Abstract
The beneficial effects of certain herbal medicines on cutaneous function have been appreciated for centuries. Among these agents, Chrysanthemum extract, apigenin, has been used for skin care, particularly in China, for millennia. However, the underlying mechanisms by which apigenin benefits the skin are not known. In the present study, we first determined whether topical apigenin positively influences permeability barrier homeostasis, and then the basis thereof. Hairless mice were treated topically with either 0.1% apigenin or vehicle alone twice-daily for 9 days. At the end of treatments, permeability barrier function was assessed with either an electrolytic water analyzer or a Tewameter. Our results show that topical apigenin significantly enhanced permeability barrier homeostasis after tape stripping, though basal permeability barrier function remained unchanged. Improved barrier function correlated with enhanced filaggrin expression and lamellar body production, which was paralleled by elevated mRNA levels for the epidermal ABCA12. The mRNA levels for key lipid synthetic enzymes also were up-regulated by apigenin. Finally, both CAMP and mBD3 immunostaining were increased by apigenin. We conclude that topical apigenin improves epidermal permeability barrier function by stimulating epidermal differentiation, lipid synthesis and secretion, as well as cutaneous antimicrobial peptide production. Apigenin could be useful for the prevention and treatment of skin disorders characterized by permeability barrier dysfunction, associated with reduced filaggrin levels, and impaired antimicrobial defenses, such as atopic dermatitis.
Keywords: Apigenin, Transepidermal Water Loss, Barrier, Differentiation, Antioxidant
Introduction
Herbal medicines have been used widely, especially in Asia, for the prevention and treatment of a variety of disorders, including inflammatory dermatoses, largely due to their considerable efficacy and improved side effect profiles in comparison to Western medicines. Well-controlled clinical studies have demonstrated that herbal medicines are effective in treating cardiovascular disease (1, 2), renal disorders(3, 4) , respiratory disease (5, 6), as well as some cancers (7, 8). Similarly, herbal medicines have shown beneficial effects in inflammatory dermatoses, such as psoriasis, acne, contact dermatitis and atopic dermatitis, not only in murine disease models, but also in humans (9–13). Our recent studies show that topical applications of several herbal extracts improve epidermal permeability barrier function, in part by stimulating keratinocyte differentiation and lipid production, in addition to up-regulating epidermal antimicrobial peptide expression in murine models (14, 15), perhaps accounting for the clinical impact of some of these preparations.
Chrysanthemum, also known as Bracteantha bracteata, is a common ingredient in some of these herbal medicines. It has proved effective in treating skin disorders in China for millennia. For instance, topical chrysanthemum extract alleviated diaper dermatitis in infants and newborns with erythema venenatum (16, 17). Improvement of certain cutaneous drug reactions also has been reported with Chrysanthemum (18). Commercialized chrysanthemum tea, as well as chrysanthemum extracts contained in skin care products, are claimed to increase skin hydration and to provide a protective barrier (www.cunan.com/magic/product/1414.html).
Apigenin is an active constituent that is present in large quantity in chrysanthemum extract. Certain benefits of apigenin on cutaneous function have already been documented. For example, apigenin exhibits preventive activity against UVB-induced skin tumors, apparently through inhibition of cyclooxygenase-2 (COX-2) expression (19–21). In a murine model, an apigenin-enriched diet attenuated the development of atopic dermatitis-like lesions (22), and one clinical study showed that an apigenin containing cream inhibited cutaneous inflammation (23). Some of these benefits of apigenin, such as UVB protection and cancer prevention, could be due to the antioxidant properties of apigenin (24, 25).
Recent studies suggest that antioxidant defense and epidermal permeability barrier could be interrelated functions. Oxidative stress inevitably occurs in skin disorders with epidermal permeability barrier abnormalities (26–28), while conversely, applications of antioxidants improve epidermal permeability barrier function (29–31, 15). However, whether the antioxidant, apigenin, improves epidermal permeability barrier function, and the underlying mechanisms responsible for such changes remain undefined. In the present study, we evaluated the effects of apigenin on epidermal permeability barrier function in normal murine skin. After first finding that apigenin improved barrier function, we assessed the responsible mechanisms, including the impact of apigenin on keratinocyte differentiation, lipid secretion, and antimicrobial peptide expression.
Materials and Methods
Materials
6–8 weeks old female hairless mice (hr/hr) were purchased from Charles River Laboratories (Wilmington, MA, USA) and fed mouse diet (Ralston-Purina Co., St Louis, MO, USA) and water ad libitum. Apigenin powder was from Sigma Chemical Co (St Louis, MO, USA). Affinity-purified, rabbit anti-mouse antibodies to loricrin, involucrin, and filaggrin were purchased from Covance (Emeryville CA, USA) for immunohistochemistry.
Experimental protocols and functional studies
All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. Both flanks of mice were treated topically with 60 µl of 0.1% apigenin or ethanol twice daily for 9 days. Basal epidermal permeability barrier function was assessed by measuring transepidermal water loss (TEWL) using TM300 connected to MPA5 (C&K, Cologne, Germany). For barrier recovery, TEWL was measured using an electrolytic water analyzer (Meeco, Warrington, PA) at 0, 2 and 4 hours after tape stripping (10–fold increase in TEWL), and percent barrier recovery was calculated as described earlier (32).
Cell culture
Second-passage keratinocytes isolated from newborn foreskins were cultured in serum-free keratinocyte growth medium containing 0.07 mM calcium (Clonetics, San Diego, California). Cells at 80%–90% confluence were switched to a medium containing 1.2 mM calcium and treated with either 10 µM apigenin or vehicle alone (0.05% ethanol). After 24 and 48 hrs of treatment, keratinocytes were collected for Western blot and Q-PCR analysis.
Western Blot Analysis of Epidermal Differentiation Proteins
Epidermis from mice treated topically with 0.1% apigenin or vehicle alone for 9 days was obtained by EDTA separation (15). Epidermis was prepared in RIPA (Radio-Immunoprecipitation Assay) buffer, and was resolved by electrophoresis on 4–12% Bis-Tris Gel (Invitrogen, Carlsbad, CA). Resultant bands were blotted onto polyvinylidene fluoride membranes, and were subsequently probed with monoclonal anti-mouse β-actin antibody (Sigma, St Louis, MO, USA), polyclonal anti-filaggrin (against the 37kD, monomer, Covance, Emeryville CA, USA), polyclonal anti-involucrin (against the 56 kD, Covance, Emeryville CA, USA) or polyclonal anti-loricrin (against the 57 kD, Covance, Emeryville CA, USA), and the corresponding bands were detected by enhanced chemiluminescence (Thermo Fisher Sci., Rockford, IL), and quantitated by scanning densitometry. For keratinocyte cultures, polyclonal anti-filaggrin (against the 37kD, monomer,), monoclonal anti-involucrin and polyclonal anti-loricrin were from Abcam, Cambrige, MA. Results were presented as percentage of vehicle-treated control, setting vehicle-treated as 100%. β-actin was used to normalize changes in expression levels. Results were presented as percentage of vehicle-treated control, setting vehicle-treated as 100%.
Q-PCR for mRNA expression of filaggrin and lipid synthetic enzymes
Total RNA was isolated from cultured human keratinocytes using TRI Reagent (Sigma). First strand cDNA was synthesized from 1ug of total RNA with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The real-time PCR contained 20 ng of reversed transcribed total RNA, 450 nM forward and reverse primers, and 10 µl of 2× LightCycler 480 SYBR Green I Master in a final volume of 20 µl in 96-well plates using Mx3000P™ Real-time PCR System (Stratagene, La Jolla, CA). Quantification was performed by the comparative CT method with Cyclophilin A used for normalization. The primers for filaggrin and lipid synthetic enzymes, including 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCoA), serine–palmitoyl transferase 1 (SPT1), fatty acid synthase (FAs), the lipid transporter [ATP-binding cassette A12 (ABCA12)], as well as cyclophilin A are listed in supplemental table 1. Relative expression of the mRNAs compared to control mRNA was calculated. Data are expressed as percentage of control (as 100%).
Immunohistochemistry
Immunohistochemical staining for changes in epidermal differentiation was performed as described previously (33). Briefly, 5 µm paraffin sections were incubated with the primary antibodies at the dilutions of 1:2000 for filaggrin, 1:1000 for involucrin, and 1:500 for loricrin overnight at 4°C. After washes ×3, sections were incubated with the secondary antibody for 30 minutes. Staining was detected with ABC-peroxidase kit from Vector Lab, and sections were then counterstained with hematoxylin. All antibodies for differentiation markers were from Covance (Emeryville CA, USA). For assessment of changes in two antimicrobial peptides, mBD3 and CAMP, expression was performed in 5 µm frozen and paraffin sections, respectively [primary antibodies to mBD3 from Alpha Diagnostics; mouse cathelicidin (CAMP) antibody was a gift from Dr. Richard Gallo (UCSD)](14). Sections were examined with a Zeiss fluorescence microscope (Jena, Germany) and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany). All pictures were taken with the same exposure times.
Electron Microscopy
Skin biopsies from both vehicle and apigenin-treated mice were taken for electron microscopy (15). Briefly, samples were minced to <0.5 mm3, fixed in modified Karnovsky's fixative overnight, and post-fixed in either 0.2% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide. After fixation, all samples were dehydrated in a graded ethanol series, and embedded in an Epon-epoxy mixture. Ultrathin sections were examined, with or without further contrasting with lead citrate, in a Zeiss 10A electron microscope (Carl Zeiss, Thornwood, NJ), operated at 60 kV. Number of lamellar body was counted in every 30 cm2 area in the first layer of stratum granulosum on micrograph with magnification of 25000 times. The data were expressed as number of lamellar body per 30 cm2 area.
Statistics
GraphPad Prism 4 software was used for all statistical analyses. An unpaired t-test with Welch’s correction was used for comparisons between two groups. Data are expressed as mean ± SEM.
Results
Topical Apigenin Improves Epidermal Permeability Homeostasis in Normal Murine Skin
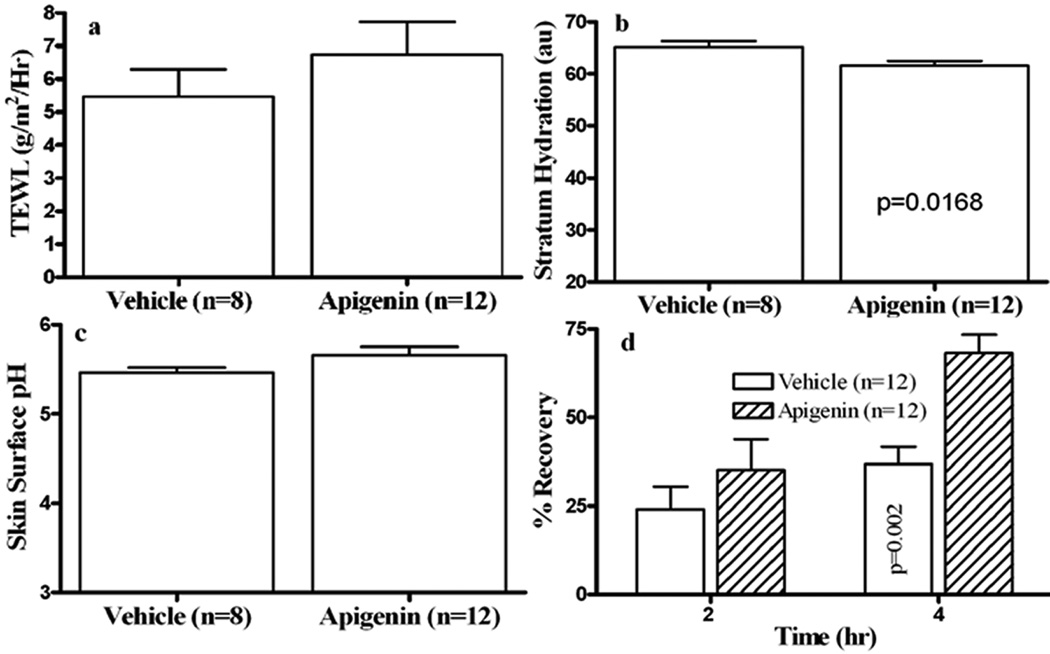
We first assessed whether topical apigenin improves epidermal permeability barrier function in normal murine skin. After 9 days of topical apigenin treatment, there were no changes in gross appearance of mouse skin. Baseline skin surface pH and transepidermal water loss rates did not differ significantly in apigenin- vs. vehicle-treated mice (Figs 1a & 1c; p=0.3411 for Fig 1a and p=0.0963 for Fig 1c). But stratum corneum hydration was slightly, but significantly lower in apigenin-treated as compared to vehicle-treated mice (Fig. 1b). In contrast to basal TEWL, topical apigenin accelerated barrier recovery, a change that was highly significant at 4 hours after acute barrier disruption (Fig 1d). These results demonstrate that topical apigenin improves epidermal permeability barrier homeostasis in normal murine skin.
Figure 1. Topical Apigenin Improves Epidermal Permeability Barrier Homeostasis in Normal Murine Skin.
Hairless mice were treated topically with 60 µl of 0.1% apigenin in 100% ethanol or ethanol alone twice daily for 9 days. Basal epidermal permeability barrier function, skin surface pH and stratum corneum (SC) hydration were assessed with a MPA5 (CK electronic GmbH, Cologne, Germany) connected to TM 300, pH905 and Corneometer 825. Two readings were taken from each mouse for basal TEWL, hydration, as well as pH. For barrier recovery, TEWL was measured with an electrolytic water analyzer (Meeco, Warrington, PA) at 0, 2 and 4 hours after tape stripping, which results in a 10-fold increase in TEWL, and percent barrier recovery rates were calculated as described earlier (32). Figure 1a is basal TEWL; 1b, SC hydration; 1c, skin surface pH; 1d, barrier recovery. Numbers and significances are indicated in the figures.
Topical Apigenin Stimulates Filaggrin Expression in the Murine Model
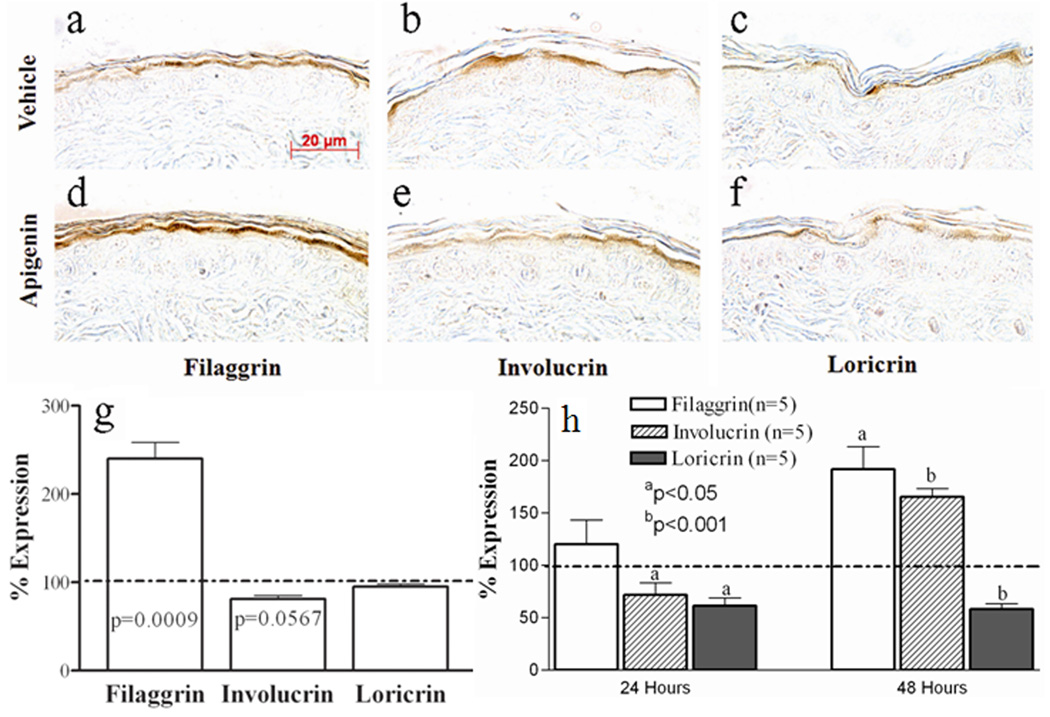
Because differentiation-related structural proteins are a key determinant of normal barrier function, we next assessed whether topical apigenin influences the expression of epidermal differentiation-related proteins, potentially providing one potential mechanism whereby apigenin could improve barrier function. As showed in Fig 2, epidermal filaggrin immunostaining became much more prominent following topical apigenin treatment (Figs 2d vs. 2a, but neither involucrin nor loricrin immunostaining differed in apigenin- vs. vehicle-treated skin (Figs 2b&c vs. 2e&f). To further confirm the immunothistochemical results, Western blotting was employed to quantitate the changes in expression of the differentiation-related proteins. Apigenin again significantly and selectively stimulated epidermal filaggrin expression, without significantly altering either involucrin or loricrin expression (Fig 2g and Suppl Fig. 1).
Figure 2. Topical Selectively Apigenin Stimulates Filaggrin Expression in Normal Mouse Epidermis and Keratinocyte Cultures.
5µm paraffin sections were incubated with respective antibodies. The sections were visualized with a Zeiss microscope. Fig. 2a and d are filaggrin staining; b and e are involucrin staining; c and f are loricrin staining. Fig. 2a, b and c are vehicle-treated samples and d, e and f are apigenin-treated samples. Magnifications are the same for all figures. Magnification bars represent 20 µm (a–f).
For Western blot analysis in vivo, differentiation-related protein from mouse epidermis was isolated and quantitated by scanning densitometry, as described in materials and methods. (N=5 for each group). For Western blot analysis in human keratinocyte cultures, Second-passage human keratinocytes isolated from newborn foreskins were cultured in serum-free keratinocyte growth medium containing 0.07 mM calcium. Cells at 80%–90% confluence were switched to medium containing 1.2 mM calcium and treated with either 10 µM apigenin or vehicle alone (0.05% ethanol). After 24 and 48 hours of treatment, keratinocytes were collected for Western blotting. The corresponding protein bands were detected by enhanced chemiluminescence and quantitated by scanning densitometry. Fig 2g & h: displays the quantitative changes of expression in mouse epidermis and human keratinocyte cultures, respectively. Results were presented as percentage of vehicle-treated control, setting vehicle-treated as 100% as indicated by dotted line. Significances are indicated in the figures.
To further validate the in vivo data, the effect of exogenous apigenin (10µM) on keratinocyte differentiation was also assessed in cultured human keratinocytes. Filaggrin expression was slightly elevated at 24 hours following exogenous apigenin treatments (Fig. 2h and Suppl Fig. 2) and significantly elevated at 48 hours (Fig. 2h and Suppl Fig. 3). In contrast to the in vivo results, apigenin induced a significant reduction in loricrin expression at both 24 and 48 hours (Fig. 2h) while involucrin expression was reduced at 24 hours, but increased at 48 hours (Fig. 2h). To determine whether the apigenin induced elevation in filaggrin occurs at a transcriptional levels, filaggrin mRNA expression was assessed in human keratinocyte cultures. The results showed that apigenin induced a significant increase in filaggrin mRNA expression at both 24 and 48 hours (vehicle 100+9.22 vs. apigenin 204+7.52, p<0.0001, for 24 hours; vehicle 100+10.20 vs. apigenin 160+16, p<0.05, for 48 hours). Together, these results indicate that topical apigenin selectively upregulates epidermal filaggrin expression, thereby providing one mechanism by which apigenin could improve epidermal permeability barrier homeostasis.
Apigenin Stimulates the mRNA Expression of Three Key Barrier-Related Lipid Synthetic Enzymes
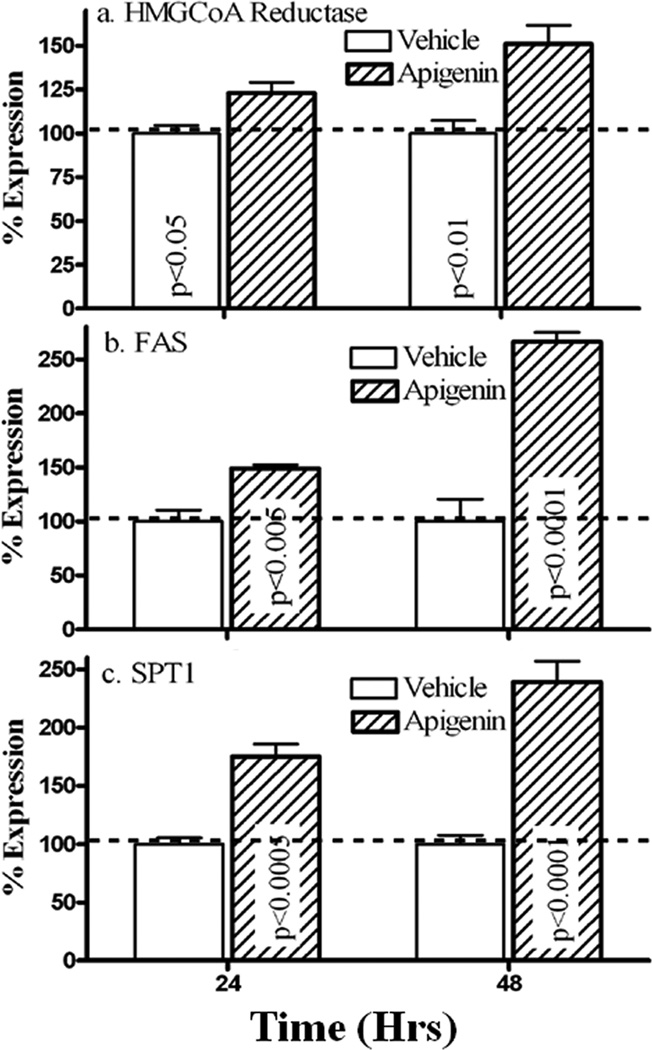
Epidermal permeability barrier function requires synthesis of three key lipids, ceramides, cholesterol and fatty acids. We next quantitated changes in mRNA levels of the three rate-limiting enzymes for synthesis each of these key lipids; i.e, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCoA), serine palmitoyltransferase 1 (SPT1), as well as fatty acid synthase (FAS), after addition of exogenous apigenin to cultured human heratinocytes. As seen in Fig 3, apigenin treatment significantly elevated the mRNA levels of HMGCoA, SPT1 and FAS. Together, these results suggest that apigenin stimulates epidermal lipid production, which could provide another mechanism by which apigenin improves epidermal permeability barrier homeostasis.
Figure 3. Apigenin Up-regulates mRNA Expression of Lipid Synthetic Enzymes In Vitro.
Total RNA was isolated from cultured human keratinocytes (detailed in Materials and Methods) and further purified by RNeasy RNA purification kit. cDNA was prepared using the reverse transcription kit. Levels of mRNA expression were measured by quantitative QPCR using SYBR Green Master Mix. Relative expression of the mRNAs compared to GAPDH control mRNA was calculated. Data are normalized to vehicle control (setting vehicle control as 100% indicated by dotted line on figures). Fig. 3a is the levels of HMGCoA mRNA expression, and Fig. 3b represents the levels of FAS mRNA expression. Fig. 3c is SPT1 mRNA expression. Significances are indicated in the figures (N=5 for each group).
Topical Apigenin Increases Production of Lamellar Bodies
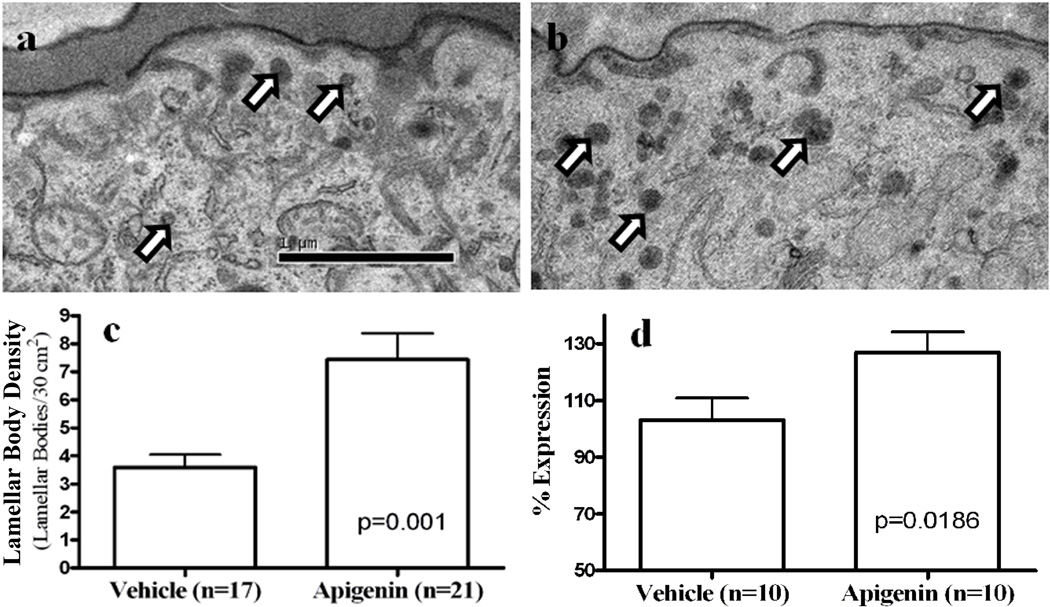
Formation of the epidermal permeability barrier function requires not only the synthesis of lipids, but also production and secretion of lamellar bodies as a means to deliver lipids to the stratum corneum (34, 35). Hence, we next evaluated whether topical apigenin accelerates lamellar body formation and secretion. Indeed, the density of lamellar bodies in intact epidermis significantly increased after topical apigenin treatment (Figs 4a-c). Since these ultrastructural studies strongly suggested that topical apigenin treatment increases lamellar body production, we next assessed changes in mRNA levels of the ATP-binding cassette transporter 12 (ABCA12), a trans-membrane glycosylceramide transporter, required for lamellar body formation (36–39). As predicted, topical apigenin induced a marked increase in ABCA12 mRNA expression in human keratinocytes cultured in 1.2mM calcium (Fig 4d, p=0.0186). Thus, apigenin appears to accelerate the delivery of newly-synthesized lipids into nascent lamellar bodies.
Figure 4. Topical Apigenin Stimulates Lamellar Body Production in Normal Mouse Epidermis, Paralleled by Increase ABCA12 mRNA Expression in Cultured Human Keratinocytes.
Skin biopsies from both vehicle- and apigenin-treated mice were taken for electron microscopy and processed as described in Materials and Methods. Fig. 4a and b display the density of lamellar bodies (see arrows) in vehicle-(a) or apigenin (b)-treated epidermis. Fig. 4c quantitatively displays changes in lamellar body density in vehicle- and apigenin-treated epidermis. Fig. 4d exhibits changes in the levels of ABCA12 mRNA expression after addition of apigenin (10 µM) to cultured keratinocytes growing under differentiating conditions (1.2 mM calcium). Magnification bars, numbers (N) and significant differences are indicated in the figures.
Topical Apigenin Increases Immunostaining for Epidermal CAMP and mBD3
Previous studies demonstrated that two antimicrobial peptides, cathelicidin-related peptide (CAMP) and mouse beta-defensin 3 (mBD3), are packaged within and secreted by lamellar bodies, and that CAMP is crucial not only for antimicrobial defense, but also for epidermal permeability barrier function (40–43). Since our recent studies showed that topical applications of another herbal extract improved epidermal permeability barrier function in parallel with increased epidermal antimicrobial peptide expression (14), we next asked whether apigenin treatment also could increase epidermal CAMP and/or mBD3 expression. As shown in Suppl Fig 4, immunostaining for both CAMP and mBD3 markedly increased following 9 days of topical apigenin treatment. These results suggest that topical apigenin enhances epidermal CAMP and mBD3 expression.
Discussion
An increasingly long line of evidence has demonstrated that certain herbal medicines benefit inflammatory dermatoses characterized and driven by abnormalities in barrier function. Yet, until recently the influence of herbal medicines on epidermal permeability barrier function has not been well studied. In the present study, we demonstrated first that topical applications of apigenin, an extract from chrysanthemum, improve epidermal permeability barrier homeostasis. Although the exact mechanisms by which apigenin accelerates epidermal permeability barrier recovery are not clear, it is a known antioxidant (25), and topical or systemic administrations of other antioxidants, such as vitamin C, vitamin E as well as epigallocatechin gallate, improve epidermal permeability barrier function (25, 29, 44, 45). Hence, it is possible that the apigenin-induced improvement in epidermal permeability barrier homeostasis could result from its antioxidant properties. Whether or not antioxidant mechanisms are involved, a most noteworthy finding in the present study was a selective, apigenin-induced upregulation of filaggrin expression, shown both in vivo and in vitro. Moreover, we show here that the increased filaggrin is likely, at least in part, to be due to upregulation of its mRNA expression. The importance of filaggrin in epidermal permeability barrier function is shown by the observation that filaggrin mutations compromise epidermal permeability barrier function (26, 46). Pertinently, increased epidermal filaggrin expression has been proposed to account in part for the enhanced epidermal permeability barrier function induced by topical peroxisome proliferator-activated receptor and liver X receptor activators (32, 47–49).
Although increased filaggrin expression could account in part for the improved epidermal permeability barrier homeostasis induced by apigenin, we show there that apigenin also stimulated epidermal lipid synthesis and lamellar body production. The epidermal lamellar body is the only known organelle that delivers lipids and their respective post-secretory processing enzymes to the extracellular space of the stratum corneum. Previous studies showed that either abrogation of lamellar body formation or blockade of their secretion disrupts epidermal permeability barrier homeostasis (34). In the present study, we show that apigenin induced a marked increase in mRNA expression of not only key lipid synthetic enzymes, but also the lipid transporter protein, ABCA12, which is required for lamellar body formation. Hence, enhanced lipid production and lamellar body formation could further explain how apigenin improves epidermal permeability barrier homeostasis. Finally, the present study revealed that topical apigenin causes a marked elevation in two critical antimicrobial peptides, epidermal CAMP and mBD3 protein expression. In addition, CAMP is crucial for epidermal permeability barrier homeostasis. Prior studies have shown that CAMP deficient mice display delayed barrier recovery and expression of both CAMP and mBD3 are regulated in paralleled with changes in epidermal permeability barrier status (42, 50). Thus, the accelerated barrier recovery could also be due in part to an up-regulation of epidermal CAMP and mBD3 expression induced by apigenin. In the present study, a remarkable reduction in stratum corneum hydration was observed following apigenin treatment. While the exact mechanism for such a reduction is not clear, it could reflect inhibition of epidermal proteasomes, induced by apigenin (51, 52). It is know that amino acid metabolites are the natural moisturizers in SC (53), and higher activity of proteasomal enzymes (which largely account for the degradation of proteins to amino acids) (Rev. in 54) presents in the epidermis (55). Apigenin is a known proteasome inhibitor (51, 52), which could progressively lower the amino acid content in SC, accounting for the low SC hydration in apigenin-treated skin. Additionally, it is also shown that filaggrin is the source of natural moisturizing factors (NMFs) and its degradation pathway to NMFs includes at least caspase-14 and bleomycin hydrolase (56,57). Caspase-14 degrades deiminated filaggrin into peptides, which is further catalyzed into NMFs by bleomycin hydrolase (56). Filaggrin deficient mice display dry skin with lower levels of NMFs (58). Therefore, it is possible that apigenin also inhibits the pathway breaking down filaggrin into NMFs. However, further studies would be needed to elucidate the mechanisms by which apigenin reduces SC hydration.
The influence of antioxidants on cutaneous structure and function varies dramatically, according to the types of agents deployed. For example, vitamin E reduces lipid peroxidation and stimulates keratinocyte differentiation (59, 60) while vitamin C stimulates both keratinocyte lipid synthesis and differentiation (61, 62). Even within the same class of antioxidants, keratinocyte function may be regulated differently. Balasubramanian, et al showed that epigallocatechin gallate, a flavonoid, increases involucrin expression (63), whereas another flavonoid, curcumin, inhibits involucrin expression induced by epigallocatechin gallate (64). In contrast, hesperdin, also a flavonoid, increases epidermal filaggrin expression, without changing involurin and loricrin expression (15), which is in agreement with data from the present study with apigenin in vivo, whereas the present in vitro study showed that apigenin reduced loricrin expression. These discrepant in vivo and in vitro results may reflect the difference in keratinocyte differentiation in vivo and in vitro (65). For example, treatment of keratinocytes with apigenin for 48 hours may not suffice to stimulate loricrin, the later differentiation protein. Nevertheless, the present study demonstrates that apigenin upregulates filaggrin expression. Taken together, these results suggest further that antioxidants differentially impact cutaneous structure and function by a variety of mechanism. Accordingly, both the types of skin condition and the specific characterics of each antioxidant should be taken into account when choosing the most appropriate agent for possible clinical deployment in dermatology. Since apigenin selectively up-regulates filaggrin expression and lipid production, it could be particularly useful for the treatment atopic dermatitis.
In summary, the present study demonstrates that topical apigenin improves epidermal permeability barrier homeostasis, stimulates lamellar body formation, upregulates filaggrin and lipid synthetic enzyme mRNA expression. Therefore, apigenin could be useful in treating skin disorders with permeability barrier dysfunction, especially those accompanied by reduced filaggrin expression, such as atopic dermatitis.
Supplementary Material
Representative Western blot images of differentiation marker protein expression in mouse epidermis. Epidermis from mice treated topically with 0.1% apigenin or vehicle alone for 9 days was obtained by EDTA separation. Western blot was carried out as described in the Materials and Methods. Hesperidin, which previously was shown to increase filaggrin expression (15), was used as positive control.
Representative Western blot images of differentiation marker protein expression in human keratinocyte cultures. Total protein was isolated from human keratinocyte culture treated with apigenin for 24 hours. The methods for western blotting analysis are detailed in the Materials and Methods.
Representative Western blot images of differentiation marker protein expression in human keratinocyte cultures. Total protein was isolated from cultured human keratinocytes treated with apigenin for 48 hours. The methods for western blotting analysis are detailed in the Materials and Methods.
Skin samples were from mice treated with either vehicle alone (Suppl Figs. 4a, c) or 0.1% apigenin (Suppl Figs. 4b, d) twice daily for 9 days. 5 µm sections were incubated with the primary antibodies (1:500 dilutions) overnight at 4°C. After 3x washing, sections were incubated with the secondary antibody for 30 min. Sections were examined with a Zeiss fluorescence confocal microscope (Jena, Germany), and digital images were captured with AxioVision software. Immunofluorescent staining (green color) for cathelicidin-related antimicrobial peptide (CAMP) (Fig. Suppl Figs. 4a, b), mouse b-defensin 3 (mBD3) (Suppl Figs. 4c, d). Suppl Figs. 4a, c are vehicle treated and b, d are apigenin. Propidium iodide was used for counterstaining in mBD3 stained sections. Magnifications are the same for all figures and magnification bars represent 40 µm (a–d).
Acknowledgement
This work was supported by grants (AR19089, PEM; AR051930, TM) from the National Institutes of Health.
Abbreviations
- ABCA12
ATP-binding cassette transporter 12
- CAMP
Cathelicidin-related peptide
- mBD3
Mouse beta-defensin 3
Footnotes
All authors have no conflicts of interest.
Author contributions
MH performed keratinocyte culture, Western blotting in vitro, prepared the graphs and figures for publication; MH performed the immunohistochemical staining; PLK performed qPCR; RS assessed barrier function, prepared samples for morphology and Western blotting in vitro; KP performed Western blotting in vivo; DC carried out the ultrastructural studies; TKL and JLS assessed basal stratum corneum function and statistical analyses; TMM and PME designed the experiment, interpreted data, critically the manuscript and approved the final version; MQM designed the experiment, interpreted data, and drafted the manuscript.
References
- 1.Yang DY, Wu XL, Xu H, Duan XZ, Wang SW, Lu ZZ. [A clinical study on manshuailing oral liquid in treating elder patients with congestive heart failure of type heart and kidney yang deficiency] Zhongguo Zhong Yao Za Zhi. 2003;28:1091–1093. [PubMed] [Google Scholar]
- 2.Liang BW, Min CY, Qiu WZ, Zhao RH, Wei GJ, Bu JM. [The summary of the clinic study of Zhi-Xin-Fang treating the heart failure resulting from cardiomyopathy] Zhong Yao Cai. 2008;31:470–472. [PubMed] [Google Scholar]
- 3.Wu XL, Li ZJ, Zhang WJ, Ao QX, Xu YW. [Clinical study on Shen Shuai Fang in treating chronic renal failure] Zhong Yao Cai. 2008;31:796–798. [PubMed] [Google Scholar]
- 4.Sheng B, He D, Zhao J, Chen X, Nan X. The protective effects of the traditional Chinese herbs against renal damage induced by extracorporeal shock wave lithotripsy: a clinical study. Urol Res. 2011;39:89–97. doi: 10.1007/s00240-010-0286-1. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh KH. Evaluation of efficacy of traditional Chinese medicines in the treatment of childhood bronchial asthma: clinical trial, immunological tests and animal study. Taiwan Asthma Study Group. Pediatr Allergy Immunol. 1996;7:130–140. doi: 10.1111/j.1399-3038.1996.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 6.Chan CK, Kuo ML, Shen JJ, See LC, Chang HH, Huang JL. Ding Chuan Tang, a Chinese herb decoction, could improve airway hyper-responsiveness in stabilized asthmatic children: a randomized, double-blind clinical trial. Pediatr Allergy Immunol. 2006;17:316–322. doi: 10.1111/j.1399-3038.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 7.Tian JH, Liu LS, Shi ZM, Zhou ZY, Wang L. A randomized controlled pilot trial of"Feiji Recipe" on quality of life of non-small cell lung cancer patients. Am J Chin Med. 2010;38:15–25. doi: 10.1142/S0192415X10007646. [DOI] [PubMed] [Google Scholar]
- 8.Telang NT, Li G, Sepkovic DW, Bradlow HL, Wong GY. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol Med Report. 2012;5:22–28. doi: 10.3892/mmr.2011.617. [DOI] [PubMed] [Google Scholar]
- 9.Yang JF. [Syndrome differentiation and typing of traditional Chinese medicine and the clinical efficacy in 148 cases of acne vulgaris] Hunan Yi Ke Da Xue Xue Bao. 2001;26:219–220. [PubMed] [Google Scholar]
- 10.Man MQ, Shi Y, Man M, Lee SH, Demerjian M, Chang S, Feingold KR, Elias PM. Chinese herbal medicine (Tuhuai extract) exhibits topical anti-proliferative and antiinflammatory activity in murine disease models. Exp Dermatol. 2008;17:681–687. doi: 10.1111/j.1600-0625.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng HM, Chiang LC, Jan YM, Chen GW, Li TC. The efficacy and safety of a Chinese herbal product (Xiao-Feng-San) for the treatment of refractory atopic dermatitis: a randomized, double-blind, placebo-controlled trial. Int Arch Allergy Immunol. 2011;155:141–148. doi: 10.1159/000318861. [DOI] [PubMed] [Google Scholar]
- 12.Man W, Man M, Hupe M, Martin-Ezquerra G, Feingold KR, Elias PM, Man MQ. Topical herbal extract (Huangdang mixture) exhibits both preventive and therapeutic effects in murine acute irritant contact dermatitis. Int J Dermatol . 2011;50:1421–1427. doi: 10.1111/j.1365-4632.2011.04970.x. [DOI] [PubMed] [Google Scholar]
- 13.May BH, Zhang AL, Zhou W, Lu CJ, Deng S, Xue CC. Oral herbal medicines for psoriasis: a review of clinical studies. Chin J Integr Med. 2012;18:172–178. doi: 10.1007/s11655-012-1008-z. [DOI] [PubMed] [Google Scholar]
- 14.Man M, Hupe M, Mackenzie D, Kim H, Oda Y, Crumrine D, Lee SH, Martin-Ezquerra G, Trullas C, Mauro TM, Feingold KR, Elias PM, Man MQ. A topical Chinese herbal mixture improves epidermal permeability barrier function in normal murine skin. Exp Dermatol. 2011;20:285–288. doi: 10.1111/j.1600-0625.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou M, Man M, Man W, Zhu W, Hupe M, Park K, Crumrine D, Elias PM, Man MQ. Topical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skin. Exp Dermatol. 2012;21:337–340. doi: 10.1111/j.1600-0625.2012.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan F. [Nursing care for babies with diaper dermatitis treated with chrysanthemum flower water] Chin Foreign Med Treat. 2010;13:117. [Google Scholar]
- 17.Sun T, Meng L, Wang S. Bathing with different concentrations of wild chrysanthemum soup for newborn erythema venenatum: comparison of the effect. J Nursing Sci. 2011;32:39–40. [Google Scholar]
- 18.Zhou M. [Treatment of drug dermatitis with chrysanthemum bathing] Jiangxi J Trad Chin Med. 2001;32:60–61. [Google Scholar]
- 19.Elmore E, Siddiqui S, Navidi M, Steele VE, Redpath JL. Correlation of in vitro chemopreventive efficacy data from the human epidermal cell assay with animal efficacy data and clinical trial plasma levels. J Cell Biochem. 2005;95:571–588. doi: 10.1002/jcb.20426. [DOI] [PubMed] [Google Scholar]
- 20.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Mol Cell Biol. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog. 2007;46:303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 22.Yano S, Umeda D, Yamashita S, Yamada K, Tachibana H. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. J Nutr Biochem. 2009;20:876–881. doi: 10.1016/j.jnutbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Arsic I, Tadic V, Vlaovic D, Homšek I, Vesić S, Isailović G, Vuleta G. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother Res. 2011;25:228–233. doi: 10.1002/ptr.3245. [DOI] [PubMed] [Google Scholar]
- 24.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Shaal L, Shegokar R, Muller RH. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int J Pharm. 2011;420:133–140. doi: 10.1016/j.ijpharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, Silva KA, Mauro TM, Hupe M, Cho S, Wu Y, Celli A, Schmuth M, Feingold KR, Elias PM. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009;124:496–506. e1–e6. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostyuk VA, Potapovich AI, Cesareo E, Brescia S, Guerra L, Valacchi G, Pecorelli A, Deeva IB, Raskovic D, De Luca C, Pastore S, Korkina LG. Dysfunction of glutathione S-transferase leads to excess 4-hydroxy-2-nonenal and H(2)O(2) and impaired cytokine pattern in cultured keratinocytes and blood of vitiligo patients. Antioxid Redox Signal. 2010;13:607–620. doi: 10.1089/ars.2009.2976. [DOI] [PubMed] [Google Scholar]
- 28.Sapuntsova SG, Lebed'ko OA, Shchetkina MV, Fleyshman MY, Kozulin EA, Timoshin SS. Status of free-radical oxidation and proliferation processes in patients with atopic dermatitis and lichen planus. Bull Exp Biol Med. 2011;150:690–692. doi: 10.1007/s10517-011-1224-0. [DOI] [PubMed] [Google Scholar]
- 29.Pasonen-Seppänen S, Suhonen TM, Kirjavainen M, Suihko E, Urtti A, Miettinen M, Hyttinen M, Tammi M, Tammi R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem Cell Biol. 2001;116:287–297. doi: 10.1007/s004180100312. [DOI] [PubMed] [Google Scholar]
- 30.Boyce ST, Supp AP, Swope VB, Warden GD. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol. 2002;118:565–572. doi: 10.1046/j.1523-1747.2002.01717.x. [DOI] [PubMed] [Google Scholar]
- 31.Vanizor Kural B, Orem A, Cimsit G, Cimşit G, Yandi YE, Calapoglu M. Evaluation of the atherogenic tendency of lipids and lipoprotein content and their relationships with oxidant-antioxidant system in patients with psoriasis. Clin Chim Acta. 2003;328:71–82. doi: 10.1016/s0009-8981(02)00373-x. [DOI] [PubMed] [Google Scholar]
- 32.Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, Moser AH, Michalik L, Desvergne B, Wahli W, Li M, Metzger D, Chambon PH, Elias PM, Feingold KR. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 33.Bourguignon LY, Ramez M, Gilad E, Singleton P, Man MQ, Crumrine DA, Elias PM, Feingold KR. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation, secretion and permeability barrier homeostasis. J Invest Dermatol. 2006;126:1356–1365. doi: 10.1038/sj.jid.5700260. [DOI] [PubMed] [Google Scholar]
- 34.Man MQ, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz G, Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- 36.Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 37.Kelsell DP, Norgett EE, Unsworth H, Teh MT, Cullup T, Mein CA, Dopping-Hepenstal PJ, Dale BA, Tadini G, Fleckman P, Stephens KG, Sybert VP, Mallory SB, North BV, Witt DR, Sprecher E, Taylor AE, Ilchyshyn A, Kennedy CT, Goodyear H, Moss C, Paige D, Harper JI, Young BD, Leigh IM, Eady RA, O'Toole EA. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet. 2005;76:794–803. doi: 10.1086/429844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama M. Pathomechanisms of harlequin ichthyosis and ABCA transporters in human diseases. Arch Dermatol. 2006;142:914–918. doi: 10.1001/archderm.142.7.914. [DOI] [PubMed] [Google Scholar]
- 39.Smyth I, Hacking DF, Hilton AA, Mukhamedova N, Meikle PJ, Ellis S, Satterley K, Collinge JE, de Graaf CA, Bahlo M, Sviridov D, Kile BT, Hilton DJ. A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet. 2008;4:e1000192. doi: 10.1371/journal.pgen.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 41.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 42.Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schröder JM, Feingold KR, Elias PM. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M, Jung M, Hong SP, Jeon H, Kim MJ, Cho MY, Lee SH, Man MQ, Elias PM, Choi EH. Topical calcineurin inhibitors compromise stratum corneum integrity and epidermal and antimicrobial barrier function. Exp Dermatol. 2010;19:501–510. doi: 10.1111/j.1600-0625.2009.00941.x. [DOI] [PubMed] [Google Scholar]
- 44.Jerajani HR, Mizoguchi H, Li J, Whittenbarger DJ, Marmor MJ. The effects of a daily facial lotion containing vitamins B3 and E and provitamin B5 on the facial skin of Indian women: a randomized, double-blind trial. Indian J Dermatol Venereol Leprol. 2010;76:20–26. doi: 10.4103/0378-6323.58674. [DOI] [PubMed] [Google Scholar]
- 45.Jeon HY, Kim JK, Kim WG, Lee SJ. Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and UV-induced skin damage. Skin Pharmacol Physiol. 2009;22:137–141. doi: 10.1159/000201562. [DOI] [PubMed] [Google Scholar]
- 46.Mildner M, Jin J, Eckhart L, Kezic S, Gruber F, Barresi C, Stremnitzer C, Buchberger M, Mlitz V, Ballaun C, Sterniczky B, Födinger D, Tschachler E. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130:2286–2294. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- 47.Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, Lau P, Fowler AJ, Chuang G, Moser AH, Brown BE, Mao-Qiang M, Uchida Y, Schoonjans K, Auwerx J, Chambon P, Willson TM, Elias PM, Feingold KR. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 48.Komuves LG, Hanley K, Lefebvre AM, Man MQ, Ng DC, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR. Stimulation of PPARalpha promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol. 2000;115:353–360. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 49.Kömüves LG, Schmuth M, Fowler AJ, Elias PM, Hanley K, Man MQ, Moser AH, Lobaccaro JM, Williams ML, Mangelsdorf DJ, Feingold KR. Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-beta in murine epidermis. J Invest Dermatol. 2002;118:25–34. doi: 10.1046/j.0022-202x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Martin M, Martin-Ezquerra G, Man MQ, Hupe M, Youm JK, Mackenzie DS, Cho S, Trullas C, Holleran WM, Radek KA, Elias PM. Expression of epidermal CAMP changes in parallel with permeability barrier status. J Invest Dermatol. 2011;131:2263–2270. doi: 10.1038/jid.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu YX, Fang X. Apigenin, chrysin, and luteolin selectively inhibit chymotrypsin-like and trypsin-like proteasome catalytic activities in tumor cells. Planta Med. 2010;76:128–132. doi: 10.1055/s-0029-1186004. [DOI] [PubMed] [Google Scholar]
- 52.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005;124:1099–1110. doi: 10.1111/j.1523-1747.2005.23726.x. [DOI] [PubMed] [Google Scholar]
- 54.Vabulas RM. Proteasome function and protein biosynthesis. Curr Opin Clin Nutr Metab Care. 2007;10:24–31. doi: 10.1097/MCO.0b013e328011645b. [DOI] [PubMed] [Google Scholar]
- 55.Suga Y, Takamori K, Ogawa H. Skin proteasomes (high-molecular-weight protease): purification, enzymologic properties, gross structure, and tissue distribution. J Invest Dermatol. 1993;101:346–351. doi: 10.1111/1523-1747.ep12365519. [DOI] [PubMed] [Google Scholar]
- 56.Kamata Y, Taniguchi A, Yamamoto M, Nomura J, Ishihara K, Takahara H, Hibino T, Takeda A. Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J Biol Chem. 2009;284:12829–1236. doi: 10.1074/jbc.M807908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233–2241. doi: 10.1038/jid.2011.153. [DOI] [PubMed] [Google Scholar]
- 58.Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, Yamada T, Amagai M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129:1538–1546. doi: 10.1016/j.jaci.2012.01.068. [DOI] [PubMed] [Google Scholar]
- 59.Shvedova AA, Tyurina YY, Tyurin VA, Kikuchi Y, Kagan VE, Quinn PJ. Quantitative analysis of phospholipid peroxidation and antioxidant protection in live human epidermal keratinocytes. Biosci Rep. 2001;21:33–43. doi: 10.1023/a:1010430000701. [DOI] [PubMed] [Google Scholar]
- 60.De Pascale MC, Bassi AM, Patrone V, Villacorta L, Azzi A, Zingg JM. Increased expression of transglutaminase-1 and PPARgamma after vitamin E treatment in human keratinocytes. Arch Biochem Biophys. 2006;447:97–106. doi: 10.1016/j.abb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Yun H, Cho Y. Analysis of ceramide metabolites in differentiating epidermal keratinocytes treated with calcium or vitamin C. Nutr Res Pract. 2011;5:396–403. doi: 10.4162/nrp.2011.5.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savini I, Catani MV, Rossi A, Duranti G, Melino G, Avigliano L. Characterization of keratinocyte differentiation induced by ascorbic acid: protein kinase C involvement and vitamin C homeostasis. J Invest Dermatol. 2002;118:372–379. doi: 10.1046/j.0022-202x.2001.01624.x. [DOI] [PubMed] [Google Scholar]
- 63.Balasubramanian S, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J Biol Chem. 2002;277:1828–1836. doi: 10.1074/jbc.M110376200. [DOI] [PubMed] [Google Scholar]
- 64.Balasubramanian S, Eckert RL. Green tea polyphenol and curcumin inversely regulate human involucrin promoter activity via opposing effects on CCAAT/enhancer-binding protein function. J Biol Chem. 2004;279:24007–24014. doi: 10.1074/jbc.M314331200. [DOI] [PubMed] [Google Scholar]
- 65.Siegenthaler G, Tomatis I, Chatellard-Gruaz D, Jaconi S, Eriksson U, Saurat JH. Expression of CRABP-I and -II in human epidermal cells. Alteration of relative protein amounts is linked to the state of differentiation. Biochem J. 1992;287:383–389. doi: 10.1042/bj2870383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative Western blot images of differentiation marker protein expression in mouse epidermis. Epidermis from mice treated topically with 0.1% apigenin or vehicle alone for 9 days was obtained by EDTA separation. Western blot was carried out as described in the Materials and Methods. Hesperidin, which previously was shown to increase filaggrin expression (15), was used as positive control.
Representative Western blot images of differentiation marker protein expression in human keratinocyte cultures. Total protein was isolated from human keratinocyte culture treated with apigenin for 24 hours. The methods for western blotting analysis are detailed in the Materials and Methods.
Representative Western blot images of differentiation marker protein expression in human keratinocyte cultures. Total protein was isolated from cultured human keratinocytes treated with apigenin for 48 hours. The methods for western blotting analysis are detailed in the Materials and Methods.
Skin samples were from mice treated with either vehicle alone (Suppl Figs. 4a, c) or 0.1% apigenin (Suppl Figs. 4b, d) twice daily for 9 days. 5 µm sections were incubated with the primary antibodies (1:500 dilutions) overnight at 4°C. After 3x washing, sections were incubated with the secondary antibody for 30 min. Sections were examined with a Zeiss fluorescence confocal microscope (Jena, Germany), and digital images were captured with AxioVision software. Immunofluorescent staining (green color) for cathelicidin-related antimicrobial peptide (CAMP) (Fig. Suppl Figs. 4a, b), mouse b-defensin 3 (mBD3) (Suppl Figs. 4c, d). Suppl Figs. 4a, c are vehicle treated and b, d are apigenin. Propidium iodide was used for counterstaining in mBD3 stained sections. Magnifications are the same for all figures and magnification bars represent 40 µm (a–d).