and detailed laboratory procedures, as described previously (9). Participants were followed till June 30, 2004, with a mean follow-up of 11.6 (standard deviation, 1.73) years. All participants gave informed consent to participate in this study, and the data collection procedures were reviewed and approved by the institutional review board of the College of Public Health, National Taiwan University.
Exposure
Body mass index
Body mass index (BMI) was defined as weight (in kilograms) divided by the square of height (in meters). A trained nurse measured body weight and height at the baseline visit. Participants were categorized into normal (<23), overweight (23–24.9), obese (25–29.9), and extremely obese (≥30) based on the published guidelines for the adult Asian population proposed by the World Health Organization (26). These weight categories are different from (and lower than) those for Western populations.
Alcohol consumption
Alcohol consumption was ascertained via a questionnaire at the baseline visit, as previously described (27). Participants were asked whether they had ever consumed alcohol (yes or no), their age at first use of alcohol, and whether they presently consumed alcohol (yes or no). Duration of alcohol use (in years) was also documented. Alcohol drinkers were defined as those who had consumed alcohol at least 4 days per week for at least 1 year.
Outcome measure: HCC
All participants who consented for the study and underwent a baseline examination were followed for the development of HCC and were accounted for in the analyses. Participants with a prior diagnosis of HCC that was either identified via the national cancer registry or diagnosed during the baseline visit or a personal interview before or at enrollment were excluded from the analysis. All participants had unique national identification numbers that were utilized to link data with the computerized national cancer registry profiles in Taiwan to identify all the new HCC cases between January 1, 1991, and December 31, 2004. The Taiwan nationwide cancer registry system was initiated in 1978 and contains updated, accurate, and complete information on cases of HCC. Because histologic confirmation of the HCC diagnosis was not reported in all of the cases identified by the national cancer registry, adjudication of HCC diagnosis was carried out by experts via review of participants’ charts. This chart review was performed on 164 HCC cases in hepatitis B surface antigen–positive carriers to further confirm and validate the primary HCC diagnosis derived from the national cancer registry. All HCC cases (n = 305) included in this study met at least 1 of the following criteria: HCC confirmed by liver histopathology (n = 141; 46.2%) or HCC confirmed by at least 2 imaging tools (ultrasonography, angiography, or computed tomography) or detected via 1 imaging diagnostic modality and a serum-α-fetoprotein level of 400 ng/mL or higher (n = 164; 53.8%).
Covariate assessment
Cirrhosis was diagnosed using abdominal ultrasonography with a systematic and quantitative scoring algorithm, which was determined by the appearance of the liver surface and the liver parenchyma, as well as by the size of the hepatic vessel and the spleen (28, 29). Laboratory tests were performed using commercial kits: hepatitis B surface antigen and hepatitis B e antigen by radioimmunoassay (Abbott Laboratories, Chicago, IL), anti-hepatitis C virus antibody by enzyme immunoassay with second-generation test kits (Abbott Laboratories), serum hepatitis B virus DNA level by polymerase chain reaction (COBAS Amplicor; Roche Diagnostics, Indianapolis, Indiana), and alanine aminotransferase level by serum chemistry autoanalyzer (model 736; Hitachi Co., Tokyo, Japan) using commercial reagents (Biomerieux, March L'Etoile, France). Each participant's history of diabetes mellitus and information on cigarette smoking were obtained from the questionnaire interview. Cigarette smokers were defined as persons who had smoked cigarettes at least 4 days per week for at least 1 year.
Statistical analysis
The cohort was divided into 4 categories based upon the presence of extreme BMI (≥30 or <30) and alcohol use. The mean values were shown for continuous variables and proportions were shown for categorical variables. Cumulative risk of incident HCC development was compared between the 4 groups using a log-rank test. Wald test for interaction was performed to assess the presence of an interaction between alcohol use and BMI. Cox proportional hazards analysis was conducted to determine the hazards of incident HCC over 14 years of follow-up and reported as relative risk estimate. Univariate, age-adjusted, and multivariable-adjusted (adjusted for age, BMI, alcohol use, smoking, serum alanine aminotransferase level, and diabetes mellitus) models were examined. Joint impact of extreme obesity (yes/no) and alcohol use (yes/no) (as well as duration and dose of alcohol use) was examined using Cox proportional hazards models. Hazard ratios and their respective 95% confidence intervals were reported.
Estimate of interaction on a multiplicative scale
To test whether the interaction is additive or multiplicative, we examined the combined impact of alcohol and obesity on HCC risk by relative excess risk due to interaction (RERI), attributable proportion (AP), and synergy index (SI) and their respective confidence intervals, as previously described (30, 31). RERI is an estimate of excess risk that is attributable to the interaction between 2 exposures, in this case alcohol use and obesity. AP is defined as the proportion of risk that is attributable to the interaction between obesity and alcohol. SI is a ratio that estimates whether a synergistic (SI > 1) or antagonistic (SI < 1) interaction exists between 2 exposures. Based on prior studies, a multiplicative interaction is suggested by the following scores: a RERI >1.5; an AP >0.25; and an SI > 1.5 (31).
Joint influence of BMI and alcohol
We measured the joint influences of World Health Organization BMI categories and alcohol use on the risk of HCC. Sensitivity analyses were conducted after excluding patients with hepatitis B virus infection. We used SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina) for all statistical analyses. A 2-tailed P ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
This study included 23,712 participants with a mean age of 47.3 (standard deviation, 10.0) years who were followed for a mean of 11.6 (standard deviation, 1.73) years. Men comprised 50.3% of the cohort, and the average BMI was 24.0. Alcohol use was reported by 10.6%, and 28.9% were smokers. The prevalences of hepatitis B surface antigen and anti-hepatitis C virus seropositivity were 17.5% and 5.5%, respectively. The baseline characteristics of the cohort and incident HCC cases were stratified by the 4 exposure categories, as shown in Table 1. Out of 305 HCC cases, 51 had chronic hepatitis C virus infection, 186 had chronic hepatitis B virus infection, 13 had hepatitis B virus/hepatitis C virus co-infection, and 52 did not have either hepatitis C virus or hepatitis B virus (Web Table 1, available at http://aje.oxfordjournals.org/).
Table 1.
Baseline Characteristics of the Cohort, Taiwan, 1991–2004
| Characteristic | All Participants |
Body Mass Indexc <30 |
Body Mass Indexc ≥30 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Alcohol Use |
Alcohol Use |
No Alcohol Use |
Alcohol Use |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of participantsa | 23,712 | 20,164 | 2,401 | 1,028 | 119 | |||||
| No. of hepatocellular carcinoma cases | 305 | 225 | 59 | 12 | 9 | |||||
| Age, yearsb | 47.3 (10.0) | 47.1 (10.0) | 48.4 (10.1) | 49.6 (9.1) | 47.3 (10.0) | |||||
| Body mass indexb,c | 24.0 (3.4) | 23.6 (2.9) | 23.8 (2.8) | 32.2 (2.4) | 31.9 (1.9) | |||||
| Alcohol use | ||||||||||
| No | 21,192 | 89.4 | 20,164 | 100.0 | 0 | 0.0 | 1,028 | 100.0 | 0 | 0.0 |
| Yes | 2,520 | 10.6 | 0 | 0.0 | 2,401 | 100.0 | 0 | 0.0 | 119 | 100.0 |
| Smokingd | ||||||||||
| No | 16,854 | 71.1 | 15,405 | 76.4 | 572 | 23.8 | 835 | 81.2 | 42 | 35.3 |
| Yes | 6,851 | 28.9 | 4,753 | 23.6 | 1,828 | 76.2 | 193 | 18.8 | 77 | 64.7 |
| Hepatitis B surface antigen status | ||||||||||
| Seronegative | 19,574 | 82.6 | 16,675 | 82.7 | 1,946 | 81.1 | 863 | 84.0 | 90 | 75.6 |
| Seropositive | 4,138 | 17.5 | 3,489 | 17.3 | 455 | 19.0 | 165 | 16.1 | 29 | 24.4 |
| Hepatitis C virus statuse | ||||||||||
| Seronegative | 22,371 | 94.5 | 19,001 | 94.4 | 2,303 | 96.0 | 954 | 93.1 | 113 | 95.0 |
| Seropositive | 1,308 | 5.5 | 1,135 | 5.6 | 96 | 4.0 | 71 | 6.9 | 6 | 5.0 |
| Alanine aminotransferase, U/L | ||||||||||
| <45 | 22,891 | 96.5 | 19,557 | 97.0 | 2,264 | 94.3 | 964 | 93.8 | 106 | 89.1 |
| ≥45 | 821 | 3.5 | 607 | 3.0 | 137 | 5.7 | 64 | 6.2 | 13 9 | 10. |
| Diabetesf | ||||||||||
| No | 23,061 | 97.49 | 19,635 | 97.6 | 2,328 | 97.2 | 985 | 96.0 | 113 | 95.0 |
| Yes | 593 | 2.51 | 479 | 2.4 | 67 | 2.8 | 41 | 4.0 | 6 | 5.0 |
a We excluded 108 subjects because of missing data on either body mass index or alcohol use.
b Values are expressed as mean (standard deviation).
c Weight (kg)/height (m)2.
d We were missing data on smoking for 7 out of 23,712 subjects.
e We were missing data on anti-hepatitis C virus for 33 out of 23,712 subjects.
f We were missing data on history of diabetes for 58 out of 23,712 subjects.
Risk of incident HCC
There were a total of 305 HCC cases over 275,125.5 person-years of follow-up. Therefore, the incidence rate of HCC was 110.9 per 100,000 person-years. We then examined the Wald test of interaction between alcohol use and BMI and found that it was statistically significant in predicting incident HCC risk (P < 0.02).
Cumulative risk
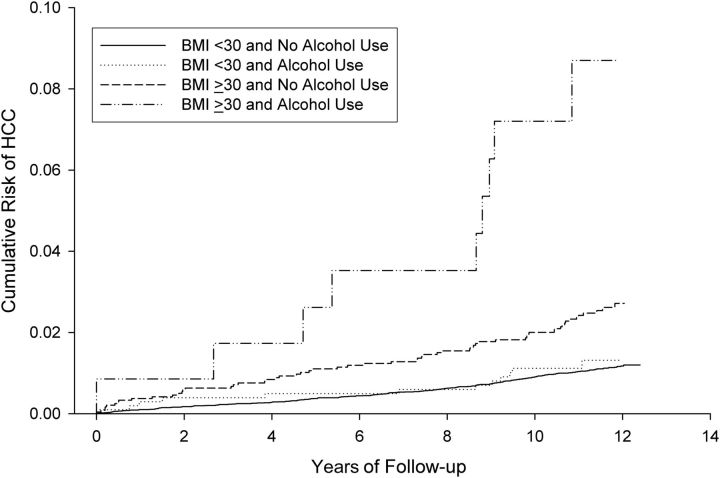
We conducted a Kaplan-Meier analysis to examine the risk of HCC among participants stratified into 4 categories based on their BMI and alcohol use at baseline (Figure 1). Table 2 shows the number of participants at risk of HCC in each of the 4 categories and the number of incident cases of HCC during follow-up. We found that risk of incident HCC was highest among obese alcohol users (P < 0.0001), followed by nonobese alcohol users. The risk of HCC was significantly lower in participants who did not consume alcohol. Among nonusers of alcohol, the cumulative risk of HCC was 1.2% and 1.3% among nonobese and obese participants, respectively. Among users of alcohol, the cumulative risk of HCC was 2.7% and 8.7% among nonobese and obese participants, respectively.
Figure 1.
Joint associations of obesity and alcohol use with incident hepatocellular carcinoma (HCC). Shown are Kaplan Meier curves for future development of hepatocellular carcinoma in 4 categories based on alcohol use and body mass index (BMI, measured as weight (kg)/height (m)2.
Table 2.
Participants at Risk and Number of Incident Cases of Hepatocellular Carcinoma in Each of the 4 Exposure Groups During Follow-up, Taiwan, 1991–2004
| Follow-up, years |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| No. of Participants at Risk | ||||||||||||||
| BMIa ≥30 and alcohol use | 119 | 119 | 118 | 116 | 114 | 112 | 110 | 109 | 109 | 104 | 103 | 101 | 56 | 2 |
| BMI <30 and alcohol use | 2,401 | 2,381 | 2,353 | 2,333 | 2,310 | 2,274 | 2,250 | 2,229 | 2,197 | 2,168 | 2,138 | 2,097 | 1,018 | 1 |
| BMI ≥30 and no alcohol use | 1,028 | 1,023 | 1,017 | 1,009 | 1,002 | 996 | 990 | 979 | 969 | 960 | 950 | 938 | 463 | 1 |
| BMI <30 and no alcohol use | 20,164 | 20,099 | 20,022 | 19,927 | 19,833 | 19,741 | 19,641 | 19,520 | 19,413 | 19,271 | 19,105 | 18,934 | 9,538 | 11 |
| No. of HCC Cases | ||||||||||||||
| BMI ≥30 and alcohol use | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | 1 | 1 | 1 | 0 |
| BMI <30 and alcohol use | 0 | 8 | 5 | 1 | 5 | 6 | 2 | 2 | 6 | 5 | 5 | 9 | 4 | 1 |
| BMI ≥30 and no alcohol use | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 4 | 0 | 2 | 0 |
| BMI <30 and no alcohol use | 0 | 19 | 15 | 11 | 10 | 19 | 13 | 18 | 18 | 22 | 32 | 26 | 20 | 2 |
Abbreviation: BMI, body mass index.
a Weight (kg)/height (m)2.
Joint influences of obesity and alcohol use on incident HCC risk
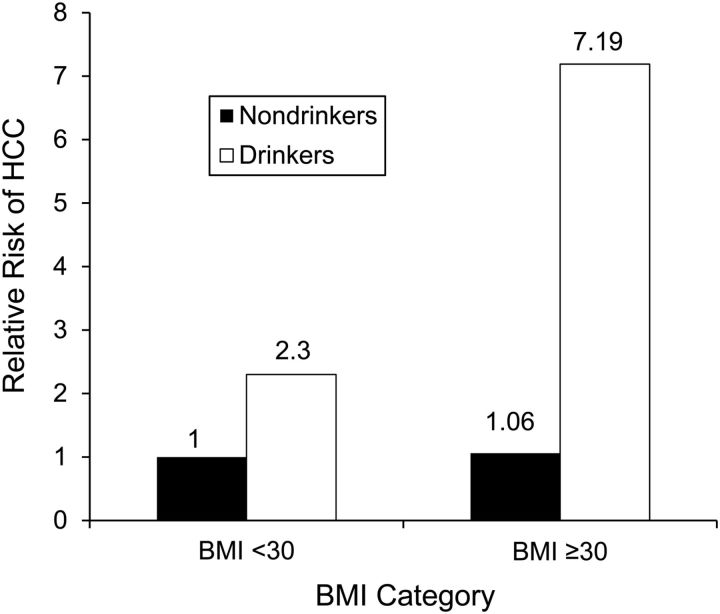
The combined impacts of extreme obesity and alcohol use showed a statistically significant and synergistic association with HCC risk, with the highest risk among extremely obese (BMI ≥30) alcohol users (hazard ratio = 7.19; 95% confidence interval (CI): 3.69, 14.00; P < 0.001), followed by alcohol users with a BMI <30 (hazard ratio = 2.30; 95% CI: 1.73, 3.07; P < 0.01), as shown in Figure 2. The risk of HCC was slightly higher among obese nondrinkers than among nonobese nondrinkers but was not statistically significant, as shown in Figures 1 and 2. The results remained consistent in multivariable-adjusted analyses, as shown in Table 3.
Figure 2.
Synergistic interaction of obesity and alcohol use with the risk of hepatocellular carcinoma (HCC). Hazard ratios showing the risk of hepatocellular carcinoma stratified by extreme obesity and alcohol use status over a mean follow-up of 11.6 years. BMI, body mass index.
Table 3.
Unadjusted and Multivariate-Adjusted Hazard Ratios for Incident Hepatocellular Carcinoma, Taiwan, 1991–2004
| Characteristic | Unadjusted |
Multivariate-Adjusted |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Sex | ||||
| Female | 1.00 | Referent | 1.00 | Referent |
| Male | 3.17 | 2.45, 4.12 | 1.94 | 1.41, 2.66 |
| Age, years | 1.07 | 1.06, 1.09 | 1.08 | 1.07, 1.10 |
| BMIa | ||||
| <30 | 1.00 | Referent | N/A | |
| ≥30 | 1.47 | 0.95, 2.30 | N/A | |
| Alcohol consumption | ||||
| No | 1.00 | Referent | N/A | |
| Yes | 2.56 | 1.96, 3.35 | N/A | |
| Cigarette smoking | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 2.24 | 1.79, 2.80 | 1.31 | 1.00, 1.72 |
| Hepatitis B surface antigen status | ||||
| Negative | 1.00 | Referent | 1.00 | Referent |
| Positive | 9.35 | 7.39, 11.84 | 9.90 | 7.77, 12.62 |
| Alanine aminotransferase, U/L | ||||
| ≤45 | 1.00 | Referent | 1.00 | Referent |
| >45 | 7.68 | 5.83, 10.14 | 3.70 | 2.75, 4.98 |
| Anti-hepatitis C virus antibody status | ||||
| Negative | 1.00 | Referent | 1.00 | Referent |
| Positive | 4.72 | 3.58, 6.21 | 3.24 | 2.42, 4.35 |
| Diabetes | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 3.11 | 1.98, 4.90 | 1.82 | 1.15, 2.88 |
| Alcohol drinkingb/BMI | ||||
| No/<30 | 1.00 | Referent | 1.00 | Referent |
| No/≥30 | 1.06 | 0.60, 1.90 | 1.17 | 0.65, 2.11 |
| Yes/<30 | 2.30 | 1.73, 3.07 | 1.46 | 1.07, 1.98 |
| Yes/≥30 | 7.19 | 3.69, 14.00 | 3.82 | 1.94, 7.52 |
| P for alcohol/BMI interaction | 0.020 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
a Weight (kg)/height (m)2.
b Alcohol drinkers were defined as those who consumed alcohol at least 4 days per week for at least 1 year.
Interaction between extreme obesity and alcohol use
We then examined the RERI, AP, and SI (measures of synergistic interaction between extreme obesity and alcohol use) and their associations with incident HCC risk. We found that the RERI, AP, and SI were 4.83 (95% CI: 0.03, 9.62), 0.67 (95% CI: 0.41, 0.93), and 4.53 (95% CI: 1.68, 12.22), respectively. These findings suggest a multiplicative interaction between extreme obesity and alcohol use in their joint association with HCC risk.
Joint impact of BMI categories and risk of HCC
We then divided the cohort into 4 categories of BMI to assess the dose-response association of BMI in modulating HCC risk. The results remained consistent, as shown in Table 4. Alcohol users in the highest BMI category had the highest risk of HCC, and findings remained consistent in both age-adjusted and multivariable-adjusted models, with hazard ratios of 7.41 (95% CI: 3.73, 14.73; P < 0.001) and 4.12 (95% CI: 2.05, 8.28; P < 0.001), respectively. To assess the dose-response relationship of alcohol use, we examined the joint impact of BMI categories and duration of alcohol use stratified into the following categories: no alcohol use, less than 1 year of alcohol use, 1–19 years of alcohol use, and 20 or more years of alcohol use. We found that the risk of incident HCC was highest among participants who were obese (highest BMI category) and had 20 or more years of alcohol use. These results remained consistent in both age-adjusted and multivariable-adjusted models, with hazard ratios of 9.23 (95% CI: 4.26, 20.00; P < 0.0001) and 5.16 (95% CI: 2.34, 11.39; P < 0.0001), respectively. We conducted further sensitivity analyses by dividing the cohort into quartiles of BMI, and the above findings remained consistent (data not shown). We conducted additional sensitivity analyses by excluding the prevalent cases of hepatitis B virus, and the results remained consistent (Web Table 2).
Table 4.
Risk of Hepatocellular Carcinoma Stratified by Body Mass Index Category, Alcohol Use Status, and Years of Alcohol Consumption, Taiwan, 1991–2004
| Variable | Age-Adjusted |
Multivariate-Adjusteda |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| BMIb categoryc by alcohol consumption category | ||||||
| No alcohol use | ||||||
| Normal weight | 1.00 | Referent | N/A | 1.00 | Referent | N/A |
| Overweight | 1.06 | 0.76, 1.48 | 0.72 | 1.14 | 0.82, 1.60 | 0.4414 |
| Obese | 1.03 | 0.76, 1.40 | 0.8283 | 1.07 | 0.79, 1.46 | 0.6545 |
| Extremely obese | 0.97 | 0.53, 1.77 | 0.9123 | 1.25 | 0.68, 2.31 | 0.4699 |
| Alcohol use | ||||||
| Normal weight | 1.68 | 1.01, 2.80 | 0.0459 | 1.22 | 0.72, 2.07 | 0.4601 |
| Overweight | 2.38 | 1.40, 4.06 | 0.0015 | 1.91 | 1.11, 3.29 | 0.0188 |
| Obese | 2.63 | 1.68, 4.11 | <0.0001 | 1.92 | 1.21, 3.03 | 0.0053 |
| Extremely obese | 7.41 | 3.73, 14.73 | <0.0001 | 4.12 | 2.05, 8.28 | <0.0001 |
| BMI category by years of alcohol consumption | ||||||
| 0 years | ||||||
| Normal weight | ||||||
| Overweight | 1.06 | 0.76, 1.48 | 0.7222 | 1.14 | 0.82, 1.60 | 0.4339 |
| Obese | 1.03 | 0.76, 1.40 | 0.8313 | 1.07 | 0.79, 1.46 | 0.6498 |
| Extremely obese | 0.97 | 0.53, 1.77 | 0.9107 | 1.26 | 0.69, 2.33 | 0.4534 |
| 1–19 years | ||||||
| Normal weight | 2.65 | 1.33, 5.28 | 0.0055 | 1.74 | 0.84, 3.62 | 0.1369 |
| Overweight | 2.38 | 0.97, 5.88 | 0.0595 | 2.15 | 0.86, 5.34 | 0.1009 |
| Obese | 2.49 | 1.15, 5.38 | 0.0206 | 1.62 | 0.75, 3.54 | 0.2224 |
| Extremely obese | 5.01 | 1.23, 20.40 | 0.0244 | 2.50 | 0.61, 10.23 | 0.2035 |
| ≥20 years | ||||||
| Normal weight | 1.11 | 0.51, 2.41 | 0.7908 | 0.89 | 0.41, 1.93 | 0.7597 |
| Overweight | 1.74 | 0.80, 3.77 | 0.1606 | 1.44 | 0.66, 3.13 | 0.357 |
| Obese | 2.68 | 1.56, 4.58 | 0.0003 | 2.05 | 1.19, 3.55 | 0.0099 |
| Extremely obese | 9.23 | 4.26, 20.00 | <0.0001 | 5.16 | 2.34, 11.39 | <0.0001 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; N/A, not applicable.
a Adjusted for sex, age, presence of hepatitis B surface antigen, anti-hepatitis C virus antibody status, serum alanine aminotransferase level, and history of diabetes.
b Weight (kg)/height (m)2.
c Participants were categorized as normal (BMI <23), overweight (BMI 23–24.9), obese (BMI 25–29.9), or extremely obese (BMI ≥30) based on the published guidelines for the adult Asian population by the World Health Organization.
DISCUSSION
Main findings
In the present large population-based prospective cohort study, we showed that alcohol use and obesity synergistically increased the risk of incident HCC among Taiwanese men and women. Furthermore, we demonstrated that the interaction between obesity and alcohol use is multiplicative based upon 3 independent measures of interaction: RERI, AP, and SI. Although the precise mechanisms underlying this synergistic interaction remain to be elucidated, previous studies have suggested that obesity and alcohol use may synergistically worsen hepatic insulin resistance and necroinflammation that can lead to progressive liver injury causing steatohepatitis, which can lead to cirrhosis and HCC in a subset of these patients (17, 32, 33). Molecular mechanisms that may underlie this interaction include cytochrome 2E1 upregulation, nuclear factor kappa-light-chain-enhancer of activated B cells activation, cytokine imbalance (upregulation of interleukin-6), activation of STAT3 by interleukin-6, and lipid peroxidation, all of which can worsen liver injury, leading to cirrhosis and HCC (33–35).
Strengths and limitations
Strengths of this study include a large prospective cohort including 23,712 men and women with over 12 years of follow-up. BMI was recorded by a trained investigator and not ascertained by self-report, and potential confounders or effect modifiers associated with HCC at baseline for adjustment in the multivariate analyses were available. However, we acknowledge the following limitations of our study. The study population was exclusively Taiwanese; therefore, further studies are needed to validate this synergism between obesity and alcohol use in diverse multiethnic populations. We did not have detailed quantification of alcohol consumption and changes in body weight either at baseline or during follow-up. Another limitation is that the alcohol use was based upon self-report, which is likely to underestimate the quantity of alcohol use. Therefore, we believe that the magnitude of synergism may actually be higher than what we found in this study. Additionally, the results were consistent with previous reports of synergism between obesity and alcohol in increasing the risk of liver injury (16, 35) and of death from liver disease conducted in diverse populations, which suggests a strong biologic plausibility (22).
In context with published literature
Recent studies and surveys have suggested that the incidence of HCC is rising in the United States and worldwide (3, 36). Given the rising rates of obesity worldwide, we believe that our findings may have strong public health significance, especially in countries with a higher per capita rate of alcohol use. Previous studies have confirmed the synergistic association of alcohol use and BMI in increasing the risk of elevated alanine aminotransferase and aspartate aminotransferase at the population level (35). Hart et al. (22) showed that alcohol use and BMI had a supra-additive interaction in increasing the risk of death from liver disease in Scottish men (n = 9,559 men). Their study did not include women, and HCC incidence was not assessed as an outcome measure. Hart et al. recommended that their findings be validated in other larger cohorts and in women (22). Ascha et al. (15) recently reported a tertiary care transplant center–based study and showed that patients with nonalcoholic steatohepatitis had an increased risk of HCC and also found that alcohol use (even social drinking) was the most important risk factor in increasing the risk of HCC in patients with nonalcoholic steatohepatitis and chronic hepatitis C infection. However, the investigators did not examine the joint contribution of alcohol use and obesity in that study. Marrero et al. (37) conducted a small case-control study comparing HCC cases with cirrhotic controls derived from a single liver transplant center and showed that alcohol use and BMI were synergistic in their interaction in increasing the odds of HCC. Although this was to our knowledge one of the first studies to report this interaction, the BMI was recorded at the clinic visit when the patients were already suffering from cirrhosis (controls) and HCC (cases) and it was limited by a small sample size and case-control design (37). We recently reported the results of our pilot study of a high-risk group of men with hepatitis B (n = 2,260) and showed that alcohol use and obesity had a multiplicative interaction on increasing the risk of incident HCC in men (23). Although the study was prospective, it was limited by a small sample size, did not include women, and was exclusively in patients with hepatitis B (23). Therefore, we conducted the present large (n = 23,820) population-based prospective cohort study including both men and women and examined the joint association of obesity and alcohol use on the risk of future development of HCC and found a supra-additive or multiplicative effect-modification of these 2 modifiable risk factors of HCC.
Implications for future research
Although our study was derived from an Asian population-based cohort, we speculate that these results might be relevant to other populations as well. We hypothesize that obesity and alcohol use could increase the risk of progressive fatty liver disease that might in turn increase the risk of HCC. Further studies are needed to examine whether cirrhosis is required for development of HCC in the setting of the fatty liver disease. We believe that our study provides robust epidemiologic evidence to conduct further research to better understand the mechanisms underlying this interaction between alcohol use and obesity.
Conclusions
Here we showed that obesity multiplies the risk of incident HCC among alcohol users. HCC incidence is rapidly increasing worldwide, and public health interventions are needed to halt this epidemic. As both alcohol use and obesity are modifiable risk factors, lifestyle interventions, such as minimizing alcohol use and maintaining a normal body weight, should be tested and utilized to reduce the incidence of HCC. Public health interventions to prevent and/or reduce obesity are important for reducing not only cardiovascular morbidity and mortality but also liver-related morbidity and mortality.
ACKNOWLEDGMENTS
Author affiliations: Division of Gastroenterology, University of California at San Diego, La Jolla, California (Rohit Loomba, David Brenner); Division of Epidemiology, University of California at San Diego, La Jolla, California (Rohit Loomba, Elizabeth Barrett-Connor); Genomics Research Center, Academia Sinica, Taipei, Taiwan (Hwai-I Yang, Chien-Jen Chen); Molecular and Genomic Epidemiology Research Center, China Medical University Hospital, Taichung, Taiwan (Hwai-I Yang); Previous Research and Development, Bristol-Myers Squibb Company, Wallingford, Connecticut (Jun Su); Research and Development, Bristol-Myers Squibb Company, Wallingford, Connecticut (Uchenna Iloeje); and Graduate Institute of Epidemiology, College of Public Health, National Taiwan University, Taipei, Taiwan (Chien-Jen Chen).
Rohit Loomba and Hwai-I Yang contributed equally to this work.
This work was supported in part by the American Gastroenterological Association Foundation–Sucampo–Association of Subspecialty Professors Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding was provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Association of Specialty Professors, the American Gastroenterological Association, and National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK090303 and National Cancer Institute grant P30CA23100-27 to Dr. Loomba. The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus Study was supported by grant from Department of Health, Academia Sinica, National Health Research Institutes in Taiwan, and Bristol-Myers Squibb Company.
Conflict of interest: none declared.
REFERENCES
- 1.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. (http://www.who.t/healthinfo/global_burden_disease/2004_report_update/en/index.html. ). (Accessed June 29, 2011). [Google Scholar]
- 2.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.C. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97(7):1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145(11):1039–1047. doi: 10.1093/oxfordjournals.aje.a009060. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie AM, Rustgi VK, Hoofnagle JH, et al. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988;108(3):390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- 7.Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85(4):498–502. [PubMed] [Google Scholar]
- 8.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160(21):3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 9.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 suppl 1):S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Marrero JA, Fontana RJ, Su GL, et al. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 12.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi S, Montella M, Polesel J, et al. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15(4):683–689. doi: 10.1158/1055-9965.EPI-05-0702. [DOI] [PubMed] [Google Scholar]
- 14.Salomao M, Yu WM, Brown RS, Jr, et al. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 34(11):1630–1636. doi: 10.1097/PAS.0b013e3181f31caa. [DOI] [PubMed] [Google Scholar]
- 15.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 16.Loomba R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30(11–12):1137–1149. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43(4):872–878. doi: 10.1002/hep.21107. [DOI] [PubMed] [Google Scholar]
- 18.Rothman K, Keller A. The effect of joint exposure to alcohol and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis. 1972;25(12):711–716. doi: 10.1016/0021-9681(72)90006-9. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ. Synergy and antagonism in cause-effect relationships. Am J Epidemiol. 1974;99(6):385–388. doi: 10.1093/oxfordjournals.aje.a121626. [DOI] [PubMed] [Google Scholar]
- 20.Kalilani L, Atashili J. Measuring additive interaction using odds ratios. Epidemiol Perspect Innov. 2006;3:5. doi: 10.1186/1742-5573-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WR, Gores GJ, Benson JT, et al. Mortality and hospital utilization for hepatocellular carcinoma in the United States. Gastroenterology. 2005;129(2):486–493. doi: 10.1016/j.gastro.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2005;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Yang HI, Su J, et al. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2005;8(10):891–898. doi: 10.1016/j.cgh.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347(3):168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 25.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang LY, You SL, Lu SN, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control. 2003;14(3):241–250. doi: 10.1023/a:1023636619477. [DOI] [PubMed] [Google Scholar]
- 28.Lin DY, Sheen IS, Chiu CT, et al. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound. 1993;21(5):303–308. doi: 10.1002/jcu.1870210502. [DOI] [PubMed] [Google Scholar]
- 29.Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11(4):797–816. doi: 10.1016/j.cld.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 31.Richardson DB, Kaufman JS. Estimation of the relative excess risk due to interaction and associated confidence bounds. Am J Epidemiol. 2009;169(6):756–760. doi: 10.1093/aje/kwn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bugianesi E, Vanni E, Marchesini G. NASH and the risk of cirrhosis and hepatocellular carcinoma in type 2 diabetes. Curr Diab Rep. 2007;7(3):175–180. doi: 10.1007/s11892-007-0029-z. [DOI] [PubMed] [Google Scholar]
- 33.Diehl AM. Nonalcoholic fatty liver disease: implications for alcoholic liver disease pathogenesis. Alcohol Clin Exp Res. 2001;25(5 suppl):8S–14S. doi: 10.1097/00000374-200105051-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wands J. Hepatocellular carcinoma and sex. N Engl J Med. 2007;357(19):1974–1976. doi: 10.1056/NEJMcibr075652. [DOI] [PubMed] [Google Scholar]
- 35.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3(12):1260–1268. doi: 10.1016/s1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 36.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 37.Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42(2):218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]