Abstract
Risk compensation associated with male circumcision has been a concern for male circumcision scale-up programs. Using posttrial data collected during 2007–2011 on 2,137 male circumcision trial participants who were uncircumcised at trial closure in Rakai, Uganda, the authors evaluated their sexual behavioral changes during approximately 3 years' follow-up after trial closure. Eighty-one percent of the men self-selected for male circumcision during the period, and their sociodemographic and risk profiles were comparable to those of men remaining uncircumcised. Linear models for marginal probabilities of repeated outcomes estimate that 3.3% (P < 0.0001) of the male circumcision acceptors reduced their engagement in nonmarital relations, whereas there was no significant change among men remaining uncircumcised. Significant decreases in condom use occurred in both male circumcision acceptors (−9.2% with all partners and −7.0% with nonmarital partners) and nonacceptors (−12.4% and −13.5%, respectively), and these were predominantly among younger men. However, the magnitudes of decrease in condom use were not significantly different between the 2 groups. Additionally, significant decreases in sex-related alcohol consumption were observed in both groups (−7.8% in male circumcision acceptors and −6.1% in nonacceptors), mainly among older men. In summary, there was no evidence of risk compensation associated with male circumcision among this cohort of men during 3 years of posttrial follow-up.
Keywords: behavior changes, behavioral disinhibition, HIV prevention, male circumcision, Rakai, risk compensation
Three randomized controlled trials (RCTs) in sub-Saharan Africa have shown that male circumcision reduces female-to-male human immunodeficiency virus (HIV) incident infection by 50%–60% (1–3). In 2007, the World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS) recommended that male circumcision be promoted as an “additional, important strategy for the prevention of heterosexually-acquired HIV infection in men,” and 13 sub-Saharan Africa countries, including Uganda, were identified as priority countries for male circumcision scale-up (4, p. 11; 5, p. 3). Although the RCTs proved the causal effect (i.e., efficacy) of male circumcision for reducing the risk of HIV infection in men, the long-term population-level impact of male circumcision on the HIV epidemic will depend on male circumcision coverage and the influence of male circumcision on sexual risk behaviors.
Risk compensation, defined as a change toward riskier sexual behaviors after adopting an HIV prevention strategy, has been a theoretical concern with male circumcision (6–23), particularly during rapid male circumcision scale-up where provision of risk reduction education, counseling, and follow-up may be limited. Simulation studies suggest that risk compensation can reduce the impact of male circumcision on HIV incidence (24, 25), and a modest level of risk compensation in men could increase female HIV infections (26). Data on behavioral changes after male circumcision acceptance have been sparse, and no empirical data are available during the current period when the efficacy of male circumcision is well known and male circumcision rollout is taking place in programmatic settings.
The Rakai Health Sciences Program conducted one of the male circumcision RCTs in Rakai, Uganda, during 2003–2006, and has continued to follow the trial participants after trial closure. Using data on the trial participants who were uncircumcised at the end of the trial, we assessed whether the adoption of male circumcision in the posttrial programmatic setting encouraged men to engage in riskier behaviors (i.e., risk compensation associated with male circumcision).
MATERIALS AND METHODS
The original Rakai male circumcision RCT has been described elsewhere (3). Briefly, HIV-negative, uncircumcised men aged 15–49 years who received their HIV test results and posttest counseling and provided consent were enrolled and randomized to undergo either immediate circumcision (intervention) or circumcision delayed for 24 months (controls). Participants were followed up at 6, 12, and 24 months to assess HIV incidence and sexual risk behaviors. At each visit, men were examined to assess circumcision status and to diagnose any penile pathology. Repeat HIV testing and individual-level health education and counseling were also provided. Free condoms were offered to all sexually active participants at all study visits and were available through community-based condom depots stocked by the Rakai program. The trial was stopped in December 2006 following an interim analysis that demonstrated the efficacy of male circumcision for HIV prevention. After trial closure, all trial participants provided consent and enrolled into a posttrial surveillance study that was integrated into the schedule of an ongoing Rakai cohort study. By July 2011, all available participants had been contacted for their second posttrial follow-up interviews. In addition, all uncircumcised, control-arm participants and intervention-arm crossovers (i.e., the population for this study) were offered free male circumcision as a service, and surgeries were conducted as expeditiously as possible. The studies were reviewed and approved by 4 institutional review boards in Uganda and the United States.
Current study design and population
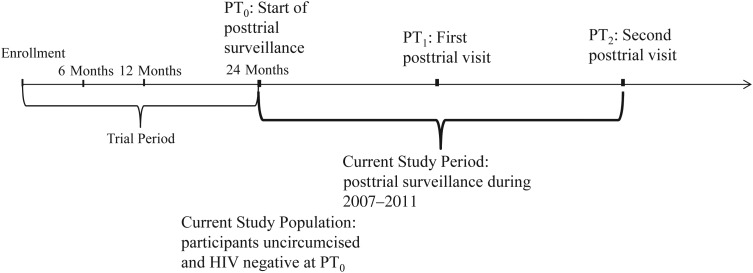
Figure 1 illustrates the current study period and population in relation to the original RCT. This current analysis is a prospective cohort study of men who were uncircumcised and HIV negative at the last trial visit. Men could opt for circumcision as their own choice during the posttrial follow-up, which represents a period when information about the efficacy of male circumcision for HIV prevention had been broadly disseminated, and free male circumcision services were widely available and promoted in Rakai.
Figure 1.
Illustration of the current study period and population in relation to the original male circumcision randomized controlled trial in Rakai, Uganda, beginning in 2007. PT0, start of posttrial surveillance; PT1, first posttrial visit; PT2, second posttrial visit.
Posttrial male circumcision service procedure
Surgery was performed by trained clinical officers in aseptic outpatient operating rooms using the dorsal slit procedure under local anesthesia. Men were followed up at 7–9 days to diagnose and manage surgery-related complications (∼1.0%) and wound healing. Men with complications were also encouraged to return for unscheduled visits or call a hotline for counseling. Prior to surgery, all men, and where applicable their wives, were educated in wound care, told to abstain from intercourse until wound healing was complete (∼4–6 weeks), and urged to consistently use condoms thereafter. All clients also received small-group health education on HIV/sexually transmitted infection (STI) prevention and were informed that male circumcision does not provide complete protection against HIV infection. Provider-initiated pretest HIV counseling was provided to all male circumcision clients, with posttest counseling for those who agreed to learn their results.
Study measurements
At each visit, men were interviewed in privacy at home or in central locations (“hubs”) in the villages to ascertain sexual behaviors. Interviews by same-sex trained interviewers used structured questionnaires with direct electronic data entry on mobile personal computers to collect detailed sociodemographic, behavioral, health, and care-seeking information in the past year, including whether, where, and when they were circumcised. Information on the characteristics of and behaviors with sexual partners in the past year (up to 4 partners) was also elicited from the respondents. Editing was conducted in the field, facilitating error correction.
In this study, we used data collected at the last trial visit (i.e., start of the posttrial surveillance (PT0)), providing information on men when they were still uncircumcised, and at the first posttrial follow-up (PT1) and the second posttrial visit (PT2). On the basis of HIV risk factors that have been identified in Rakai studies (27, 28), 6 variables were defined to quantify sexual behaviors reported for the past 12 months prior to interview, including being sexually active (yes/no), number of sex partners, number of nonmarital sex partners (i.e., partners not in a marital or long-term consensual relationship), condom use with all partners (always/sometimes/never), condom use with nonmarital partners (always/sometimes/never), and alcohol use before sex (sometimes/never). Variables for condom- and sex-associated alcohol use were evaluated on the basis of partner-specific information, including partner type and condom and alcohol use frequency. Only if the man reported using condoms with all partners (or all nonmarital partners) were the condom-use variables coded as “always.”
In addition, in consideration of potential recall lapse over 12 months, we defined 2 variables for condom use at last sex (yes/no) with any type of partner and with nonmarital partners, where the value was “yes” only if the man reported using a condom in the last sex act with each of his partners (nonmarital partners). Other important variables considered were circumcision status and self-perceived risk of HIV infection (likely/unlikely) at each visit, as well as the age, religion, education, occupation, and marital status recorded at PT0.
Statistical analysis
We first assessed possible self-selection of male circumcision by comparing men's sociodemographic characteristics and sexual behaviors reported at PT0 by their subsequent circumcision acceptance during posttrial follow-up using the Pearson χ2 test. Linear models for modeling the marginal distributions of repeated outcomes were used (29) to compare the within-individual behavior changes in relation to acceptance of male circumcision. Specifically, because the timeframe of risk behaviors referred to the 12 months prior to interview, we evaluated the behavior changes from PT0 to PT2, by which time the majority of men opting for male circumcision had been circumcised for more than 1 year. In addition, we compared the men observed with those lost to follow-up at PT2 and used logistic regression with stepwise selection of predictors (measured at PT0) to model the probability of loss to follow-up. Hosmer and Lemeshow goodness-of-fit tests were performed to check on the fitness of the logistic model for loss to follow-up. The inverse probability weighting method was then used in the aforementioned linear models to account for loss to follow-up at PT2 (30–32).
For behavior variables that changed significantly during the posttrial follow-up, we further repeated the linear model analyses stratifying by age group, in order to investigate whether behavior changes or risk compensation associated with male circumcision occurred among men of certain age groups. Inverse probability weighting was applied if there was informative loss to follow-up within an age group. All hypothesis tests were 2 sided with a significance level of 0.05. Analyses were conducted in SAS, version 9.2, statistical software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Study population
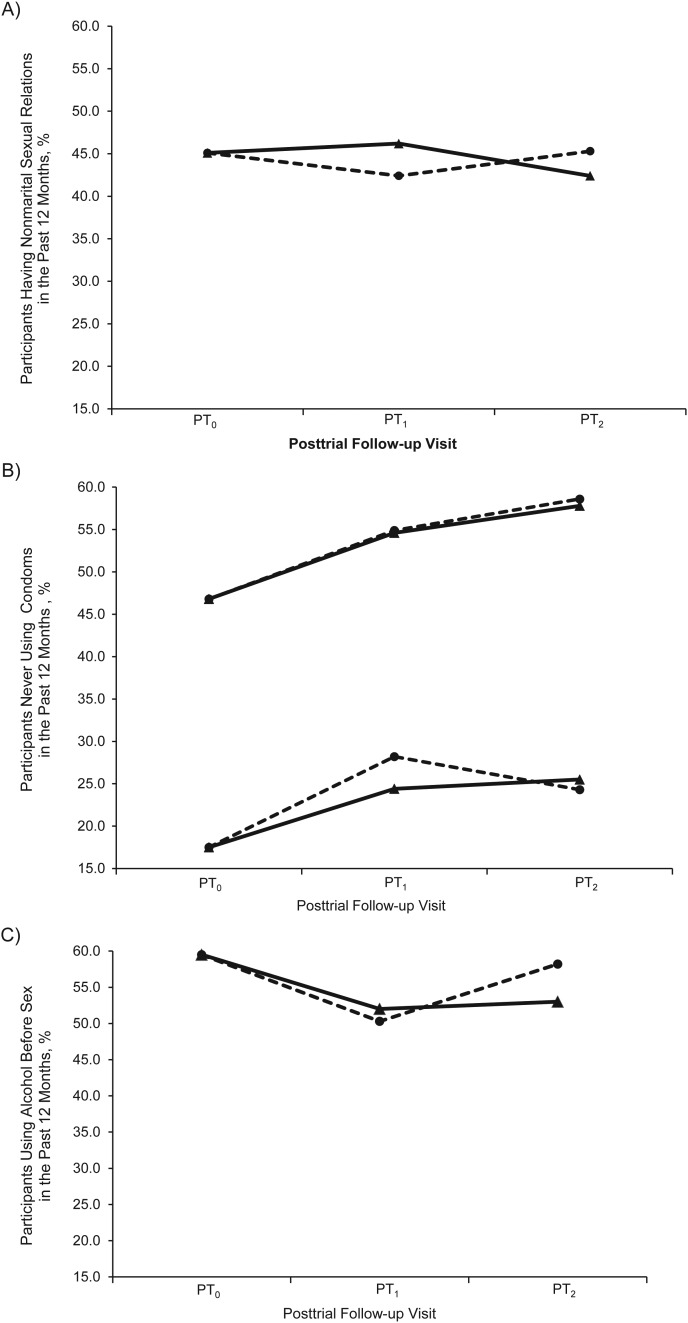
There were 2,137 uncircumcised and HIV-negative men at PT0 who constituted the study population. Their sociodemographic and risk behavior profiles at PT0 are presented in Table 1. From PT0, the median time to PT1 was 1.63 (interquartile range: 1.28–2.17) years and to PT2, 3.13 (interquartile range: 2.85–3.69) years. There were 1,534 men observed at PT1 (retention rate, 71.8%), of whom 1,211 (78.9%) were circumcised. At PT2, 1,597 men were observed (retention rate, 74.7%), of whom 1,297 (81.2%) had been circumcised. Figure 2 summarizes selected risk behaviors by using the cross-sectional data observed at each visit. There were no statistically significant differences at either PT1 or PT2 between circumcised and uncircumcised men with respect to nonmarital relations, condom use, or alcohol consumption and sex in the past 12 months.
Table 1.
Sociodemographic Characteristics and Risk Behaviors of Rakai, Uganda, Trial Participants Who Were Uncircumcised and HIV Negative at PT0 beginning in 2007
| No.a | % | |
|---|---|---|
| Age, years | ||
| 16–20 | 401 | 18.8 |
| 21–25 | 634 | 29.7 |
| 26–30 | 446 | 20.9 |
| 31–35 | 308 | 14.4 |
| 36–51 | 348 | 16.3 |
| Education | ||
| No education | 122 | 5.7 |
| Primary school | 1,417 | 66.3 |
| Secondary school or higher | 598 | 28.0 |
| Religion | ||
| Catholic | 1,483 | 69.4 |
| Protestant | 525 | 24.6 |
| Other | 129 | 6.0 |
| Occupation | ||
| Trading | 475 | 22.2 |
| Student | 292 | 13.7 |
| Fishing | 122 | 5.7 |
| Agriculture for selling | 186 | 8.7 |
| Agriculture for home | 449 | 21.0 |
| Other (e.g., motorcycle/taxi driver or trucker) | 613 | 28.7 |
| Currently married, yes | 1,236 | 57.8 |
| Sexually active past year, yes | 1,839 | 86.1 |
| Self-perceived HIV risk, likely | 600 | 28.1 |
| No. of sex partners in past year | ||
| 0 | 298 | 13.9 |
| 1 | 1,122 | 52.5 |
| ≥2 | 717 | 33.6 |
| No. of nonmarital partners in past year | ||
| 0 | 1,139 | 53.3 |
| 1 | 657 | 30.7 |
| ≥2 | 341 | 16.0 |
| Alcohol use before sex in past year, yes (i.e., sometimes)b | 1,094 | 59.5 |
| Condom use with all partners in past yearb | ||
| Never | 860 | 46.8 |
| Sometimes | 695 | 37.8 |
| Always | 284 | 15.4 |
| Condom use with nonmarital partners in past yearc | ||
| Never | 169 | 17.5 |
| Sometimes | 347 | 36.0 |
| Always | 448 | 46.5 |
| No condom use at last sex with any partnerb | 1,446 | 78.6 |
| No condom use at last sex with nonmarital partnersc | 427 | 44.3 |
Abbreviations: HIV, human immunodeficiency virus; PT0, start of posttrial surveillance.
a Total no. = 2,137.
b Among sexually active men.
c Among men reporting nonmarital/consensual sexual relations in the partner-specific blocks of the questionnaire.
Figure 2.
Trend of selected behavior variables by circumcision status using cross-sectional data at each posttrial follow-up visit (2007–2011) of the Rakai, Uganda, male circumcision trial participants uncircumcised at trial closure. PT0, start of posttrial surveillance; PT1, first posttrial visit; PT2, second posttrial visit. A, proportion of participants reporting nonmarital sexual relations in the past 12 months. Pearson's χ2 test comparing the circumcised (solid line) with the uncircumcised (broken line): P = 0.22 at PT1 and P = 0.36 at PT2. B, proportion never using condoms with all partners (the top lines in the figure) and with nonmarital partners (the bottom lines in the figure) in the past 12 months. Pearson's χ2 test comparing the circumcised (solid line) with the uncircumcised (broken line) for no condom use with all partners: P = 0.68 at PT1 and P = 0.91 at PT2; and for no condom use with nonmarital partners: P = 0.20 at PT1 and P = 0.99 at PT2. C, proportion of sex-associated alcohol drinking in the past 12 months. Pearson's χ2 test comparing the circumcised (solid line) with the uncircumcised (broken line): P = 0.69 at PT1 and P = 0.07 at PT2.
Because our behavior variables generally refer to a 12-month recall period during which most uncircumcised men underwent the procedure, behaviors reported at PT1 may not fully reflect the behaviors of circumcised men. Therefore, from here on, we focus on PT2 where 99.1% of the circumcised men (n = 1,285) had been circumcised for more than 1 year.
Men lost to follow-up at PT2 were significantly different from men observed at PT2 by being more likely to be students, of younger age, with secondary or higher education, unmarried, and having nonmarital sex partners, less sex-related alcohol use, and less condom use in the past year. These variables were used to construct the logistic regression model for loss to follow-up using the stepwise procedure, and the final model retained marital status, age, religion, and education with reasonable goodness of fit (Hosmer and Lemeshow test, P = 0.79). Among men followed at PT2, those opting for circumcision were not significantly different from those remaining uncircumcised in either sociodemographic characteristics or risk behaviors at PT0 (i.e., when they were all uncircumcised), except for occupation where the male circumcision acceptors were slightly more frequently students than the nonacceptors (Table 2).
Table 2.
Sociodemographic Characteristics and Risk Behaviors at PT0 in Men Opting for Male Circumcision and Men Remaining Uncircumcised at PT2, Rakai, Uganda, 2007–2011
| Male Circumcision Acceptors (n = 1,297) |
Male Circumcision Nonacceptors (n = 300) |
P Valuea | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | 0.63 | ||||
| ≤20 | 227 | 17.5 | 43 | 14.3 | |
| 21–25 | 362 | 27.9 | 91 | 30.3 | |
| 26–30 | 281 | 21.7 | 61 | 20.3 | |
| 31–35 | 195 | 15.0 | 50 | 16.7 | |
| ≥36 | 232 | 17.9 | 55 | 18.3 | |
| Education | 0.96 | ||||
| No education | 70 | 5.4 | 17 | 5.7 | |
| Primary school | 885 | 68.2 | 206 | 68.7 | |
| Secondary school or higher | 342 | 26.4 | 77 | 25.7 | |
| Religion | 0.62 | ||||
| Catholic | 73 | 5.6 | 18 | 6.0 | |
| Protestant | 913 | 70.4 | 218 | 72.7 | |
| Other | 311 | 24.0 | 64 | 21.3 | |
| Occupation | 0.01 | ||||
| Trading | 312 | 24.1 | 72 | 24.0 | |
| Student | 165 | 12.7 | 21 | 7.0 | |
| Fishing | 65 | 5.0 | 25 | 8.3 | |
| Agriculture for selling | 120 | 9.3 | 20 | 6.7 | |
| Agriculture for home | 280 | 21.6 | 67 | 22.3 | |
| Other (e.g., motorcycle/taxi driver or trucker) | 355 | 27.4 | 95 | 31.7 | |
| Current marital status, yes | 806 | 62.1 | 190 | 63.3 | 0.70 |
| Sexually active in past year, yes | 1,132 | 87.3 | 269 | 89.7 | 0.26 |
| Self-perceived HIV risk, likely | 364 | 28.1 | 79 | 26.3 | 0.55 |
| No. of sex partners in past year | 0.40 | ||||
| 0 | 165 | 12.7 | 31 | 10.3 | |
| 1 | 688 | 53.1 | 168 | 56.0 | |
| ≥2 | 444 | 34.2 | 101 | 33.7 | |
| No. of nonmarital partners in past year | 0.45 | ||||
| 0 | 706 | 54.4 | 166 | 55.3 | |
| 1 | 399 | 30.8 | 83 | 27.7 | |
| ≥2 | 192 | 14.8 | 51 | 17.0 | |
| Alcohol use before sex in past year, yesb | 692 | 61.1 | 172 | 63.9 | 0.39 |
| Condom use with all partners in past yearb | 0.86 | ||||
| Never | 554 | 48.9 | 127 | 47.2 | |
| Sometimes | 427 | 37.7 | 106 | 39.4 | |
| Always | 151 | 13.3 | 36 | 13.4 | |
| Condom use with nonmarital partners in past yearc | 0.13 | ||||
| Never | 116 | 20.2 | 16 | 12.6 | |
| Sometimes | 204 | 35.5 | 47 | 37.0 | |
| Always | 254 | 44.3 | 64 | 50.4 | |
| No condom use at last sex with any partnerb | 912 | 80.6 | 220 | 81.8 | 0.65 |
| No condom use at last sex with nonmarital partnersc | 272 | 47.4 | 52 | 40.9 | 0.19 |
Abbreviations: HIV, human immunodeficiency virus; PT0, start of the posttrial surveillance; PT2, the second posttrial visit.
a P value based on 2-sided χ2 test.
b Among sexually active men.
c Among men reporting nonmarital/nonconsensual relations in the partner-specific blocks of the questionnaire.
Changes in risk behaviors
Table 3 presents the changes in key sexual behavior variables and self-perceived risk of HIV infection between PT0 and PT2 for men who became circumcised and men who remained uncircumcised during the period (after adjustment for informative loss to follow-up). At PT2, more men became married (15.0% increase in male circumcision acceptors and 18.4% increase in nonacceptors), and both groups reported an increase in sexual activity in the past year. Correspondingly, the distribution of number of sexual partnerships in the past year changed significantly in both groups: Among male circumcision acceptors, the proportion with single partnership increased, and there was no significant change in the proportion of multiple partnerships; whereas among the nonacceptors, the proportion of single partnership did not change significantly, but the proportion of multiple partnerships increased. The overall difference between the 2 groups in the change of number of partnerships was statistically significant, with uncircumcised men having a greater increase in multiple partnerships (Table 3).
Table 3.
Changes in Sexual Behavior and HIV Risk Perception From PT0 to PT2 Among Male Circumcision Acceptors and Nonacceptors, Rakai, Uganda, 2007–2011
| Male Circumcision Acceptors (n = 1,297) |
Male Circumcision Nonacceptors (n = 300) |
P Value Testing Ha,b0: Δac = Δnac | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT0 |
PT2 |
Δaac | 95% CI | P Value Testing Ha,b0: Δac = 0 | PT0 |
PT2 |
Δanac | 95% CI | P Value Testing Ha,b0: Δnac = 0 | ||||||
| No. | % | No. | % | No. | % | No. | % | ||||||||
| Sexually active in past year, yes | 1,132 | 87.3 | 1,218 | 93.9 | 7.7 | 6.1, 9.4 | <0.0001 | 269 | 89.7 | 283 | 94.3 | 5.4 | 2.2, 8.6 | <0.01 | 0.21 |
| No. of sex partners in past year | 0.04c | ||||||||||||||
| 0 | 165 | 12.7 | 79 | 6.1 | −7.7 | −9.4, −6.1 | <0.0001 | 31 | 10.3 | 17 | 5.7 | −5.4 | −8.6, −2.2 | <0.01 | 0.21 |
| 1 | 688 | 53.1 | 750 | 57.8 | 5.3 | 2.3, 8.2 | <0.01 | 168 | 56.0 | 157 | 52.3 | −3.1 | −8.9, 2.7 | 0.30 | 0.01 |
| 2 | 444 | 34.2 | 468 | 36.1 | 2.5 | −0.2, 5.2 | 0.08 | 101 | 33.7 | 126 | 42.0 | 8.5 | 3.0, 13.9 | 0.002 | 0.05 |
| No. of nonmarital partners in past year | 0.45c | ||||||||||||||
| 0 | 706 | 54.4 | 747 | 57.6 | 3.3 | 0.5, 6.2 | 0.02 | 166 | 55.3 | 164 | 54.7 | 0.1 | −5.8, 6.0 | 0.98 | 0.33 |
| 1 | 399 | 30.8 | 388 | 29.9 | −0.9 | −3.9, 2.0 | 0.53 | 83 | 27.7 | 94 | 31.3 | 3.3 | −2.6, 9.2 | 0.28 | 0.21 |
| ≥2 | 192 | 14.8 | 162 | 12.5 | −2.4 | −4.6, −0.3 | 0.03 | 51 | 17.0 | 42 | 14.0 | −3.4 | −8.0, 1.3 | 0.16 | 0.72 |
| Alcohol use before sex in past year, yesd | 674 | 61.2 | 584 | 53.0 | −7.8 | −10.5, −5.0 | <0.0001 | 169 | 64.8 | 152 | 58.2 | −6.1 | −11.3, −1.0, | 0.02 | 0.58 |
| Condom use with all partners in past yeard | 0.55c | ||||||||||||||
| Never | 543 | 49.3 | 636 | 57.8 | 9.1 | 6.2, 12.0 | <0.0001 | 123 | 47.1 | 153 | 58.6 | 12.4 | 6.2, 18.6 | <0.0001 | 0.35 |
| Sometimes | 418 | 38.0 | 389 | 35.3 | −2.1 | −5.1, 0.9 | 0.17 | 106 | 40.6 | 89 | 34.1 | −6.1 | −12.7, 0.4 | 0.07 | 0.28 |
| Always | 140 | 12.7 | 76 | 6.9 | −7.0 | −8.9, −5.2 | <0.0001 | 32 | 12.3 | 19 | 7.3 | −6.3 | −10.5, −2.0 | <0.01 | 0.75 |
| Condom use with nonmarital partners in past yeare | 0.28c | ||||||||||||||
| Never | 59 | 17.9 | 84 | 25.5 | 7.0 | 2.6, 11.4 | <0.01 | 8 | 10.8 | 18 | 24.3 | 13.5 | 4.4, 22.5 | <0.01 | 0.21 |
| Sometimes | 130 | 39.4 | 123 | 37.3 | −1.2 | −6.9, 4.5 | 0.69 | 27 | 36.5 | 28 | 37.8 | 0.8 | −10.4, 12.1 | 0.89 | 0.76 |
| Always | 141 | 42.7 | 123 | 37.3 | −5.8 | −11.3, −0.3 | 0.04 | 39 | 52.7 | 28 | 37.8 | −14.3 | −25.1, −3.4 | 0.01 | 0.17 |
| No condom use at last sex with any partnerd | 895 | 81.3 | 977 | 88.7 | 8.7 | 6.5, 11.0 | <0.0001 | 216 | 82.8 | 228 | 87.4 | 6.1 | 1.1, 11.2 | 0.02 | 0.36 |
| No condom use at last sex with nonmarital partnere | 155 | 47.0 | 176 | 53.3 | 6.4 | 0.9, 11.8 | 0.02 | 29 | 39.2 | 43 | 58.1 | 18.8 | 8.0, 29.7 | 0.01 | 0.04 |
| Self-perceived HIV risk, likely | 364 | 28.1 | 678 | 52.3 | 24.5 | 21.8, 27.3 | <0.0001 | 79 | 26.3 | 164 | 54.7 | 27.8 | 21.9, 33.8 | <0.0001 | 0.32 |
Abbreviations: CI, confidence interval; H0, null hypothesis; HIV, human immunodeficiency virus; PT0, start of the posttrial surveillance; PT2, the second posttrial visit; Δac, change in percentage from PT0 to PT2 within the male circumcision acceptors; Δnac, change in percentage from PT0 to PT2 within the male circumcision nonacceptors.
a After adjustment for loss to follow-up at PT2 using the inverse probability weighting method. Results are similar to those without accounting for the informative loss to follow-up.
b P value based on the Wald test using linear models for modeling the marginal distributions of repeated outcomes.
c If the behavior variable has more than 2 categories (e.g., number of partners, condom use), the P value is provided for the global test for testing the overall difference in change of the behavior between the 2 groups (i.e., df = no. of categories − 1).
d Among men reporting being sexually active at both PT0 and PT2.
e Among men reporting nonmarital/nonconsensual relations at both PT0 and PT2.
Overall, men opting for circumcision and men remaining uncircumcised did not differ in change of the number of nonmarital partnerships (Table 3). Specifically, the number of nonmarital partnerships did not change significantly in the male circumcision nonacceptors, whereas male circumcision acceptors reduced their engagement in nonmarital partnerships. Both groups significantly reduced their sex-associated alcohol consumption, but there was no differential change in alcohol use before sex between the 2 groups.
Condom use with all partners and with nonmarital partners in the past 12 months decreased significantly in both male circumcision acceptors and nonacceptors, but the decreases in condom use were not significantly different between the 2 groups, suggesting that getting circumcised did not make men more likely to reduce use of condoms. Similar decreasing trends were observed in condom use at last sex with any partner and with nonmarital partners in both groups. In particular, the decrease within the nonacceptors with nonmarital partners was significantly greater than that in the male circumcision acceptors (Table 3).
Corresponding to the sexual behavioral changes during the posttrial follow-up, men's self-perceived risk of HIV infection also increased significantly: 24.5% more male circumcision acceptors and 27.8% more male circumcision nonacceptors thought that it was “likely” that they had been exposed to HIV, but the increase in self-perceived risk did not differ significantly between the 2 groups.
Changes in risk behaviors by age group
For alcohol use before sexual intercourse and condom use that changed significantly in both groups, we further evaluated the behavior changes stratified by age. For simplicity of presentation, alcohol use in the past 12 months and condom use at the last sex act with any partner and with a nonmarital partner are shown in Table 4. In summary, in no age group were there significant differences in changes of alcohol or condom use between the male circumcision acceptors and nonacceptors. The only exception was for condom use with nonmarital partners among younger men aged 16–20 years, where men remaining uncircumcised had significantly greater decrease in condom use than men opting for male circumcision (Table 4).
Table 4.
Changes in Alcohol Use and Nonuse of Condoms by Age From PT0 to PT2 Among Male Circumcision Acceptors and Nonacceptors, Rakai, Uganda, 2007–2011
| Male Circumcision Acceptors |
Male Circumcision Nonacceptors (n = 300) |
P Value Testing Ha,b0: Δac = Δnac | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT0 |
PT2 |
Δaac | 95% CI | P Value Testing Ha,b0: Δac = 0 | PT0 |
PT2 |
Δanac | 95% CI | P Value Testing Ha,b0: Δnac = 0 | ||||||
| No. | % | No. | % | No. | % | No. | % | ||||||||
| Alcohol use before sex in past year by age, yearsc | |||||||||||||||
| 16–20 | 41 | 33.3 | 39 | 31.7 | 1.6 | −11.2, 7.9 | 0.74 | 9 | 36 | 9 | 36 | 0 | −22.2, 22.2 | 1 | 0.9 |
| 21–25 | 152 | 48 | 136 | 42.9 | −5.5 | −10.7, −3.2 | 0.04 | 37 | 46.8 | 48 | 60.8 | −7.3 | −16.7, 2.2 | 0.13 | 0.75 |
| 26–30 | 162 | 62.3 | 143 | 55 | −5.7 | −11.5, 0.2 | 0.06 | 38 | 64.4 | 38 | 64.4 | 1.4 | −9.6, 12.5 | 0.8 | 0.27 |
| 31–35 | 146 | 78.5 | 117 | 62.9 | −15.6 | −21.5, −9.6 | <0.0001 | 38 | 80.9 | 34 | 72.3 | −7.8 | −18.1, 2.4 | 0.13 | 0.2 |
| 36–51 | 173 | 80.5 | 149 | 69.3 | −11 | −17.0, −5.0 | <0.001 | 47 | 92.2 | 40 | 78.4 | −14 | −25.0, −2.9 | 0.01 | 0.64 |
| No condom use at last sex with any partner by age, yearsc | |||||||||||||||
| 16–20 | 65 | 52.9 | 87 | 70.7 | 17.9 | 7.8, 28.0 | <0.001 | 13 | 52 | 21 | 84 | 32 | 13.7, 50.3 | <0.001 | 0.19 |
| 21–25 | 225 | 71 | 281 | 88.6 | 18.3 | 13.6, 22.9 | <0.0001 | 58 | 73.4 | 66 | 83.5 | 11.3 | 1.3, 21.4 | 0.03 | 0.22 |
| 26–30 | 233 | 89.6 | 238 | 91.5 | 3.2 | −1.1, 7.4 | 0.14 | 54 | 91.5 | 52 | 88.1 | −2.6 | −12.6, 7.3 | 0.6 | 0.29 |
| 31–35 | 173 | 93 | 174 | 93.6 | 0.8 | −3.1, 4.8 | 0.67 | 42 | 89.4 | 42 | 89.4 | 0.5 | −10.6, 11.7 | 0.93 | 0.96 |
| 36–51 | 199 | 92.6 | 197 | 91.6 | −0.9 | −4.6, 2.8 | 0.63 | 49 | 96.1 | 47 | 92.2 | −3.9 | −12.3, 4.6 | 0.37 | 0.53 |
| No condom use at last sex with nonmarital partnerd | |||||||||||||||
| ≤20 | 39 | 46.4 | 40 | 47.6 | 1.2 | −12.2, 14.6 | 0.86 | 9 | 47.4 | 14 | 73.7 | 26.3 | 6.5, 46.1 | <0.01 | 0.04 |
| 21–25 | 59 | 48.8 | 70 | 59.9 | 9.3 | 1.0, 17.6 | 0.03 | 8 | 30.8 | 12 | 46.2 | 16.4 | −4.9, 37.7 | 0.13 | 0.54 |
| 26–30 | 29 | 43.3 | 36 | 53.7 | 12.5 | −0.8, 25.7 | 0.07 | 6 | 46.2 | 8 | 61.5 | 11.4 | −15.6, 38.4 | 0.41 | 0.96 |
| 31–35 | 16 | 57.1 | 16 | 57.1 | 0.3 | −21.0, 21.6 | 0.98 | 4 | 44.4 | 6 | 66.7 | 23.8 | 0.0, 47.6 | 0.05 | 0.15 |
| ≥36 | 12 | 40 | 14 | 46.7 | 4.9 | −14.5, 24.3 | 0.62 | 2 | 28.6 | 3 | 42.9 | 14.3 | −28.8, 57.4 | 0.52 | 0.7 |
Abbreviations: CI, confidence interval; H0, null hypothesis; PT0, start of the posttrial surveillance; PT2, the second posttrial visit; Δac, change in percentage from PT0 to PT2 within the male circumcision acceptors; Δnac, change in percentage from PT0 to PT2 within the male circumcision nonacceptors.
a After adjustment for loss to follow-up at PT2 using the inverse probability weighting method. Results are similar to those without accounting for the informative loss to follow-up.
b P value based on the Wald test using the linear models for modeling the marginal distributions of repeated outcomes.
c Among men reporting being sexually active at both PT0 and PT2.
d Among men reporting nonmarital/nonconsensual relations at both PT0 and PT2.
Alcohol use before intercourse was lower at younger ages in both male circumcision acceptors and nonacceptors, whereas reduced alcohol consumption largely occurred in older age groups (Table 4). Regarding condom use at the last sex act with any partner, no significant decrease in condom use was observed in men older than 25 in either male circumcision acceptors or nonacceptors. The decrease in condom use centers on young men (age, ≤25 years) in both groups. Condom use with nonmarital partners declined in most age groups, especially among the male circumcision nonacceptors, although the reductions may not be statistically significant (Table 4). Similar findings were observed for condom use in the past 12 months (data not shown, available upon request).
DISCUSSION
After closure of the Rakai randomized trial of male circumcision, the majority of uncircumcised participants opted for male circumcision during the posttrial follow-up. Men who accepted the procedure were similar to men who remained uncircumcised in most sociodemographic characteristics and risk behaviors at their last trial visit (Table 2), suggesting minimal self-selection.
During the approximately 3 years of posttrial follow-up, we observed significant sexual behavior changes in both men who opted for male circumcision and men who remained uncircumcised. Nearly half the study population were young men (<25 years), and they progressively became sexually active over time. There was no significant increase in the number of nonmarital partnerships among men remaining uncircumcised; likewise, there was a slight decrease in nonmarital relationships among the male circumcision acceptors (Table 3). Alcohol use before sex declined significantly in both groups, mainly among the older men who originally had more frequent sex-associated drinking (Table 4). Condom use with all partners decreased significantly in both male circumcision acceptors and nonacceptors, especially among younger men. This is probably in part because more young men became married, and condom use within marriage is generally low in this population. Condom use with nonmarital partners also declined in both groups, where a decreasing trend was present in all age groups of the nonacceptors and was also shown in the male circumcision acceptors at ages 21–30 years.
The magnitudes of behavior changes were generally comparable between the male circumcision acceptors and nonacceptors, but the nonacceptors reported more multiple partnerships and greater reduction in condom use with nonmarital sex partners. The nonacceptors, compared with the acceptors, received less intensive counseling and fewer free condoms that were provided when men came for surgery. It is also possible that, although we did not find a significant difference in risk behaviors between the 2 groups when they were all uncircumcised at trial closure, the male circumcision nonacceptors may be more prone to risk-taking behaviors when follow-up and health education were less frequent after the trial.
Several factors may have contributed to the significant changes in sexual behaviors in both the circumcised and uncircumcised men. The first is that the population was growing older and, thus, more likely to marry and engage in sex. The second is that, during the 2-year trial period, all participants received repeated intensive education and counseling and free condoms that, of necessity, diminished in the posttrial setting where follow-up was also less frequent.
Although our empirical data do not show evidence of risk compensation associated with circumcision, recent qualitative studies in sub-Saharan Africa provide mixed findings. Qualitative studies on circumcised men reported no risk compensation among the majority of subjects, while a minority of men reported increased sexual risk behaviors (33) with the motivation to “test out” their circumcised penis or be “rewarded” for their abstinence during the healing period (34, p. 250). Another qualitative study among uncircumcised men in Nyanza Province of Kenya reported frequent opinions such as, “Male circumcision acts as a ‘natural condom’,” and for this reason circumcised men can enjoy sex ‘skin-on-skin’ without needing a latex condom.” Participants also expressed fear that “male circumcision will make a man promiscuous” and, in a community, “male circumcision might create a generation of men, especially young men, who think that they can have sex without any risk” (35, p. 4). Our age-stratified analysis did not show that circumcised young men engaged in riskier behaviors than their uncircumcised peers 1 year or more after circumcision, but we do not know whether their sexual behaviors may have changed soon after wound healing postsurgery.
Among data prior to male circumcision rollout, the South African trial noted that circumcised men “had significantly more sexual contacts” (but no data on follow-up behaviors were provided) (1, p. 1121). Both the Ugandan and Kenyan trials reported increased condom use during trial follow-up. The increase was similar between the intervention and control arms in the Ugandan trial, whereas the Kenyan trial found a lower increase of condom use in the intervention arm (2, 3). However, a substudy on the Kenyan trial participants during the RCT period concluded that there was no risk compensation associated with male circumcision on the basis of analysis of a summary behavioral risk score and the incidence of 3 nonviral STIs (10). Outside the RCT context, early cross-sectional studies in South Africa and Uganda reported that circumcision was associated with higher sexual risk behaviors (36–38), which may be because men with riskier behaviors were more likely to acquire STIs and to be circumcised for medical reasons. Another longitudinal study from 2003 to 2004 (i.e., before the RCT results) in western Kenya found no risk compensation 1 year after circumcision (22).
To our knowledge, this is the first analysis to assess risk compensation associated with male circumcision in a posttrial setting, where the health benefits of circumcision were widely known and men voluntarily chose to accept or decline free circumcision services. A range of sexual behaviors were examined, and we found no evidence of risk compensation associated with circumcision.
However, our study has important limitations that preclude a definitive statement on postcircumcision risk compensation. The study participants were self-selected and motivated to enroll into the RCT, and they received intensive health education and counseling during the 2-year trial period. Consequently, although it is reassuring that no male circumcision-associated risk compensation was found during the posttrial follow-up, the results may not be generalizable to the general male population who receive male circumcision through routine services. Moreover, if the male circumcision nonacceptors were more prone to riskier behaviors than the acceptors, using their behavioral changes as a caliber may underestimate the magnitude of risk compensation associated with circumcision. Additionally, we evaluated behavioral changes during an approximately 3-year period, where the majority of circumcised men had been circumcised for over a year, but it is unknown whether risk compensation would occur soon after wound healing among circumcised men where, on one hand, men may seek more nonmarital sex partners to “test out” their circumcised penis or be “rewarded” for their abstinence during the healing period (34, p. 250). On the other hand, the education component in the male circumcision service package may help to prevent risk compensation, particularly for a short term.
Another limitation is the use of self-reported sexual behaviors that may be vulnerable to social desirability and recall bias. Nevertheless, prior Rakai studies repeatedly showed that self-reported risk behaviors are associated with HIV/STI acquisition (27, 33, 34, 39–41). In addition, our recent analysis of posttrial HIV incidence among the Rakai RCT participants (42, 43) found that the effectiveness of male circumcision on HIV prevention (67%) was maintained after the trial, thus supporting our observation based on self-reported risk behaviors.
In summary, we observed significant risk behavior changes in both circumcised and uncircumcised men but found no evidence of behavioral risk compensation associated with male circumcision during the approximately 3 years of posttrial surveillance. Future studies of risk compensation with male circumcision should focus on the general population of men in programmatic settings and behavior changes within the short term after male circumcision (e.g., 3 or 6 months after male circumcision). The significant decrease in condom use observed in all men also indicates the importance of behavior interventions that can be incorporated into male circumcision scale-up programs to synergistically combat the HIV epidemic in sub-Saharan Africa.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Xiangrong Kong, Maria Wawer, Ronald Gray, Carl Latkin); Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Xiangrong Kong); Department of Health, Behavior, and Society, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Carl Latkin); Rakai Health Sciences Program, Entebbe, Uganda (Godfrey Kigozi, Fred Nalugoda, Richard Musoke, Joseph Kagaayi, Robert Ssekubugu, Tom Lutalo, Betty Nantume, Iga Boaz, David Serwadda); and School of Public Health, Makerere University, Kampala, Uganda (David Serwadda).
This study was supported by the Division of Allergy and Infectious Diseases, National Institutes of Health (grants U1AI51171 and 1UO1AI075115-O1A1 to R. G.), and the Johns Hopkins Center for Global Health (2009 faculty grant to X. K.).
Conflict of interest: none declared.
REFERENCES
- 1.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):pe298. doi: 10.1371/journal.pmed.0020298. doi:10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.WHO/UNAIDS technical consultation, 6–8 March 2007. Conclusions and recommendations (excerpts) Reprod Health Matters. 2007;15(29):11–14. doi: 10.1016/S0968-8080(07)29307-3. World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS). Male circumcision for HIV prevention: research implications for policy and programming. [DOI] [PubMed] [Google Scholar]
- 5.Progress in Male Circumcision Scale-up: Country Implementation and Research Update. Geneva, Switzerland: World Health Organization; 2010. World Health Organization. [Google Scholar]
- 6.Eaton L, Kalichman SC. Behavioral aspects of male circumcision for the prevention of HIV infection. Curr HIV/AIDS Rep. 2009;6(4):187–193. doi: 10.1007/s11904-009-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4(4):165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padian NS, Buvé A, Balkus J, et al. Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008;372(9638):585–599. doi: 10.1016/S0140-6736(08)60885-5. [DOI] [PubMed] [Google Scholar]
- 9.Brooks RA, Etzel M, Klosinski LE, et al. Male circumcision and HIV prevention: looking to the future. AIDS Behav. 2010;14(5):1203–1206. doi: 10.1007/s10461-009-9523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattson CL, Campbell RT, Bailey RC, et al. Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PLoS One. 2008;3(6):pe2443. doi: 10.1371/journal.pone.0002443. doi:10.1371/journal.pone.0002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermund SH, Allen KL, Karim QA. HIV-prevention science at a crossroads: advances in reducing sexual risk. Curr Opin HIV AIDS. 2009;4(4):266–273. doi: 10.1097/COH.0b013e32832c91dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss HA, Dickson KE, Agot K, et al. Male circumcision for HIV prevention: current research and programmatic issues. AIDS. 2010;24(suppl 4):S61–S69. doi: 10.1097/01.aids.0000390708.66136.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurth AE, Celum C, Baeten JM, et al. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bingenheimer JB, Geronimus AT. Behavioral mechanisms in HIV epidemiology and prevention: past, present, and future roles. Stud Fam Plann. 2009;40(3):187–204. doi: 10.1111/j.1728-4465.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 15.Sawires SR, Dworkin SL, Fiamma A, et al. Male circumcision and HIV/AIDS: challenges and opportunities. Lancet. 2007;369(9562):708–713. doi: 10.1016/S0140-6736(07)60323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler D, Odling-Smee L. Circumcision for HIV needs follow-up. Nature. 2007;447(7148):1040–1041. doi: 10.1038/4471040a. [DOI] [PubMed] [Google Scholar]
- 17.Laurence J. Suppressing HIV transmission—through behavior and biology. AIDS Read. 2006;16(5):231–232. [PubMed] [Google Scholar]
- 18.Baeten JM, Celum C, Coates TJ. Male circumcision and HIV risks and benefits for women. Lancet. 2009;374(9685):182–184. doi: 10.1016/S0140-6736(09)61311-8. [DOI] [PubMed] [Google Scholar]
- 19.Behavioral approaches overlooked in HIV prevention. AIDS Read. 2008;18(11):554. [PubMed] [Google Scholar]
- 20.Kalichman S, Eaton L, Pinkerton S. Circumcision for HIV prevention: failure to fully account for behavioral risk compensation. PLoS Med. 2007;4(3):pe138. doi: 10.1371/journal.pmed.0040138. doi:10.1371/journal.pmed.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassell MM, Halperin DT, Shelton JD, et al. Risk compensation: the Achilles' heel of innovations in HIV prevention? BMJ. 2006;332(7541):605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agot KE, Kiarie JN, Nguyen HQ, et al. Male circumcision in Siaya and Bondo districts, Kenya: prospective cohort study to assess behavioral disinhibition following circumcision. J Acquir Immune Defic Syndr. 2007;44(1):66–70. doi: 10.1097/01.qai.0000242455.05274.20. [DOI] [PubMed] [Google Scholar]
- 23.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past present future of HIV prevention: integrating behavioral biomedical structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143–167. doi: 10.1146/annurev.clinpsy.032408.153530. doi:10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White RG, Glynn JR, Orroth KK, et al. Male circumcision for HIV prevention in sub-Saharan Africa: who, what and when? AIDS. 2008;22(14):1841–1850. doi: 10.1097/QAD.0b013e32830e0137. [DOI] [PubMed] [Google Scholar]
- 25.Andersson KM, Owens DK, Paltiel AD. Scaling up circumcision programs in Southern Africa: the potential impact of gender disparities and changes in condom use behaviors on heterosexual HIV transmission. AIDS Behav. 2011;15(5):938–948. doi: 10.1007/s10461-010-9784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallett TB, Singh K, Smith JA, et al. Understanding the impact of male circumcision interventions on the spread of HIV in southern Africa. PLoS One. 2008;3(5):pe2212. doi: 10.1371/journal.pone.0002212. doi:10.1371/journal.pone.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wawer MJ, Sewankambo NK, Berkley S, et al. Incidence of HIV-1 infection in a rural region of Uganda. BMJ. 1994;308(6922):171–173. doi: 10.1136/bmj.308.6922.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zablotska IB, Gray RH, Serwadda D, et al. Alcohol use before sex and HIV acquisition: a longitudinal study in Rakai, Uganda. AIDS. 2006;20(8):1191–1196. doi: 10.1097/01.aids.0000226960.25589.72. [DOI] [PubMed] [Google Scholar]
- 29.Agresti A. Categorical Data Analysis. Hoboken, NJ: Wiley-Interscience; 2002. [Google Scholar]
- 30.Hernán MA, Robins JM. Estimating causal effects from epidemiological. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23(9):1455–1497. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 32.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc. 1995;90(429):106–121. [Google Scholar]
- 33.Riess TH, Achieng MM, Otieno S, et al. “When I was circumcised I was taught certain things”: risk compensation and protective sexual behavior among circumcised men in Kisumu, Kenya. PLoS One. 2010;5(8):pe12366. doi: 10.1371/journal.pone.0012366. doi:10.1371/journal.pone.0012366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grund JM, Hennink MM. A qualitative study of sexual behavior change and risk compensation following adult male circumcision in urban Swaziland. AIDS Care. 2012;24(2):245–251. doi: 10.1080/09540121.2011.596516. [DOI] [PubMed] [Google Scholar]
- 35.Herman-Roloff A, Otieno N, Agot K, et al. Acceptability of medical male circumcision among uncircumcised men in Kenya one year after the launch of the national male circumcision program. PLoS One. 2011;6(5):pe19814. doi: 10.1371/journal.pone.0019814. doi:10.1371/journal.pone.0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagarde E, Dirk T, Puren A, et al. Acceptability of male circumcision as a tool for preventing HIV infection in a highly infected community in South Africa. AIDS. 2003;17(1):89–95. doi: 10.1097/00002030-200301030-00012. [DOI] [PubMed] [Google Scholar]
- 37.Bailey RC, Neema S, Othieno R. Sexual behaviors and other HIV risk factors in circumcised and uncircumcised men in Uganda. J Acquir Immune Defic Syndr. 1999;22(3):294–301. doi: 10.1097/00126334-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 38.Gray R, Azire J, Serwadda D, et al. Male circumcision and the risk of sexually transmitted infections and HIV in Rakai, Uganda. AIDS. 2004;18(18):2428–2430. [PubMed] [Google Scholar]
- 39.Kong X, Gray RH, Moulton LH, et al. A modeling framework for the analysis of HPV incidence and persistence: a semi-parametric approach for clustered binary longitudinal data analysis. Stat Med. 2010;29(28):2880–2889. doi: 10.1002/sim.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serwadda D, Wawer MJ, Musgrave SD, et al. HIV risk factors in three geographic strata of rural Rakai District, Uganda. AIDS. 1992;6(9):983–989. doi: 10.1097/00002030-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Konde-Lule JK, Wawer MJ, Sewankambo NK, et al. Adolescents, sexual behaviour and HIV-1 in rural Rakai District, Uganda. AIDS. 1997;11(6):791–799. doi: 10.1097/00002030-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26(5):609–615. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong X, Kigozi G, Ssempija V, et al. Longer-term effects of male circumcision on HIV incidence and risk behaviors during post-trial surveillance in Rakai, Uganda [abstract]. Presented at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 27 to March 2. 2011 [Google Scholar]