Abstract
Simvastatin (SV), a competitive inhibitor of 3-hydroxy-3-methylglutaryl CoA reductase and a widely prescribed treatment for hypercholesterolemia, exerts numerous positive pleiotropic effects that are thought to occur independent of its cholesterol-lowering properties. In previously published work, we have shown that chronic SV treatment rescues cognitive function in a transgenic mouse model of Alzheimer’s disease, and enhances learning and memory in non-transgenic mice without affecting total brain cholesterol and amyloid-beta levels. More recently, we demonstrated the ability of SV to enhance long-term potentiation (LTP) in the CA1 region of the hippocampus in slices from wild type C57BL/6 mice via a mechanism dependent upon phosphatidylinositol 3-kinase (PI3-K)/Akt activation during LTP induction. The present study was conducted to better understand the molecular mechanisms underlying SV-induced enhancement of LTP. Specifically, it was found that inhibiting production of isoprenoid intermediates in the biosynthetic pathway for cholesterol triggers the downstream events leading to enhanced LTP. Interestingly, two major isoprenoid intermediates exhibit differential effects. Replenishment of farnesyl pyrophosphate, but not geranylgeranyl pyrophosphate, abolished the LTP-enhancing ability of SV. In parallel to this finding, inhibiting farnesylation, but not geranylgeranylation replicated the enhancement of LTP caused by SV. Finally, inhibiting farnesylation promotes the activation of Akt during the induction phase. Together, these results suggest that SV enhances LTP in CA1 by modulating isoprenylation-dependent molecular pathways downstream of farnesyl transferase. These findings will aid in the identification of novel therapeutic targets that modulate synaptic and cognitive function.
Keywords: statins, isoprenoids, protein prenylation, synaptic plasticity, hippocampus, long-term potentiation
INTRODUCTION
Statins directly inhibit the rate-limiting enzyme of the cholesterol biosynthetic pathway, 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, which converts HMG-CoA to mevalonate (Endo, 2004). Inhibiting this reaction not only reduces de novo cholesterol biosynthesis, but also reduces production of non-sterol intermediates, known as isoprenoids, downstream of mevalonate and preceding cholesterol. Isoprenoids, such as farnesyl-pyrophosphate (FPP) and geranylgeranyl-pyrophosphate (GGPP) serve as lipid attachments for all members of the small GTPase superfamilies which include the well-known Ras, Rho and Rac (McTaggart, 2006). The isoprenylation state of GTPases alters their intracellular trafficking, subcellular localization and interactions with substrates and, therefore, modifies their function and functions of downstream effectors (McTaggart, 2006). Consequently, reducing isoprenoid availability or the process of isoprenylation itself can affect a diverse group of intracellular signaling pathways and processes. Some lipophilic statins, such as simvastatin and lovastatin, are capable of crossing the blood-brain barrier (Jones, 2003). The effects of statins are diverse and extend across several disciplines. Thus, it is imperative to understand how statins affect cognitive function, and a number of experimental and epidemiological studies have been conducted to this end (Cole and Vassar, 2006).
Statins have been shown, to some extent, to be therapeutic and neuroprotective in humans, though these results are not without controversy. Some epidemiological studies indicate a reduced prevalence of Alzheimer’s disease (AD) or dementia in statin-prescribed populations (Jick et al., 2000; Wolozin et al., 2000). However, there are conflicting reports that statins are not neuroprotective (Collins et al., 2002; Shepherd et al., 2002), and some statin users suffer memory loss that is ameliorated by withdrawal from statin treatment (Wagstaff et al., 2003). In support of a neuroprotective role for statins, it has been observed that statins reduce pro-inflammatory responses of microglia after amyloid-β peptide exposure in vitro (Cordle and Landreth, 2005) and in vivo (Clarke et al., 2008), and protect cultured cortical neurons from excitotoxicity after exposure to N-methyl D-aspartate (NMDA) (Zacco et al., 2003) and monosodium glutamate (Bosel et al., 2005). Also, a recent study found elevated levels of FPP and GGPP in the brains of AD patients suggesting that reducing isoprenoid production may prove therapeutic (Eckert et al., 2009). Indeed, in previously published work from our laboratory, a simvastatin (SV)-supplemented diet rescued learning and memory in a transgenic mouse model of AD independent of changes in amyloid beta pathology (Li et al., 2006). Interestingly, dramatic memory improvements are also observed in non-transgenic wild type (WT) littermate controls. A similar effect has been observed in adult rats administered SV for twenty-five days prior to testing in passive avoidance or object-in-place tasks (Douma et al., 2011). It is therefore possible that SV can augment the processes underlying learning and memory in normal, non-diseased brains. Chronic statin treatment stimulates production of brain-derived neurotrophic factor (BDNF) (Wu et al., 2008), increases levels of NMDA receptors (Wang et al., 2009), promotes neurogenesis and increases cerebral blood flow (Chen et al., 2003). Additionally, we recently reported that treatment of hippocampal slices for several hours with SV increases the magnitude of NMDA receptor-dependent long-term potentiation (LTP), a mechanism thought to mediate memory at the cellular level, in the CA1 region in the brains of young adult C57BL/6 mice (Mans et al., 2010). Many of the pleiotropic effects described above have been attributed to reduced isoprenoid production and subsequent altered small GTPase function.
In the current study, we investigate the mechanism by which SV augments LTP in the CA1 region of the hippocampus of C57BL/6 mice. We test the hypothesis that SV-induced reduction of isoprenoid production leads to enhanced LTP. We also test the related hypothesis that inhibition of isoprenylation contributes to the SV-induced LTP enhancement we observe. In support of these hypotheses, evidence is presented demonstrating that replenishing FPP, but not GGPP, abolishes SV-induced LTP enhancement. Furthermore, we find that inhibiting farnesylation, but not geranylgeranylation, mimics the LTP-enhancing property of SV. Lastly, we present data suggesting that inhibiting farnesylation augments the recruitment of PI3-K activity during LTP induction.
EXPERIMENTAL PROCEDURES
Animals
Three- to four-month-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME; stock no. 000664) were used in this study. The mice were housed in a specific-pathogen-free facility under veterinary supervision at an ambient temperature of 22–23 °C and under a 12:12-hr light/dark cycle. The mice were allowed ad libitum access to food and water. All animal procedures used for this study were prospectively reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Slice preparation and electrophysiology
Hippocampal slices (400 µM) were prepared from male C57BL/6 mice using methods described previously (Mans et al., 2010) with modifications. Briefly, mice were anesthetized with isoflurane and decapitated. Their brains were removed and immersed in ice-cold “high-sucrose” artificial cerebral spinal fluid (aCSF) composed of (in mM): NaCl 85; KCl 2.5; MgSO4 4; CaCl2 0.5; NaH2PO4 1.25; NaHCO3 25; glucose 25; sucrose 75; 290–300 mOsm. This solution contains less Na+ and Ca2+ and higher Mg2+ than sodium-based aCSF and promotes neuronal survival during the slicing procedure by reducing excitotoxicity (Kuenzi et al., 2000). Coronal slices of dorsal hippocampi were cut on a vibratome (Leica) and incubated in high-sucrose aCSF for ten min, then for ≥ one hr in regular aCSF containing (in mM): NaCl 119; KCl 2.5; CaCl2 2.5; MgSO4 1.3; NaH2PO4 1; NaHCO3 26; and glucose 10 saturated with 95% O2–5% CO2 (pH 7.4). To record field excitatory postsynaptic potentials (fEPSPs), slices were placed in a submersion recording chamber and continuously perfused at approximately 1.5–2.0 ml/min with aCSF warmed to 26–28 °C and recirculated via peristaltic perfusion pump. CA1 extracellular dendritic fEPSPs were recorded (Axopatch 200B,Molecular Devices, Sunnyvale, CA) using standard methods (Mans et al., 2010). Stimulus frequency was 0.1 Hz (100 µs duration), and stimulus intensity was adjusted to yield fEPSPs with amplitudes of 0.5–0.8 mV. Schaffer collaterals were stimulated with a bipolar tungsten stimulating electrode placed in CA1 stratum (s.) radiatum, and fEPSPs were recorded using a glass microelectrode filled with aCSF, also placed in CA1 s. radiatum. If stable fEPSPs were maintained for at least 15 minutes (min), NMDA receptor-dependent LTP was induced with a high-frequency stimulation (HFS) protocol (four 0.5-s trains of 100 Hz stimulation applied at 20 s intervals) (Mans et al., 2010). The stimulus intensity was increased to 1.5 times the baseline intensity during the HFS to ensure strong postsynaptic depolarization and NMDA receptor activation, and was returned to baseline intensity immediately after HFS.
Preparation of solutions for electrophysiology experiments
Simvastatin (SV)
Simvastatin was purchased from Calbiochem (Cat#567020). Prior to its use in experiments, SV was converted from its inactive lactone prodrug form to its active dihydroxy open acid form by alkaline hydrolysis as described previously (Mans et al., 2010) (first dissolving 50 mg of the compound in 1 ml of ethanol (100%) and then adding 0.813 ml of l N NaOH). This stock solution was stored in aliquots at −20 °C (for up to 1 month). On the day of use, the SV stock solution was neutralized with l N HCl to pH of 7.4 and diluted in aCSF. The final concentration of SV in the recording solution was 10 µM. In addition, a vehicle solution lacking SV was added to aCSF to serve as control.
Mevalonate (Mev)
Mevalonate was purchased from Sigma (Cat # M-4667) and prepared according to previously published methods (Essig et al., 1998; Wagner et al., 2000). Briefly, mevalonate was dissolved before undergoing alkaline hydrolysis in 1N NaOH (heated at 50 C for 2 hrs), and 0.2 M stock solutions (pH 7.0) were stored at −20 °C.
Farnesol (FOH)
Trans, trans-farnesol (96%) was purchased from Sigma (Cat# 27754). To prepare 0.2 mM FOH, 1.85 µL of farnesol were initially pipetted into 4 µL of ethanol to improve solubility. This solution was then diluted into 40 mL of aCSF to reach a final concentration of 0.2 mM. 0.01% ethanol was used for vehicle control.
Geranylgeraniol (GGOH)
Geranylgeraniol (≥ 85%) was purchased from Sigma (Cat# G3278). To prepare 0.2 mM GGOH, 2.42 µL of GGOH were pipetted into 4 µL of ethanol to improve solubility. This solution was then diluted into 40 mL of aCSF to reach a final concentration of 0.2mM. Control solutions contained 0.01% ethanol.
Farnesyl transferase inhibitor (FTI)
FTI-277 was purchased from Calbiochem (Cat# 344555). A 1 mM stock solution was prepared by reconstituting 250 µg FTI in 559 µL dimethylsulfoxide. This stock solution was aliquoted and stored at −80 °C for a maximum of one week. On the day of use, 40 µL of stock solution were dissolved in 40 mL of aCSF to reach a final concentration of 1 µM. Vehicle solutions contained 0.1% DMSO and served as controls.
Geranylgeranyl transferase inhibitor type-1 (GGTI)
GGTI-2133 was purchased from Calbiochem (Cat# 345884). A 1 mM stock solution was prepared by reconstituting 250 µg GGTI in 547 µL dimethylsulfoxide. This stock solution was aliquoted and stored at −80 °C for a maximum of one week. On the day of use, 40 µL of stock solution were dissolved in 40 mL of aCSF to reach a final concentration of 1 µM. Vehicle solutions contained 0.1% DMSO and served as controls.
Simvastatin treatment
Slices were treated with aCSF containing 10 µM simvastatin (SV-aCSF) or vehicle (Veh-aCSF) for a minimum of 100 min before HFS. The 100-min treatment entailed two steps: 1) a 60-min incubation at room temperature in a beaker containing oxygenated 10 µM SV- or Veh-aCSF; 2) continuous perfusion in the recording chamber with SV- or Veh-aCSF warmed to 26–28 °C for at least 40 min. Beaker incubations in SV or vehicle for subsequent experiments began immediately after HFS of the preceding experiment to ensure consistent incubation times between groups. Control experiments were performed in an interleaved fashion, and the order of experiments was reversed over multiple days.
Mevalonate, farnesyl-diphosphate or geranylgeranyl-diphosphate replenishment
An initial 60 min inhibition of the cholesterol biosynthetic pathway with 10 µM oxygenated SV-aCSF occurred in a beaker at room temperature. This was followed by a metabolite replacement period lasting a minimum of 40 min pre-HFS. Metabolite replacement entailed continuous perfusion in the recording chamber with SV- or Veh-aCSF supplemented with the metabolite of interest (or its vehicle) warmed to 26–28 °C. Therefore, the minimum SV treatment was 100 min, and the minimum period of metabolite replacement lasted 40 min. Baseline responses were obtained during treatment in the recording chamber, and the recording solution was unaltered after HFS.
Immunoblot analysis
Hippocampal slices were prepared as described above for LTP experiments. For each mouse, six hippocampal slices were obtained. In interleaved trials, slices were moved to the recording chamber after a 40 min preincubation in FTI (1 µM), GGTI (µM) or DMSO (0.1%) at 26 °C. Once moved to the recording chamber, bath application of FTI, GGTI or DMSO continued during approximately 20 min of baseline stimulation. Slices then underwent either HFS or continued baseline stimulation, and region CA1 was harvested 5 min after this point, then immediately homogenized in SDS sample buffer (Invitrogen, Carlsbad, CA) containing protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany) and phosphatase inhibitor cocktail (Sigma, St. Louis, MO). The entire sample (15 µL) from each experiment was separated by SDS-PAGE, and blotted to PVDF membranes. The membranes were incubated with primary antibody for p-Akt (Ser473) (Cell Signaling) followed by HRP-conjugated secondary antibodies. Signal was detected by the Western Lightning™ Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Boston, MA) and quantified by densitometric scanning using the LabWorks image acquisition and analysis software (UVP Inc., Upland, CA). For a loading control, the blots were stripped and re-probed with a mouse anti-pan Akt antibody (Cell Signaling).
Data analysis
Data were expressed as mean ± standard error of the mean (SEM). Comparison of data from different treatment groups was performed by Student’s t test, and P < 0.05 was considered statistically significant. Data from electrophysiology experiments were filtered at 3 kHz, digitized at 10 kHz, and acquired using LabVIEW data acquisition software (Richard Mooney, Duke University). The slope of the rising phase of the fEPSP was measured and plotted versus time. Each point represents the average of five raw data points. To determine the magnitude of LTP, the slopes of the rising phase of the fEPSPs were normalized to baseline, and the fEPSPs between thirty-five and forty minutes post-HFS were averaged. Comparisons between treatment groups were not made unless all treatments were successfully completed from the same mouse brain. This requirement controlled for inter-animal variability as well as potential differences in slice health between batches of slices.
RESULTS
Mevalonate enrichment suppresses SV-mediated LTP enhancement
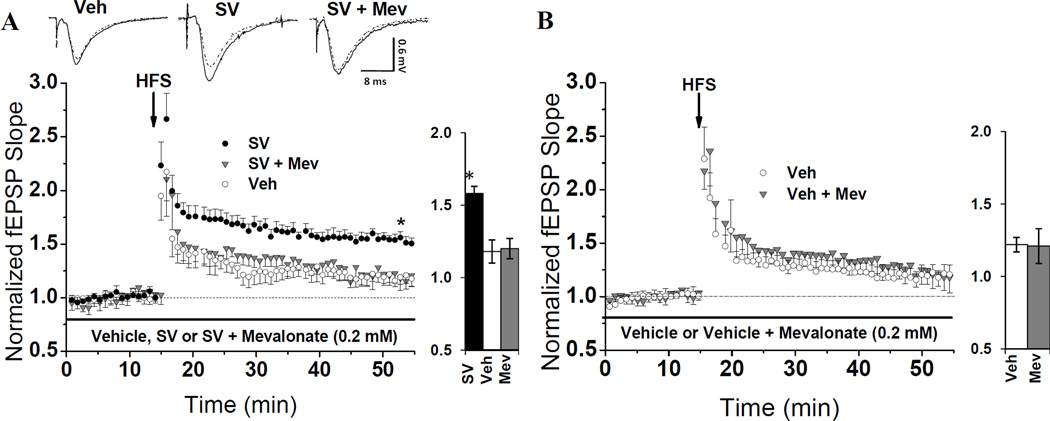
The pleiotropic effects of statins are often attributed to the reduced production of metabolites downstream of HMG-CoA reductase and upstream of cholesterol in the cholesterol biosynthetic pathway, also known as the mevalonate pathway. We therefore hypothesized that SV’s ability to enhance LTP in CA1 (Mans et al., 2010) could be suppressed by replenishing products downstream of HMG-CoA reductase. To test this hypothesis, we employed a two-step in vitro treatment protocol. Initially, hippocampal slices were incubated in SV for one hour. Mevalonate (Mev; 0.2 mM) was then bath applied in the recording chamber in the presence of SV for at least 40 min, and a stable baseline fEPSP was evoked. After the treatments with SV (100 min) and mev (40 min) were completed, a high frequency-stimulation protocol induced LTP in CA3-CA1 synapses. To establish the enhancement of LTP caused by SV, interleaved experiments from seven animals were performed on slices treated with SV or vehicle (Veh). Consistent with previous findings (Mans et al., 2010), SV-treated slices achieved significantly more (P=0.003) LTP (Fig. 1A), than Veh-treated slices (158 ± 0.05% vs. 118 ± 0.05%, respectively). To test if Mev could reverse the SV-mediated enhancement of LTP, SV + Mev-treated slices from four animals were interleaved with SV and Veh control experiments. Mev successfully reversed the LTP enhancement by SV (P=0.002), as SV + Mev-treated slices achieved only 120 ± 0.07% potentiation, a magnitude statistically indifferent from vehicle (P > 0.05) (Fig. 1A). To verify that Mev specifically reversed the effect of SV as opposed to non-specifically inhibiting LTP, interleaved control experiments were conducted in Veh + Mev-treated slices and slices treated with Veh alone. The magnitude of LTP was not reduced (P = 0.97) (Fig. 1B) in Veh + Mev-treated slices (121 ± 0.12%) compared to those treated with Veh alone (122 ± 0.05%), indicating that the LTP-suppressing effect of mevalonate was specific to SV-treated slices. These results support our hypothesis that replenishing metabolites in the mevalonate pathway reverses the ability of SV to enhance LTP in CA1.
Fig. 1. Mevalonate (mev) enrichment specifically suppresses SV-mediated LTP enhancement.
(A) Hippocampal slices were pre-incubated in SV (10 µM) or veh for one hr, then moved to the recording chamber and treated with SV or Veh in the presence or absence of Mev (0.2 mM) for at least 40 min prior to HFS. The average magnitude of LTP from SV-treated slices (158 ± 0.05%, n=7 slices/7 animals) was significantly higher (* P=0.003) than that from Veh-treated slices from the same animals (118 ± 0.05%, n=7 slices/7 animals). To test if Mev enrichment can suppress this enhancement, SV + Mev-treated slices were interleaved with SV and Veh. Mev reduced (P=0.002) the LTP magnitude from 157 ± 0.04% (n=4 slices/4 animals) in SV-treated slices to 120 ± 0.07% (n=4 slices/4 animals) in slices from the same animals. (B) Mean LTP from slices pre-incubated in Veh for one hour, then treated with Veh containing either, 0.2 mM Mev or Mev's vehicle for at least 40 min pre-HFS. LTP suppression following mev enrichment is specific to SV-treated slices: LTP in Veh + Mev-treated slices averaged 121 ± 0.12% (n=6 slices/6 animals) compared to 122 ± 0.05% (n=6 slices/6 animals) LTP in Veh-treated slices (P=0.97). Waveforms show fEPSPs during baseline (dotted) and 40-min post-tetanus (solid) from slices in different treatment groups. Bar charts show LTP magnitude within each treatment group.
Farnesyl-pyrophosphate enrichment suppresses SV-mediated LTP enhancement
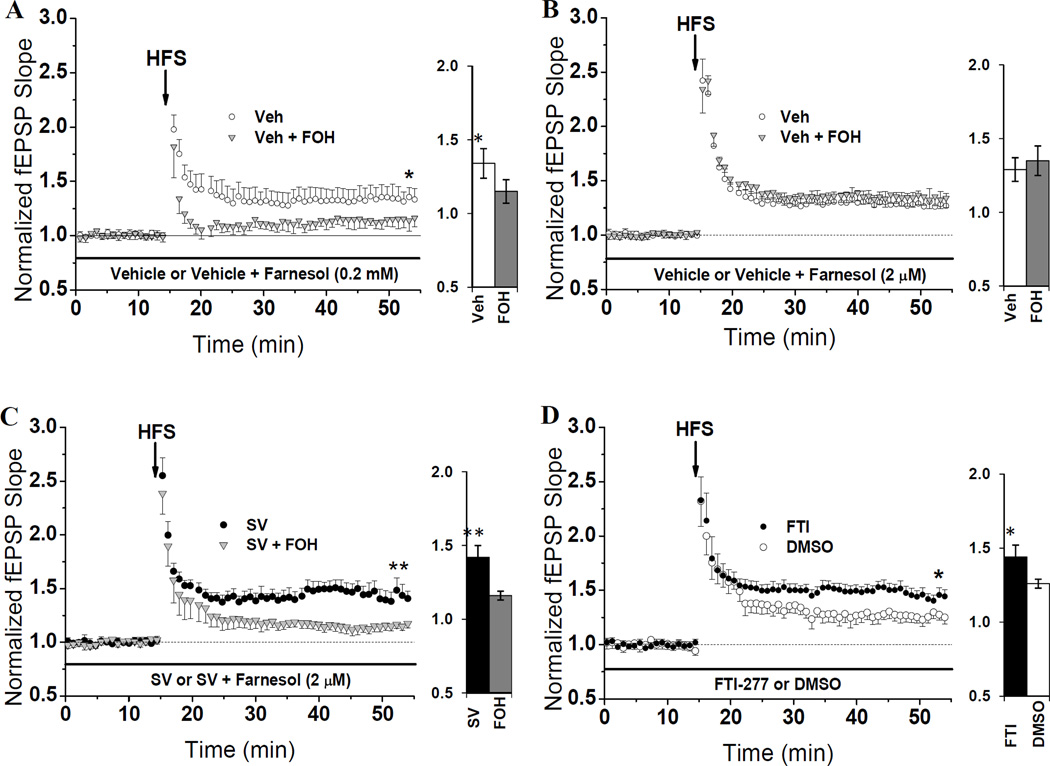
Given our positive findings in the mevalonate-replenishment experiments, we began testing if restoring isoprenoids with known regulatory effects downstream of Mev could also reverse SV-induced increases in LTP. To this end, we coupled the same two-step treatment protocol used for Mev with an established method for resupplying isoprenoids (Crick et al., 1997) in which farnesol (FOH) is bath applied, incorporated into the intracellular space, then converted to FPP via endogenous salvage mechanisms. It has been demonstrated that little if any FPP formed from FOH is converted to GGPP when this protocol is implemented (Crick et al., 1997). When LTP was induced after this treatment, it was found that a high dose (0.2 mM) of FOH caused a significant reduction (P = 0.046) of LTP, even in the absence of SV. As seen in Fig. 2A, the amount of LTP in slices treated with FOH-supplemented Veh (115 ± 0.08%) was lower than LTP in slices treated with Veh + ethanol (FOH’s vehicle), which averaged 134 ± 0.10%. These data suggest that the amount of intracellular FPP can bidirectionally modulate LTP induction. Namely, reduced FPP, which occurs during SV treatment, can lead to enhanced LTP induction, but high levels of FPP can suppress LTP.
Fig. 2. SV-mediated LTP enhancement is suppressed by restoring FPP and mimicked by inhibiting farnesylation.
(A) Mean LTP from slices pre-incubated in Veh for one hour, then treated with Veh containing either, 0.2 mM farnesol (FOH) or 0.01% ethanol for at least 40 min in the recording chamber pre-HFS. This high dose of FOH significantly (*P=0.046) lowered LTP in Veh-treated slices (115 ± 0.08%, n=4 slices/4 animals) compared to LTP in slices treated with Veh alone (134 ± 0.10%, n=4 slices/4 animals). (B) Mean LTP from slices pre-incubated in Veh for one hour then treated with Veh containing either, 2 µM FOH or 0.01% ethanol for at least 40 min in the recording chamber pre-HFS. The lower dose of FOH did not reduce (P>0.05) LTP in Veh-treated slices: 129 ± 0.08% (n=6 slices/6 animals) achieved in Veh + ethanol-treated slices, and 135 ± 0.10% (n=6 slices/6 animals) achieved in Veh + FOH-treated slices. (C) Mean LTP from slices pre-incubated in SV (10 µM) for one hour then treated with SV containing either, 2 µM FOH or 0.01% ethanol for at least 40 min in the recording chamber pre-HFS. Unlike in Veh-treated slices, 2 µM FOH reduced LTP in slices pre-treated with SV. LTP was reduced (*P=0.005) from 142 ± 0.08% (n=5 slices/5 animals) in SV + ethanol-treated slices to 116 ± 0.03% (n=5 slices/5 animals) in SV + FOH-treated slices. (D) Inhibiting farnesyl transferase mimics the LTP-enhancing property of SV. Hippocampal slices were treated in the recording chamber with FTI-277 (1 µM) or 0.1% DMSO for at least one hour-pre HFS. FTI significantly (*P=0.011) enhanced LTP (144 ± 0.06%, n=8 slices/6 animals) compared to DMSO (126 ± 0.04%, n=6 slices/6 animals). Bar charts show LTP magnitude within each treatment group.
To test this hypothesis further, we lowered the FOH dosage by 100 fold. Our goal was to increase FPP production to a degree that would restore FPP levels in SV-treated slices, but not appreciably affect total FPP in Veh-treated slices. In interleaved trials, we found that a 2 µM dose of FOH does not reduce LTP in Veh-treated slices. As seen in Fig. 2B, the LTP in slices treated with FOH-supplemented Veh (135 ± 0.10%) was not different (P > 0.05) from that achieved in slices treated with Veh + ethanol (129 ± 0.08%). However, treatment with 2 µM FOH does reduce (P = 0.005) LTP in statin-incubated slices, reducing the average magnitude of potentiation from 142 ± 0.08% in SV + ethanol-treated slices to only 116 ± 0.03% in slices treated with FOH-supplemented SV (Fig. 2C). These results strongly suggest that FOH specifically reverses SV’s ability to enhance LTP, and that it does so by reversing the limitation on FPP availability caused by SV treatment.
SV-induced LTP enhancement is mimicked by inhibiting farnesyl transferase (FTase)
Prenylation, an important regulatory mechanism conducted by prenyl transferase enzymes, entails the covalent attachment of prenyl moieties, such as FPP, to a specific CAAX motif (McTaggart, 2006). Our finding that FPP enrichment suppresses LTP in CA1 raises the possibility that prenylation, and specifically farnesylation, might modulate LTP induction. To test this hypothesis, we inhibited farnesylation for at least 60 min pre-HFS via bath application of a FTase inhibitor (FTI-277), and asked if FTI-277 can mimic the LTP enhancement we observe in slices treated with SV. Interleaved treatments with FTI (1 µM) or 0.1% DMSO revealed that inhibiting FTase for at least 60 min does indeed mimic (P = 0.011) the effect of SV treatment (Fig. 2D): the average LTP from FTI-treated slices reached 144 ± 0.06%, but the average LTP from DMSO-treated slices reached only 126 ± 0.04%. These data suggest that the increase in LTP we observe after SV incubation may arise from reduced farnesylation of downstream proteins.
Geranylgeranyl-pyrophosphate replacement does not suppress LTP in SV-treated slices
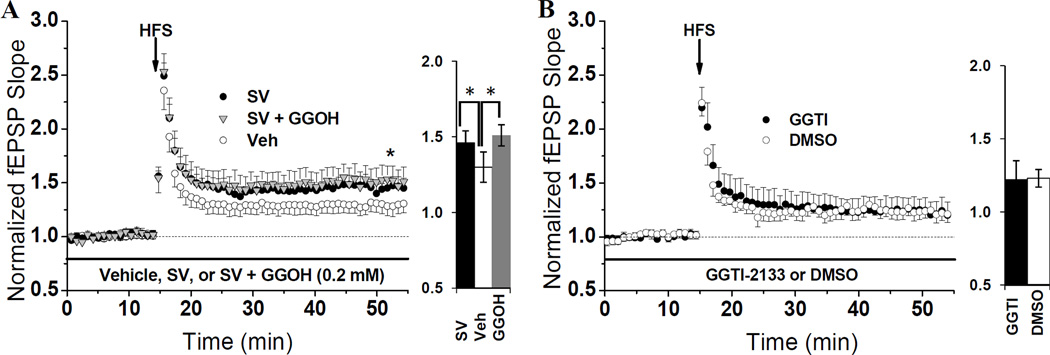
Similar to FPP, the amount of geranylgeranyl-pyrophosphate (GGPP) produced via the mevalonate pathway can exert regulatory changes on molecules subject to geranylgeranylation. To test if GGPP can also suppress LTP, 0.2 mM GGOH was bath-applied in the same two-step treatment protocol used for FPP, and we relied upon endogenous salvage pathways to convert GGOH to GGPP (Crick et al., 1997). In contrast to FOH treatment, GGOH did not reverse the enhancement of LTP caused by SV. As seen in Fig. 3A, the average LTP in SV + GGOH-treated slices (151 ± 0.13%) was significantly (P = 0.035) enhanced over the average LTP from slices treated with vehicle (130 ± 0.08%). Furthermore, the magnitude of LTP in SV + GGOH-treated slices was similar to the LTP from slices treated with SV alone (146 ± 0.08%). From these data we conclude that the LTP enhancement we observe in SV-treated slices does not arise due to reduced production of GGPP, and that the LTP-suppressing effects of FOH treatment are specific to the farnesyl pathway.
Fig. 3. SV-mediated LTP enhancement is not affected by GGPP or mimicked by inhibiting geranylgeranylation.
(A) Hippocampal slices were pre-incubated in SV (10 µM) or Veh for one hr, then moved to the recording chamber and treated with SV or Veh in the presence or absence of GGOH (0.2 mM) for at least 40 min prior to HFS. The average magnitude of LTP from SV-treated slices (146 ± 0.08%, n=5 slices/5 animals) was significantly higher (*P=0.009) than that from Veh-treated slices from the same animals (130 ± 0.08%, n=5 slices/5 animals). To test if GGPP enrichment can suppress this enhancement, SV + GGOH-treated slices were interleaved with SV and Veh controls. GGOH did not (P>0.05) reverse the SV-induced LTP enhancement, as LTP in SV + GGOH-treated slices averaged 151 ± 0.13% (n=5 slices/5 animals). (B) Inhibiting geranylgeranyl transferase type 1 does not affect LTP. Hippocampal slices were treated in the recording chamber with GGTI-2133 (1 µM) or 0.1% DMSO for at least one hour-pre HFS. LTP in the presence of GGTI (122 ± 0.13%, n= 6 slices/5 animals) was not different (P>0.05) from LTP in DMSO (123 ± 0.06%, n= 6 slices/5 animals). Bar charts show LTP magnitude within each treatment group.
Inhibiting geranylgeranyl transferase (GGTase) type I does not change LTP magnitude
To confirm the negative result of the GGPP-enrichment experiments, we utilized a GGTase type-I inhibitor (GGTI-2133) to inhibit geranylgeranylation. It was expected that reducing geranylgeranylation would have no effect on the magnitude of potentiation. GGTI or 0.1% DMSO were bath applied for at least one hour pre-HFS. In agreement with our hypothesis, LTP in GGTI-treated slices (122 ± 0.13%) did not differ (P > 0.05) from LTP in DMSO-treated slices (123 ± 0.06%) (Fig. 3B). This negative result, in conjunction with the inability of GGPP to suppress SV’s LTP-enhancing abilities, leads us to conclude that SV enhances potentiation using mechanisms independent of GGPP production and type I geranylgeranylation.
Inhibiting farnesylation promotes recruitment of the PI3-K/Akt signaling cascade during LTP induction
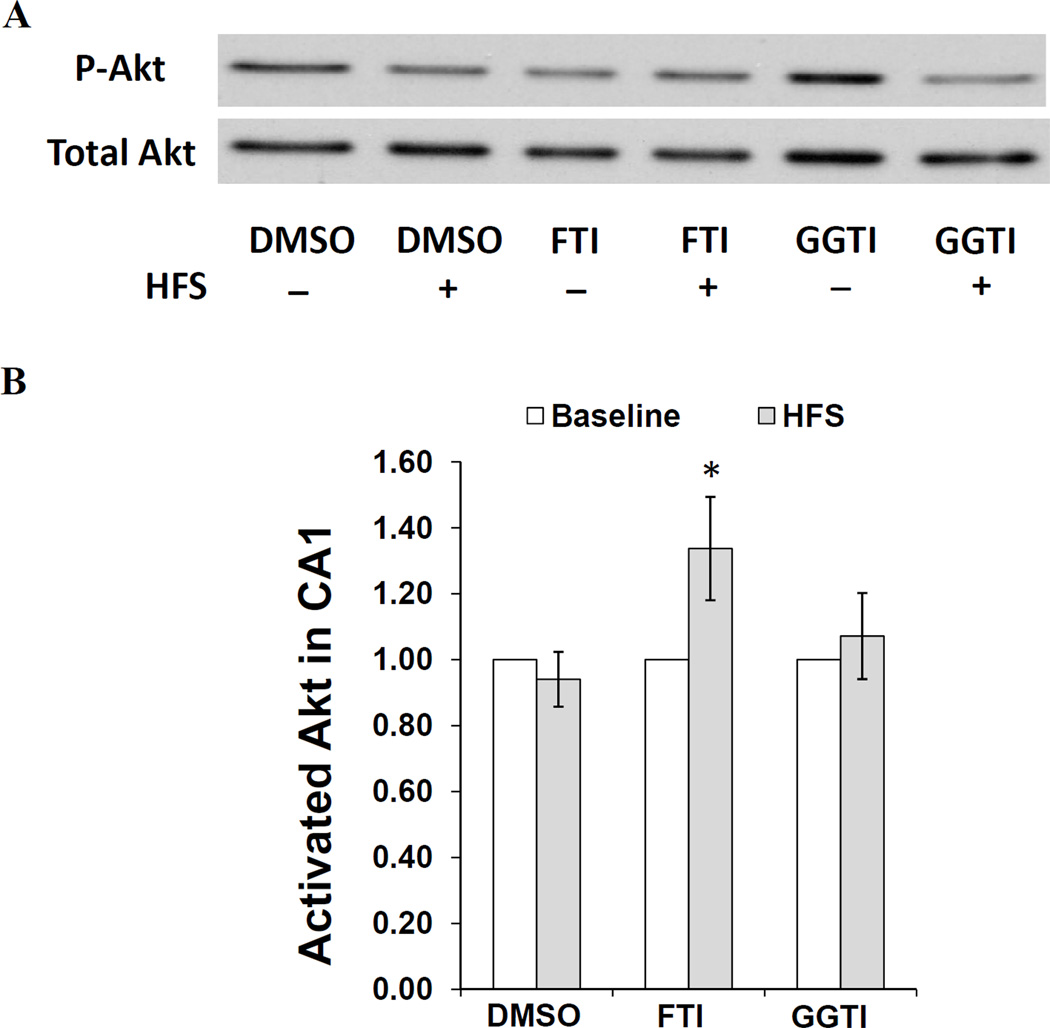
In a previous study, we showed that a PI3-K inhibitor, LY294002 (LY), prevents SV from enhancing LTP in CA1 (Mans et al., 2010) if applied for at least 40 min pre-HFS. Interestingly, we also found that LY did not decrease the magnitude of LTP in vehicle-treated slices. This finding suggests that under our experimental conditions, PI3-K/Akt signaling is only recruited for LTP induction in the presence of SV. Since blocking farnesylation mimics the LTP-enhancing property of SV, we hypothesized that FTI-277 might facilitate the recruitment of PI3-K during LTP induction. To test this hypothesis, we treated hippocampal slices with FTI, GGTI or DMSO for one hour, tetanized the Schaffer-collateral pathway in CA1, then homogenized the CA1 subfield for Western blot analysis five minutes post-HFS. We chose this time-point based on the work of Racaniello et al. (Racaniello et al., 2010) in which tetanus-induced phosphorylation of Akt, an indirect measure of PI3-K activity, peaked at five-minutes post-tetanus. To generate interleaved internal controls from non-tetanized slices from the same animals, CA1 subfields were also harvested from slices treated with FTI, GGTI or DMSO for one hour prior to stimulation at baseline intensity. These slices served as estimators of the basal p-Akt level under each treatment condition per animal. All p-Akt measurements were normalized to total Akt. Also, the ratio of activated Akt/total Akt in baseline-stimulated slices was set to 1 within each treatment group. We used this experimental design to test the hypothesis that only slices treated with FTI would display an increase in p-Akt after tetanus. Relative to their non-tetanized controls, we found the level of post-HFS p-Akt in DMSO-, FTI- and GGTI-treated slices to be 0.94 ± 0.08 (n=13), 1.34 ± 0.16 (n=12) and 1.07 ± 0.13 (n=12), respectively (Fig. 4B). After a paired, one-tailed t-test, only FTI-treated slices showed a significant increase in p-Akt following tetanus (P= 0.027). We interpret this result as an indication that FTI facilitates the recruitment of PI3-K/Akt during LTP induction.
Fig. 4. HFS-induced activation of Akt in FTI-treated slices.
(A) Representative immunoblot images of p-Akt and total Akt in the homogenate of CA1 region of hippocampal slices treated with FTI-277 (FTI), GGTI-2133 (GGTI) or DMSO for one hour, then harvested 5 min after HFS or baseline stimulation. (B) Densitometric analysis of immunoblots from tetanized slices (normalized to total Akt) with non-tetanized slices set as 1. Data represent means ± SEM (n=12 slices/12 animals). The results show that the ratio of activated Akt increased (*P=0.027) after HFS compared to non-tetanized controls in FTI-treated slices only. Relative to their non-tetanized controls, we found the level of post-HFS p-Akt in DMSO-, FTI- and GGTI-treated slices to be 0.94 ± 0.08, 1.34 ± 0.16 and 1.07 ± 0.13, respectively.
DISCUSSION
Previously, we showed that chronically administered SV enhances spatial learning and memory in both a transgenic mouse model of AD and non-transgenic controls (Li et al., 2006), and our subsequent study demonstrated that incubation in SV for 2–4 hours enhances LTP in hippocampal slices from young adult C57BL/6 mice (Mans et al., 2010). The SV-induced increase in LTP magnitude is dependent upon PI3-K activity during the induction phase (Mans et al., 2010). In the present study we dissect the mevalonate pathway to identify the key metabolite(s) contributing to this effect. Specifically, we test the hypothesis that SV’s ability to inhibit isoprenoid production is driving the SV-induced LTP enhancement in CA1. Our results demonstrate that: (1) modulating levels of FPP, but not GGPP, increases the magnitude of early LTP in CA1 region of mature C57BL/6 mice, (2) inhibiting FTase, but not GGTase Type I for one hour preceding HFS mimics the LTP-enhancing property of SV, and (3) FTase inhibition increases the recruitment of PI3-K during LTP induction.
These findings strongly implicate farnesylated proteins and/or FPP itself as modulators of LTP induction in area CA1. The small GTPases, which include the two major families Ras and Rho, are differentially regulated by farnesylation and geranylgeranylation, respectively, though some exceptions do occur (McTaggart, 2006). By facilitating the coupling of extracellular signals to intracellular kinase activity, both families of GTPases regulate intracellular functions. It is well established that statins inhibit function of small GTPases by reducing isoprenoid availability. It is in this vein that statins are currently studied to combat oncogenic Ras and Rho GTPases in a variety of cancers (Dai et al., 2007; Fritz, 2009). Also, in a mouse model of neurofibromatosis type 1 mental retardation, in which excessive p21 Ras activity impairs LTP (Li et al., 2005), lovastatin normalizes p21Ras activity and reverses deficits in LTP and spatial learning (Li et al., 2005). Consistent with Li et al., 2005, there are several lines of evidence that Ras negatively regulates NMDAR transmission. Specifically, it has been shown that H-Ras overexpression decreases NR2A tyrosine phosphorylation and LTP (Thornton et al., 2003), and inhibiting a Ras effector protein, RACK1, increases NMDAR currents in hippocampal neurons (Yaka et al., 2002). Conversely, H-Ras-deficient mice display enhanced tyrosine phosphorylation of NMDAR NR2A and NR2B subunits and an accompanying increase in NMDAR conductance and LTP magnitude (Manabe et al., 2000). The aforementioned findings strongly suggest that Ras inhibition can augment NMDAR-dependent plasticity. In the current investigation, LTP is enhanced following incubation in the FTase inhibitor, FTI-277, a known H-Ras inhibitor which blocks farnesylation of H-Ras. We also find that the LTP-enhancing property of SV is specifically blocked by enriching the recording solution with substrate for farnesylation using 2 µM farnesol. Our observations corroborate reports (Manabe et al., 2000; Yaka et al., 2002; Thornton et al., 2003; Li et al., 2005) demonstrating a negative relationship between NMDAR-dependent synaptic plasticity and the farnesylation pathway. Based on our results, it is likely that FTI-277 and SV both enhance plasticity by inhibiting the farnesylation pathway. Certainly, further studies are needed to identify particular farnesylated protein(s) that modulate synaptic and cognitive function. It is interesting to note that the brains of Alzheimer's disease patients produce elevated amounts of FPP and GGPP (Eckert et al., 2009), further corroborating the possibility that elevated isoprenoid production may be directly detrimental to cognitive function.
Our previous investigation into SV-mediated LTP enhancement revealed that a PI3-K inhibitor, LY-294002, selectively reduced LTP in SV-treated slices (Mans et al., 2010), suggesting that PI3-K signaling is necessary for SV-induced LTP enhancement. This led us to hypothesize that inhibition of FTase mimics SV treatment by facilitating the recruitment of PI3-K during induction. We also tested the accompanying hypothesis that PI3-K is not recruited in the presence of DMSO or GGTI-2133. In an attempt to explore the molecular signaling mediating FTI-induced enhancement of LTP, we assayed for activation/phosphorylation of Akt as an indirect measure of PI3-K activity five minutes after HFS. When homogenates from CA1 were subjected to Western blot analysis, we found that pre-treatment with FTI for one hour pre-HFS promotes the phosphorylation of Akt after tetanus, but treatment with DMSO or GGTI does not. While these results are in agreement with our hypotheses, they should be interpreted conservatively. The primary limitation is the use of whole homogenate, rather than synaptic fractions. Using whole-homogenates prevents the need to pool tissue from several animals to generate sufficient protein volume, but it also includes nuclear and cytosolic Akt that likely does not participate in early LTP signaling. The post-HFS increase in Akt activation we observe may therefore be independent of LTP induction mechanisms and may explain why we did not observe a robust activation of PI3-K in the presence of DMSO or GGTI similar to that observed in Racaniello et al. (Racaniello et al., 2010), in which a pure synaptic fraction was assayed. It is also possible that PI3-K is not appreciably activated during tetanus under our experimental conditions, unless SV or FTI are present. This observation is in agreement with the findings in Mans et al. (Mans et al., 2010) and Opazo et al. (Opazo et al., 2003), in which LY294002 did not reduce LTP under control conditions. PI3-K may, in fact, act as a modulatory enzyme to supplement other kinases during the induction process. It is known that insulin modulates hippocampal plasticity in a PI3-K-dependent manner (van der Heide et al., 2005). Based on our results, SV may enhance LTP by a similar mechanism--namely, the recruitment of PI3-K during induction. Certainly, further experimentation will be required to fully answer this question as it relates to statins and other prenylation inhibitors. Also, the activity of PDK1, the intermediary between PI3-K and Akt, may have its own regulatory influence not explored here.
CONCLUSION
The present study demonstrates that the SV-induced enhancement of LTP we observe in hippocampal slices from adult C57BL/6 mice is due to reduced production of FPP, but not GGPP, in the mevalonate pathway. Additionally, inhibition of farnesylation, but not geranylgeranylation type 1 can enhance LTP, and this effect is associated with the recruitment of Akt during the induction phase. The fact that over three hundred proteins have been identified as prenylation targets complicates any prediction of how statins and other prenylation inhibitors affect intracellular signaling. Predictions are further confounded by an incomplete understanding of how the two classes of prenylation, farnesylation and geranylgeranylation, regulate the vast number of small GTPases in the context of multiple cellular compartments and interacting processes. Nevertheless, the present study provides further evidence that isoprenylation, and particularly farnesylation, plays an important role in regulating synaptic plasticity, presents a ground-work for future explorations into how statins enhance cognition at the cellular level, and motivates the development of novel FTIs as a potential treatment for cognitive disorders.
Highlights.
-
-
Simvastatin (SV) enhances hippocampal LTP via inhibition of isoprenoid production;
-
-
Levels of farnesyl-PP but not geranylgeranyl-PP affect hippocampal LTP.
-
-
Inhibiting farnesyl transferase (FTase) mimics the LTP-enhancing property of SV;
-
-
FTase inhibition increases the activation of PI3-K/Akt during LTP induction;
-
-
These data suggest that SV enhances LTP by modulating pathways downstream of FTase.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health NIA Award AG-031846 and a grant from an anonymous philanthropic foundation to L. Li.
ABBREVIATIONS
- aCSF
artificial cerebral spinal fluid;
- AD
Alzheimer’s disease;
- Akt
protein kinase B;
- fEPSPs
field excitatory postsynaptic potentials;
- FPP
farnesyl pyrophosphate;
- FTase
farnesyl transferase;
- FTI
farnesyl transferase inhibitor;
- GGPP
geranylgeranylpyrophosphate;
- GGTase
geranylgeranyl transferase;
- GGTI
geranylgeranyl transferase inhibitor;
- HFS
high-frequency stimulation;
- HMG-CoA
3-hydroxy-3-methylglutaryl Coenzyme A;
- LTP
long-term potentiation;
- Mev
mevalonate;
- NMDA
N-methyl D-aspartate;
- PI3-K
phosphatidylinositol 3-kinase;
- PPF
paired-pulse facilitation;
- SV
simvastatin;
- Veh
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Clarke RM, Lyons A, O'Connell F, Deighan BF, Barry CE, Anyakoha NG, Nicolaou A, Lynch MA. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J Biol Chem. 2008;283:1808–1817. doi: 10.1074/jbc.M707442200. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. Isoprenoids and Alzheimer's disease: a complex relationship. Neurobiol Dis. 2006;22:209–222. doi: 10.1016/j.nbd.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- Cordle A, Landreth G. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25:299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick DC, Andres DA, Waechter CJ. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma TN, Borre Y, Hendriksen H, Olivier B, Oosting RS. Simvastatin improves learning and memory in control but not in olfactory bulbectomized rats. Psychopharmacology Berl. 2011;216:537–544. doi: 10.1007/s00213-011-2245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Muller WE, Wood WG. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis. 2009;35:251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A. The discovery and development of HMG-CoA reductase inhibitors. 1992. Atheroscler Suppl. 2004;5:67–80. doi: 10.1016/j.atherosclerosissup.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Essig M, Vrtovsnik F, Nguyen G, Sraer JD, Friedlander G. Lovastatin modulates in vivo and in vitro the plasminogen activator/plasmin system of rat proximal tubular cells: role of geranylgeranylation and Rho proteins. J Am Soc Nephrol. 1998;9:1377–1388. doi: 10.1681/ASN.V981377. [DOI] [PubMed] [Google Scholar]
- Fritz G. Targeting the mevalonate pathway for improved anticancer therapy. Curr Cancer Drug Targets. 2009;9:626–638. doi: 10.2174/156800909789057033. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Jones PH. Comparing HMG-CoA reductase inhibitors. Clin Cardiol. 2003;26:I15–I20. doi: 10.1002/clc.4960261306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzi FM, Fitzjohn SM, Morton RA, Collingridge GL, Seabrook GR. Reduced long-term potentiation in hippocampal slices prepared using sucrose-based artificial cerebrospinal fluid. J Neurosci Methods. 2000;100:117–122. doi: 10.1016/s0165-0270(00)00239-9. [DOI] [PubMed] [Google Scholar]
- Li L, Cao D, Kim H, Lester R, Fukuchi K. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60:729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]
- Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, Cannon TD, Silva AJ. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans RA, Chowdhury N, Cao D, McMahon LL, Li L. Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience. 2010;166:435–444. doi: 10.1016/j.neuroscience.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O'Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello M, Cardinale A, Mollinari C, D'Antuono M, De Chiara G, Tancredi V, Merlo D. Phosphorylation changes of CaMKII, ERK1/2, PKB/Akt kinases and CREB activation during early long-term potentiation at Schaffer collateral-CA1 mouse hippocampal synapses. Neurochem Res. 2010;35:239–246. doi: 10.1007/s11064-009-0047-0. [DOI] [PubMed] [Google Scholar]
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-D-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem. 2003;278:23823–23829. doi: 10.1074/jbc.M302389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–880. doi: 10.1592/phco.23.7.871.32720. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zengin A, Deng C, Li Y, Newell KA, Yang GY, Lu Y, Wilder-Smith EP, Zhao H, Huang XF. High dose of simvastatin induces hyperlocomotive and anxiolytic-like activities: The association with the up-regulation of NMDA receptor binding in the rat brain. Exp Neurol. 2009;216:132–138. doi: 10.1016/j.expneurol.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3- methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacco A, Togo J, Spence K, Ellis A, Lloyd D, Furlong S, Piser T. 3-hydroxy-3- methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]