Abstract
In a series of 105 patients with polycythemia vera, we retrospectively determined whether the JAK2V617F mutation correlated with severity of disease phenotype. Higher JAK2V617F allele burden correlated with more advanced myelofibrosis, greater splenomegaly, and higher white blood cell count, but not with age, gender, hematocrit level, or frequency of phlebotomy prior to cytoreductive therapy. Although a subgroup at increased risk for thrombosis was not clearly defined, there was a suggestion that frequency of thrombosis increased as the JAK2V617F allele burden increased. The JAK2V617F allele burden did not change significantly in treated patients with serial JAK2 analyses.
Keywords: Myeloproliferative disorders, Treatment of polycythemia vera, JAK2V617F mutation, Allele burden, Splenomegaly, Myelofibrosis
1. Introduction
Almost all patients with the classical phenotypic characteristics of polycythemia vera (PV) carry either the JAK2V617F or exon 12 mutations [1,2]. Whether or not the JAK2 mutant allele correlates with all phenotypic characteristics of the disease remains unresolved. In fact, its clinical relevance has been questioned [3].
In retrospective studies, JAK2V617F allele burden has been performed using archived DNA derived from marrow [3] and peripheral blood granulocytes [4–6]. Although it was reported in three studies that leukocytosis correlated with higher JAK2V617F allele burden, differences were noted with respect to age, hemoglobin concentration, spleen size, and disease duration [3–5]. In a subsequent prospective multi-center study, Vannucchi et al. [6] observed a correlation between higher JAK2V617F allele burden and leukocytosis, higher hematocrit values, larger spleen size, and thrombosis, in addition to other parameters of disease activity.
In the aforementioned studies of Tefferi et al. [3,4] and Vannucchi et al. [5,6], the clinical diagnosis of PV was made using the 2002 World Health Organization (WHO) criteria [7], and in the case of Vannucchi et al. [5,6], clinical data were collected from multiple centers. The limitations of using the WHO criteria for the diagnosis of PV have been reviewed in detail [8]. Because of these reasons, and because the issue of JAK2V617F allele burden and PV phenotype has not been completely resolved, we decided to examine results from our 105 polycythemia patients whose clinical diagnosis was rendered according to Polycythemia Vera Study Group (PVSG) criteria, in a single institution, and where JAK2V617F determinations were performed by two collaborating laboratories [9]. We aimed to determine whether JAK2V617F allele burden correlated with specified clinical and laboratory disease parameters: white blood cell (WBC) count, hematocrit value, platelet count, spleen size, disease duration, grade of myelofibrosis, and past history of thrombotic events, including arterial or venous thrombosis. Since therapy could have influenced JAK2V617F expression, we carefully evaluated the effects of various treatments on the JAK2V617F allele burden.
2. Design and methods
The clinical diagnosis of polycythemia vera initially was based upon the demonstration of an increased Cr51 red blood cell mass and simultaneously determined I125 plasma volume, and other PVSG criteria [10]. Cr51 red blood cell studies were not performed in patients with a hematocrit value of ≥60%. Patients were eligible to enter this study after the results of the blood sample for JAK2 mutation analysis confirmed the molecular diagnosis of PV (n = 105 patients). Written informed consent and IRB approval were obtained. White blood cell count (WBC), hematocrit level (HCT), platelet count (PLT), and spleen size were measured at the time of JAK2 analysis, which in most cases was after the time of diagnosis. Spleen size was measured in centimeters below the midpoint of the left costal margin in the mid-clavicular line and categorized as not enlarged (<1 cm), slightly (1–3 cm), moderately (4–9 cm), or grossly enlarged (>9 cm). Thrombotic events were recorded within 5 years of JAK2V617F determination. Bone marrow trephine biopsies were performed from the posterior iliac crest and evaluated by board-certified hematopathologists. All samples were processed in the usual fashion and by standard techniques. Marrow biopsy slides were prepared with Giemsa, hematoxylin and eosin stain, argyrophilic fibers, and reticulin and collagen were demonstrated using the silver impregnation method, or Gomori stain. The degree of fibrosis was estimated as absent, mild (Grades 1–2), moderate (Grade 3), or severe (Grade 4), by routine examination, according to the Manohoran method [11].
In general, patients were phlebotomized to maintain a HCT ≤45% in men, and ≤42% in women. All patients received aspirin, 81 mg daily. All had received some form of myelosupressive therapy, preferentially interferon [12], but also hydroxyurea, anagrelide, imatinib or dasatinib, thalidomide, P-32, phlebotomy-only, or experimental protocols conforming to our treatment philosophy [12,13]. All had at least one JAK2 allele determination, irrespective of specific treatment or the time of disease onset. Fifty-four patients (51.4%) had JAK2 analysis performed within 5 years of diagnosis [3]. We addressed the possibility that treatment may have influenced JAK2V617F allele burden by conducting separate statistical analyses for those patients treated with imatinib, hydroxyurea, or recombinant interferon alfa-2b (rIFNα-2b). Patients treated with anagrelide, dasatinib, thalidomide, P-32, and phlebotomy-only were too few in number to permit statistical analysis. For those patients (n = 51) who had two or more sequential JAK2V617F assays while receiving imatinib (n = 13), hydroxyurea (n = 13), or rIFNα-2b (n = 25) therapy, molecular response was evaluated according to the criteria of the European LeukemiaNet [14]. Major cardiovascular events were defined by Landolfi et al. [15].
2.1. JAK2 mutation analysis
The DNA used for genotyping in our analysis was purified from total white blood cells. Genotyping was carried out using a qualitative ARMS-PCR assay with a sensitivity of 0.1%, as described previously [2,16,17]. JAK2V617F levels were determined by pyrosequencing, a method that quantifies JAK2V617F when the mutant allele is >5% [18].
2.2. Statistical analysis
The JAK2 mutant allele burden was evaluated both as a continuous variable and as an ordered categorical variable based on five groups: 0.1–20%, 21–40%, 41–60%, 61–80%, and 81–100%. The chi-square or Fisher’s exact test was used to evaluate the association between clinical categorical variables and allele burden category. The analysis of variance (ANOVA) or Kruskal–Wallis test was used to compare mean and median JAK2 allele burden, respectively, between levels of the clinical variables of interest (i.e., spleen size category, myelofibrosis category, and type of thrombosis). Similarly, the ANOVA test was used to compare mean disease duration and mean WBC between ordinal categories of JAK2 allele burden. The Spearman-rank correlation coefficient was used to assess the correlation between JAK2 allele burden (continuous) and clinical continuous variables of interest. All p-values were two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SPSS Version 18.0 (SPSS Inc., Chicago, IL) [19].
3. Results
3.1. JAK2V617F allele burden and clinicohematologic findings
The demographics of the 105 patients are shown in Table 1. There were 52 men and 53 women. At initial evaluation, the median age was 52 years (range 27–77 years), and at the time of JAK2 determination, the median age was 60.0 years (range 35.0–88.0 years). The median duration of disease prior to JAK2 determination was 4.6 years (range 0.0–34.0 years), and the median duration of follow-up after JAK2 determination was 1.0 year (range 0.1–3.6 years). The median phlebotomy requirement (0.5 L/phlebotomy) after diagnosis was 6 per year (range 0–16 per year). The clinical and laboratory findings at the time of JAK2V617F determination are shown in Table 1. At diagnosis, the median HCT values, and WBC and PLT counts were 55.2%, 12.0 × 109/L, and 549.0 × 109/L, respectively. At the time of the JAK2V617F determination, the median HCT values, and WBC, and PLT counts were, respectively, 42.5%, 9.0 × 109/L, and 385.0 × 109/L. Sixty-two (59.0%) of the patients did not have splenomegaly at the time of JAK2 analysis, and 43 (41.0%) did. One of the 43 patients had a splenectomy because of massive splenomegaly prior to JAK2V617F analysis (JAK2V617F allele burden: 100.0%). Of the patients with palpable splenomegaly, the spleen was slightly enlarged in 26 and significantly enlarged in 16. The mean JAK2V617F allele burden at initial evaluation was 46.0% (standard deviation (s.d.) ± 29.7%) (Table 2). The mean allele burden of the highest (fifth) quintile (81–100%) was 90.2% (s.d. ± 5.8%), and of the lowest quintile (0.1–20%), 9.9% (s.d. ± 6.3%) (Table 2). Twenty-five patients experienced a single thrombotic event (16 patients had a single arterial thrombosis and 9 had a single venous thrombosis). Two patients experienced both arterial and venous thromboses (Table 1). There was not a significant difference in disease duration between those patients with an allele burden of <50% versus those with an allele burden ≥50%, although as Vannucchi et al. [5] has described, disease duration tended to be higher in patients with an allele burden ≥75%.
Table 1.
Demographic characteristics, laboratory values and clinical features of PV patients at time of first JAK2V617F determination, n = 105.
| Men, no. (%) | 52 (49.5) |
| Women no. (%) | 53 (50.4) |
| Age: mean ± s.d., median age (range), years | 60.0 ± 12.0, 60.0 (35.0–88.0) |
| Median time between diagnosis and JAK2 analysis in years (range) | 4.6 (0.0–34.0) |
| Median duration follow-up after JAK2 analysis in years (range) | 1.0 (0.1–3.6) |
| HCT (%) mean ± s.d., median (range) | 42.3 ± 4.3, 42.5 (25.4–55.9) |
| WBC (× 109/L) mean ± s.d., median (range) | 11.4 ± 8.4, 9.0 (2.9–65.6) |
| PLT(× 109/L) mean ± s.d., median (range) | 426.2 ± 245.6, 385.0 (59.2–1090.0) |
| Splenomegaly, no. (%)1 | 42 (40.4) |
| Spleen size, cm below the left costal margin, mid-clavicular line, no. (%) | |
| 0 cm | 62 (59.0) |
| >0–3 cm | 26 (24.8) |
| 4–9 cm | 9 (8.6) |
| >9 cm | 7 (6.7) |
| Mean ± s.d., median (range) | 2 ± 4, 0 (0–13) |
| Thromboses, no. (%) | 27 (25.7) |
| Single arterial thrombosis | 16 (15.2) |
| Single venous thrombosis | 9 (8.6) |
| Both arterial and venous thromboses | 2 (1.9) |
| Myelofibrosis, no. (%) | 64 (61.0) |
| Grade 1 | 27 (25.7) |
| Grade 2 | 19 (18.1) |
| Grade 3 | 14 (13.4) |
| Grade 4 | 4 (3.8) |
Table 2.
JAK2V617F allele burden at time of first determination, n = 105.
| % Allele burden | No. of patients (%) | Mean allele burden ± s.d. |
|---|---|---|
| 0.1–20 | 27 (25.7) | 9.9 ± 6.3 |
| 21–40 | 22 (21.0) | 29.3 ± 5.3 |
| 41–60 | 20 (19.0) | 49.8 ± 5.6 |
| 61–80 | 19 (18.1) | 73.0 ± 5.6 |
| 81–100 | 17 (16.2) | 90.2 ± 5.8 |
| All | 105 (100) | 46.0 ± 29.7 |
3.2. JAK2V617F allele burden correlates with degree of myelofibrosis
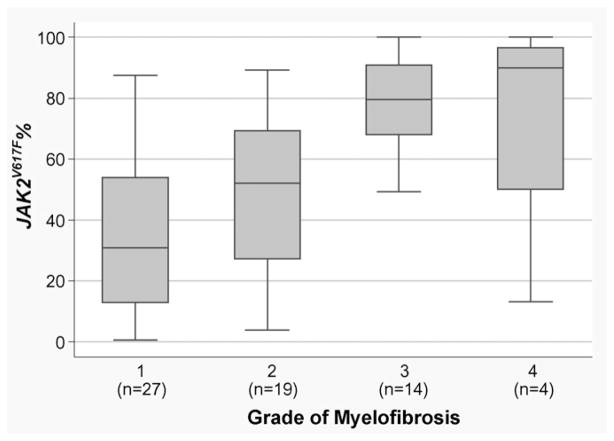
Of 64 evaluable patients who had bone marrow biopsies done, 27 patients had grade 1 myelofibrosis, and 37 patients had grade two to four myelofibrosis, of which 19 patients were grade 2, 14 grade 3, and 4 grade 4 (Table 1). None of the patients had a phenotypic post-PV myelofibrosis syndrome. A quantified relation between JAK2V617F allele burden and degree of fibrosis is shown in Fig. 1 by a box-and-whisker plot, and in Table 3.
Fig. 1.
Box-and-whisker plot of JAK2V617F percent values by grade of myelofibrosis. On the x-axis of this box plot, the grade of myelofibrosis is presented. JAK2V617F percent values are given on the y-axis. The boxes represent the interquartile distances. The upper and lower limits of the boxes indicate the 75th and 25th percentile, respectively. The horizontal lines in the boxes represent the median. The box and the whiskers together indicate the area in which all observations are found, unless outliers are present. When a given observation is located more than 1.5 times the interquartile distance (i.e., above the 75th or below the 25th percentile), then this observation is called an outlier. Grade 1: median JAK2V617F = 30.9 (range 0.7–87.4); Grade 2: median JAK2V617F = 52.1 (range 3.9–89.1); Grade 3: median JAK2V617F = 79.6 (range 12.7–100.0); Grade 4: median JAK2V617F = 90.0 (range 23.4–100.0); p < 0.0001 by Kruskal–Wallis test.
Table 3.
JAK2V617F allele burden and clinical correlates at time of first JAK2V617F determination, n = 105.
| JAK2V617F allele burden% | Mean disease duration (years)* | Mean WBC (×109/L)† | No. of patients |
|---|---|---|---|
| 0–20% | 8.6 ± 6.0 | 6.6 ± 2.0 | 27 |
| 21–40% | 8.3 ± 5.4 | 8.6 ± 3.9 | 22 |
| 41–60% | 5.6 ± 3.8 | 10.7 ± 3.7 | 20 |
| 61–80% | 8.0 ± 5.6 | 16.1 ± 10.1 | 19 |
| 81–100% | 14.6 ± 9.1 | 18.3 ± 13.4 | 17 |
| Spleen size | Mean JAK2V617F%‡ | No. of patients | |
|
| |||
| 0 cm | 36.3 ± 25.7 | 62 | |
| 1–3 cm | 48.8 ± 27.9 | 26 | |
| 4–9 cm | 69.6 ± 23.3 | 9 | |
| >9 cm | 81.8 ± 30.8 | 7 | |
| Patients with myelofibrosis | Mean JAK2V617F%§ | No. of patients | |
|
| |||
| Grades 2–4 only | 63.0 ± 26.7% | 37 | |
| None–Grade 1 | 32.2 ± 23.4% | 42 | |
| Type of thrombosis | Mean JAK2V617F %|| | No. of patients | |
|
| |||
| Arterial | 37.4 ± 28.5 | 13 | |
| Venous | 47.7 ± 24.2 | 14 | |
p = 0.001 by ANOVA.
p < 0.0001 by ANOVA.
p < 0.0001 by ANOVA.
p < 0.0001 by t-test.
p = 0.32 by t-test (trend not statistically significant).
3.3. JAK2V617F allele burden clinical correlates
ANOVA analysis at the time of JAK2V617F determination revealed that overall, higher JAK2V617F burden did correlate with higher WBC count (p < 0.0001), greater degree of splenomegaly (p < 0.0001), more advanced myelofibrosis (p < 0.0001), and longer duration of disease (p = 0.001) (Table 3). Higher JAK2V617F burden also correlated retrospectively with higher white blood cell count at diagnosis (p = 0.02) (data not shown). There was a slight trend for a higher JAK2V617F allele burden among patients with venous compared to arterial thromboses, which was not, however, statistically significant (p = 0.32). JAK2V617F allele burden did not correlate with age, gender, rate of phlebotomy prior to cytoreductive therapy, or hematocrit or platelet counts when measured either at the time of the JAK2V617F determination or at diagnosis. Due to the small sample sizes for various levels of the clinical variables of interest, we could not perform reliable multivariate analysis to assess the independent relationship between JAK2 allele burden and the clinical variables of interest. Adjustment for disease duration would have been helpful but any such multivariate model did not yield stable effect estimates due to collinearity and small sample size issues. When we re-analyzed our data excluding patients with JAK2 values less than 10% (n = 11), all of the findings were consistent and the statistical significance of the findings did not change (data not shown). Similarly, the observed trend of higher JAK2 allele burden with greater degree of fibrosis (Fig. 1) also remained the same after the exclusion of patients with JAK2 values less than 10% (p = 0.001).
3.4. Minimal effects of treatment on JAK2V617F allele burden
Most of our patients received one kind of treatment or another, as previously discussed. The median disease duration before genotyping was 4.6 years. Knowing this could have influenced the level of JAK2V617F mutant allele, we conducted separate statistical analyses at the time of JAK2 evaluation for each group of patients, depending on type of treatment. In patients treated with imatinib mesylate (IM, n = 24), hydroxyurea (HU, n = 25), and recombinant interferon alfa-2b (rIFNα-2b, n = 33), higher JAK2V617F allele burden correlated with elevated WBC count (IM p = 0.004, HU p = 0.02, rIFNα-2b p = 0.002), greater spleen size (IM p = 0.06, HU p = 0.05, rIFNα-2b p = 0.002), and more advanced myelofibrosis (IM p = 0.005, HU p = 0.02, rIFNα-2b p = 0.07), but not with age, gender, hematocrit level, or incidence of thrombotic events. These findings were similar to those observed in the analysis of the entire study group (n = 105). In the group of patients treated with imatinib, patients with a higher JAK2V617F allele burden tended to have elevated platelet counts, but this trend was not statistically significant (IM p = 0.12), and was not seen in patients treated with hydroxyurea or rIFNα-2b. Although there was no direct correlation between JAK2V617F allele burden and thrombosis, the JAK2V617F allele burden in rIFNα-2b-treated PV patients tended to be lower in those who had experienced thrombotic events (p = 0.09). The median time and range, in years, between treatment start and JAK2V617F analysis was 1.5 (0.0–4.5), 3.3 (0.0–17.0), and 3.4 (0.0–16.0) for the imatinib, hydroxyurea, and rIFNa-2b groups, respectively. At the time of evaluation, there were 13, 13, and 25 patients treated with IM, HU, and rIFNα-2b, respectively, who had two or more sequential JAK2 analyses. Two (15.4%) of 13 IM-treated patients, 2 (15.4%) of the 13 HU-treated patients, and 4 (16.0%) of the 25 rIFNα-2b-treated patients achieved a partial molecular response (PMolR) [14].
3.5. Exceptional cases
When the patients who had JAK2 testing performed within 5 years of diagnosis were examined (n = 54), the associations between JAK2V617F allele burden and the clinical variables were similar to those reported for all 105 patients, except for thrombosis, suggesting the duration of disease is not a major confounding factor (data not shown). However, given that this subset represents only half of our cohort, these results should be interpreted appropriately.
Although JAK2V617F burden correlated with severity of disease phenotype in general, exceptions occurred. Of 27 patients in the first quintile with an allele burden of 0.1–20%, 13 patients had a phenotype reflecting extensive disease, characterized by two or more of the following: splenomegaly, bone marrow fibrosis, phlebotomy requirement >4 per year, elevated white blood cell count, elevated hematocrit level, and/or elevated platelet count. Conversely, of 17 patients with an allele burden of 81–100%, 5 had a mild disease phenotype. These patients had minimal or no splenomegaly, an absence of bone marrow fibrosis, lower phlebotomy requirement, and lower white blood cell, platelet, and hematocrit values.
4. Discussion
Our findings, along with those of Tefferi et al. [3,4] and Vannucchi et al. [5,6], are summarized in Table 4. In our series, nearly half of our PV patients had an allele burden of more than 50%, similar to that reported by others [20–22]. Severity of clinical phenotype usually, but not always, correlated with JAK2V617F allele burden. It is noteworthy that some patients with a high allele burden had minimal phenotypic evidence of disease, and conversely, some patients with a low burden had evidence of significant phenotypic disease of PV. The cause and/or genetic basis for this dichotomy is currently under investigation.
Table 4.
JAK2V617F clinical correlates: a review of the literature.
| Summary | Tefferi et al., Leukemia, 2007 | Tefferi et al., Cancer, 2006 | Vannuchi et al., Blood, 2007 | Vannuchi et al., Leukemia, 2007 | Silver et al., Leukemia Research, 2010 |
|---|---|---|---|---|---|
| Criteria for PV diagnosis | WHO | WHO | WHO + PVSG | WHO | PVSG |
| Multi-center study? | No | No | Yes | Yes | No |
| Sample source | Bone Marrow | Peripheral Blood | Peripheral Blood | Peripheral Blood | Peripheral Blood |
| Method of JAK2 analysis | QPCR using DNA | QPCR using DNA | ASO-PCR using DNA | ARMS-PCR using cDNA and QRT-PCR | ARMS-PCR, and Pyrosequencing |
| Expression of JAK2 results | Mut/(wt + mut) | Mut/(wt + mut) | Mut/(wt + mut) | Mut/(wt + mut), quantified | Mut/(wt + mut), quantified |
| Patient population | 77 patients with JAK2 done within 5 years of diagnosis. | 63 patients evaluated either at or after diagnosis | 323 PV patients evaluated at or after diagnosis | 173 patients evaluated at diagnosis (untreated except for aspirin). | 105 patients evaluated at or after diagnosis |
| Clinical parameters | Correlation between clinical parameter and JAK2V617F allele burden? | ||||
|
| |||||
| Higher WBC count | Yes | Yes (after diagnosis) | Yes (at diagnosis) | Yes | Yes |
| Higher hemoglobin level | No | Yes (at diagnosis) | – | – | – |
| Higher HCT level | – | – | – | Yes | – |
| Higher platelet count | No | No | – | No | No |
| Older age | No | No | Yes | – | No |
| Gender | No | No | – | – | No |
| Spleen size | No | – | Yes | Yes | Yes |
| Pruritus | No | Yes | Yes | Yes | – |
| Marrow fibrosis | – | – | – | – | Yes |
| Fibrotic transformation | – | Yes | Yes | – | Yes |
| Disease duration | Yes | No | – | – | Yes |
| Thrombosis | No | No | No | Yes | No* |
trend not statistically significant (p = 0.32).
In our study, an allele burden more than 80% taken at various times during the course of the illness identified a subgroup of patients with more symptomatic disease, an increased degree of splenomegaly, and an increased degree of myelofibrosis. Because of the retrospective nature of this study, we could not quantify fibrosis occurring at diagnosis, since the great majority of patients were not seen at our institution at that time. However, when the patient was referred to our institution, and a bone marrow biopsy and JAK2 determination were performed, there was a statistically significant correlation between these two parameters (Fig. 1). We did not attempt to relate aquagenic pruritus to JAK2 allele burden, as did others [3–6], since, in our experience, this is an amorphous symptom, and difficult to quantify.
Finding a high allele burden may identify patients, in general, who may be at risk for morbidity associated with the disease or its progression, and thus may be candidates for intervention with myelosuppressive or other kinds of treatment, for example, anti-JAK therapy or interferon [12]. Currently, however, we do not treat patients with a high allele burden in the absence of significant phenotypic evidence of disease, and conversely, patients with morbidity due to PV are treated regardless of their allele burden. However, we believe that the assay of JAK2V617F allele burden using a quantitative, rather than qualitative, assay can be clinically useful, and should be performed both at diagnosis and during the course of the disease. In our experience, treatment with recombinant interferon alfa-2b, hydroxyurea, or imatinib did not appear to have a significant effect on the JAK2V617F allele burden, as most treated patients with serial JAK2 analyses did not achieve a molecular response. Although sequential changes in JAK2V617F allele burden following pegylated interferon alfa-2a have been reported [22,23], we have observed no significant change in JAK2V617F allele burden in our patients with PV treated with recombinant interferon alfa-2b [24]. Whether this represents a qualitative difference between these two agents remains to be determined. Reduction in JAK2V617F allele burden with hydroxyurea therapy has also been reported [25,26], but we noted a partial molecular response in only 2 patients who received HU therapy. Similarly, only two of our patients treated with imatinib have had a partial molecular response. Whereas our study did not clearly define a subgroup of patients who were at increased risk from a cardiovascular standpoint, there was a suggestion that the frequency of both arterial and venous thromboses were increased as the JAK2 allele burden increased. The importance of the JAK2 burden is emphasized, since other factors such as age, previous thrombotic risk, and leukocytosis were eliminated as potential confounders.
5. Conclusion
In PV, higher JAK2V617F allele burden correlates with significant disease phenotype including degree of myelofibrosis. In contrast to results reported with pegylated interferon [22,23], the allele burden of patients treated with recombinant interferon alfa-2b (rIFNα-2b) did not change significantly despite excellent clinical and hematologic response. Therefore, whether patients with a high JAK2V617F allele burden should be candidates for earlier therapeutic intervention can be clarified only by a prospective study.
Acknowledgments
This work was supported in part by a grant from the Judy and William Higgins Memorial Trust of the Cancer Research and Treatment Fund, Inc. New York, NY. Dr. Paul Christos was partially supported by the Clinical Translational Science Center (CTSC) (UL1-RR024996). Drs. Attilio Orazi, Wayne Tam, and Amy Chadburn independently reviewed marrow biopsies for this study. We would like to thank Dr. Susan Mathew and Dr. Vesna Najfeld for their review of cytogenetic evaluations for this study. Presented in part at the 2007 and 2009 Annual Meetings of the American Society of Hematology. This study was approved by the New York Presbyterian Hospital - Weill Cornell Medical College Internal Review Board (IRB). All patients participating in this study have signed informed consent. This study is in compliance with the principles of the Declaration of Helsinki, and with national and local ethical guidelines.
Footnotes
Conflict of interest statement
None of the authors have any commercial affiliations, consultancies, stock or equity interests, or patent-licensing arrangements that could be considered to pose a conflict of interest regarding the submitted article.
References
- 1.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YL, Vandris K, Jones A, Cross NC, Christos P, Adriano F, et al. JAK2 mutations are present in all cases of polycythemia vera. Leukemia. 2008;22:1289. doi: 10.1038/sj.leu.2405047. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Strand JJ, Lasho TL, Knudson RA, Finke CM, Gangat N, et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia. 2007;21:2074–5. doi: 10.1038/sj.leu.2404724. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Lasho TL, Schwager SM, Strand JS, Elliott M, Mesa R, et al. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2V617F in polycythemia vera. Cancer. 2006;106:631–5. doi: 10.1002/cncr.21645. [DOI] [PubMed] [Google Scholar]
- 5.Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840–6. doi: 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- 6.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Harris NL, Brunning JD. The WHO classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 8.Spivak JL, Silver RT. The revised World Health Organization diagnostic criteria for polycythemia vera, essential thrombocytosis, and primary myelofibrosis: an alternative proposal. Blood. 2008;112:231–9. doi: 10.1182/blood-2007-12-128454. [DOI] [PubMed] [Google Scholar]
- 9.Silver RT, Vandris K, Wang YL, Christos PJ, Adriano F, Jones AV, et al. JAK2V617F mutational load in patients with polycythemia vera (PV) measured by peripheral blood DNA is associated with disease severity. Blood (ASH Annual Meeting Abstracts) 2007 Nov;110:2530. [Google Scholar]
- 10.Peterson P, Wasserman LR. The natural history of polycythemia vera. In: Wasserman LR, Berk PD, Berlin NI, Saunders WB, editors. Polycythemia and the myeloproliferative diseases. Philadelphia: WB Saunders; 1995. pp. 14–21. [Google Scholar]
- 11.Manoharan A, Horsley R, Pitney WR. The reticulin content of bone marrow in acute leukaemia in adults. Br J Haematol. 1979;43:185–90. doi: 10.1111/j.1365-2141.1979.tb03740.x. [DOI] [PubMed] [Google Scholar]
- 12.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-α. Cancer. 2006;107:451–8. doi: 10.1002/cncr.22026. [DOI] [PubMed] [Google Scholar]
- 13.Silver RT. Treatment of polycythemia vera. Semin Thromb Hemost. 2006;32:437–42. doi: 10.1055/s-2006-942765. [DOI] [PubMed] [Google Scholar]
- 14.Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829–33. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- 15.Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major risk factor in patients with polycythemia vera. Blood. 2007;109:2446–52. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Lu P, Jones AV, Cross NCP, Silver RT, Wang YL. Amplification refractory mutation system, a highly sensitive and simple polymerase chain reaction assay, for the detection of JAK2V617F mutation in chronic myeloproliferative disorders. J Mol Diagn. 2007;9:272–6. doi: 10.2353/jmoldx.2007.060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YL, Lee JW, Kui JS, Chadburn A, Cross NC, Knowles DM, et al. Evaluation of JAK2 in B and T cell neoplasms: identification of JAK2(V617F) mutation of undetermined significance (JMUS) in the bone marrow of three individuals. Acta Haematol. 2007;118:209–14. doi: 10.1159/000111532. [DOI] [PubMed] [Google Scholar]
- 18.Jones AV, Silver RT, Waghorn K, Curtis C, Kreil S, Zoi K, et al. Minimal molecular response in polycythemia vera patients treated with imatinib or interferon alpha. Blood. 2006;107:3339–41. doi: 10.1182/blood-2005-09-3917. [DOI] [PubMed] [Google Scholar]
- 19.SPSS for Windows, Rel. 18.0.1. Chicago: SPSS Inc; 2009. [Google Scholar]
- 20.Baxter EJ, Scott LM, Campbell P, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 21.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 22.Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–72. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 23.Kiladjian JJ, Chomienne C, Fenaux P. Interferon-alfa therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia. 2008;22:1990–8. doi: 10.1038/leu.2008.280. [DOI] [PubMed] [Google Scholar]
- 24.Silver RT, Vandris K, Goldman JJ, Adriano F, Wang YL, Jones AV, et al. Decrease in JAK2V617F allele burden is not a prerequisite to clinical response in patients with polycythemia vera (PV) Blood (ASH Annual Meeting Abstracts) 2009 Nov;114:1908. [Google Scholar]
- 25.Girodon F, Schaeffer C, Cleyrat C, Mounier M, Lafont I, Dos Santos F, et al. Frequent reduction or absence of detection of the JAK2-mutated clone in JAK2V617F-positive patients within the first years of hydroxyurea therapy. Haematologica. 2008;93:1723–7. doi: 10.3324/haematol.13081. [DOI] [PubMed] [Google Scholar]
- 26.Sirhan S, Lasho TL, Hanson CA, Mesa RA, Pardanani A, Tefferi A. The presence of JAK2V617F in primary myelofibrosis or its allele burden in polycythemia vera predicts chemosensitivity to hydroxyurea. Am J Hematol. 2008;83:63–5. doi: 10.1002/ajh.21149. [DOI] [PubMed] [Google Scholar]