Abstract
The authors prospectively evaluated the association of soy food intake with lung cancer risk, overall and by tumor aggressiveness, and performed a meta-analysis of published data. Included in the analysis were 71,550 women recruited into the Shanghai Women's Health Study (Shanghai, China) in 1997–2000. Usual soy food intake was assessed at baseline and reassessed during follow-up through in-person interviews. During a mean follow-up period of 9.1 years, 370 incident lung cancer cases were identified; 340 patients were lifetime never smokers. After adjustment for potential confounders, soy food intake was inversely associated with subsequent risk of lung cancer (Ptrend = 0.004); the hazard ratio for the highest quintile of intake compared with the lowest was 0.63 (95% confidence interval: 0.44, 0.90). This inverse association appeared predominately among women with later age at menopause (Pinteraction = 0.01) and for aggressive lung cancer as defined by length of survival (<12 months vs. ≥12 months; Pheterogeneity = 0.057). Meta-analysis of 7 studies conducted among nonsmokers found a summary relative risk of 0.59 (95% confidence interval: 0.49, 0.71) for the highest categories of soy or isoflavone intake versus the lowest. This study suggests that soy food consumption may reduce lung cancer risk in nonsmoking women, particularly for aggressive tumors, and its effect may be modified by endogenous estrogens.
Keywords: cohort studies, lung neoplasms, meta-analysis, risk, soy foods, women
Lung cancer is the leading cause of cancer death in women worldwide (1). Several lines of evidence suggest that estrogens may be involved in the development and progression of lung cancer. Of all nonreproductive tissues, the lung possesses some of the highest levels of estrogen receptor transcripts (2), especially for estrogen receptor β, in both normal lung tissue and lung tumor cell lines (3, 4). Estrogens, particularly estradiol, can stimulate the proliferation of lung adenocarcinoma cell lines; this estrogen-induced proliferation can be blocked by estrogen receptor antagonists or aromatase inhibitors (5, 6). The evidence that most strongly supports a potential causal role for estrogens in the pathogenesis of lung cancer comes from a recent clinical trial, the Women's Health Initiative (7). Use of estrogen-plus-progestin therapy significantly increased the risk of developing aggressive lung cancer (poorly differentiated and metastatic tumors), resulting in an elevated risk of death from lung cancer, particularly from non-small-cell lung cancer (7). In contrast, in a large breast cancer follow-up study (8), use of the antiestrogen tamoxifen was linked to a reduction in lung cancer deaths.
Soy foods are rich in phytoestrogens, mainly in the form of isoflavones, the most common phytoestrogens in the human diet. Like selective estrogen receptor modulators such as tamoxifen, soy isoflavones have been shown to bind competitively to estrogen receptors, preferentially to estrogen receptor β, and they exert weak estrogenic activity in some tissues and antiestrogenic activity in others (9–12). They also have non-estrogen-receptor-mediated effects, including modulation of multiple signaling pathways in neoplastic transformation, stimulation of the immune system, and antioxidative and antiinflammatory activity (13–16). These multifaceted biologic properties may inhibit cell transformation and tumor growth, induce apoptosis and cell-cycle arrest, and inhibit tumor invasion and angiogenesis (17–22). In vivo animal models of lung carcinogenesis have shown that administration of isoflavones significantly decreases the incidence of tumor development and increases the life span of the tumor-bearing animals (23, 24), particularly in female mice (25). The relevance of these findings to humans, however, remains to be determined.
We prospectively evaluated the association of soy food intake with the risk of lung cancer in the Shanghai Women's Health Study (SWHS), a population-based, prospective cohort study. We also examined whether the soy food–lung cancer association differs by tumor aggressiveness or is modified by indicators of exposure to endogenous sex hormones. To help put the new data in context, we also performed a meta-analysis that combined our results with those published previously.
MATERIALS AND METHODS
Study population
The design and methods of the SWHS have been described in detail elsewhere (26). Briefly, the cohort includes 74,942 women who were recruited between 1997 and 2000 in Shanghai, China, and were aged 40–70 years at study enrollment. The participation rate was 92.7%. The study was approved by the relevant institutional review boards for human research in both China and the United States. Written informed consent was obtained from all study participants.
The cohort was followed for occurrence of cancer and death through a combination of biennial home visits and annual record linkage to the Shanghai Cancer Registry and death certificates in the Shanghai vital statistics database. Nearly all cohort members were successfully followed (response rates for in-person follow-up surveys were over 96%). For deceased participants, an adult family member (next of kin) was interviewed. All possible incident cancer cases were verified by means of home visits. Inpatient medical charts were reviewed to verify the diagnosis, and clinicopathologic characteristics of the tumor were recorded.
In this analysis, we excluded participants who reported a history of cancer at baseline (n = 1,576), had extreme total energy intake (<500 kcal/day or >3,500 kcal/day; n = 44), used postmenopausal hormones (n = 1,653), or were lost to follow-up immediately after study enrollment (n = 5). Additionally, we omitted the first year of observation, which resulted in 23 incident cases and 165 deaths being excluded (not mutually exclusive), in order to minimize the potential influence of preclinical disease on the study results. After these exclusions, a total of 71,550 women remained for the present analysis.
Data collection
All study participants completed a detailed baseline survey that collected information on demographic characteristics, lifestyle and dietary habits, medical history, family history of cancer, and other exposures (26). Anthropometric measurements were also taken at baseline. Information on secondhand exposure to tobacco smoke, both at home during adolescence and adulthood and in the workplace, was collected for 91.6% of the cohort in the first follow-up survey (2–3 years after baseline).
Usual dietary intake over the 12 months prior to the interview was assessed at baseline for all cohort members and was reassessed 2–3 years after the baseline survey for approximately 91% of cohort members through an in-person interview (26). We used a comprehensive, quantitative food frequency questionnaire (FFQ) that included 11 soy food items (27), covering virtually all soy foods commonly consumed in Shanghai. Our validation study showed that the validity of the FFQ was at least as good as that of the FFQs used in other major cohort studies. Soy food intakes assessed by means of the FFQ and by means of multiple 24-hour dietary recalls were moderately correlated (r = 0.49) (28). To improve the assessment of usual dietary intake, we used the average of the intakes from the first FFQ completed at baseline and the second FFQ completed 2–3 years after the baseline survey. For persons who did not complete the second FFQ survey, who reported having diabetes diagnosed between the two FFQ surveys, or whose cancer was diagnosed between the baseline FFQ and the first year after the second FFQ survey, only baseline dietary intake was used as the exposure (n = 7,772; 10.9%). Nutrient intakes were calculated according to the Chinese Food Composition Tables (29). Because the water content of soy foods varies widely, we also calculated total intake of the dry weight of soy foods (29).
In addition to the FFQ survey, which asked about dietary habits in the year preceding the survey, a 7-day recall was also administered in person by trained interviewers when biospecimens were procured for 54,648 women (76.4%) at baseline. Information on the frequency of consumption of selected foods was collected.
Statistical analysis
Cox proportional hazards modeling was used, with age as the time scale, to compute hazard ratios and 95% confidence intervals for development of lung cancer according to soy food intake and to adjust for potential confounders. Entry time was defined as age at the second year of enrollment (the first year of observation was excluded from the analysis), and exit time was defined as the date of lung cancer diagnosis, the date of death, or December 31, 2008, whichever came first. The dietary intakes of interest were categorized into quintiles based on distributions of intake in the entire cohort, with the lowest quintile serving as the reference group. Continuous variables were used to evaluate linear trends. We also used a restricted cubic spline Cox regression analysis to evaluate the association between lung cancer and soy food intake on a continuous basis (30).
Potential confounders adjusted for in multivariable models included age, education, cigarette smoking status, pack-years of smoking, alcohol consumption, body mass index, physical activity, lung cancer among first-degree relatives, menopausal status, and dietary intakes of total calories, red meat, fruits, and nonsoy vegetables; analyses were also stratified on calendar year of birth (31). Postmenopausal women were defined as those in whom menstruation had stopped for at least 12 months, including natural and surgically induced menopause. Menopausal status as updated during follow-up was considered a time-varying covariate. Additional adjustments for tea consumption, dietary pattern (32), B vitamin intake (vitamins B1, B2, B6, and B12, folic acid, and niacin), status and years of exposure to secondhand smoke at home and in the workplace, and occupational exposures (33) did not appreciably alter the results; therefore, these variables were not included in the final model.
We examined heterogeneity in risk by tumor aggressiveness, as defined by length of survival (<12 months vs. ≥12 months), with the Wald statistic. Multiplicative interactions with some lifestyle and reproductive factors were determined using the likelihood ratio test. The “proportionality assumption” that underlies the Cox model was checked by including time-dependent covariates in the Cox model and was found not to have been violated.
We also performed meta-analyses of studies on consumption of soy, soy foods, and/or isoflavones in relation to lung cancer risk, identified through searches of MEDLINE up to November 30, 2010. Summary relative risks and 95% confidence intervals were estimated on the basis of the study-specific most-adjusted odds ratio or relative risk for the highest category of soy or isoflavone intake versus the lowest. The DerSimonian and Laird random-effects model was used (34) when significant heterogeneity was present across studies. A fixed-effects model was used in a few subgroup analyses (35), such as studies conducted among nonsmokers where the heterogeneity was not significant. We also calculated a summary relative risk for lung cancer associated with a 1-g/day increment of soy protein intake (36). Heterogeneity among studies was evaluated using Cochran's Q test and was considered significant if the P value was less than 0.10 (37). Publication bias was evaluated using Egger's regression test (38).
Statistical analyses were carried out using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina), and Stata, version 11.0 (StataCorp LP, College Station, Texas). All statistical tests were based on 2-sided probability, and P < 0.05 was considered statistically significant.
RESULTS
Associations between soy food intake and lung cancer risk in the SWHS
The distributions of selected baseline age-adjusted characteristics of the study population by quintile category of soy food intake are presented in Table 1. The mean age at baseline was 52.0 years (standard deviation, 9.0). Mean soy food intakes based on dry weight, soy protein, and isoflavone content were 19.1 g/day, 9.3 g/day, and 31.4 mg/day, respectively. Women with higher soy food intakes were slightly older. Few women in this cohort reported having ever smoked at least 1 cigarette per day for more than 6 consecutive months (2.8%).
Table 1.
Characteristics of Study Participants According to Soy Food Intake, Shanghai Women's Health Study, 1997–2008a
| All Participants (n = 71,550) |
Average Soy Food Intake, g/dayb |
||||
|---|---|---|---|---|---|
| % | Mean (SD) | Q1 (n = 14,582) | Q2–Q4 (n = 42,873) | Q5 (n = 14,095) | |
| Baseline characteristics | |||||
| Age, years | 52.0 (9.0) | 51.5 | 51.8 | 53.0 | |
| High school education or above | 40.7 | 38.9 | 41.2 | 40.9 | |
| Ever smoking cigarettes | 2.8 | 3.6 | 2.6 | 2.8 | |
| Current smoker | 2.4 | 3.2 | 2.2 | 2.4 | |
| Usual no. of cigarettes smoked per dayc | 9.4 (7.3) | 9.9 | 9.3 | 8.8 | |
| Years of smokingc | 22.2 (15.1) | 22.0 | 22.1 | 22.6 | |
| Exposure to SHS at homed | 66.8 | 67.9 | 66.9 | 65.7 | |
| Exposure to SHS at workd | 38.4 | 37.0 | 38.7 | 38.8 | |
| Ever drinking alcohol | 2.3 | 2.2 | 2.2 | 2.7 | |
| Physical activity (>100 MET-hours/week per year) | 50.6 | 46.3 | 50.6 | 55.4 | |
| Body mass indexe | 24.0 (3.4) | 23.7 | 24.0 | 24.4 | |
| First-degree family history of lung cancer | 4.8 | 4.5 | 5.0 | 4.6 | |
| Menopausal status | 47.8 | 47.5 | 47.9 | 48.1 | |
| Age at menopause, yearsf | 48.6 (4.3) | 48.4 | 48.7 | 48.8 | |
| Years of fertilityf | 33.5 (4.6) | 33.1 | 33.5 | 33.6 | |
| Daily dietary intakeb | |||||
| Fruits, g | 249.4 (151.6) | 243.1 | 251.0 | 251.1 | |
| Vegetables, g | 303.8 (152.6) | 254.0 | 297.7 | 374.1 | |
| Red meat, g | 48.1 (30.1) | 47.8 | 48.5 | 47.0 | |
| Total calories, kcal | 1,651.5 (351.1) | 1,457.1 | 1,641.8 | 1,882.4 | |
| Isoflavones, mg | 31.4 (19.3) | 12.2 | 29.0 | 58.6 | |
Abbreviations: MET, metabolic equivalent; Q, quintile; SD, standard deviation; SHS, secondhand smoke.
aExcept for mean age, the data shown according to quintiles of soy food intake were standardized to the age distribution of the cohort at baseline; data on dietary variables, except for total calories, were further standardized to total caloric intake.
b Average of intakes from the baseline and second food frequency questionnaires; soy food intake was assessed on a dry-weight basis.
c Among ever smokers.
dInformation on SHS was collected at the first follow-up for 91.6% of the cohort; data on home SHS exposure were missing for 6,005 participants and on work SHS exposure for 5,976 participants.
e Weight (kg)/height (m)2.
fAmong postmenopausal women at baseline (n = 34,214).
During a mean follow-up period of 9.1 years, we identified 370 incident cases of malignant neoplasm of the bronchus or lung, of which 340 cases were in lifetime never smokers. After adjustment for age, cigarette smoking, and other lifestyle and dietary factors, lung cancer risk significantly decreased with increasing soy food intake (Table 2), with an approximately linear trend (see Web Figure 1 (http://aje.oxfordjournals.org/)). Compared with women in the lowest quintile of soy food intake, women in the highest quintile of intake had a hazard ratio of 0.63 (95% confidence interval (CI): 0.44, 0.90; Ptrend = 0.004). Each 5-g/day increment of intake of dry-weight soy foods (approximately equivalent to 1 ounce of tofu per day) was associated with a reduction in risk of 7% (hazard ratio (HR) = 0.93, 95% CI: 0.88, 0.98) (Table 2). A similar inverse association with lung cancer risk was also found for dietary intake of isoflavones (Table 2) and soy protein (data not shown).
Table 2.
Hazard Ratios for Lung Cancer Associated With Intake of Soy Foods and Isoflavones, Shanghai Women's Health Study, 1997–2008a
| Person-Years of Follow-up | No. of Cases | Age- and Energy-Adjusted HR | 95% CI | Multivariable HRb | 95% CI | Multivariable HR Among Never Smokersb,c | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Quintile of soy food intake, g/dayd | ||||||||
| ≤9.95 | 132,263 | 90 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 9.96–14.61 | 130,690 | 76 | 0.88 | 0.65, 1.20 | 0.89 | 0.65, 1.21 | 0.79 | 0.56, 1.08 |
| 14.62–19.56 | 130,413 | 70 | 0.78 | 0.57, 1.08 | 0.78 | 0.57, 1.08 | 0.73 | 0.52, 1.02 |
| 19.57–26.66 | 128,998 | 72 | 0.79 | 0.57, 1.08 | 0.78 | 0.56, 1.08 | 0.77 | 0.55, 1.08 |
| >26.66 | 127,176 | 62 | 0.64 | 0.45, 0.91 | 0.63 | 0.44, 0.90 | 0.58 | 0.40, 0.85 |
| Petrend | 0.006 | 0.004 | 0.006 | |||||
| Per 5-g/day increment | 0.92 | 0.88, 0.98 | 0.93 | 0.88, 0.98 | 0.93 | 0.87, 0.98 | ||
| Quintile of isoflavone intake, mg/dayd | ||||||||
| ≤15.92 | 130,606 | 88 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 15.93–23.86 | 130,767 | 73 | 0.88 | 0.64, 1.20 | 0.88 | 0.65, 1.21 | 0.84 | 0.61, 1.17 |
| 23.87–32.43 | 130,353 | 66 | 0.77 | 0.56, 1.06 | 0.77 | 0.56, 1.07 | 0.70 | 0.50, 0.99 |
| 32.44–44.23 | 129,349 | 72 | 0.80 | 0.58, 1.11 | 0.79 | 0.58, 1.10 | 0.76 | 0.55, 1.07 |
| >44.23 | 128,466 | 71 | 0.74 | 0.53, 1.04 | 0.72 | 0.51, 1.02 | 0.68 | 0.47, 0.97 |
| Petrend | 0.03 | 0.02 | 0.02 | |||||
| Per 10-mg/day increment | 0.93 | 0.88, 0.99 | 0.93 | 0.87, 0.99 | 0.93 | 0.87, 0.99 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a The first year of observation was omitted from the analysis.
b Multivariable Cox proportional hazards models were adjusted for age, education, cigarette smoking, alcohol consumption, physical activity, body mass index, menopausal status, family history of lung cancer among first-degree relatives, and average intakes of total calories, fruits, nonsoy vegetables, red meat, and nonsoy calcium and were stratified on birth year.
c Restricted to never smokers (n = 69,536), with 340 incident lung cancer cases.
d Average of intakes from the baseline and second food frequency questionnaires; soy food intake was assessed on a dry-weight basis.
e Continuous variables were used to evaluate linear trends.
To assess the effect of assigning values to the data missing from the second FFQ (10.9% of the cohort), we restricted analyses to participants (n = 63,778) who completed both the first and second FFQs and were free of diabetes at the second FFQ survey. The multivariable hazard ratio for the comparison of extreme quintiles was 0.62 (95% CI: 0.40, 0.94), similar to that observed in the entire population. Likewise, results were comparable when only baseline intake was analyzed for the entire cohort (HR = 0.68, 95% CI: 0.48, 0.95). Furthermore, a similar inverse association was observed in a nutrient residual (energy-adjusted) model (HR = 0.63, 95% CI: 0.46, 0.88) for the highest quintile of soy food intake versus the lowest.
We also evaluated the association of lung cancer with the frequency of soy food consumption assessed by means of a 7-day recall at baseline. The frequency of soy food consumption was inversely associated with the risk of lung cancer (Ptrend = 0.01). Women who had consumed soy food at least 3 times during the preceding week versus none had a multivariable hazard ratio of 0.64 (95% CI: 0.45, 0.90).
We further examined whether the soy–lung cancer association varied by tumor aggressiveness (Table 3). Among 370 incident cases, 171 patients survived for less than 12 months and were classified as having more aggressive tumors; 165 patients survived for 12 months or more and were classified as having less aggressive tumors; and 34 surviving patients with a follow-up period of less than 12 months were excluded from the analysis. The inverse association between soy food intake and lung cancer risk was more pronounced for more aggressive tumors. Results derived from the age- and energy-adjusted model (data not shown) were essentially identical to results derived from the multivariable model (Table 3). The multivariable hazard ratio for the comparison of extreme quintiles of intake was 0.49 (95% CI: 0.28, 0.86; Ptrend = 0.005), as compared with 0.76 (95% CI: 0.46, 1.27; Ptrend = 0.31) for less aggressive tumors (Pheterogeneity = 0.057).
Table 3.
Association Between Soy Food Intake and Lung Cancer Risk According to Tumor Aggressiveness, Shanghai Women's Health Study, 1997–2008a
| Quintile of Soy Food Intake, g/dayb | Person-Years of Follow-up | More Aggressive Lung Cancerc |
Less Aggressive Lung Cancerc |
||||
|---|---|---|---|---|---|---|---|
| No. of Cases | HRd | 95% CI | No. of Cases | HRd | 95% CI | ||
| ≤9.95 | 131,997 | 40 | 1.00 | Reference | 40 | 1.00 | Reference |
| 9.96–14.61 | 130,464 | 34 | 0.90 | 0.57, 1.43 | 31 | 0.79 | 0.49, 1.27 |
| 14.62–19.56 | 130,211 | 33 | 0.82 | 0.51, 1.31 | 34 | 0.84 | 0.53, 1.34 |
| 19.57–26.66 | 128,820 | 34 | 0.80 | 0.50, 1.29 | 33 | 0.80 | 0.49, 1.29 |
| >26.66 | 126,990 | 24 | 0.49 | 0.28, 0.86 | 33 | 0.76 | 0.46, 1.27 |
| Petrend | 0.005 | 0.31 | |||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a The first year of observation was omitted from the analysis.
b Average of intakes from the first and second food frequency questionnaires, assessed on a dry-weight basis.
c More aggressive cases were defined as those among patients with a survival time of <12 months; less aggressive cases were defined as those among patients with a survival time of ≥12 months. The P value for heterogeneity in lung cancer risk associated with soy food intake by tumor aggressiveness (survival time <12 months vs. ≥12 months) was 0.057, derived from a logistic regression model of lung cancer cases only after adjusting for tumor stage, histologic type, and other potential confounders.
d Multivariable Cox proportional hazards models were adjusted for age, education, cigarette smoking, alcohol consumption, physical activity, body mass index, menopausal status, family history of lung cancer among first-degree relatives, and average intakes of total calories, fruits, nonsoy vegetables, and red meat and were stratified on birth year.
e Continuous variables were used to evaluate linear trends.
The inverse association between soy food intake and lung cancer risk appeared predominantly among women with a later age at menopause (Pinteraction = 0.01) or more years of reproduction (Pinteraction = 0.04) (Table 4). Years of reproduction were calculated by subtracting age at menarche from age at menopause. Additionally, the inverse association tended to be slightly stronger among never smokers (Table 2); however, we had limited statistical power to examine potential effect modification by cigarette smoking (regular smokers accounted for only 2.8% of this cohort).
Table 4.
Multivariable Hazard Ratio for Lung Cancer Associated With Soy Food Intake (per 5-g/day Increment), According to Selected Covariates, Shanghai Women's Health Study, 1997–2008a
| No. of Cases | Hazard Ratiob | 95% Confidence Interval | P Value | Pinteraction | |
|---|---|---|---|---|---|
| Age at baseline, years | |||||
| <50 (median, 50) | 76 | 0.93 | 0.82, 1.05 | 0.26 | 0.53 |
| ≥50 | 294 | 0.92 | 0.87, 0.98 | 0.01 | |
| Age at menopause at baseline, yearsc | |||||
| <49 (median, 49) | 148 | 0.98 | 0.91, 1.05 | 0.57 | 0.01 |
| ≥49 | 138 | 0.88 | 0.80, 0.97 | 0.008 | |
| Years of reproduction at baselinec | |||||
| <34 (median, 34) | 169 | 0.98 | 0.91, 1.05 | 0.49 | 0.04 |
| ≥34 | 117 | 0.86 | 0.78, 0.96 | 0.006 |
a The first year of observation was omitted from the analysis.
b Multivariable Cox proportional hazards models were adjusted for age, education, cigarette smoking, alcohol consumption, physical activity, body mass index, menopausal status, family history of lung cancer among first-degree relatives, and average intakes of total calories, fruits, nonsoy vegetables, red meat, and nonsoy calcium and were stratified on birth year. Usual soy food intake on a dry-weight basis was measured by averaging intakes from the first and second frequency food questionnaires.
c Restricted to postmenopausal women.
Meta-analysis
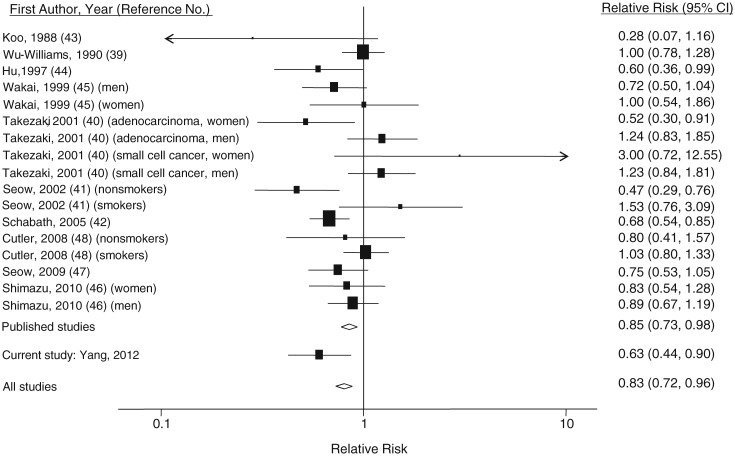
We conducted a meta-analysis of 11 studies (7 case-control studies (39–45) and 3 published cohort studies (46–48), plus the present study) involving a total of 231,494 participants and 6,811 lung cancer cases. Two studies were conducted in the United States (42, 48) and 9 in Asian countries (39–41, 43–47). All but one early study (43) reported results adjusted for cigarette smoking. Previous studies have generally suggested an inverse association of lung cancer with intake of soy or its isoflavones. However, a positive but statistically nonsignificant association was found among smokers in 2 studies (41, 48). The summary relative risk from the 10 published studies was 0.85 (95% CI: 0.73, 0.98); after combining our results with the others, the summary relative risk was 0.83 (95% CI: 0.72, 0.96) (Figure 1).
Figure 1.
Results from a meta-analysis of the association between soy/isoflavone intake and lung cancer risk. The size of each square is proportional to the study's weight (inverse of variance). The diamonds represent the summary risk estimates for the analyses including and excluding the current study. Bars, 95% confidence interval (CI).
Sensitivity analyses suggested that exclusion of studies with the largest (40) and smallest (43) relative risks did not appreciably alter the pooled risk estimates (summary relative risk (RR) = 0.82 (95% CI: 0.71, 0.94) and RR = 0.84 (95% CI: 0.73, 0.97), respectively). We also conducted analyses excluding 1 study with the largest weight (42) and 1 study that did not adjust for smoking status (43); no material changes in the pooled risk estimates were found (RR = 0.85 (95% CI: 0.72, 0.99) and RR = 0.84 (95% CI: 0.73, 0.97), respectively). Thus, the pooled results appear robust to potential influential observations. Weighted meta-regression revealed no association between the log relative risk and year of publication (b = −0.00081, P = 0.80) or year of study enrollment (b = −0.03, P = 0.10). Both the funnel plot and Egger's test (b = −0.14, P = 0.88) suggested no publication bias.
In subgroup analyses (Table 5), pooled risk estimates did not significantly differ by sex, study design (case-control study vs. cohort study), or measure of soy intake (soy foods vs. isoflavones). However, the inverse association was primarily confined to nonsmokers (summary relative risk = 0.59, 95% CI: 0.49, 0.71), as compared with a summary relative risk of 0.91 (95% CI: 0.78, 1.05) for smokers (Pheterogeneity = 0.0003). The standardized summary relative risks for lung cancer associated with a 1-g/day increment of soy protein intake were 0.96 (95% CI: 0.93, 0.99) among nonsmokers and 1.00 (95% CI: 0.99, 1.01) among smokers.
Table 5.
Pooled Risk Estimates for Lung Cancer Associated With Soy/Isoflavone Intake in Subgroup Meta-Analysis
| Factor | No. of Studies | Relative Riska | 95% Confidence Interval | I2, %b | Test for Heterogeneityc |
Pdheterogeneity | |
|---|---|---|---|---|---|---|---|
| χ2 | P Value | ||||||
| All studies | 11 | 0.83 | 0.72, 0.96 | 54.6 | 37.44 | 0.003 | |
| Sex | 0.89 | ||||||
| Male | 6 | 0.80 | 0.61, 1.06 | 67.9 | 18.72 | 0.005 | |
| Female | 11 | 0.79 | 0.67, 0.94 | 49.9 | 25.93 | 0.02 | |
| Ever smoker | 0.0003 | ||||||
| Yes | 5 | 0.91 | 0.78, 1.05 | 38.4 | 9.74 | 0.14 | |
| No | 7 | 0.59 | 0.49, 0.71 | 0.0 | 4.58 | 0.71 | |
| Study design | 0.84 | ||||||
| Case-control | 7 | 0.83 | 0.67, 1.04 | 65.6 | 31.94 | <0.001 | |
| Cohort | 4 | 0.85 | 0.74, 0.97 | 8.4 | 5.46 | 0.36 | |
| Study population | 0.71 | ||||||
| Asian | 9 | 0.83 | 0.70, 0.99 | 55.8 | 31.69 | 0.004 | |
| American | 2 | 0.83 | 0.60, 1.14 | 64.3 | 34.69 | 0.06 | |
| Measure of soy intake | 0.54 | ||||||
| Soy/tofu | 8 | 0.82 | 0.66, 1.01 | 62.0 | 31.55 | 0.002 | |
| Isoflavones | 6 | 0.80 | 0.71, 0.89 | 29.0 | 11.26 | 0.19 | |
a Summary relative risks were based on the odds ratio or relative risk for the highest category of soy/isoflavone intake versus the lowest in each original study, using the DerSimonian and Laird random-effects model (34), with the exception of summary relative risks for nonsmokers and cohort studies, for which a fixed-effects model was used.
b I2 represents the percentage of total variation across studies that is attributable to heterogeneity rather than to chance (54).
c Test for heterogeneity among studies in the category.
d Test for heterogeneity between categories of the factor stratified.
DISCUSSION
Concerns about the adverse effects of hormone therapy have led to increased interest in naturally occurring plant-based estrogens (7, 49), especially soy phytoestrogens, as alternative approaches to optimize postmenopausal health (10, 50). In this large, population-based, prospective cohort study of women, 97.2% of whom were never smokers, we found that increasing intake of soy foods was associated with a substantially reduced risk of lung cancer. This association was independent of traditional risk factors for lung cancer and was not explained by differences in consumption of other dietary components, such as fruits and vegetables.
In this study, we found that the soy–lung cancer association varied significantly by tumor behavior; the inverse association with soy food intake was more pronounced for aggressive lung cancer, a form of lung cancer that generally has a short survival time. This finding is in direct contrast to the Women's Health Initiative finding of a more significantly increased risk of aggressive lung cancer with use of combined hormone therapy (7). Emerging evidence suggests that estrogen signaling promotes lung cancer proliferation and progression (5–8), and as mentioned above, estrogen receptor antagonists such as tamoxifen may counteract the detrimental effect of hormone therapy on lung cancer (5, 8). Soy phytoestrogens have been shown to act as natural estrogen antagonists and have diverse cancer-inhibitory activity, including induction of apoptosis and cell-cycle arrest and inhibition of tumor invasion and angiogenesis (17–20, 22). This novel finding of a protective effect of soy foods against aggressive lung cancer merits further investigation, particularly among users of hormone therapy.
Another novel finding from this study is that the soy–lung cancer association may be modified by endogenous estrogens. Soy isoflavones have a diphenolic structure similar to that of 17β-estradiol and can compete with endogenous estrogens in the binding of estrogen receptors (11). The biologic behavior of isoflavones may thus be modulated by individuals' endogenous estrogen levels. It has been shown that isoflavones act primarily as estrogen agonists in a low-estrogen environment, whereas they act like estrogen antagonists in a high-estrogen environment (51). Our finding of an inverse association of soy consumption with lung cancer risk was predominantly observed among women with indicators of longer and higher exposure to endogenous hormones, which suggests that the effect of soy on lung cancer may, at least in part, be due to its estrogen antagonist-like effect. However, we did not find that risk estimates associated with isoflavone intake were stronger than risk estimates associated with soy food intake. This may be because measurement errors in the assessment of isoflavone intake are larger than measurement errors related to soy food intake (52). On the other hand, soy foods are also rich in calcium, folic acid, and fiber and contributed 26%, 18%, and 15% of the total intake of these nutrients, respectively, in this study population. It is possible that these and probably many other phytochemicals found in soy foods are also responsible for the observed cancer-preventive effect of soy foods.
The observed inverse association between soy food intake and lung cancer risk in the SWHS was further supported by our meta-analysis of 7 studies that had specifically estimated lung cancer risk for nonsmokers (6 published studies plus the present study) (39–48), with a summary relative risk of 0.59 (95% CI: 0.49, 0.71). Results from previous cohort studies carried out among nonsmokers have been rather consistent (46, 47), although heterogeneity in the association is present across studies of smokers (Table 5). In 2 Asian cohort studies (46, 47), isoflavone intake was found to be inversely associated with lung cancer risk in both male (46) and female (47) lifetime nonsmokers but not in current or former cigarette smokers. A similar but statistically nonsignificant protective effect of isoflavones was also suggested in the Iowa Women's Health Study (48). However, that study, like other studies of soy/isoflavones and health outcomes conducted in Western populations, was limited by the generally very low intake of isoflavones (median intake was 0.25 mg/day, as compared with 27.9 mg/day in our study population).
Several features distinguish this study from previous investigations. This study population was well suited to examination of the soy–lung cancer association given its high yet diverse soy food intakes. The median intakes of isoflavones for the highest and lowest quintile categories (58.6 mg/day vs. 12.2 mg/day) varied nearly 5-fold. Particularly noteworthy characteristics of this cohort are the extremely low prevalences of use of tobacco products (2.8%) and hormone therapy (2.0%; users of hormone therapy were excluded from the analysis); thus, the potential for effect masking by cigarette smoking or confounding by postmenopausal hormone use should have been minimal. Other strengths of our study include a prospective design, high rates of participation (93% at enrollment) and follow-up (over 96% for active, in-person follow-up), use of repeated dietary assessments with a validated FFQ, and use of an in-person interview.
As with all nutritional epidemiology studies, measurement error in assessing soy food intake is a possible concern, although the FFQ used in this study had been previously evaluated and found to have reasonably good validity for the measurement of usual dietary intake of soy foods (28). The validity of the FFQ for assessing soy food intake is also supported by our recent work showing moderate correlation (r = 0.48) between soy food intake derived from the FFQ and the mean level of isoflavone excretion measured in 4 spot urine samples collected quarterly over a 1-year period (53). In addition, the inverse association was consistently observed for all of the analytic approaches used in the study, including the analysis of baseline FFQ data alone, the cumulative average of the baseline and second FFQs, and the 7-day dietary recall administered at baseline. Furthermore, because the exposure assessment was conducted prospectively and prior to cancer diagnosis, errors in measurement of soy consumption are likely to have been nondifferential, which would have tended to attenuate the true association between soy consumption and lung cancer. Another concern is that we could not completely rule out the possibility of residual confounding due to unmeasured or inaccurately measured covariates, although we carefully adjusted for a wide range of dietary and nondietary factors that are potential confounders of the soy–lung cancer association.
The direction and magnitude of risk estimates for lung cancer associated with soy food intake in this study were generally similar to risk estimates in our meta-analysis of previous studies. Therefore, although our study was conducted in a relatively homogeneous population (Chinese women with very low prevalences of tobacco-product and postmenopausal hormone use), the present findings should have wider relevance.
In conclusion, this prospective cohort study, reinforced by a meta-analysis, provides strong evidence that soy food consumption may confer protection against lung cancer, particularly aggressive lung cancer, among nonsmoking women. Given the fact that incidence of lung cancer among women is increasing steadily worldwide and that soy can be readily incorporated into most diets, our findings, if replicated in other populations, would have important public health implications for the prevention of this common, fatal malignancy.
ACKNOWLEDGMENTS
Author affiliations: Division of Epidemiology, Department of Medicine, School of Medicine, Vanderbilt University, Nashville, Tennessee (Gong Yang, Xiao Ou Shu, Xianglan Zhang, Hui Cai, Shenghui Wu, Wei Zheng); Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, Tennessee (Gong Yang, Xiao Ou Shu, Xianglan Zhang, Hui Cai, Shenghui Wu, Wei Zheng); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Wong-Ho Chow, Bu-Tian Ji); and Department of Epidemiology, Shanghai Cancer Institute, Shanghai, China (Hong-Lan Li, Yu-Tang Gao).
This work was supported by the US National Cancer Institute, National Institutes of Health (grants R37CA070867 to W. Z. and RFP N02 CP1101066 to X. O. S.).
The authors are grateful to the research staff of the Shanghai Women's Health Study for their contributions. They also thank Bethanie Rammer and Jacqueline Stern for their assistance in preparing the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Couse JF, Lindzey J, Grandien K, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138(11):4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty SM, Mazhawidza W, Bohn AR, et al. Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocr Relat Cancer. 2006;13(1):113–134. doi: 10.1677/erc.1.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CT, Chang YL, Shih JY, et al. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130(4):979–986. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62(7):2141–2150. [PubMed] [Google Scholar]
- 6.Weinberg OK, Marquez-Garban DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65(24):11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374(9697):1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchardy C, Benhamou S, Schaffar R, et al. Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer. 2011;117(6):1288–1295. doi: 10.1002/cncr.25638. [DOI] [PubMed] [Google Scholar]
- 9.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29(2):95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 10.Taylor CK, Levy RM, Elliott JC, et al. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67(7):398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 13.Duffy C, Perez K, Partridge A. Implications of phytoestrogen intake for breast cancer. CA Cancer J Clin. 2007;57(5):260–277. doi: 10.3322/CA.57.5.260. [DOI] [PubMed] [Google Scholar]
- 14.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98(18):1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 15.Dia VP, Berhow MA, Gonzalez De Mejia E. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J Agric Food Chem. 2008;56(24):11707–11717. doi: 10.1021/jf802475z. [DOI] [PubMed] [Google Scholar]
- 16.Hämäläinen M, Nieminen R, Vuorela P, et al. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. doi:10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian F, Li Y, Bhuiyan M, et al. p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer. 1999;33(2):125–131. doi: 10.1207/S15327914NC330202. [DOI] [PubMed] [Google Scholar]
- 18.Lian F, Bhuiyan M, Li YW, et al. Genistein-induced G2-M arrest, p21WAF1 upregulation, and apoptosis in a non-small-cell lung cancer cell line. Nutr Cancer. 1998;31(3):184–191. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- 19.Whyte L, Huang YY, Torres K, et al. Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res. 2007;67(24):12007–12017. doi: 10.1158/0008-5472.CAN-07-2464. [DOI] [PubMed] [Google Scholar]
- 20.Tallett A, Chilvers ER, Hannah S, et al. Inhibition of neuropeptide-stimulated tyrosine phosphorylation and tyrosine kinase activity stimulates apoptosis in small cell lung cancer cells. Cancer Res. 1996;56(18):4255–4263. [PubMed] [Google Scholar]
- 21.Ryan-Borchers TA, Park JS, Chew BP, et al. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 2006;83(5):1118–1125. doi: 10.1093/ajcn/83.5.1118. [DOI] [PubMed] [Google Scholar]
- 22.Zou H, Zhan S, Cao K. Apoptotic activity of genistein on human lung adenocarcinoma SPC-A-1 cells and preliminary exploration of its mechanisms using microarray. Biomed Pharmacother. 2008;62(9):583–589. doi: 10.1016/j.biopha.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Menon LG, Kuttan R, Nair MG, et al. Effect of isoflavones genistein and daidzein in the inhibition of lung metastasis in mice induced by B16F-10 melanoma cells. Nutr Cancer. 1998;30(1):74–77. doi: 10.1080/01635589809514644. [DOI] [PubMed] [Google Scholar]
- 24.Lee YS, Seo JS, Chung HT, et al. Inhibitory effects of biochanin A on mouse lung tumor induced by benzo(a)pyrene. J Korean Med Sci. 1991;6(4):325–328. doi: 10.3346/jkms.1991.6.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallo D, Zannoni GF, De Stefano I, et al. Soy phytochemicals decrease nonsmall cell lung cancer growth in female athymic mice. J Nutr. 2008;138(7):1360–1364. doi: 10.1093/jn/138.7.1360. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W, Chow WH, Yang G, et al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 27.Yang G, Shu XO, Li H, et al. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89(2):577–583. doi: 10.3945/ajcn.2008.26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu XO, Yang G, Jin F, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur J Clin Nutr. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 29.Yang YX, Wang GY, Pan XC, editors. China Food Composition Tables 2002. Beijing, China: Beijing University Medical Press; 2002. [Google Scholar]
- 30.Harrell FJ., Jr . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag New York; 2001. [Google Scholar]
- 31.Collett D. Non-proportional hazards. In: Collett D, editor. Modelling Survival Data in Medical Research. 1st ed. New York, NY: Chapman & Hall, Inc; 1994. pp. 267–270. [Google Scholar]
- 32.Cai H, Shu XO, Gao YT, et al. A prospective study of dietary patterns and mortality in Chinese women. Epidemiology. 2007;18(3):393–401. doi: 10.1097/01.ede.0000259967.21114.45. [DOI] [PubMed] [Google Scholar]
- 33.Pronk A, Coble J, Ji BT, et al. Occupational risk of lung cancer among lifetime non-smoking women in Shanghai, China. Occup Environ Med. 2009;66(10):672–678. doi: 10.1136/oem.2008.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: John Wiley & Sons, Inc; 1981. [Google Scholar]
- 36.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Juni P, Bartlett C, et al. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7(1):1–76. [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu-Williams AH, Dai XD, Blot W, et al. Lung cancer among women in north-east China. Br J Cancer. 1990;62(6):982–987. doi: 10.1038/bjc.1990.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takezaki T, Hirose K, Inoue M, et al. Dietary factors and lung cancer risk in Japanese: with special reference to fish consumption and adenocarcinomas. Br J Cancer. 2001;84(9):1199–1206. doi: 10.1054/bjoc.2001.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seow A, Poh WT, Teh M, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int J Cancer. 2002;97(3):365–371. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 42.Schabath MB, Hernandez LM, Wu X, et al. Dietary phytoestrogens and lung cancer risk. JAMA. 2005;294(12):1493–1504. doi: 10.1001/jama.294.12.1493. [DOI] [PubMed] [Google Scholar]
- 43.Koo LC. Dietary habits and lung cancer risk among Chinese females in Hong Kong who never smoked. Nutr Cancer. 1988;11(3):155–172. doi: 10.1080/01635588809513983. [DOI] [PubMed] [Google Scholar]
- 44.Hu J, Johnson KC, Mao Y, et al. A case-control study of diet and lung cancer in northeast China. Int J Cancer. 1997;71(6):924–931. doi: 10.1002/(sici)1097-0215(19970611)71:6<924::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 45.Wakai K, Ohno Y, Genka K, et al. Risk modification in lung cancer by a dietary intake of preserved foods and soyfoods: findings from a case-control study in Okinawa, Japan. Lung Cancer. 1999;25(3):147–159. doi: 10.1016/s0169-5002(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 46.Shimazu T, Inoue M, Sasazuki S, et al. Isoflavone intake and risk of lung cancer: a prospective cohort study in Japan. Japan Public Health Center-based Prospective Study Group. Am J Clin Nutr. 2010;91(3):722–728. doi: 10.3945/ajcn.2009.28161. [DOI] [PubMed] [Google Scholar]
- 47.Seow A, Koh WP, Wang R, et al. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2009;18(3):821–827. doi: 10.1158/1055-9965.EPI-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cutler GJ, Nettleton JA, Ross JA, et al. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. Int J Cancer. 2008;123(3):664–671. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 50.Nedrow A, Miller J, Walker M, et al. Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Arch Intern Med. 2006;166(14):1453–1465. doi: 10.1001/archinte.166.14.1453. [DOI] [PubMed] [Google Scholar]
- 51.Hwang CS, Kwak HS, Lim HJ, et al. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006;101(4-5):246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302(22):2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SA, Wen W, Xiang YB, et al. Assessment of dietary isoflavone intake among middle-aged Chinese men. J Nutr. 2007;137(4):1011–1016. doi: 10.1093/jn/137.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]