Abstract
Diagnosing gastroesophageal reflux disease (GERD) often entails using a combination of patient symptoms, response to proton pump inhibitors (PPI), upper endoscopy, and ambulatory reflux testing. Each of these has limitations which the clinician must be aware of when managing patients with reflux symptoms. Ambulatory reflux monitoring, in particular, can potentially document the true presence of pathologic GERD. Consequently, reflux testing is often necessary in our evaluation of patients with reflux symptoms and can be useful in distinguishing etiologies driving a lack of response to PPI therapy. Reflux testing results can be also used to guide appropriate PPI prescribing and clinical decision making for appropriate or unnecessary therapy. This review focuses on the limitations of our current diagnostic paradigm and highlights how reflux testing can be helpful in the diagnosis and management of patients with poor response to PPI therapy.
Keywords: gastroesophageal reflux disease, reflux monitoring, proton pump inhibitor, heartburn, pH testing, impedance, endoscopy
I. INTRODUCTION
The requirement of reflux testing in gastroesophageal reflux disease (GERD) can be determined by analyzing the Montreal consensus definition which describes a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.1 In order to diagnose and distinguish GERD one must be able to confidently identify pathologic gastroesophageal reflux using a gold standard to either “rule in” or “rule out” GERD as the cause of the current symptoms. Unfortunately, there is no gold standard in GERD diagnosis and what we are left with is our best guess based on a combination of factors: patient symptoms, response to PPI, endoscopy and reflux testing. Each of these factors is helpful in specific scenarios and they may be useful by themselves in instances where patients present with endoscopic findings that are synonymous with GERD, including severe erosive esophagitis and/or long segment Barrett’s esophagus. However, abnormal endoscopic findings are less common these days due to widespread PPI use and we are more commonly faced with the dilemma of patients who are not responding to PPI therapy in the context of a normal endoscopy. The current paradigm for evaluating these patients revolves around reflux testing as this tool can potentially document the presence of pathologic gastroesophageal reflux and distinguish functional heartburn from GERD. Consequently, reflux testing is necessary in our evaluation of patients with GERD symptoms, but it is not a gold standard and should never be considered as a substitute for good clinical judgment. This review will focus on the limitations of our current diagnostic paradigm and highlight how reflux testing can be helpful in the diagnosis and management of patients with poor response to PPI therapy (PPI non-responders).
II. CURRENT DIAGNOSTIC PARADIGM FOR REFLUX SYMPTOMS
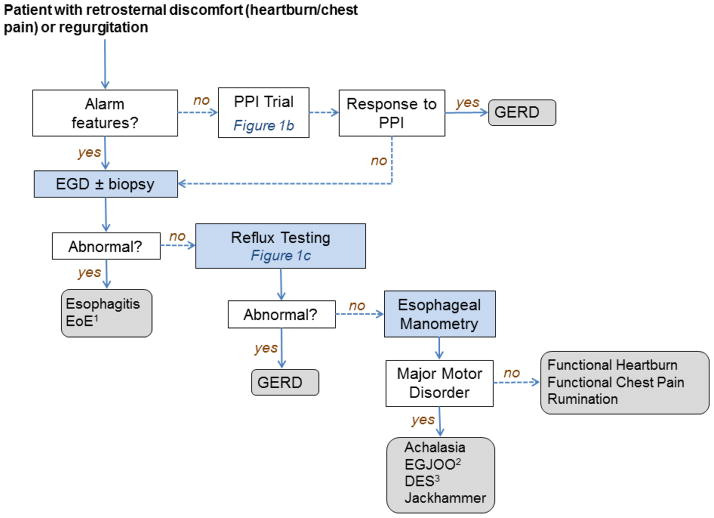
The algorithm for managing patients with retrosternal discomfort/chest pain and/or regurgitation is presented in Figure 1a and represents a modification of the diagnostic paradigm presented by Kahrilas and Smout in 2010.2 The diagnoses are determined by a combination of the presenting symptoms and various diagnostic tests ranging from a simple empiric PPI trial to invasive tests. Components of this paradigm focused on reflux testing will be evaluated separately regarding the strengths and limitations of the approach and how the methodology can be leveraged to help improve diagnosis and management.
Figure 1.
Figure 1a: Diagnostic approach for patients presenting with reflux symptoms (modified from Kahrilas and Smout2 ) 1 EOE=eosinophilic esophagitis; 2 EGJOO=esophagogastric junction outflow obstruction; 3 DES=diffuse esophageal spasm
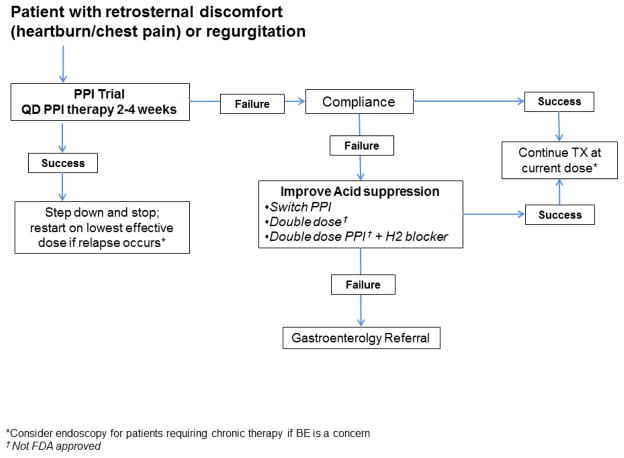
Figure 1b: Empiric proton pump inhibitor therapy for patients with reflux symptoms (modified from Tytgat et al.9)
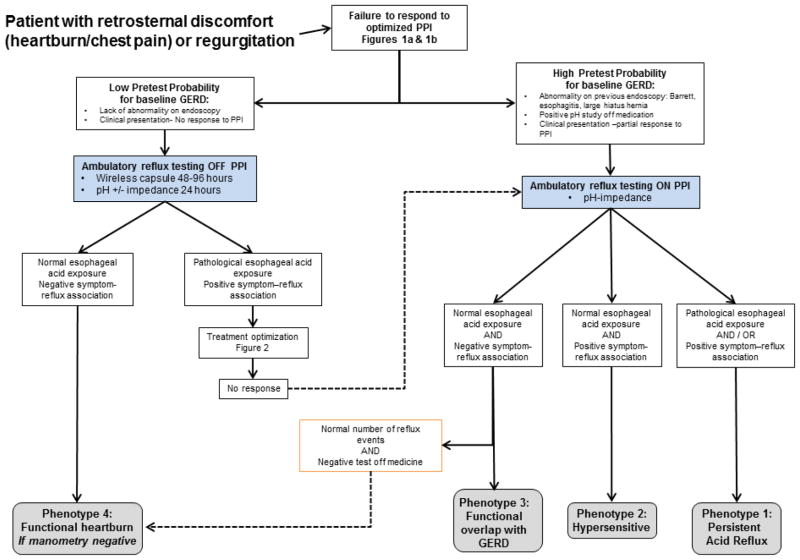
Figure 1c: Ambulatory reflux monitoring algorithm for PPI non-responders (modified from Richter et al.48)
IIa. Symptom Presentation
Symptoms of heartburn and regurgitation have a much lower specificity than previously believed. Dent et al. evaluated the accuracy of a validated questionnaire (Reflux Disease Questionnaire - RDQ), family practitioners, gastroenterologists, and a trial of PPI therapy in diagnosing GERD.3 Patients received endoscopy and 48hr pH monitoring to determine the presence or absence of GERD. Patients were given a diagnosis of GERD if they had at least one of the following: 1) Any grade of reflux esophagitis 2) esophageal pH <4 for >5.5% time 3) positive symptom association probability (SAP >95%) with reflux and 4) borderline acid (pH<4 3.5–5.5% time) AND positive response to esomeprazole treatment.3 Overall, the RDQ, family physicians, and gastroenterologists had similar sensitivity (62%, 63%, 67% respectively) and specificity (67%, 63%, 70% respectively) in diagnosing GERD. Notably, symptom response to PPI therapy did not improve diagnostic accuracy, indicating that a symptom based assessment in patients with reflux symptoms is important in making the diagnosis.
Another recent study has evaluated the association between a widely used symptom based diagnostic questionnaire (the GerdQ) and 48-hr wireless pH monitoring in patients both off and on PPI therapy.4 Strengths of this study include a prospective design and large number of enrolled patients (175 patients on PPI and 178 off PPI therapy). Higher GerdQ scores were predictive of an abnormal pH study in patients off PPI therapy, but overall, the symptom based questionnaire had only modest accuracy in diagnosing GERD. Of note, the questionnaire was not associated with an abnormal pH study or high symptom association probability (SAP 95%) in any patients on PPI therapy.4 The authors concluded that the GerdQ is not an accurate tool for diagnosing GERD and recommended that pH testing should be performed only off PPI therapy in patients with a low pre-test probability of GERD.
Other investigators have evaluated the additional use of low distal baseline impedance measurements in evaluating the specificity and sensitivity of symptoms in diagnosing GERD. A small study of 70 consecutive NERD (44 PPI responders, 26 PPI non-responders, 10 healthy volunteers) patients with MII-pH testing evaluated the correlation of symptoms with low baseline esophageal impedance measurements.5 They found that these baseline measurements did not predict PPI response or symptom perception. Of note, Heard et al. found in a retrospective review of 2809 patients that only 38 patients (1.4%) had low distal baseline impedance measurements raising concerns about the clinical utility of this measurement for diagnostic purposes.6
A robust clinical history is important when evaluating patients for a possible GERD diagnosis. Despite the limitations described above, the use of validated questionnaires and focused questions provides structured assessment of symptoms with little harm to the patient, minimal cost, and with performance characteristics on par with medical specialty care. However, clinicians should be mindful that symptoms alone are not good enough to predict a definitive GERD diagnosis or response to therapy.
IIb. Response to empiric PPI therapy
A very recent study reevaluated data from the well-known DIAMOND study3 to assess the ability of the PPI “test” to identify patients with GERD. The authors included patients diagnosed with GERD according to study protocol (criterion included pH testing and positive response to esomeprazole treatment).7 Of the 203 patients diagnosed with GERD, only 9 patients had a diagnosis based exclusively on response to PPI therapy. Also, a substantial number of patients (up to 62%) without GERD had symptom response with PPI therapy and physicians were not able to predict which patients would respond to a PPI. The authors conclude that the PPI test adds very little information in distinguishing patients with and without GERD.7 Interestingly, in those patients with symptomatic relief from a PPI the time to response was seen predominantly in 5–7 days. The AGA position statement on GERD treatment does not actually specify an exact time duration for empiric therapy but common practice is 4–8 weeks.8,9 We have found that many patients continue empiric PPI therapy even after negative results from reflux testing.10 As the most recent data suggests that a PPI test is equivocal in the majority of patients, shorter durations of empiric therapy are not unreasonable. As shown in Figure 1b, a reasonable daily PPI trial would be 2–4 weeks. If symptoms are still present, physicians should assess patient compliance and increase the PPI dose for a short period of time (2 weeks) prior to further evaluation and diagnostic testing. A primary issue of this algorithm is that the burden falls on the physician to assess response in a timely fashion so patients do not continue medications indiscriminately without appropriate follow up.
Even before one assesses the level of response, one would also have to document compliance to medical therapy at an appropriate dose sufficient to treat most grades of reflux severity. In evaluating the dose of proton pump inhibitor therapy that would reasonably be seen as a failure, one would likely utilize a dose that is higher than the current FDA-approved doses for the various available PPIs. Based on current treatment guidelines8 and physiologic testing data available on patients on single dose therapy and on double dose therapy, a reasonable approach would be to consider patients who fail twice the FDA-approved dose in either a single dose or split (bid.) dose regimen as a failure. Most guidelines advocate for physiologic reflux testing after patients have attempted an escalation of PPI therapy to double dose.8 In addition, studies assessing abnormal acid exposure on PPI therapy in patients with continued symptoms suggest that up to 30% of symptomatic patients on single-dose PPI therapy will have abnormal acid exposure. In contrast, less than 10% of symptomatic patients on double-dose PPI therapy will have abnormal acid exposure. Given that the primary mechanism of PPI therapy is to reduce overall acid reflux and reflux burden, it would appear that the yield of reducing acid burden by increasing the PPI dose to double the FDA-approved dose is significant and would warrant this degree of escalation as the threshold for someone to be considered a PPI failure. Thus, we would not recommend endoscopy or reflux testing in most patients with symptoms on standard daily doses as a proportion of patients will receive benefit from increased dosing.
IIc. Upper Gastrointestinal Endoscopy
GERD symptoms are the most common indication for upper endoscopy11 and there has a been up to a 40% increase in the use of endoscopy among Medicare beneficiaries.12 Upper endoscopy can be revealing and guide management in patients with GERD and alarm symptoms (bleeding, dysphagia, anemia, weight loss, vomiting). Barrett’s esophagus (BE) and esophageal adenocarcinoma are also concerns in certain patients (older age, male, white race) with chronic reflux symptoms.12 However, symptoms alone do not predict BE, as men without symptoms have a much higher risk of BE than women with chronic reflux symptoms.13 Also, follow up or repeat endoscopy is only recommended in patients with severe erosive esophagitis to document healing.12 Prior work has shown that upper endoscopy is completely normal in 39% of patients with reflux symptoms and reveals erosive esophagitis in only ~28% of patients.14 In addition, over 50% of patients who continue to have heartburn symptoms on once daily PPI therapy have been shown to have a normal endoscopy.15 Upper endoscopy solely performed for reflux symptoms (with or without PPI therapy) is often negative and rarely changes the clinical management of these patients. Along with clinical predictors (symptoms) and empiric therapy, upper endoscopy falls well short of providing adequate sensitivity or specificity for diagnosing GERD.
One caveat to the use of upper endoscopy is our evolving understanding of the relationship between GERD, eosinophilic esophagitis (EoE), and PPI-responsive eosinophilia as recently reviewed.16 The consensus recommendations for the diagnosis and management of EoE were recently updated to reflect the recognition of the overlap and potential co-existence of EoE and GERD.17 Patients with a clinical history and symptoms suspicious for EoE, regardless of GERD symptoms, should undergo upper endoscopy with proximal and distal biopsies.17 Patients with only refractory reflux symptoms may undergo endoscopy and it is certainly reasonable to rule out EoE, especially if endoscopic features are suggestive of the diagnosis. However, practitioners should be aware solid evidence is lacking to support endoscopic biopsies in every patient with refractory reflux symptoms to rule out EoE. Poh et al. retrospectively evaluated 105 Veterans who underwent endoscopy with biopsies for PPI “failure”.18 They found that EoE was found in in only 0.9% of patients. Other prevalence estimates of EoE in the US population range from 43–53/100,000 (~0.05%).19 Miller et al. recently designed a Markov decision model and concluded that in patients with refractory reflux symptoms, upper endoscopy with biopsies to rule out EoE was cost effective when the prevalence of disease was ≥8%, which is significantly greater than current prevalence estimates.20 In addition, the utility of ambulatory reflux monitoring (pH or pH-impedance) in EoE patients needs further study before this can be routinely recommended.
IId. Ambulatory Reflux Monitoring
As PPIs are extremely effective at healing virtually all esophagitis grades and virtually all patients referred for refractory GERD symptoms are taking or have taken PPI therapy, many patients will have a negative endoscopy as described above. Reflux monitoring can be performed to document pathologic acid gastroesophageal reflux in patients requiring evaluation for symptoms compatible with GERD (esophageal/extra-esophageal) in several circumstances as outlined previously21 and described below.
Ambulatory reflux testing OFF medication
Patients not fully responding to PPI therapy without a diagnosis of GERD based on endoscopy or a positive ambulatory test confirming pathologic acid gastroesophageal reflux.
Pre-operative evaluation of patients prior to anti-reflux procedures (endoscopic/surgical)
Patients with persistent GERD symptoms after anti-reflux surgery.
Ambulatory reflux testing ON medication
To assess adequacy of acid control in patients with complicated GERD, such as strictures and severe esophagitis.
To assess a potential role of non-acid reflux in patients with a previously established diagnosis of GERD based on endoscopy or a positive ambulatory test confirming pathologic acid gastroesophageal reflux.
In particular, as shown in Figure 1c, when the pretest probability of GERD is low, wireless pH monitoring off medication is suitable and may aid in the diagnosis of functional heartburn. Patients that are able, should stop their PPIs 5–7 days prior to testing.21 When probability of GERD is high, combined pH-impedance on PPI should be the preferred method as the main question now focuses on why the medicine is not working.
III. Diagnostic and treatment algorithm for PPI non-responders
The real management issue in current practice is focused on dealing with refractory symptoms in patients who are on optimized PPI therapy with a negative endoscopy. A more accurate definition of this clinical dilemma should focus on the lack of response to therapy and thus, “PPI non-responder” is an appropriate term. The mechanism behind PPI non-response may be related to non-reflux pathophysiology or to refractory gastroesophageal reflux and therefore, the latter is actually a sub-category of PPI non-response causes.
The evaluation of patients that are not responding to PPI therapy begins by first documenting that the patient is compliant with medical management (Figure 1b). A recent systematic review of PPI adherence in GERD found that ~40–50% of patients on PPI therapy are not compliant with their medication.22 In addition to daily compliance, less than 50% of patients take their PPI optimally (timing, frequency, and dose).23 Most of the current guidelines support empiric treatment with FDA-approved single dose PPI therapy for a 4–8 week period for a patient presenting with typical GERD symptoms.8 Patients should also be taking their medications 30–60min before meals for optimal acid suppression, although this can be liberalized if the patient is taking formulations such as dexlansoprazole or omeprazole sodium-bicarbonate.24,25 If the patient fails single dose therapy, it is reasonable to escalate therapy to double dose as there is little risk to this practice and a small group of patients may respond.
One caveat to empiric treatment focuses on the presence or absence of warning signs. Although there is controversy regarding the predictive value of warning symptoms, an upfront endoscopy is reasonable if there is evidence of dysphagia, odynophagia, GI bleeding, unintentional weight loss, early satiety or age at presentation greater than 55 to rule out significant complications and malignancy.12 In the absence of warning signs, patients are typically not referred for endoscopy unless they have failed a course of optimized PPI therapy. The timing of endoscopy in the algorithm and the dose of PPI that is considered a failure which warrants endoscopic evaluation is unclear, however, we would recommend a trial of double dose therapy.
As mentioned above, patients who fail initial single-dose FDA-approved PPI therapy will have their acid suppression therapy increased to at least double the FDA-approved dose.9 The data to support the yield of an escalation of PPI therapy to double dose in patients not responding to single dose is marginal and likely in the range of 10–20%.26 Once patients are documented to have a poor or inadequate response to optimize PPI therapy (double dose PPI), the next most important steps are a) to document whether or not the patients actually have abnormal gastro-esophageal reflux and b) to document whether or not their symptoms experienced on medication are associated with reflux. Other diagnoses should also be considered including gastroparesis which can contribute to refractory reflux symptoms. Updated comprehensive clinical guidelines have been recently published regarding the diagnosis and management of gastroparesis.27 By focusing on these specific issues, further physiologic testing can distinguish four specific phenotypes of PPI non-responders. The four specific phenotypes of PPI non-responders are described in Table 1.
Table 1.
Phenotypes of PPI non-responders based on physiologic testing
| Phenotype 1 | Phenotype 2 | Phenotype 3 | Phenotype 4 | |
|---|---|---|---|---|
| Persistent acid reflux | Hypersensitive | Functional overlap with GERD | Functional heartburn | |
| Acid esophageal exposure off PPI* | + | +/− | + | − |
| Acid esophageal exposure on PPI* | + | − | − | − |
| Excessive number of reflux events with impedance on PPI | +/− | +/− | + | − |
| Positive reflux- symptom association with impedance on PPI | +/− | + | − | − |
prolonged wireless pH monitoring both off and on PPI may be used to evaluate esophageal acid exposure in a single examination 8
The first distinction in the phenotypes is focused on determining whether or not the patient has baseline abnormal gastro-esophageal reflux (Figure 1c). Phenotypes 1 through 3 are patients who have abnormal gastro-esophageal reflux off PPI therapy, but continued to have symptoms that are either partially treated or secondary complaints that may (Phenotypes 1 & 2) or may not (Phenotype 3) be related to reflux. Phenotypes 1 and 2 have continued symptoms that are related to reflux and these subtypes are truly refractory GERD. Phenotype 1 will have evidence of abnormal acid exposure on ambulatory pH reflux testing and/or a positive symptom reflux correlation in the context of overt abnormal acid exposure or normal acid exposure associated with an acid hypersensitivity. Similarly, phenotype 2 will also have a positive symptom reflux correlation; however, the correlation is not associated with overt abnormality in distal esophageal acid exposure and these patients are likely hypersensitive to a) volume, b) other components of the gastric refluxate or c) refluxate with a pH above 4. These particular phenotypes may respond to an escalation of anti-reflux therapy focused on reducing acid burden and the overall number of reflux events. Alternative options for therapy include baclofen, which has been shown to reduce transient lower esophageal sphincter relaxation28, although evidence from studies to date has involved small numbers of patients.29,30 Surgical therapy (fundoplication) may also be discussed with the patient and local surgical team, including minimally invasive approaches which have shown some promise.31 Prokinetic agents are sometimes attempted but when previously compared to PPI therapy, cisapride was found to be no more effective than placebo32 and significantly less effective than omeprazole.33 A systematic Cochrane review found no recent quality placebo controlled trials evaluating the efficacy of prokinetics for endoscopy negative reflux disease.34 In addition, a recent randomized controlled trial evaluating the addition of mosapride to omeprazole showed no benefit over omeprazole alone in NERD.35
Phenotypes 3 and 4 are important to distinguish from phenotypes 1 and 2 because they should exhibit a lack of response to more aggressive anti-reflux therapy. However, phenotype 3 patients do have baseline reflux disease and many require PPI therapy to maintain control of other symptoms that are related to abnormal reflux. This particular group of patients will exhibit pathologic acid reflux off PPI therapy and normalization on PPI therapy with a negative symptom correlation with all types of reflux events. Ambulatory reflux testing on PPI therapy incorporating impedance may reveal an increased number of overall reflux events suggesting underlying baseline GERD. Thus, these patients will be unable to discontinue PPI therapy and will require an evaluation for alternative causes and therapy beyond reflux suppression. In contrast, phenotype 4 patients will have no evidence of abnormal reflux or a symptom-reflux correlation at baseline or on PPI therapy. This group of patients can be labeled as functional heartburn once an endoscopy has ruled out alternative causes and manometry has not revealed an underlying esophageal motor disorder. These patients should have their PPI therapy discontinued and will likely require therapy focused beyond acid suppression and reflux inhibition.
Evidence to support this phenotypic classification can be found in recent studies assessing large series of referral patients for combined pH-impedance testing both off and on PPI therapy. Savarino et al. noted in a series of 200 patients with non-erosive reflux disease that 27% had normal esophageal acid exposure and negative symptom association probability on 24-h pH-impedance monitoring performed off PPI (phenotype 4).36 Eleven percent of the patients presented with a positive association between symptoms and non-acid reflux events only in the absence of PPI therapy. Mainie et al. also observed different phenotypes of PPI non- responders in a series of 168 patients who underwent 24-h pH-impedance monitoring on PPI for refractory GERD symptoms.37 Eleven percent of subjects had a persistent pathological esophageal acid exposure despite PPI twice daily (phenotype 1), 31% had a positive association between symptoms and non-acid reflux (phenotype 2) and 58% had no evidence of pathological reflux and/or positive association on PPI (phenotypes 3 and 4). As data on esophageal pH monitoring off PPI were not available for these patients it is not possible to differentiate phenotypes 3 and 4.
IV. Should PPI therapy be stopped based on ambulatory reflux testing results?
The treatment of refractory reflux symptoms remains challenging and identification of PPI non-responders phenotypes may provide the pathophysiological basis to help guide therapy. Improving reflux inhibition may be proposed in patients with phenotypes 1 and 2, whereas treatment strategies targeting visceral hypersensitivity may be more relevant in phenotypes 3 and 4. In these patients, it would be reasonable to recommend decreasing or stopping therapy. This is easier said than done in clinical practice as we recently found that up ~42% of patients with negative results from reflux tests continued PPI therapy and very few were ever told to attempt to stop therapy.10 Proton pump inhibitors are often the last line of therapy provided to patients with refractory symptoms and due to widespread availability are difficult to stop in many patients. There is also very limited research on effective interventions to stop prescribing of unwarranted medications, including PPIs.38 The American Gastroenterological Association and American Board of Internal Medicine have taken one step by highlighting PPI use in the widely publicized “Choosing Wisely” campaign which states that if empiric or escalated PPI dosing does not control GERD symptoms within the recommended 4–8 weeks, the medication should be stopped and alternative options assessed.39 However, the effects of this campaign on prescribing habits and patient behavior are not yet known and the campaign also does not specifically address the use of ambulatory reflux monitoring to guide clinical decision making.
Another dilemma is long term continuation of therapy in patients with symptom response but normal ambulatory reflux testing. The individual risk of PPI use is small but there is data linking PPIs to a higher risk of fracture, Pneumonia, and Clostridium difficile diarrhea.40–42 Although the absolute risks of these conditions is small for individual patients, due to the large number of PPI prescriptions it is estimated that >30,000 patients could be harmed annually by one of these conditions.43 Recently, the FDA announced a safety alert for PPI use and associated risk of C. Diff infection.44 In their review of 28 observational studies, 23 showed that patients treated with PPIs had a 1.4 to 2.75 times higher risk of C difficile infection or disease.44 Although the strength of these associations is variable and the overall safety profile remains good, efforts should be made to decrease unnecessary long term PPI use in the general population, especially in those patients without any objective evidence (i.e. positive ambulatory reflux monitoring results) of reflux disease.
In the case of persistent symptoms despite active PPI treatment and negative ambulatory reflux testing, systematic efforts should be made to evaluate other potential causes of symptoms and alternative approaches to therapy.21 As stated above, select patients may respond to baclofen29,30 There is also modest evidence that anti-depressants (e.g. tricyclic anti-depressants, selective serotonin reuptake inhibitors) can modulate pain, especially non-cardiac chest pain, although rigorous trials and definitive evidence is lacking.45
Non-pharmaceutical therapies are also an option. There has been promising recent research showing that abdominal breathing exercises can improve GERD symptoms.46 Our group has also shown a benefit of hypnotically assisted relaxation therapy for globus sensation47, and work is ongoing to evaluate if similar results can be obtained in patients with functional heartburn. Overall, therapeutic options are still limited and further studies are required to evaluate and provide evidence for alternative treatment strategies that could replace or augment PPI therapy.
CONCLUSION
Reflux testing is important in documenting the primary pathophysiologic outcome responsible for GERD and thus, is an important component of the current management strategy. Symptoms, empiric PPI therapy, and endoscopy play a role but are often not sufficient for making a diagnosis of GERD or other syndromes (i.e. functional heartburn). Ambulatory reflux monitoring, when used appropriately, is useful in distinguishing etiologies driving a lack of response to PPI therapy. Reflux testing results can be used to guide appropriate PPI prescribing and clinical decision making for appropriate or unnecessary therapy. The question of whether or not to stop therapy in PPI non-responders should be supplemented by objective evidence of the likelihood of true reflux disease. Ambulatory reflux monitoring provides valuable information to help physicians and patients choose therapeutic options, and perhaps even more importantly, stop unnecessary therapy. Therapeutic options for PPI non-responders are limited and alternative therapies should be sought and investigated in larger, well designed randomized controlled trials. Future work is also needed to determine the most efficient approach to diagnostic testing to avoid inappropriate long term PPI therapy and overuse of endoscopy.
Acknowledgments
This work was supported by R01 DK079902 (JEP) from the Public Health Service. Dr. Gawron is supported by a NRSA award from the Agency for Research and Quality, T-32 HS 000078 (PI: Jane L Holl, MD MPH).
Footnotes
Dislcosure
John E. Pandolfino has been a consultant for Given Imaging; Andrew J. Gawron: none.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Smout AJPM. Esophageal disorders. Am J Gastroenterol. 2010;105:747–56. doi: 10.1038/ajg.2010.65. [DOI] [PubMed] [Google Scholar]
- 3.Dent J, Vakil N, Jones R, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut. 2010;59:714–21. doi: 10.1136/gut.2009.200063. [DOI] [PubMed] [Google Scholar]
- 4**.Lacy BE, Chehade R, Crowell MD. A Prospective Study to Compare a Symptom-Based Reflux Disease Questionnaire to 48-h Wireless pH Monitoring for the Identification of Gastroesophageal Reflux (revised 2-26-11) Am J Gastroenterol. 2011;106:1604–11. doi: 10.1038/ajg.2011.180. This study provides comparative data between a widely used diagnostic questionnairea and pH testing for making a GERD diagnosis. [DOI] [PubMed] [Google Scholar]
- 5*.Ribolsi M, Emerenziani S, Borrelli O, et al. Impedance baseline and reflux perception in responder and non-responder non-erosive reflux disease patients. Scand J Gastroenterol. 2012;47:1266–73. doi: 10.3109/00365521.2012.722674. References 5 and 6 provide data suggesting a limited ability of baseine impedance measurements to diagnose GERD. [DOI] [PubMed] [Google Scholar]
- 6*.Heard R, Castell J, Castell DO, et al. Characterization of patients with low baseline impedance on multichannel intraluminal impedance-pH reflux testing. J Clin Gastroenterol. 2012;46:e55–7. doi: 10.1097/MCG.0b013e318247c319. References 5 and 6 provide data suggesting a limited ability of baseine impedance measurements to diagnose GERD. [DOI] [PubMed] [Google Scholar]
- 7**.Bytzer P, Jones R, Vakil N, et al. Limited Ability of the Proton-Pump Inhibitor Test to Identify Patients With Gastroesophageal Reflux Disease. Clin Gastrol Hep. 2012;10:1360–1366. doi: 10.1016/j.cgh.2012.06.030. This recent paper calls into question the utility of the PPI test for diagnosing GERD. [DOI] [PubMed] [Google Scholar]
- 8.Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterol. 2008;135:1383–1391. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Tytgat GN, McColl K, Tack J, et al. New algorithm for the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2008;27:249–56. doi: 10.1111/j.1365-2036.2007.03565.x. [DOI] [PubMed] [Google Scholar]
- 10**.Gawron AJ, Rothe J, Fought AJ, et al. Many patients continue using proton pump inhibitors after negative results from tests for reflux disease. Clin Gastroenterol Hepatol. 2012;10:620–5. doi: 10.1016/j.cgh.2012.02.012. This study found that a large proportion of patients continue PPI therapy even after negative reflux tests and most are never told to attempt to stop therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg A, Amorosi SL, Lacey MJ, et al. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–496. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 12*.Shaheen NJ, Weinberg DS, Denberg TD, et al. Upper Endoscopy for Gastroesophageal Reflux Disease: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2012:808–816. doi: 10.7326/0003-4819-157-11-201212040-00008. This paper outlines the best practices of using upper endoscopy in efforts to avoid over-utilization. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc. 2010;71:21–7. doi: 10.1016/j.gie.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickman R, Mattek N, Holub J, et al. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol. 2007;102:1173–1179. doi: 10.1111/j.1572-0241.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 15.Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71:28–34. doi: 10.1016/j.gie.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:1066–78. doi: 10.1016/j.cgh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21–2. [DOI] [PubMed] [Google Scholar]
- 18.Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71:28–34. doi: 10.1016/j.gie.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SM, Goldstein JL, Gerson LB. Cost-effectiveness model of endoscopic biopsy for eosinophilic esophagitis in patients with refractory GERD. Am J Gastroenterol. 2011;106:1439–45. doi: 10.1038/ajg.2011.94. [DOI] [PubMed] [Google Scholar]
- 21.Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc. 2009;69:917–30. 930, e1. doi: 10.1016/j.gie.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Hungin APS, Hill C, Molloy-Bland M, et al. Systematic review: Patterns of proton pump inhibitor use and adherence in gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:109–16. doi: 10.1016/j.cgh.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Gunaratnam NT, Jessup TP, Inadomi J, et al. Sub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2006;23:1473–7. doi: 10.1111/j.1365-2036.2006.02911.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee RD, Mulford D, Wu J, et al. The effect of time-of-day dosing on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR: evidence for dosing flexibility with a Dual Delayed Release proton pump inhibitor. Aliment Pharmacol Ther. 2010;31:1001–11. doi: 10.1111/j.1365-2036.2010.04272.x. [DOI] [PubMed] [Google Scholar]
- 25.Howden CW, Ballard ED, Koch FK, et al. Control of 24-hour intragastric acidity with morning dosing of immediate-release and delayed-release proton pump inhibitors in patients with GERD. J Clin Gastroenterol. 2009;43:323–6. doi: 10.1097/MCG.0b013e31818a386e. [DOI] [PubMed] [Google Scholar]
- 26.Hershcovici T, Fass R. An algorithm for diagnosis and treatment of refractory GERD. Best Pract Res Clin Gastroenterol. 2010;24:923–936. doi: 10.1016/j.bpg.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of Gastroparesis. Am J Gastroenterol. 2012;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340–54. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 29.Ciccaglione AF, Marzio L. Effect of acute and chronic administration of the GABA agonist baclofen on 24 hour pH metry and symptoms in control subjects and in patients with gastro-oesophageal reflux disease. Gut. 2003;52:464–471. doi: 10.1136/gut.52.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koek GH, Sifrim D, Lerut T, et al. Effect of the GABA agonsit baclofen in patients with symptoms and duoedeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut. 2003;52:1397–1402. doi: 10.1136/gut.52.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RCW, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215:794–809. doi: 10.1016/j.jamcollsurg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Hatlebakk JG, Hyggen A, Madsen PH, et al. Heartburn treatment in primary care: randomised, double blind study for 8 weeks. BMJ. 1999;319:550–3. doi: 10.1136/bmj.319.7209.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galmiche JP, Barthelemy P, Hamelin B. Treating the symptoms of gastro-oesophageal reflux disease: a double-blind comparison of omeprazole and cisapride. Aliment Pharmacol Ther. 1997;11:765–73. doi: 10.1046/j.1365-2036.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Pinxteren B, Sigterman KE, Bonis P, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD002095.pub4. [DOI] [PubMed] [Google Scholar]
- 35.Miwa H, Inoue K, Ashida K, et al. Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:323–32. doi: 10.1111/j.1365-2036.2010.04517.x. [DOI] [PubMed] [Google Scholar]
- 36.Savarino E, Tutuian R, Zentilin P, et al. Characteristics of Reflux Episodes and Symptom Association in Patients With Erosive Esophagitis and Nonerosive Reflux Disease: Study Using Combined Impedance-pH Off Therapy. Am J Gastroenterol. 2009 doi: 10.1038/ajg.2009.670. [DOI] [PubMed] [Google Scholar]
- 37.Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy. A multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006 doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostini R, Jackson C, Hegney D, et al. How Is Medication Prescribing Ceased? A Systematic Review Medical Care. 2011;49:24–36. doi: 10.1097/MLR.0b013e3181ef9a7e. [DOI] [PubMed] [Google Scholar]
- 39.American Gastroenterological Association. [Accessed April 6, 2012];Choosing Wisely: Five Things Physicians and Patients Should Question. 2012 Available at: http://www.choosingwisely.org/
- 40.Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772–778. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 41.Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–90. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 42.Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richter JE, Penagini R, Pohl D, et al. Barrett’s esophagus: proton pump inhibitors and chemoprevention II. Annals of the New York Academy of Sciences. 2011;1232:114–139. doi: 10.1111/j.1749-6632.2011.06048.x. [DOI] [PubMed] [Google Scholar]
- 44.FDA Drug Safety Communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs) 2012. Safety Announcement [02–08–2012] [Google Scholar]
- 45*.Nguyen TMT, Eslick GD. Systematic review: the treatment of noncardiac chest pain with antidepressants. Aliment Pharmacol Ther. 2012;35:493–500. doi: 10.1111/j.1365-2036.2011.04978.x. This paper provides the available, albeit limited data, on treating non-cardiac chest pain with anti-depressants. [DOI] [PubMed] [Google Scholar]
- 46*.Eherera J, Netolitzky F, Högenauer C, et al. Positive Effect of Abdominal Breathing Exercise on Gastroesophageal Reflux Disease: A Randomized, Controlled Study. Am J Gastroenterol. 2012;107:372–8. doi: 10.1038/ajg.2011.420. This paper provides data suggesting a non-pharmacologic approach may be beneficial in certain patients with GERD. [DOI] [PubMed] [Google Scholar]
- 47.Kiebles JL, Kwiatek MA, Pandolfino JE, et al. Do patients with globus sensation respond to hypnotically assisted relaxation therapy? A case series report. Dis Esophagus. 2010;23:545–553. doi: 10.1111/j.1442-2050.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 48.Richter JEE, Pandolfino JEE, Vela MFF, et al. Utilization of wireless pH monitoring technologies: a summary of the proceedings from the Esophageal Diagnostic Working Group. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01384.x. [DOI] [PubMed] [Google Scholar]